Abstract
Kidney dysfunction, including chronic kidney disease and acute kidney injury, is a globally prevalent health problem. However, treatment regimens are still lacking, especially for conditions involving kidney fibrosis. Stem cells hold great promise in the treatment of chronic kidney disease and acute kidney injury, but success has been hampered by insufficient incorporation of the stem cells in the injured kidney. Thus, new approaches for the restoration of kidney function after acute or chronic injury have been explored. Recently, kidney organoids have emerged as a useful tool in the treatment of kidney diseases. In this review, we discuss the mechanisms and approaches of cell therapy in acute kidney injury and chronic kidney disease, including diabetic kidney disease and lupus nephritis. We also summarize the potential applications of kidney organoids in the treatment of kidney diseases.
Impact statement
Stem cells hold great promise in regenerative medicine. Pluripotent stem cells have been differentiated into kidney organoids to understand human kidney development and to dissect renal disease mechanisms. Meanwhile, recent studies have explored the treatment of kidney diseases using a variety of cells, including mesenchymal stem cells and renal derivatives. This mini-review discusses the diverse mechanisms underlying current renal disease treatment via stem cell therapy. We postulate that clinical applications of stem cell therapy for kidney diseases can be readily achieved in the near future.
Keywords: Cytokine therapy, chronic kidney disease, acute kidney injury, mechanism, kidney organoids
Introduction
Kidney diseases are prevalent all over the world, with chronic kidney disease (CKD)1 comprising more than 10% of kidney disease diagnoses, and acute kidney disease (AKI) a wide range between 1% and 66% in different regions globally.2 In the case of AKI, approximately 41% of patients not recovering renal function before hospital discharge will progress into CKD.3 Treatment regimens for CKD and AKI are currently limited. Current efforts have focused on improving outcomes and reducing comorbidities for both CKD and AKI.
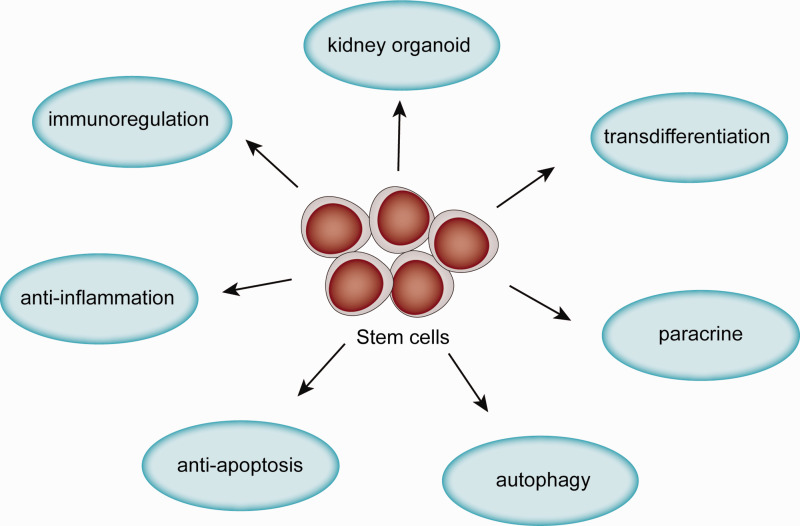
Stem cells are a class of cells that maintain the ability to self-renew and differentiate into multiple cell lineages. They can be divided into three categories: (a) pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs); (b) multipotent stem cells with partial differentiation ability; and c) unipotent stem cells, which can only differentiate into one kind of cell lineage. PSCs have been differentiated into kidney organoids,4 which are useful for kidney functional replacements in vivo and in vitro. Multipotent stromal cells such as mesenchymal stem cells (MSCs) that are derived from organs or tissues, such as bone marrow (BM-MSCs), amniotic fluid (AFSCs), urine (USCs) and umbilical cord (UC-MSCs), are used to treat kidney disease through mechanisms including paracrine signaling, initiation of autophagy, anti-apoptotic, or anti-inflammatory effects. Thus, stem cell treatment is a very promising therapy for kidney diseases. In this article, we review the mechanisms underlying the effects of stem cells on different kidney diseases (Figure 1, Table 1 and Table 2). We also summarize the progress in the development of kidney organoids for research and clinical application.
Figure 1.
