Abstract
Objectives
To describe the prevalence of adjuvants to opioid therapy and changes in these agents for pharmacologic management in nursing home residents with cancer.
Methods
We included Medicare beneficiaries with cancer and documented opioid use at nursing home admission in 2011–2013 (N=3,268). The Minimum Data Set 3.0 provided information on sociodemographic and clinical characteristics. Part D claims provided information on opioids and adjuvants use during 7 days after admission and 90 days later. Proportions of changes in these agents were estimated. Separate logistic models estimated for associations between resident characteristics and 1) use of adjuvants at admission; and 2) intensification of pharmacologic management at 90 days.
Results
Nearly 20% received adjuvants to opioids at admission. Among those receiving adjuvants, gabapentin was most common (34.4%). After 90 days, approximately25% had maintained or intensified pharmacologic management. While advanced age (≥85 versus 65–74, adjusted odds ratio (aOR): 0.80; 95% Confidence Interval (CI): 0.63 to 1.02) and comorbidities including dementia (aOR: 0.65; 95% CI: 0.53 to 0.82) and depression (aOR: 1.55; 95% CI: 1.29 to 1.87) were associated with adjuvants use at admission, worse cognitive impairment (severe versus no/mild, aOR: 0.80; 95% CI: 0.64 to 0.99) and presence of more severe pain (moderate/severe versus no pain, aOR: 1.60; 95% CI: 1.26 to 2.03) were associated with intensification of drug regimen.
Conclusion
Given aging-related changes and the presence of comorbid conditions in older adults, safety studies of these practices are warranted.
1. Introduction
In nursing homes, 8.8% of residents have a cancer diagnosis [1]. One third of Medicare beneficiaries with cancer spend time in a nursing home in the last 90 days of life [2]. Nursing home residents with cancer need effective strategies to manage and relieve the symptoms of pain and ensure dignity in care [3]. Pain is common in this setting and can be effectively managed in many patients using clinical guidelines [4–9].
Opioids are often used for treatment of cancer pain, but may increase the risk of falls/fractures, constipation, gastrointestinal bleeds, insomnia, and depression [10,11]. Regimens that include the use of adjuvants (e.g., gabapentinoids and other anticonvulsants, duloxetine and other antidepressants) attenuate pain perception and thus enhance analgesia, potentially reducing opioid use [12]. Yet, little is known about the extent to which adjuvants to analgesia are used in nursing home settings. Furthermore, the longitudinal descriptive studies regarding the regimens for adjuvants use in nursing homes are also lacking. Since nursing homes are of increasing importance as a care site for patients with cancer, understanding the extent to which these adjuvants are used to supplement opioid use in this setting is important. In addition, with the identification of the correlates and common regimens for pain management using adjuvants, we will then be able to evaluate the extent to which such approaches reduce potential untoward effects of medications (e.g., falls/fractures, gastrointestinal events) relative to opioid use without adjuvants.
Using a national database including all nursing home residents in the United States, this study sought to describe the prevalence of adjuvants analgesics to opioid use including antidepressants and/or anticonvulsants for pain management in persons who have cancer and who are living in nursing homes. A secondary objective of the study was to evaluate changes in pharmacologic management during the 90-days following nursing home admission among residents with cancer who were initially prescribed opioids shortly after admission.
2. Methods
2.1. Data sources
We linked three data sources including Minimum Data Set (MDS) version 3.0 (2011–2013), Master Beneficiary Summary Files (that determine Medicare enrollment), and Medicare Part D prescription drug transaction data. The MDS 3.0 is a standardized assessment tool required by the federal government for residents living in Medicare- and Medicaid-approved nursing homes. Assessments are conducted by registered nurses at admission, annually, and when a resident experiences a significant change in health status (e.g., enrollment into hospice). A subset of items is assessed quarterly [13]. The MDS 3.0 provides a systematic and comprehensive record that includes both sociodemographic and clinical information (e.g., functional status, behavior and medical conditions, treatment provided, and measures for signs and symptoms) [14–17]. The MDS is completed using information from medical records, direct resident interviews, and conversations with residents and/or their family members.
