Abstract
Idiopathic pulmonary fibrosis is a relentless fibrotic disease with largely unknown etiologies. Currently, the crosstalk between alveolar epithelial cells and lung-resident mesenchymal cells (especially [myo]fibroblasts) is considered to be the central pathogenesis to initiate and propagate the fibrotic process. Unfortunately, the master switch hidden in the profibrotic milieu that mediates pathogenic epithelial-mesenchymal interactions is still not well elucidated. Thus, the definite treatment target that can block and cure idiopathic pulmonary fibrosis is now lacking. Based on the previous studies, we proposed the notion that epithelium-derived triple type 2 cytokines, i.e. interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) are important pro-fibrotic mediators in idiopathic pulmonary fibrosis via two possible mechanisms: (1) paracrine pathway: directly acting on (myo)fibroblast. There may exist a structural and functional axis of (IL-25/IL-33/TSLP)+alveolar epithelial cells-(IL-25R/IL-33R/TSLPR)+ (myo)fibroblasts in fibroblastic foci of idiopathic pulmonary fibrosis patients. The crosstalk between alveolar epithelium and the adjacent mesenchymal compartment is well established by the binding of IL-25/IL-33/TSLP expressed on alveolar epithelial cells with their corresponding receptors (i.e. IL-17BR/sT2L/TSLPR) expressed on (myo)fibroblasts; (2) autocrine pathway: directly acting on alveolar epithelial cells. Alveolar epithelial cells may act as both cellular sources and targets of IL-25/IL-33/TSLP. Autocrine IL-25/IL-33/TSLP causes salient injury and phenotypic changes of alveolar epithelial cells. Thus, epithelium-derived IL-25/IL-33/TSLP may be the novel promising treatment target for the cure of idiopathic pulmonary fibrosis.
Impact statement
We suggest a novel modality in terms of IL-25/IL-33/TSLP’s pro-fibrotic role in IPF. First, IL-25/IL-33/TSLP fully activates (myo)fibroblasts in fibroblastic foci (FF) in a paracrine-dependent manner. (IL-25/IL-33/TSLP)+alveolar epithelial cells-(IL-25R/IL-33R/TSLPR)+ (myo)fibroblasts axis may contribute greatly to the abnormal epithelial-mesenchymal crosstalk and lung fibrosis. Second, IL-25/IL-33/TSLP causes significant injury and phenotypic changes of alveolar epithelial cells in an autocrine-dependent manner. By acting directly on the two most important cells in the fibrotic process, i.e. alveolar epithelial cells and (myo)fibroblasts, we support the notion that biological therapies targeting IL-25/IL-33/TSLP will shed new light on the cure of IPF patients.
Keywords: Epithelial-mesenchymal crosstalk, fibroblastic foci, idiopathic pulmonary fibrosis, IL-25/IL-33/TSLP
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease with unknown etiology and dismal prognosis.1 Since the late 1960s that Liebow and Carrington first defined IPF, there is no available pharmacological treatment for IPF patients for the longest time.2 The advent of “two drugs era,” i.e. pirfenidone and nintedanib, has brought us brand new promise in slowing disease progression of IPF. Thus, these two drugs were recommended for the treatment of IPF patients in the 2015 ATS/ERS/JRS/ALAT guidelines.2–4 However, the definite cure pharmacology for IPF is still lacking. The novel insights gained from the underlying pathophysiology of IPF offer us new hopes for the promising intervention targets and more efficacious therapies.2,5 In this review, we hypothesize that the epithelium-derived triple cytokines, i.e. interleukin(IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) are the master switch hidden in the fibrotic milieu by mediating alveolar epithelial cells (AECs) injury and the abnormal epithelial-mesenchymal interaction. Targeted interventions on IL-25/IL-33/TSLP in further studies will show great promise for preventing or halting the continuous progressive nature of IPF.
What are IL-25, IL-33, and TSLP?