Mechanisms of stem cells in treatment of kidney disease.(A color version of this figure is available in the online journal.)
Table 1.
Sources of stem cells and their roles in the treatment of kidney diseases.
| Sources of stem cells | Disease model | Mechanisms | References |
|---|---|---|---|
| BMSCs | Acute kidney injury | Transdifferentiation | Wong et al.;8 |
| hUCMSC-exosome, ADSCs-exosome, BMSCs-exosome, hAD-MSCs-exosome, UC-MSCs-exosome. | Cisplatin-induced Nephrotoxicity; Diabetic nephropathy; Acute kidney injury; AKI-CKD transmission; High phosphorus-induced vascular smooth muscle cells calcification; Unilateral ureteral obstruction. |
paracrine | Guo et al.;28 Zhang et al.;29 Zhu et al.;30 Wang et al.;33 Jia et al.;35 Jin ;36 Kilpinen et al.;80 Zhong et al.;106 Nagaishi et al.107 |
| hUC-MSCs, BMSCs, hUCMSC-exosomes, ASCs, ADSCs-exosome. | Acute kidney injury; Cr (VI)-injured kidney; Diabetic nephropathy; Cisplatin-induced AKI. |
Autophagy | Ebrahim et al.;24 Wang et al.;33 Jin et al.;36 Yin et al.;38 Rashed et al.;39 Xiang et al.;40 Tang et al.41 |
| BMSCs, PSC-derived endothelial progenitor cells, uADSCs, hUCMSCs, USCs. | Chromium-injured kidney; Acute kidney injury; Cisplatin-induced acute kidney injury; Rhabdomyolysis-induced acute kidney injury model; Adriamycin nephropathy; Diabetic nephropathy; Unilateral ureteral obstruction; Obesity-associated kidney injury; Renal artery stenosis; Chronic kidney disease. |
Anti-apotosis | Jin et al.;36 Yin et al.;38 Rashed et al.;39 Li et al.;48 Song et al.;54 Liu et al.;55 Xie et al.;56 Shen et al.;59 Zhang et al.;60 Zhang et al.;61 Jiao et al.;62 Tian et al.;63 Geng et al.;64 Zhu et al.79 |
| hU-MSCs, iPS-MSCs, BMSCs, hAFS, USCs, ADSCs. | Lupus nephritis; Obesity-associated kidney injury; Cardiorenal syndrome type II; Acute kidney injury. |
Anti-inflammation | Li et al.;48 Zhang et al.;60 Tian et al.;63 Vescovo et al.;67 Liu et al.68 |
| MSCs, ADSCs | Acute kidney injury; Unilateral ureteral obstruction; Chronic kidney disease; Diabetic nephropathy; Systemic lupus erythematosus. |
Immunoregulation | Shi et al.;73 Vasandan et al.;74 Wang et al.;75 Mittal et al.;76 Xing et al.;77 Rota et al.;78 Li et al.;79 Hamza et al.;80 He et al.81 |
Table 2.
Application of mesenchymal stem cells and exosomes in the treatment of kidney diseases.