2.2. Study sample
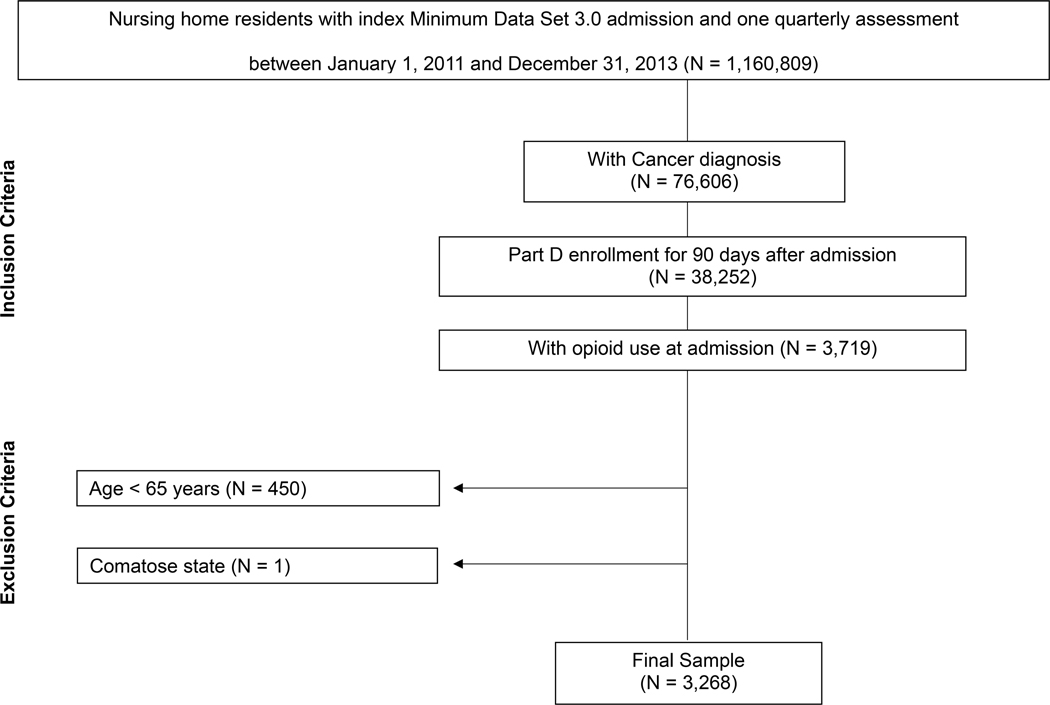
Figure 1 shows the development of the study sample. We first included nursing home residents with an admission assessment performed between January 1, 2011, and December 31, 2013 (N=1,160,809). For residents with multiple admission records, the first record was used. The inclusion criteria were: (1) active cancer diagnosis on MDS admission assessment; (2) continuous Part D enrollment for ≥ 90 days after admission; (3) ≥ one Part D claim for an opioid within 7 days of admission. We excluded residents who were < 65 years of age (N=450) or who were in a comatose state (N=1). The analytic sample included 3,268 residents.
Figure 1.
Flowchart of the study sample.
2.3. Opioid use
Medicare Part D prescription drug claims between January 1, 2011 to December 31, 2013 were used to identify residents with prescribed opioids within the nursing home. Medicare Part D claims included information such as the generic and brand names of the drug prescribed to the residents, product identification code using National Drug Code, date of service, days of drug supply, drug form, and drug strength. Opioid analgesics were categorized by the duration of action (i.e., long- versus short-acting) according to recent clinical guidelines [12,18,19]. Information on route of administration included oral, injected, transdermal, or other. Given that residents could have been prescribed different opioids concurrently, the total number of unique opioids prescribed during the 90-day follow up period was determined using the prescription fill dates and days’ supply during the 90-day period.
We calculated the average daily dose in oral morphine equivalents (OME) dispensed to each resident using the following formula: strength per unit x (number of units/day) x OME conversion factor = OME units per day [20]. For instance, for a resident prescribed oxycodone 40mg tablet taken orally in the morning and at night, the OMEs was calculated as 40mg x 2 (doses per day) x conversion factor (1.5) = 120mg OME per day.
2.4. Use of adjuvants
We adopted the National Comprehensive Cancer Network (NCCN) clinical guidelines for pharmacological management of neuropathic cancer pain in adults to identify agents for adjuvant analgesics [12]. In general, adjuvant drugs included categories such as anticonvulsants, antidepressants, and other agents such as corticosteroid and topical agents (e.g., lidocaine).
2.5. Evaluation of changes in medication use
We evaluated medication use at two time points: 1) at admission period (within 7 days of admission); and 2) 90 days after admission. We believe that changes in pharmacologic management during this period is important as medication use at admission likely reflects pain management practices in the community or the hospital setting, and that 90 days provides sufficient time for the nursing home clinicians to evaluate the residents and make appropriate adjustments to pain management regimens. For each time period, a 7-day window was used (time between admission date and admission date + 7 days was the admission period; time between admission date + 83 days and admission date + 90 days was the period of the 90 days after admission). During each time period, we determined the opioids prescribed, dose in OME, and adjuvants prescribed based on Medicare Part D prescription fill dates and days’ supply. We assumed that medications were used as prescribed and started on the prescription fill date. To avoid undercounting opioids prescribed during the second time period, we incorporated information from both claims during the 7-day window and claims preceding this window but having days’ supplies extending into the time period. We were unable to determine whether medications were used on a scheduled basis or as needed from claims.
For opioids, we calculated the differences between OMEs at these two time points and further created four categories of changes in opioid use: 1) intensified (changes in OMEs > 0); 2) no change (changes in OMEs = 0); 3) decrease (changes in OMEs < 0); and dropped (no new treatments or depleted days of supply were observed). For adjuvants to opioid therapy, we calculated the numbers and types of prescribed adjuvants during the two different time periods. We then created four categories to define changes in adjuvants use between these two time points: 1) Added (no adjuvants at admission but had ≥ one at follow-up); 2) no change, had adjuvants use at both admission and follow-up periods; 3) dropped (had at least one adjuvant at admission but no adjuvants during the follow-up), and 4) no use (no adjuvants at both admission and follow-up periods). We then created a binary variable (yes/no) to indicate the intensification of pharmacologic management which we operationalized as having either increase in OMEs in opioids or added adjuvants at 90 days.