IL-25 (i.e. IL-17E), IL-33, and TSLP are pluripotent cytokines that belong to IL-17, IL-1, and IL-2 cytokine family, respectively. Multiple cells are the potential cellular sources of these cytokines, including AECs. (Myo)fibroblasts, as well as other cell types respond to IL-25, IL-33, and TSLP by expressing receptor complex consisting of IL-17-receptor A and B (IL-17RA/IL-17RB), receptor complex composed of IL-1RL1 (i.e. ST2L) and IL-1RAcP, and receptor complex that consists of TSLPR and an IL-7Rα subunit, respectively. Despite from different cytokine families, IL-25, IL-33, and TSLP are allocated together because they share significant similarities in the biological functions. Specifically, they all regulate a broad spectrum of the innate and adaptive immune response and drive pro-fibrotic type 2 cytokine reactions in a highly milieu-dependent manner. The detailed introduction on the biological functions of these triple cytokines is out of the focus of the review and can be founded elsewhere.6–10
IL-25, IL-33, and TSLP in allergic airway diseases
Allergic airway diseases (AADs) are an array of type 2 immunity-mediated disorders characterized by chronic airway inflammation and remodeling, including allergic asthma, allergic rhinitis (AR), and chronic rhinosinusitis (CRS). First, studies show that the expression level of serum and/or tissue specific IL-25, IL-33, and TSLP is increased in peripheral blood, bronchial mucosal epithelium, nasal polyp epithelium or nasal epithelial cells in patients with asthma, CRS, and AR, respectively. Second, the expression level of IL-25, IL-33, and TSLP may reflect disease severity of patients with AADs as indicated by their significant correlation with FEV1 (forced expiratory volume in 1 second) % predicted, Sinus CT score, and upper airway inflammation/remodeling biomarkers. Third, murine models show that over-expression and/or exogenous administration of IL-25, IL-33, and TSLP mimics features of human AADs, including type 2 immunity response, nasal/bronchial eosinophilic inflammation, and sub-epithelial fibrosis. By contrast, blockade of IL-25, IL-33, and TSLP reduces T-helper type 2 cytokine production and nasal/bronchial inflammation/remodeling in allergen-induced murine models of AADs. Collectively, current studies support the notion that epithelium-derived triple cytokines (IL-25/IL-33/TSLP) significantly contribute to allergic nasal/airway inflammation and remodeling. Important original studies and reviews about the role of IL-25/IL-33/TSLP in AADs are listed here.11–18
IL-25, IL-33, and TSLP in IPF
In our opinion, IPF is a non-allergen driven, type 2 immunity-high disease characterized by immunologic injury of AECs and abnormal remodeling of lung parenchyma. AECs can respond to a variety of pathogen-associated molecular patterns (PAMPs) by their constitutively expressed toll-like receptors (TLRs). When AECs are activated, they can produce various mediators that act on different kinds of potential-targeted cells in the surrounding environment in a paracrine-dependent fashion. However, AECs-derived pro-fibrotic cytokine milieu is largely uncovered.
Hams et al.19 firstly reported that bronchoalveolar lavage fluid (BALF) level of IL-25 was significantly increased in IPF patients. Also, they found that the group 2 innate lymphoid cells (ILC2s) may mediate lung fibrosis induced by intra-nasally instillation of IL-25. Tajima et al.20 showed that IPF patients with acute exacerbation had higher serum level of ST2 (sST2), which was negatively correlated with the percentage of predicted vital capacity (VC% pred.). They also found that bleomycin (BLM) significantly augmented soluble ST2 mRNA expression.21 Similarly, Luzina et al.22 found that human (h)IL-33 was much higher in the lungs of IPF patients. Intratracheally instillation with IL-33 induced remarkable collagen deposition, and exhibited a synergistic effect on extracellular matrix (ECM) production induced by BLM.23 Li et al.24 also showed that BLM-induced lung fibrosis was significantly reduced in ST2L−/− mice or by using anti-IL-33 antibody. They first showed that lung M2-macrophages (CD11b+F4/80+CD206+) and lineage−ICOS[inducible costimulator]−ST2L+ILC2 may mediate IL-33’s pro-fibrotic effects. Datta et al.25 showed that TSLP/TSLPR axis was remarkably upregulated in patients with IPF, especially in AECs and myofibroblasts. Taken together, the representative studies listed here highlight the potential pro-fibrotic role and mechanism of IL-25, IL-33, and TSLP in IPF.