| Treatment regimens | Protocols | References |
|---|---|---|
| EVs | MSCs were incubated with 10% exosome-depleted fetal bovine serum for 24–72 h and then collected to be centrifuged at 3000g for 20 min to remove cell debris. The supernatant was ultracentrifuged at 100,000g for 1–2 h at 4°C. Exosomes were then washed once with serum-free M199 or PBS and stored at −80°C | Ebrahim et al.;24 Guo et al.;28 Zhu et al.;30 Wang et al.;33 Jin et al.; Kilpinen et al.;80 Nagaishi etal.;107 Zhong et al.111 |
| BMSCs | Femurs and tibiae were flushed with a 20 g needle containing DMEM nutrient mixture F-12 and then cultured in 10% FBS incubated at 37°C and 5% CO2 | Yin et al.;38 Rashed et al.39 Song et al.;5 Xie et al.56 |
| ASCs and AFSCs | ASCs of iguinnal adipose tissue were isolated from C57 background mice, while AFSCs were obtained via amniocentesis from healthy pregnant mice with a C57 background. Both of them were cultured in StemPro MSC SFM supplemented with 10% FBS incubating at 37°C and 5% CO2 | Tang et al.;41 Zhang et al.60 |
| USCs | The centrifugation of urine sample was at 400g for 10 min at room temperature. Then discard the supernatant and wash the sediment with PBS. After centrifugation again, use DMEM to resuspend with 2% FBS, 10 ng/mL human epidermal growth factor, 2 ng/mL platelet-derived growth factor, 1 ng/mL transforming growth factor-β, 2 ng/mL basic fibroblast growth factor, 0.5 mM cortisol, 25 mg/mL insulin, 20 mg/mL transferrin, 549 ng/mL adrenaline, 50 ng/mL triiodothyronine and L-glutamine | Tian et al.63 |
| hUC-MSCs | Umbilical cord was washed by PBS containing antibiotics and minced into 1 mm three pieces. Thereafter, the pieces were seeded into cell culture dishes with low-glucose DMEM-containing 10% FBS incubated at 37°C and 5% CO2 | Liu et al.;56 Wang et al.75 |
Note: Clinical use of stem cells has focused on applications for lupus nephritis100 and monoclonal immunoglobulin-related renal disease,101 including AL amyloidosis, light chain deposition disease, heavy chain deposition disease, and myeloma-related renal disease. Because of immunogenicity, autologous peripheral blood stem cells are used in treatment. Study has shown using stem cells could improve remission rate and patient survival in monoclonal immunoglobulin-related renal disease.102 Studies on CKD have shown that infusing BMSCs or autologous CD34+ cells was safe and tolerable for CKD patients, with no significant change of kidney function.103,104 However, in AKI after cardiac surgery, using allogeneic MSCs did not improve the time to kidney function recovery, indicating the mechanisms of action behind the use of stem cell treatments in AKI patients have yet to be fully elucidated.
Differentiation of stem cells into functional kidney cells
Kidney cells contribute to many different physiological functions. For example, podocyte processes prevent proteinuria and tubule cells reabsorb filtrated materials. Damage to these cells induces proteinuria or kidney dysfunction.5–7 Studies have shown that culturing MSCs with injured mesangial cells induced by hydrogen peroxide in vitro allowed MSCs to differentiate into mesangial cells,8 and that MSCs injected into the suprarenal aorta could trans-differentiate into podocyte-like cells in a puromycin aminonucleoside induced acute proteinuria model in rat.9 However, stem cells have not been shown to trans-differentiate into tubule cells.10 Using iPSCs in adriamycin nephropathy revealed labeled-iPSCs were trapped in the lungs,11 and very few donor cells were observed in the kidney.10,12–14 This was attributed to the fact that only few stem cells infused into the soma could home into the injured areas during kidney injury and multiple reasons may contribute to these challenges.15–17 First, detrimental environment of stem cells may affect their differentiation abilities. Second, targeting stem cells to the location of injury is a major challenge in clinical applications. Third, the internal environment in AKI and CKD, including oxidative stress, inflammation, uremic toxin, hypoxia and other factors, can influence stem cell vitality.
Extracellular vesicles (EVs) from stem cells: Paracrine effects
EVs or exosomes in the size of approximately 40–200 nm can be secreted from endosomal compartments by most cell types and have been reported to be involved in the cell-to-cell communication and delivery of biomolecules including proteins, DNA, messenger RNAs (mRNAs), microRNAs (miRNAs) and lipids to recipient cells.18 Exosomes from MSCs have been reported to be a new regimen for AKI and CKD.19 Treatment with EVs via caudal vein after reperfusion could improve cortical microvascular and peritubular capillary density as well as the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF)20 and hepatocyte growth factor.21 VEGF subsequently activated Notch122 in human arterial endothelial cells to trigger arteriogenesis and angiogenesis,23 which improves microcirculation through more efficient delivery of oxygen. As a result, oxidative stress and apoptosis were attenuated, resulting in better renal function and histological conditions.24–27
Furthermore, EVs exhibited anti-calcification and anti-fibrosis characteristics. In the end stage renal disease, vascular calcification is commonly attributed to high phosphorus-induced vascular smooth muscle cell calcification which lacks of effective treatment, while the disease could induce sudden death, increase fragility of blood vessels and remote ischemia. It has been reported that EVs could secret miRNAs to activate Wnt, mammalian target of the rapamycin (mTOR), and mitogen-activated protein kinase pathways to inhibit vascular calcification.28 Therefore, using EVs to ameliorate the calcification would be a promising method in end stage renal disease patients. In addition, kidney fibrosis is a final common pathology of CKD attributed to hypoxia and ischemia. In the fibrosis model of unilateral ureteral obstruction, miRNA-26a inhibited the levels of connective tissue growth factor and transforming growth factor-β (TGF-β1).29 Also, exosomes from MSCs activate tubular Sox9 to prevent the transition of tubular epithelium cells into a pro-fibrotic phenotype induced by TGF-β130; however, this transition model has been challenged31 and whether EVs can inhibit kidney fibrosis needs to be validated.