2.6. Characteristics of nursing home residents
Information on resident characteristics were obtained from the MDS admission assessments. Sociodemographic characteristics included age (65–74, 75–84, ≥85 years), gender, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other). Clinical characteristics included physical function limitations, cognitive function impairments, comorbidities, and the presence of pain. Information on physical function limitations were extracted from items of MDS activity of daily living (ADL) short-form including personal hygiene, toilet use, locomotion on unit, and eating. Using these 4 items, we created a seven-category scale for the ADL Self-Performance Hierarchy (range: 0–6) and then categorized residents as no to mild limitation (0–2), moderate limitation (3–4), or severe limitation (5–6) [16,21]. Cognitive function impairments were measured using the Brief Interview of Mental Status (BIMS, range: 0–15) if residents could be interviewed [22]. For residents who were unable to be interviewed, information from the Cognitive Performance Scale (CPS, range: 0–6) was used [23]. We then categorized residents as no to mild impairment (BIMS 13–15 or CPS 0–2), moderate impairment (BIMS 8–12 or CPS 3–4), or severe impairment (BIMS 0–7 or CPS 5–6). Information about comorbidities associated with pain and/or adjuvants was obtained including fractures, surgical wounds, osteoporosis, and depression were evaluated.
2.7. Assessment of pain
The information of pain was obtained from the pain interview of the MDS assessment including both self-reported and staff assessed pain. If residents were able to self-report their pain, they were asked if they had pain in the last 5 days [15]. For those who responded yes, information on the frequency (rarely, occasionally, frequently, or constantly) and severity of the worst pain were collected. The severity of pain was assessed using the 4-level verbal descriptor scale (VDS, mild, moderate, severe, or very severe/horrible) or the numeric rating scale (NRS, range 0–10). Using information on the frequency and severity of the worst pain, we then classified residents’ self-reported pain into no pain, mild /infrequent, or moderate/severe pain [24,25]. For residents who could not self-report their pain, a pain assessment was conducted by the staff using medical records and direct observation. Pain indicators including crying, moaning, and grimaces as well as pain frequency in the previous 5 days were documented. We then grouped the staff-assessed pain into no pain, infrequent pain (1–2 days), or frequent pain (≥3 days) [26]. For the purpose of this analysis, we combined self-reported and staff-assessed pain into three categories: moderate/severe (self-reported moderate/severe pain or staff-assessed frequent pain), mild/infrequent (self-reported mild/infrequent pain or staff-assessed infrequent pain), or no pain (no self-reported pain or no staff-assessed pain) [15].
2.8. Statistical analysis
We first conducted descriptive statistics including median and interquartile range for continuous variables and percentages for categorical variables to characterize sociodemographic and clinical factors in nursing home residents with cancer by their use of opioid and adjuvants status during the admission period. The numbers and percentages for each types of adjuvants agents were used to summarize adjuvants use to opioids therapy in nursing home residents with cancer during the admission period. Lastly, proportions of residents with changes in opioid and/or adjuvants use over the study period were estimated to evaluate changes in pharmacological management. Logistic regression models were used to conduct exploratory analyses and estimate crude and adjusted odds ratio (aOR) with 95% confidence intervals (CIs) for resident characteristics and: 1) use of adjuvants at admission; and 2) intensification of drug regimen at 90 days. For the model building process for each outcome, we used an iterative, but not computer-driven, approach for developing the final model.
3. Results
Of the newly admitted nursing home residents with cancer, nearly20% had opioids use plus any adjuvants during the 7-day admission period. Characteristics of nursing home residents using opioids with and without adjuvants at admission are presented in Table 1. Most newly admitted nursing home residents with cancer receiving opioids were aged ≥ 75, women, non-Hispanic white, and had moderate to severe physical limitation and cognitive impairment. Approximately a third of residents had some chronic condition such as arthritis, and diabetes mellitus. Relative to residents with opioids used without adjuvants at admission, those with adjuvants use had a higher prevalence of depression (51.3% versus 40.4%). The distribution of presence of pain was similar between groups. The median daily OME was 53 mg/day among these nursing home residents who received opioids and adjuvants at admission and 45 mg/day among those receiving opioids without an adjuvant.
Table 1.