However, these studies failed to uncover whether IL-25, IL-33, and TSLP contribute to the pathogenesis of IPF by modulating the phenotypic changes of AECs and (myo)fibroblasts, two of the most important target cells for IPF. It is well known that fibroblastic foci (FF) and honeycomb cysts are the most remarkable histopathological feature in IPF patients.26–28 FF are the interstitial aggregates of fully activated fibroblasts and/or myofibroblasts embedded into a basophilic myxoid stroma. (Myo)fibroblasts in FF are typically arranged parallel to the alveolar epithelium surface. The epithelial layer often exhibits a cuboidal appearance, indicating the hyperplasia of type 2 AECs. Spatial proximity of AECs and (myo)fibroblasts within FF indicates there must be a close functional connection between them.2,29 The dysfunction of AECs is involved in the initiation of the fibrotic process, while abnormal AECs-(myo)fibroblasts crosstalk and the activation of (myo)fibroblasts are involved in the maintenance and progression of lung fibrosis.2,5,29 The “young” active fibroblastic areas in lung are initiated by FF. Progressive development of FF will eventually lead to the marked distortion of alveolar structure and the formation of microscopic honeycombing characterized by clusters of small cysts. Honeycomb cysts are typically covered by phenotypic abnormal, columnar-shaped type 2 AECs (bronchiolization of alveolar septa).30,31 It has been proposed that bronchiolization of enlarged alveolar ducts, cysts, and alveoli maybe the result of aberrant autonomous remodeling of AECs in response to chronic/repetitive micro-injury.30,31
In a recent study, we try to test whether IL-25, as the potential AECs-derived cytokine, can promote lung fibrosis by mediating AECs injury/phenotypic changes and AECs-(myo)fibroblasts interaction. We first found that compared with normal human subjects, IL-25 was over-expressed by phenotypic abnormal AECs that cover the surface of FF in IPF patients.32 On the other hand, IL-25 receptor (IL-17RB) was found to be highly expressed in (myo)fibroblasts underneath AECs in FF.32 This indicates that there may exist a structural and functional axis of IL-25+AECs-IL-25R(IL-17BR)+(myo)fibroblasts in FF. This was initially confirmed by our in vitro cell study which showed that administration of exogenous IL-25 can significantly facilitate the proliferation, differentiation, and activation of human lung fibroblasts.32 Collectively, we suggest that IL-25 has the potential to fully activate (myo)fibroblasts in fibroblastic foci (FF) in a paracrine-dependent manner.
Interestingly, we found that IL-17RB staining was also prominent in AECs lining the surface of FF, indicating that AECs are both the potential cellular sources and targets of IL-25. We also showed that nasal instillation of IL-25 significantly changed the phenotype of murine AECs. Specifically, the immunoreactivity for collagen I/III, fibronectin, and connective tissue growth factor (CTGF) was evident in injured AECs after intranasal instillation of IL-25. Thus, AECs-derived IL-25 may have the potential in promoting the injury and pro-fibrotic phenotypic changes of AECs in an autocrine-dependent manner. This may eventually lead to the formation of bronchiolized alveolar septa.
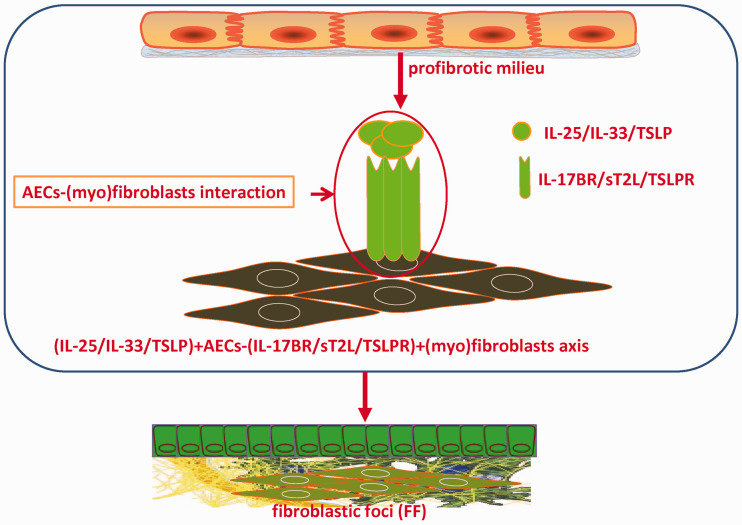
Herein, based on the redundancy of cytokine’s action, we proposed a novel modality that epithelium-derived IL-25, IL-33, and TSLP are important pro-fibrotic mediators in IPF via two possible mechanisms: (1) directly acting on (myo)fibroblast. Damaged AECs express and secrete high level of IL-25/IL-33/TSLP, and act on the adjacent (myo)fibroblasts within FF by binding with their corresponding receptors (i.e. IL-17RB/sT2L/TSLPR). There may exist a structural and functional axis of (IL-25/IL-33/TSLP)+AECs-(IL-25R/IL-33R/TSLPR)+ (myo)fibroblasts in FF of IPF patients (Figure 1); (2) directly acting on AECs. AECs may act as both cellular sources and targets of IL-25/IL-33/TSLP. Autocrine-IL-25/IL-33/TSLP causes salient injury and phenotypic changes of AECs in favor of bronchiolar-like and more pro-fibrotic pattern.