The effect of stem cells on autophagy
Autophagy is a self-clearance process in which excess or damaged organelles are selectively degraded mediated by autophagosome. There are usually three types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy. Mutation of autophagy-related protein, ATG5 or ATG7, resulted in mild dysfunction of podocytes and tubules within two months and severe glomerular and tubular changes by four months, and then organ failure by six months.32 Similar changes were observed in human idiopathic focal segmental glomerular sclerosis kidney biopsy specimens,32 suggesting that dysregulated autophagy could influence kidney structure. Transmission electron microscopy has revealed large amounts of autophagosomes in MSC-exosome-treated rat kidney epithelial cells,33 indicating autophagy was activated marked by elevated expressions of LC3B.34 The mechanisms of activating autophagy include: a. Increasing autophagy proteins ATG5 and ATG7 to activate the mTOR pathway34; b. 14–3-3ζ protein contained in MSC-exosomes interacts with ATG16L to promote the localization of ATG16L at autophagosome precursors, resulting in activation of autophagy35; c. Secreting miRNA-486 inhibits mTOR activation, resulting in the increase of autophagy and the reduction of podocyte apoptosis via decreasing smad1.36 But whether autophagy improves or perturbs kidney function in AKI and CKD remains unclear. It is known that rapamycin can inhibit mTOR pathway to activate mitophagy. In ischemia and cisplatin-induced kidney injury, rapamycin did not enhance autophagy or ameliorate kidney injury although it blocks the mTOR pathway.37 In parallel, BMSCs inhibited mitophagy, manifesting as decreased Beclin1, PINK1, Parkin, p-Parkin, and LC3B, to restore chromium-injured kidney via upregulating phosphorylation of extracellular signal-regulated kinases and downregulating phosphorylation of p38 and c-Jun-N-terminal kinase.38 On top of that, decreased beclin1 was also observed in diabetic nephropathy rats treated with MSCs.39 In contrast, human UC-MSCs could secrete miRNA-145 to induce HK-2 cellular autophagy via inhibition of the phosphoinositide-3-kinase (PI3K)/AKT/mTOR signaling pathway.40 Moreover, LC3B and ATG5 as well as ATG7 were increased in cisplatin-induced AKI rats after receiving the treatment of MSC-exosomes,33 suggesting autophagy was activated to preserve kidney function after injury. This kind of protection effect is not only on cisplatin-induced AKI but also in diabetic nephropathy model. It has been shown that glucose metabolism and kidney fibrosis could be improved41 by using ASCs via decreasing mTOR pathway.
Based on these studies, autophagy should be an important mechanism correlating with different kidney injury models. Previous studies show that MSC delivery via peripheral veins results in decreased autophagy,38,39 while MSCs via kidney injection33 or subrenal capsule increase autophagy.41 Although peripheral delivery of MSCs also show some labeled stem cells homing into kidney tissue, it is still unclear why different injecting methods influence the activity of autophagy in injured kidney.