Characteristics of newly admitted nursing home residents by opioid therapy only and opioids plus adjuvants used at admission (N=3,268).
| Opioid therapy only (N = 2,712) | Opioids plus adjuvant therapy (N = 556) | |
|---|---|---|
| Age, years | Percentage | |
| 65–74 | 27.3 | 30.4 |
| 75–84 | 35.5 | 40.3 |
| ≥85 | 37.2 | 29.3 |
| Women | 65.9 | 68.4 |
| Race/ethnicity | ||
| Non-Hispanic white | 82.1 | 86.3 |
| Non-Hispanic black | 11.4 | 8.6 |
| Hispanic | 4.4 | 3.5 |
| Other | 2.1 | 1.7 |
| Physical function | ||
| No/mild limitation | 22.0 | 24.8 |
| Moderate limitation | 51.6 | 49.3 |
| Severe limitation | 26.4 | 25.9 |
| Cognitive function | ||
| No/mild impairment | 47.1 | 54.9 |
| Moderate impairment | 28.9 | 27.6 |
| Severe impairment | 23.9 | 17.5 |
| Comorbidities | ||
| Dementia | 31.8 | 23.4 |
| Arthritis | 34.2 | 34.8 |
| Osteoporosis | 16.7 | 17.6 |
| Hip fracture | 4.2 | 3.6 |
| Other fracture | 9.5 | 10.3 |
| Surgical wounds | 11.3 | 8.5 |
| Pressure ulcers | 17.7 | 15.7 |
| Diabetes mellitus | 33.2 | 37.1 |
| Parkinson disease | 3.5 | 3.4 |
| Anxiety disorder | 27.7 | 32.7 |
| Depression | 40.4 | 51.3 |
| Presence of pain | ||
| No pain | 29.1 | 26.7 |
| Mild/infrequent | 44.6 | 46.2 |
| Moderate/severe | 26.4 | 27.1 |
| Average daily dose of oral morphine equivalent dosage, mg/d (median, interquartile range) | 45 (24 – 51) | 53 (24 – 59) |
Table 2 displays the types of adjuvants agents used at admission among nursing home residents with cancer who received opioid therapy at admission. Overall, 14.2% of the analytic sample had more than one adjuvant during the admission period. Among residents with adjuvants used during the admission period, gabapentin was the most commonly prescribed agent (34.4%), followed by corticosteroids (29.3%), duloxetine (12.1%), and lidocaine (18.0%).
Table 2.
Types of adjuvant use at admission among nursing home residents with cancer (N=556).
| Use of adjuvant analgesic agents§ | No. (%) using the drug |
|---|---|
| Anticonvulsants | |
| Pregabalin | 38 (6.8) |
| Gabapentin | 191 (34.4) |
| Carbamazepine | 10 (1.8) |
| Lamotrigine | 8 (1.4) |
| Antidepressants | |
| Amitriptyline | 17 (3.1) |
| Nortriptyline | 3 (0.5) |
| Desipramine | 1 (0.2) |
| Imipramine | 6 (1.1) |
| Duloxetine | 67 (12.1) |
| Venlafaxine | 38 (6.8) |
| Corticosteroid¶ | 163 (29.3) |
| Topical agents: Lidocaine | 100 (18.0) |
Residents could have more than one adjuvant. Overall, 14.2% of residents with cancer who used opioids during the admission period had more than one adjuvant.
Systematic corticosteroids including prednisone, dexamethasone, betamethasone, hydrocortisone etc.
Characteristics associated with use of adjuvants during admission period are shown in Table 3. Advanced age was less likely to be associated with use of adjuvants (≥85 versus 65–74, aOR: 0.80; 95% CI: 0.63 to 1.02). Presence of pain was not one of the factors associated with adjuvants use, however, while comorbidities such as dementia (aOR: 0.65; 95% CI: 0.53 to 0.82) and surgical wound (aOR: 0.70; 95% CI: 0.50 to 0.97) were inversely associated with adjuvants use, depression (aOR: 1.55; 95% CI: 1.29 to 1.87) were positively correlated with adjuvants use during the admission period.
Table 3.
Characteristics* associated with adjuvant use at admission among newly admitted nursing home residents with cancer (N=3,268).
| Characteristic | Use of adjuvants (%) | Crude | Adjusted¶ |
|---|---|---|---|
| Odds ratio (95% Confidence Interval) | |||
| Age, years | |||
| 65–74 | 18.6 | Reference | Reference |
| 75–84 | 18.9 | 1.02 (0.82 to 1.27) | 1.08 (0.86 to 1.35) |
| ≥85 | 13.9 | 0.71 (0.56 to 0.90) | 0.80 (0.63 to 1.02) |
| Women | 17.5 | 1.12 (0.92 to 1.36) | 1.11 (0.91 to 1.35) |
| Physical function | |||
| No/mild limitation | 18.8 | Reference | Reference |
| Moderate limitation | 16.4 | 0.85 (0.68 to 1.06) | 0.90 (0.72 to 1.34) |
| Severe limitation | 16.7 | 0.87 (0.67 to 1.12) | 0.92 (0.71 to 1.19) |
| Dementia | 13.1 | 0.65 (0.53 to 0.81) | 0.65 (0.53 to 0.82) |
| Surgical wound | 13.4 | 0.73 (0.53 to 1.01) | 0.70 (0.50 to 0.97) |
| Depression | 20.7 | 1.55 (1.29 to 1.87) | 1.55 (1.29 to 1.87) |
| Presence of pain | |||
| No pain | 15.9 | Reference | Reference |
| Mild/infrequent | 17.6 | 1.13 (0.90 to 1.41) | 1.04 (0.83 to 1.30) |
| Moderate/severe | 17.4 | 1.12 (0.87 to 1.43) | 1.07 (0.83 to 1.38) |
Reference group for the outcome included residents who did not use adjuvants.