Figure 1.
IL-25/IL-33/TSLP are the key mediators involving abnormal AECs-(myo)fibroblasts interaction. Impaired AECs express and secrete abundant IL-25/IL-33/TSLP, which directly act on and activate the adjacent interstitial (myo)fibroblasts by binding their corresponding receptors, i.e. IL-17BR/sT2L/TSLPR. Thus, the structural and functional axis of (IL-25/IL-33/TSLP)+AECs-(IL-25R/IL-33R/TSLPR)+ (myo)fibroblasts within fibroblastic foci (FF) is developed. Ultimately, IL-25/IL-33/TSLP promotes the constant formation of FF and progressive pulmonary fibrosis by modulating AECs- (myo)fibroblasts crosstalk. Intervention targeting IL-25/IL-33/TSLP may have the great promise to block the abnormal epithelial-mesenchymal communication and the progression of pulmonary fibrosis. (A color version of this figure is available in the online journal.)
Future directions
In this review, we propose a novel paradigm that epithelium-derived IL-25/IL-33/TSLP may be the master switch for IPF by mediating AECs injury and the abnormal AECs-(myo)fibroblasts interactions in FF. However, a lot of further work should be done to verify this hypothesis. First, double immunohistochemistry assay should be conducted on lung sections from large IPF cohort to further confirm that AECs express high level of IL-25, IL-33, and TSLP, while (myo)fibroblasts adjacent to AECs express the receptors of IL-25/IL-33/TSLP, i.e. IL-17BR, sT2L, and TSLPR. This will confirm the actual existence of the basic structural and functional unit mediated by (IL-25/IL-33/TSLP)+AECs-(IL-25R/IL-33R/TSLPR)+ (myo)fibroblasts axis in FF. Second, BLM-induced mice lung fibrosis models (or other lung fibrosis murine models) should be used to further certificate whether IL-25/IL-33/TSLP promotes lung fibrosis via mediating AECs injury and AECs-(myo)fibroblasts interaction. Third, in vitro cell study should be used to confirm the following issues: (1) whether AECs can express and secrete excessive IL-25/IL-33/TSLP following different kinds of damage (e.g. BLM, smoke and pathogenic microorganism); (2) whether AECs respond to exogenous administration of IL-25/IL-33/TSLP; (3) whether injured AECs can directly activate the nearby fibroblasts by paracrine secretion of abundant IL-25/IL-33/TSLP.
Conclusions
In this review, we suggest a novel modality in terms of IL-25/IL-33/TSLP’s pro-fibrotic role in IPF. Specifically, IL-25/IL-33/TSLP contributes to lung fibrosis by acting directly on the two most important cells in the fibrotic process, i.e. AECs and (myo)fibroblasts. We support the notion that biological therapies targeting IL-25/IL-33/TSLP will shed new light on the definite cure of IPF patients.
Authors’ contributions
DH conceived the hypothesis, XX drafted the manuscript, ZJ revised the article, and all authors contributed to the final approval for the version to be published.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the General Program of National Nature Science Foundation of China (No.81870056), CAMS Innovation Fund for Medical Sciences (CIFMS, No. 2018–12 M-1–001).