The anti-apoptosis effects on AKI models
The initiation of apoptosis is induced by BAK and BAX42 via mitochondrial outer membrane permeabilization,43 which subsequently releases pro-apoptotic protein, cytochrome c, which activates caspase-9 protease in the cytosol.44 Thereafter, caspase-9 activates caspase-3,-6,-7 to induce the cascade that culminates in apoptotic cell death. Apoptosis is a common pathophysiological process in AKI.45 It has been shown that intraluminal debris appeared after AKI,45 which are apoptotic tubule cells induced by oxidative stress and endoplasmic reticulum stress (ER). Oxidative stress can induce mitochondria to transmit apoptotic signals via release of cytochrome c into cytoplasm.46 ER could induce unfolded protein response (UPR). Mildly enhanced UPR protects against glomerular injury,47 while severe ER stress results in overwhelming UPR and UPR-related apoptosis emerges.48,49 Studies have shown using MSCs could inhibit oxidative stress50–56 and ER stress57,58 to attenuate apoptosis. Also, previous studies have revealed the effect of MSCs on anti-apoptosis in different kidney injury models to improve kidney outcomes.36,38,59–64 In the chromium-injured kidney, BMSCs treatment revealed decreased apoptosis-related proteins Bax, cytochrome c, and caspase-3,38 which were also observed in ischemia AKI after dealing with PSC-derived endothelial progenitor cells59 via decreasing indoxyl sulfate and interleukin-1β (IL-1β).63 Furthermore, this kind of anti-apoptosis could be enhanced by resveratrol to promote MSCs to secrete platelet-derived growth factor-DD, activating extracellular signal-regulated kinases, which inhibit renal tubular cells apoptosis.61 In addition, cisplatin-induced nephropathy also exhibits reduced tubular apoptosis after treating with BMSCs through activating Wnt/β-catenin pathway.62 Wnt/β-catenin pathway has also been defined to induce kidney fibrosis.65 Therefore, the effect of MSCs on kidney fibrosis needs to be validated. Rhabdomyolysis-induced AKI model is another kidney injury model, manifesting as muscle pain and hematuria as well as kidney dysfunction requiring dialysis. Collagen graft packed with MSCs has been reported to ameliorate outcome of rhabdomyolysis-induced AKI via activating PI3K/Akt pathway to inhibit apoptosis.64 Delivery of MSCs via biological membrane provides a useful tool in the treatment of AKI and CKD. Potentially, it would be more useful if biological membrane can be made into small capsule to deliver MSCs as subrenal capsule.
Anti-inflammation and immunoregulation
Aseptic inflammation, a common pathophysiology process in AKI and CKD, is induced by multiple signaling pathways that are activated by the binding of ligands, termed “damage-associated molecular patterns,” to toll-like receptors. These pathways include the nuclear factor kappa B, mitogen-activated protein kinase, and type I interferon pathways.66 The activated inflammatory pathways release IL-1β, tumor necrosis factor-α (TNF-α), interferon-γ to recruit chemokines resulting the migration of mononuclear leucocytes into the injured areas inducing local inflammation. Inflammation can result from different pathogenesis. Ischemia kidney injury could induce hypoxia and increase release of inflammatory factors by necrotic proximal tubule cells, which could be reversed by treatment with USCs,63 adipose-derived mesenchymal stem cells (ADSCs),60 and human AFSCs67 via decreasing the mRNA expression levels of interferon-γ and IL-1β and increasing IL-10 and TGF-β1, resulting in decreased lumen expansion and loss of tubular epithelial cells.63 Another common etiology of CKD, lupus nephritis, induces kidney injury by immune system activation resulting in elevation of inflammatory markers which could also be inhibited by human UC-MSCs via suppressing nuclear factor kappa B pathway to downregulate the expression of TNF-α, intercellular cell adhesion molecule-1, and plasminogen activator inhibitor-1.68 Besides the classical kidney injury model, there are still chronic infiltration inflammation in kidney, such as obesity-associated kidney injury manifesting as elevated inflammation markers of IL-6, chemokine (C-X-C motif) ligand 1 and 2 which were also mitigated after treatment with MSCs.48
During ischemia–reperfusion injury, T cells accumulation in kidney tissues is observed to reside in the infarction boundary zone for about 14 days. The CD4+ and CD8+ T cells have a detrimental role in the kidneys after ischemia–reperfusion injury.69 In contrast, T-regulatory cells exhibit a protective role in ischemia and reperfusion by secreting IL-10 to reduce the ischemia–reperfusion injury.70 On top of that, plenty of innate immune cells, including mast cells, neutrophils, macrophages, myeloid-derived suppressor cells, dendritic cells and natural killer cells are engaged in ischemia–reperfusion injury regulated by MSCs.71 MSCs can secret prostaglandin E2,72 kynurenic acid,73 TNF-stimulated gene 674 to promote macrophage polarization from an M1 phenotype towards an M2 phenotype to ease inflammatory status. MSCs also inhibited the infiltration of macrophages in kidney tissue.75,76 In addition, MSCs inhibit lipopolysaccharide-stimulated rat peritoneal macrophages by downregulating inflammation-related cytokines such as IL-6, TNF-α, IL-1β,77 and IL-878 to prevent diabetic nephropathy. Moreover, decreasing IL-17 and increasing CD4+CD25+Foxp3+Tregs by ADSCs79 or EVs80 could relieve immune inflammation.