Odds ratios shown were adjusted for all resident characteristics displayed in the table.
Table 4 shows the proportion of residents with changes in opioids and/or adjuvants use from admission to 90 days after admission among nursing home residents with cancer. In terms of opioids, discontinuation was the most frequent change in therapy as defined by no new Part D claims for opioids observed and their days supply was depleted by 90 days. However, one in four residents either maintained or intensified the opioid therapy at 90 days after admission. For adjuvants use, while most residents did not use adjuvants overall, approximately17% of residents had either maintained or added use of adjuvants to opioid therapy at 90days after admission.
Table 4.
Proportion of residents with changes in opioid and/or adjuvant use from admission to 90 days after admission among nursing home residents with cancer (N=3,268).
| Opioid* therapy | Proportion (%) |
|---|---|
| Intensified | 14.9 |
| No change | 8.5 |
| Decrease | 13.0 |
| Dropped+ - no new treatments or depleted days of supply | 63.7 |
| Adjuvants¶ use | |
| Added | 10.8 |
| No change | 6.6 |
| Dropped+ | 10.5 |
| No use | 72.2 |
Opioid use was calculated and converted using morphine equivalents table.
Added: No adjuvants at admission but had ≥ 1 at the end of follow-up period
No change: Had adjuvants use during both periods
Dropped: Had at least one adjuvant at admission but no adjuvants during the follow-up period
No use: No adjuvants at both admission and follow-up periods.
Did not include information from pro re nata.
Table 5 shows the factors that are associated with intensification of pharmacologic management among residents receiving opioids at admission during the 90-day study period. While advanced age (≥85 versus 65–74, aOR: 0.82; 95% CI: 0.66 to 1.01) and worse cognitive impairment (severe versus no/mild, aOR: 0.80; 95% CI: 0.64 to 0.99) were less likely to be associated with intensification, residents with more severe pain were more likely to have intensification of drug regimen (moderate/severe versus no pain, aOR: 1.60; 95% CI: 1.26 to 2.03). Comorbidities such as anxiety (aOR: 1.20; 95% CI: 1.00 to 1.44) and diabetes (aOR: 1.17; 95% CI: 0.98 to 1.39) were associated with pharmacological intensification at 90-days after admission.
Table 5.
Factors* at admission period associated with pharmacologic pain management during the 90-day observation period among newly admitted nursing home residents with cancer (N=3,268).
| Characteristic | Pharmacologic intensification (%) | Crude | Adjusted¶ |
|---|---|---|---|
| Odds ratio (95% Confidence Interval) | |||
| Age, years | |||
| 65–74 | 27.3 | Reference | Reference |
| 75–84 | 22.4 | 0.77 (0.63 to 0.94) | 0.83 (0.67 to 1.02) |
| ≥85 | 21.0 | 0.71 (0.58 to 0.87) | 0.82 (0.66 to 1.01) |
| Women | 24.0 | 0.89 (0.75 to 1.06) | 1.12 (0.94 to 1.34) |
| Cognitive function | |||
| No/mild impairment | 26.4 | Reference | Reference |
| Moderate impairment | 21.0 | 0.74 (0.61 to 0.90) | 0.79 (0.65 to 0.97) |
| Severe impairment | 19.7 | 0.68 (0.55 to 0.84) | 0.80 (0.64 to 0.99) |
| Diabetes | 25.5 | 1.20 (1.02 to 1.43) | 1.17 (0.98 to 1.39) |
| Anxiety | 26.1 | 1.24 (1.04 to 1.48) | 1.20 (1.00 to 1.44) |
| Presence of pain | |||
| No pain | 16.0 | Reference | Reference |
| Mild/infrequent | 27.5 | 1.99 (1.61 to 2.45) | 1.85 (1.49 to 2.29) |
| Moderate/severe | 24.0 | 1.66 (1.31 to 2.10) | 1.60 (1.26 to 2.03) |
Reference group for the outcome included residents who did not have an increase in OMEs and added adjuvants.
Odds ratios shown were adjusted for all resident characteristics displayed in the table.
4. Discussion
This purpose of this study was to describe the prevalence of adjuvant use and the intensification of pharmacologic regimens for pain in nursing home residents with cancer. To our knowledge, we are the first to describe the changes in pharmacologic management for pain among newly admitted nursing home residents with cancer in the 90 days after admission. We found that nearly one in five newly admitted nursing home residents with cancer had not only opioids but also adjuvant use during the admission period. In addition, roughly25% of residents had intensification or no change in opioids use and nearly17% had adjuvant use at the end of the 90-day study period. We also observed several resident characteristics that appeared to be inversely associated with use of adjuvants at the admission period (e.g., advanced age and comorbidities including dementia) and overall pharmacologic intensification (e.g., advanced age, cognitive impairment). Pain severity at baseline was associated with intensification of pain medications.