ORCID iD
Xuefeng Xu https://orcid.org/0000-0002-1749-6765
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018; 378:1811–23 [DOI] [PubMed] [Google Scholar]
- 2.Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol 2018; 71-72:112–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richeldi L, Baldi F, Pasciuto G, Macagno F, Panico L. Current and future idiopathic pulmonary fibrosis therapy. Am J Med Sci 2019; 357:370–3 [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schunemann HJ; American Thoracic S, European Respiratory s, Japanese Respiratory S, Latin American Thoracic A. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192:e3–19 [DOI] [PubMed] [Google Scholar]
- 5.Winters NI, Burman A, Kropski JA, Blackwell TS. Epithelial injury and dysfunction in the pathogenesis of idiopathic pulmonary fibrosis. Am J Med Sci 2019; 357:374–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell PD, O’Byrne PM. Epithelial-derived cytokines in asthma. Chest 2017; 151:1338–44 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PD, O’Byrne PM. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol Ther 2017; 169:104–12 [DOI] [PubMed] [Google Scholar]
- 8.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol 2015; 15:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest 2019; 129:1441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015; 43:29–40 [DOI] [PubMed] [Google Scholar]
- 11.Yao XJ, Liu XF, Wang XD. Potential role of interleukin-25/interleukin-33/thymic stromal lymphopoietin-fibrocyte axis in the pathogenesis of allergic airway diseases. Chin Med J 2018; 131:1983–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, Kim DW. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2015; 135:1476–85 [DOI] [PubMed] [Google Scholar]
- 13.Liao B, Cao PP, Zeng M, Zhen Z, Wang H, Zhang YN, Hu CY, Ma J, Li ZY, Song J, Liu JX, Peng LY, Liu Y, Ning Q, Liu Z. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy 2015; 70:1169–80 [DOI] [PubMed] [Google Scholar]
- 14.Akasaki S, Matsushita K, Kato Y, Fukuoka A, Iwasaki N, Nakahira M, Fujieda S, Yasuda K, Yoshimoto T. Murine allergic rhinitis and nasal Th2 activation are mediated via TSLP- and IL-33-signaling pathways. Int Immunol 2016; 28:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata Y, Maruoka S, Gon Y, Mizumura K, Kishi H, Nomura Y, Hikichi M, Hashimoto S, Oshima T. Expression of IL-25, IL-33, and thymic stromal lymphopoietin in nasal polyp gland duct epithelium in patients with chronic rhinosinusitis. Am J Rhinol Allergy 2019; 33:378–87 [DOI] [PubMed] [Google Scholar]
- 16.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, Lv Z, An Y, Wang YH, Liu YJ, Kay AB, Lee TH, Ying S. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol 2011; 128:116–24 [DOI] [PubMed] [Google Scholar]
- 17.Yao X, Sun Y, Wang W, Sun Y. Interleukin (IL)-25: pleiotropic roles in asthma. Respirology 2016; 21:638–47 [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Li Y, Lv Z, Chen Y, Li Y, Huang K, Corrigan CJ, Ying S. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol 2018; 201:2221–31 [DOI] [PubMed] [Google Scholar]
- 19.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, Voehringer D, McKenzie AN, Donnelly SC, Fallon PG. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A 2014; 111:367–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest 2003; 124:1206–14 [DOI] [PubMed] [Google Scholar]
- 21.Tajima S, Bando M, Ohno S, Sugiyama Y, Oshikawa K, Tominaga S, Itoh K, Takada T, Suzuki E, Gejyo F. ST2 gene induced by type 2 helper T cell (Th2) and proinflammatory cytokine stimuli may modulate lung injury and fibrosis. Exp Lung Res 2007; 33:81–97 [DOI] [PubMed] [Google Scholar]
- 22.Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, Hasday JD, Todd NW, Atamas SP. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol 2013; 49:999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, Papadimitriou JC, McKenzie AN, Atamas SP. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol 2012; 189:403–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY, McSharry C, Xu D. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol 2014; 134:1422–32 e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datta A, Alexander R, Sulikowski MG, Nicholson AG, Maher TM, Scotton CJ, Chambers RC. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J Immunol 2013; 191:4867–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, Vancheri C. Idiopathic pulmonary fibrosis: an update. Ann Med 2015; 47:15–27 [DOI] [PubMed] [Google Scholar]
- 27.Noble PW, Homer RJ. Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2005; 33:113–20 [DOI] [PubMed] [Google Scholar]
- 28.Spagnolo P, Rossi G, Cavazza A. Pathogenesis of idiopathic pulmonary fibrosis and its clinical implications. Expert Rev Clin Immunol 2014; 10:1005–17 [DOI] [PubMed] [Google Scholar]
- 29.Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta 2013; 1832:911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, Whitsett JA. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax 2011; 66:651–7 [DOI] [PubMed] [Google Scholar]
- 31.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, Schwartz DA. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 2016; 96:1567–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Luo S, Li B, Dai H, Zhang J. Feature article: IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp Biol Med 2019; 244:770–80 [DOI] [PMC free article] [PubMed] [Google Scholar]