Kidney organoids open a new avenue for stem cell therapy
The kidney organoids are developed from ESCs or iPSCs,81,82 and transcriptional congruence of nephron cell type-specific markers, as well as stromal and endothelial markers were similar to human fetal kidney.83 The induced organoids begin from the primitive streak, induced by Wnt pathway agonist, CHIR99021, to intermediate mesoderm.84 Thereafter, ureteric epithelium, metanephric mesenchyme, progenitors of renal interstitium, and endothelium are induced from intermediate mesoderm by using a transwell filter. The above progenitors then aggregate at an air-media interface.4,85 After about 20 days, kidney organoids can be obtained. During this procession, exosomes from stem cells are involved in the impaction of kidney organoids by entering the cytoplasm and nucleus via fluorescently labeled exosomal RNA.86 Kidney organoids contain all kinds of kidney cell lineages. However, lacking of capillary loops suggests that kidney organoids may be insufficient to filtrate urine yet.87 It has been reported that modulating Wnt pathway activity could generate capillary loops that is adding CHIR99021 at different time period.88 Although a vascular network is developed, no significant filtration ability was still observed by the organoids.88,89 There should be other factors or microenvironment influencing the function of kidney organoids. Therefore, the kidney organoids were transplanted into vivo resulting functionally vascular filtration,88,89 suggesting a soft environment promotes the maturation of kidney organoids.88,90 Likewise, high fluidic shear stress also revealed generation of capillary loops using a millifluidic chips in vitro.87 This study indicates a future direction to combine kidney organoids and dialyzer together to use fluidic shear stress to promote the maturation of kidney organoids. The matured kidney organoids then could replace dialysis membrane to serve as kidney replacement therapy.
Based on the similarity to kidney structure and function, kidney organoids have been used to screen drugs. Taking cisplatin kidney injury model as an example, after introduction of 5 µM cisplatin into the culture medium with kidney organoids, acute apoptosis of mature proximal tubular cells emerged at only one day.91 In addition, kidney organoids can also be used as kidney disease models. For example, polycystic kidney disease is an autosomal dominant hereditary kidney disease that lacks an ideal disease model. Cruz et al.92 have used kidney organoids to generate a polycystic kidney disease model with decreased cystogenes of polycystin-1 after removing stoma, while increased cysts when adding cyclic adenosine monophosphate. Furthermore, kidney organoids from iPSCs of a congenital nephrotic patient with a missense mutation of Nephrin revealed impaired silt diaphragm which could be restored by genetic correction of mutation in the single amino acid, resulting in normal silt diaphragm formation.93,94 Apart from establishing disease models, exploring gene expression in kidney intrinsic cell lines is available. Single cell transcriptional signature analysis of podocytes disease in kidney organoids revealed LYPD1, PRSS23, and CDH6 correlated with human glomerular disease, which had not been reported before as it is difficult to study the gene expression signatures of podocytes in vivo.95
When it comes to transplantation application of kidney organoid, many questions remain to be resolved. First, the source of PSCs needs to be secured. While ESCs are viewed with potential ethic concerns, iPSC banks with MHC matching are not. Recently, urine-derived renal progenitor cells has been published that showed renal stem cell markers -SIX2, CITED1, WT1, CD24, and CD106 and pluripotency-associated proteins- TRA-1–60, TRA-1–81, SSEA4, C-KIT, and CD133 indicating a potential source for kidney organoids.96 Second, current kidney organoids are too small to provide sufficient filtration ability. Therefore, kidney organoids shall contain a sufficient amount of nephrons for future transplantation; Third, as blood perfusion is essential to promote the maturation of kidney organoids,88–90 the transplantation site should be adjacent to great vessels and ureter. It has been reported using omentum97 and retroperitoneal fat tissue98 as the transplantation site. Retroperitoneal fat tissue resulted in better glomerular filtration rate. However, both studies transplanted metanephroi not kidney organoids into rats. A recent study99 showed transplanted kidney organoids beneath kidney capsule could survive for several months and also be vascularized from endothelial cells of host mice. Finally, although kidney organoids exhibit better nephron-like structures after transplantation, structure of tubules brush border, filtration barrier, and capillary lumens were not as organized as in the mature kidney tissues, requiring further improvement of kidney organoids differentiation.