In our study, we observed that gabapentin was one of the most commonly prescribed adjuvants in the US nursing home setting. While this is consistent with evidence regarding the use of gabapentin in patients with cancer pain among other settings [27–29], one study showed 70% of patients received gabapentin in an inpatient palliative unit [30]. Gabapentinoids are suggested to treat bone and visceral pain [31–33] given that cancer induced pain may involve neuropathic mechanisms [34]. Despite the evidence to support the use of adjuvants for pain management, these findings often come from studies of non-malignant pain and which have explicitly excluded frail patients with multiple comorbidities such as nursing home residents [3,35,36]. Evidence regarding analgesic effectiveness and adverse effects of gabapentin for patients with cancer pain in the nursing home setting are scarce. A systematic review that included patients with cancer-related neuropathy suggested that although the use of gabapentin provided some pain relief, adverse events were common [37]. Given the prevalent use of gabapentin in the nursing home setting, more studies to evaluate its analgesic effectiveness and adverse effects as an adjuvant to opioids among nursing home residents are needed.
Although nearly one in four residents had pharmacological intensification for opioids therapy over time, our study also found a substantial proportion of residents had discontinued opioids use 90 days after nursing home admission. Given concerns of opioid abuse and misuse in the general population [38,39], prescribers may be more cautious on using opioids to potentially reduce pain and improve function since the risks may outweigh the benefits [40]. In the nursing home setting, which is a more controlled care environment, the focus of using opioid medications is often the uncertainty about the safety [41]. Nevertheless, pain has been historically undertreated in nursing home residents with cancer [42]. Though potential improvements in pain management have been documented among residents with and without cancer [26,43], the risk and consequences of untreated and undertreated pain must carefully be considered in this treatment setting. Furthermore, these findings must be also cautiously evaluated given our limitations in ascertaining as-needed medication use, which may have been undercounted during follow-up due to limitations of teasing out whether medications were used on as needed or on a scheduled basis using claims data. In addition, the extent to which these medications were discontinued because the residents no longer required analgesia could not be identified with our administrative data analysis.
Prior studies examining cancer pain management at nursing home admission have consistently documented that advanced age and severe cognitive impairment are associated with undertreated or untreated pain [42,44]. Our study findings suggested that these characteristics were inversely associated with pharmacologic intensification despite that we have limited information to evaluate the quality of care (i.e., pain management) among nursing residents with longer stays. On the other hand, we also observed that residents with documented severe pain were more likely to receive pharmacologic intensification 90 days after admission. These data may suggest that nursing home residents are changing drug regimens to address the needs of residents with cancer. This was not observed in previous work [44]. However, longitudinal studies to evaluate how changes in treatment regimens may affect symptom relief or health-related outcomes are still needed in this setting.
Strengths and limitations of this present study are acknowledged. A national sample of Medicare beneficiaries living in nursing homes was used to evaluate adjuvants along with opioids use at admission and changes in these agents over time. Using assessments from MDS 3.0 linked to Medicare Part D claims, we were able to identify resident characteristics that were associated with adjuvant use at admission. The work herein can be used to inform future studies to evaluate whether the use of these agents relieves pain and whether or not they are associated with adverse drug events. Despite that the MDS 3.0 provides direct resident interviews and thus may offer more complete information on resident characteristics, there may be other factors related to use of these agents that were not readily available in these data. For instance, we were not able to disentangle whether patients who had intensified opioid therapy were due to advancing cancer. Furthermore, the severity of cancer and type of pain (e.g., musculoskeletal versus neuropathic pain) may be associated with the pain experienced due to cancer. Despite that the focus of this present study is cancer pain, the adjuvants may be used for multiple reasons especially diabetic neuropathy which many residents in this study sample had the condition. However, we were not able to examine whether the use of these adjuvants were indeed for cancer pain. In addition, the agents for adjuvant analgesics adopted from NCCS guidelines were for neuropathic pain only and thus we may underestimate the use of adjuvants [12]. However, we found that the percentages of other adjuvants use in our included sample were small (data not shown). Although Medicare Part D claims were used, residents may have had access to drugs outside of observation window. For instance, residents may give the as-needed medication use or have over the counter use for adjuvants [45]. As such, there is a potential for misclassification of drug use. However, we expect that this information bias would be non-differential which would have diluted the observed associations. Prescribing patterns for adjuvants use might have changed since the time that the data were collected, particularly after the most recent NCCN guidelines were issued.
In conclusion, we found that nearly one in five nursing home residents with cancer and prescribed opioids at admission used adjuvants. In addition, despite that the majority of residents did not use any adjuvants and had dropped opioids use over time, the changes in pharmacological management of these agents were not uncommon. We also identified several resident characteristics that were associated with pharmacological intensification of either increasing OMEs in opioids or adding adjuvants during the 90-day study period. Despite these findings, empirical evidence quantifying the effectiveness of such pain management strategies in nursing home settings are lacking. Given that nursing home residents are mostly old, frail, and with multiple comorbidities, understanding the beneficial and/or adverse effects among these commonly administered drugs on symptoms management are critical and remain to be established.