Conclusions
In our review, we focused on the mechanisms by which stem cells could help treat kidney diseases, as well as the use of kidney organoids as an emerging treatment approach and research resource. Although MSCs are versatile with many mechanisms during the treatment, exosomes may be an alternative to fulfill the therapeutic effects of MSCs. As transdifferentiation of MSCs into tubule cells is not successful in vivo,10 MSCs may restore kidney injury via paracrine pathways including secreting exosomes. In fact, exosomes are being used in a clinical trial (NCT04173650).
Clinically, stem cells have been used to treat lupus nephritis patients, with good complete recovery rates. However, clinical trials using MSCs in diabetic nephropathy did not show sufficient effect and the treatment effects of stem cells on AKI are still controversial. As lupus nephritis or primary amyloidosis are systemic diseases, MSCs exhibit effective treatment results clinically. Based on current clinical methods, delivering exosomes into injured kidney through renal artery could be a more feasible and useful procedure. In addition, injection of exosomes subrenal capsule may also be a clinically relevant delivery method.
Although many studies have shown the safety of MSCs treatment, the side effects of stem cell treatment of kidney diseases remain to be investigated. Nevertheless, we foresee a great future for stem cell therapy of nephropathy. Through better understanding the pathophysiology of CKD and AKI, we will further clarify the protective mechanisms of stem cells in the treatment of kidney diseases.
Supplemental Material
Supplemental material, EBM915901 Supplemental Material for Stem cell-based treatment of kidney diseases by Binbin Pan and Guoping Fan in Experimental Biology and Medicine
Authors’ contributions
All authors contributed to writing the article. All authors reviewed and approved the final submission.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors acknowlege the financial support from NIH RO1 DE 025474 for the research, authorship, and publication of this article.
ORCID iD
Guoping Fan https://orcid.org/0000-0001-5235-6410
References
- 1.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379:815–22 [DOI] [PubMed] [Google Scholar]
- 2.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018; 14:607–25 [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014; 371:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morizane R, Bonventre JV. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc 2017; 12:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou XJ, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy 2019; 15:908–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 2017; 13:483–95 [DOI] [PubMed] [Google Scholar]
- 7.Lazzeri E, Angelotti ML, Peired A, Conte C, Marschner JA, Maggi L, Mazzinghi B, Lombardi D, Melica ME, Nardi S, Ronconi E, Sisti A, Antonelli G, Becherucci F, De Chiara L, Guevara RR, Burger A, Schaefer B, Annunziato F, Anders HJ, Lasagni L, Romagnani P. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun 2018; 9:1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CY, Tan EL, Cheong SK. In vitro differentiation of mesenchymal stem cells into mesangial cells when co-cultured with injured mesangial cells. Cell Biol Int 2014; 38:497–501 [DOI] [PubMed] [Google Scholar]
- 9.Rangel EB, Gomes SA, Kanashiro-Takeuchi R, Saltzman RG, Wei C, Ruiz P, Reiser J, Hare JM. Kidney-derived c-kit+ progenitor/stem cells contribute to podocyte recovery in a model of acute proteinuria. Sci Rep 2018; 8:14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 2005; 68:1613–7 [DOI] [PubMed] [Google Scholar]
- 11.Wu HJ, Yiu WH, Wong DWL, Li RX, Chan LYY, Leung JCK, Zhang Y, Lian Q, Lai KN, Tse HF, Tang S. Human Induced pluripotent stem cell-derived mesenchymal stem cells prevent adriamycin nephropathy in mice. Oncotarget 2017; 8:103640–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezquer F, Giraud-Billoud M, Carpio D, Cabezas F, Conget P, Ezquer M. Proregenerative microenvironment triggered by donor mesenchymal stem cells preserves renal function and structure in mice with severe diabetes mellitus. Biomed Res Int 2015; 2015:164703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, Liu G, Su M. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract 2014; 104:143–54 [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract 2012; 98:465–73 [DOI] [PubMed] [Google Scholar]