Key Points.
Of the newly admitted nursing home residents with cancer, nearly20% used adjuvants to opioid therapy within the week following admission. Gabapentin was the most commonly prescribed adjuvant, but the use of corticosteroids and lidocaine were also common.
Advanced age and dementia were inversely associated with the use of adjuvants to opioid therapy at admission and inversely associated with maintenance of pharmacologic management at 90 days.
Acknowledgments
Funding: This study was funded by the National Cancer Institute (Grant number: R21CA198172-02 awarded to Dr. Kate Lapane). The funder had no roles in design, methods, data collection, analysis, or preparation of this manuscript.
Footnotes
Conflict of interests: All authors declare that they have no conflicts of interest directly relevant to the content of this study.
Compliance with Ethical Standards: This study used routinely-collected administrative and claims data and was approved by the University of Massachusetts Medical School Institutional Review Board.
5 References
- 1.Johnson VMP, Teno JM, Bourbonniere M, Mor V. Palliative Care Needs of Cancer Patients in U.S. Nursing Homes. J. Palliat. Med. 2005;8:273–279. [DOI] [PubMed] [Google Scholar]
- 2.Teno M, Gozalo L, Bynum PW, Leland E, Miller C, Morden E, et al. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA J. Am. Med. Assoc. 2013;309:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Allcock N. Pain in Older People: Reflections and experiences from an older person’s perspective. London: British Pain Society, Help the Aged, 2008. [Google Scholar]
- 4.van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:12–20. [DOI] [PubMed] [Google Scholar]
- 5.Lanser P, Gesell S. Pain management: the fifth vital sign. Healthc. Benchmarks. 2001;8:62,68–70. [PubMed] [Google Scholar]
- 6.Levy MH. Pharmacologic treatment of cancer pain. N. Engl. J. Med. 1996;335:1124–32. [DOI] [PubMed] [Google Scholar]
- 7.Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63:65–76. [DOI] [PubMed] [Google Scholar]
- 8.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J. Am. Geriatr. Soc. 2009;57:1331–46. [DOI] [PubMed] [Google Scholar]
- 9.Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the Management of Chronic Severe Pain in the Elderly: Consensus Statement of an International Expert Panel with Focus on the Six Clinically Most Often Used World Health Organization step III Opioids (Buprenorphine, Fentanyl, Hydromorphone, Met. Pain Pract. Blackwell Publishing Inc; 2008;8:287–313. [DOI] [PubMed] [Google Scholar]
- 10.Dowell D, TM H, Chou R. Cdc guideline for prescribing opioids for chronic pain—united states, 2016. JAMA. 2016;315:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krashin D, Murinova N, Jumelle P, Ballantyne J. Opioid risk assessment in palliative medicine. Expert Opin. Drug Saf. 2015;14:1023–33. [DOI] [PubMed] [Google Scholar]
- 12.Oncology NCPG in. NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain [Internet]. [cited 2018 Apr 10]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx
- 13.U.S. Centers for Medicare and Medicaid Services. MDS 3.0 RAI Manual. 2017. [cited 2017 Jul 12]; Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual.html
- 14.Saliba D, Buchanan J. Making the Investment Count: Revision of the Minimum Data Set for Nursing Homes, MDS 3.0. J. Am. Med. Dir. Assoc. 2012;13:602–10. [DOI] [PubMed] [Google Scholar]
- 15.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of Significant Changes in the Minimum Data Set for Nursing Homes Version 3.0. J. Am. Med. Dir. Assoc. 2012;13:595–601. [DOI] [PubMed] [Google Scholar]
- 16.Morris JN, Fries BE, Morris SA. Scaling ADLs Within the MDS. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 1999;54:M546–53. [DOI] [PubMed] [Google Scholar]
- 17.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med. Care. 2017;55:e68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Practice guidelines for cancer pain management: A report by the American Society of Anesthesiologists Task Force on Pain Management, cancer pain section. Anesthesiology. 1996;84:1243–57. [PubMed] [Google Scholar]
- 19.Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F. Management of cancer pain: ESMO clinical practice guidelines. Ann. Oncol. 2012. October;23 Suppl 7:vii139–54. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol. Drug Saf. 2016;25:733–7. [DOI] [PubMed] [Google Scholar]
- 21.Wysocki A, Thomas KS, Mor V. Functional Improvement Among Short-Stay Nursing Home Residents in the MDS 3.0. J. Am. Med. Dir. Assoc. 2015;16:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saliba D, Buchanan J, Edelen MO, Streim J, Ouslander J, Berlowitz D, et al. MDS 3.0: Brief Interview for Mental Status. J. Am. Med. Dir. Assoc. 2012;13:611–7. [DOI] [PubMed] [Google Scholar]
- 23.Morris JN, Fries BE, Mehr DR, Hawes C, Phillips C, Mor V, et al. MDS Cognitive Performance Scale. J. Gerontol. 1994;49:M174–82. [DOI] [PubMed] [Google Scholar]
- 24.Edelen MO, Saliba D. Correspondence of verbal descriptor and numeric rating scales for pain intensity: An item response theory calibration. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2010;65 A:778–85. [DOI] [PubMed] [Google Scholar]
- 25.Center for Medicare & Medicaid services. Nursing Home Data Compendium 2015. Edition. 2015; Available from: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf
- 26.Hunnicutt JN, Tjia J, Lapane KL. Hospice Use and Pain Management in Elderly Nursing Home Residents With Cancer. J. Pain Symptom Manage. 2017;53:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitera G, Zeiadin N, Kirou-Mauro A, DeAngelis C, Wong J, Sanjeevan T, et al. Retrospective Assessment of Cancer Pain Management in an Outpatient Palliative Radiotherapy Clinic Using the Pain Management Index. J. Pain Symptom Manage. 2010;39:259–67. [DOI] [PubMed] [Google Scholar]
- 28.K C B, Binti Mohd Yusoff Z, Alrasheedy AA, Othman S. The Characteristics and the Pharmacological Management of Cancer Pain and Its Effect on the Patients’ Daily Activities and their Quality of Life: A Cross – Sectional study from Malaysia. J. Clin. Diagn. Res. [Internet]. Delhi, India: JCDR Research and Publications (P) Limited; 2013;7:1408–13. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3749647/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra S, Bhatnagar S, Gupta D, Goyal GN, Jain R, Chauhan H. Management of Neuropathic Cancer Pain Following WHO Analgesic Ladder: A Prospective Study. Am. J. Hosp. Palliat. Med. [Internet]. 2009;25:447–51. Available from: 10.1177/1049909108322288 [DOI] [PubMed] [Google Scholar]
- 30.Shinde S, Gordon P, Sharma P, Gross J, Davis MP. Use of non-opioid analgesics as adjuvants to opioid analgesia for cancer pain management in an inpatient palliative unit: does this improve pain control and reduce opioid requirements? Support. Care Cancer. 2014;23:695–703. [DOI] [PubMed] [Google Scholar]
- 31.Caraceni A, Zecca E, Martini C, Pigni A, Bracchi P. Gabapentin for breakthrough pain due to bone metastases. Palliat. Med. 2008. p. 392–3. [DOI] [PubMed] [Google Scholar]
- 32.Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–40. [DOI] [PubMed] [Google Scholar]
- 33.Meymandi MS, Keyhanfar F. Assessment of the antinociceptive effects of pregabalin alone or in combination with morphine during acetic acid-induced writhing in mice. Pharmacol. Biochem. Behav. 2013;110:249–54. [DOI] [PubMed] [Google Scholar]
- 34.Honoré P, Schwei MJ, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, et al. Cellular and neurochemical remodeling of the spinal cord in bone cancer pain. Prog. Brain Res. 2000;129:389–97. [DOI] [PubMed] [Google Scholar]
- 35.Kurita GP, Tange UB, Farholt H, Sonne NM, Strömgren AS, Ankersen L, et al. Pain characteristics and management of inpatients admitted to a comprehensive cancer centre: A cross-sectional study. Acta Anaesthesiol. Scand. 2013;57:518–25. [DOI] [PubMed] [Google Scholar]
- 36.Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat. Med. 2011;25:553–9. [DOI] [PubMed] [Google Scholar]
- 37.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice ASC. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane database Syst. Rev. 2014;4:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okie S A Flood of Opioids, a Rising Tide of Deaths. N. Engl. J. Med. [Internet]. 2010;363:1981–5. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMp1011512 [DOI] [PubMed] [Google Scholar]
- 39.Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R. Increases in Drug and Opioid Overdose Deaths - United States, 2000–2014. Am. J. Transplant. 2016;16:1323–7. [DOI] [PubMed] [Google Scholar]
- 40.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR. Recomm. Reports. 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 41.Jennings AA, Linehan M, Foley T. The knowledge and attitudes of general practitioners to the assessment and management of pain in people with dementia. BMC Fam. Pract. 2018;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pimentel CBC, Briesacher BAB, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen X, Zuckerman IH, Palmer JB, Stuart B. Trends in prevalence for moderate-to-severe pain and persistent pain among medicare beneficiaries in nursing homes, 2006–2009. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2015;70:598–603. [DOI] [PubMed] [Google Scholar]
- 44.Monroe TB, Carter MA, Feldt KS, Dietrich MS, Cowan RL. Pain and hospice care in nursing home residents with dementia and terminal cancer. Geriatr. Gerontol. Int. 2013;13:1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veitonmäki T, Murtola TJ, Talala K, Taari K, Tammela T, Auvinen A. Non-steroidal anti-inflammatory drugs and cancer death in the finnish prostate cancer screening trial. PLoS One. 2016. April 21;11(4):e0153413. [DOI] [PMC free article] [PubMed] [Google Scholar]