- 15.Han YS, Kim SM, Lee JH, Jung SK, Noh H, Lee SH. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC-dependent enhancement of the mitochondrial function. J Pineal Res 2019; 66:e12535. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhang Y, Liu S, Li F, Wang B, Wang J, Cao L, Xia T, Yao Q, Chen H, Zhang Y, Zhu X, Li Y, Li G, Wang J, Li X, Ni S. Melatonin enhances proliferation and modulates differentiation of neural stem cells via autophagy in hyperglycemia. Stem Cells 2019; 37:504–15 [DOI] [PubMed] [Google Scholar]
- 17.Yoon YM, Han YS, Yun CW, Lee JH, Kim R, Lee SH. Pioglitazone protects mesenchymal stem cells against P-Cresol-Induced mitochondrial dysfunction via up-regulation of PINK-1. Int J Mol Sci 2018; 19:2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 2018; 9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 2012; 3:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res 2016; 8:4289–99 [PMC free article] [PubMed] [Google Scholar]
- 21.Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY, Gu D, Miao S, Zhu YJ, Sun J, Du T. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One 2015; 10:e0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 2003; 23:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal stem Cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant 2018; 27:1080–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells 2018; 7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, Biancone L, Gontero P, Frea B, Camussi G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 2017; 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragni E, Banfi F, Barilani M, Cherubini A, Parazzi V, Larghi P, Dolo V, Bollati V, Lazzari L. Extracellular vesicle-shuttled mRNA in mesenchymal stem cell communication. Stem Cells 2017; 35:1093–110 [DOI] [PubMed] [Google Scholar]
- 27.Zhu G, Pei L, Lin F, Yin H, Li X, He W, Liu N, Gou X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol 2019; 234:23736–49 [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Bao S, Guo W, Diao Z, Wang L, Han X, Guo W, Liu W. Bone marrow mesenchymal stem cell-derived exosomes alleviate high phosphorus-induced vascular smooth muscle cells calcification by modifying microRNA profiles. Funct Integr Genomics 2019; 19:633–43 [DOI] [PubMed] [Google Scholar]
- 29.Zhang A, Wang H, Wang B, Yuan Y, Klein JD, Wang XH. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB J 2019; 33:13590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, , Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget 2017; 8:70707–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 2014; 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakami T, Gomez IG, Ren S, Hudkins K, Roach A, Alpers CE, Shankland SJ, D’Agati VD, Duffield JS. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol 2015; 26:1040–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther 2017; 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N, Yoshimori T, Ohsumi Y. The role of atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27:107–32 [DOI] [PubMed] [Google Scholar]
- 35.Jia H, Liu W, Zhang B, Wang J, Wu P, Tandra N, Liang Z, Ji C, Yin L, Hu X, Yan Y, Mao F, Zhang X, Yu J, Xu W, Qian H. HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res 2018; 10:101–13 [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther 2019; 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrianova NV, Zorova LD, Babenko VA, Pevzner IB, Popkov VA, Silachev DN, Plotnikov EY, Zorov DB. Rapamycin is not protective against ischemic and cisplatin-induced kidney injury. Biochemistry 2019; 84:1502–12 [DOI] [PubMed] [Google Scholar]
- 38.Yin F, Yan J, Zhao Y, Guo KJ, Zhang ZL, Li AP, Meng CY, Guo L. Bone marrow mesenchymal stem cells repair Cr (VI)- injured kidney by regulating mitochondria-mediated apoptosis and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol Environ Saf 2019; 176:234–41 [DOI] [PubMed] [Google Scholar]
- 39.Rashed LA, Elattar S, Eltablawy N, Ashour H, Mahmoud LM, El-Esawy Y. Mesenchymal stem cells pretreated with melatonin ameliorate kidney functions in a rat model of diabetic nephropathy. Biochem Cell Biol 2018; 96:564–71 [DOI] [PubMed] [Google Scholar]
- 40.Xiang J, Jiang T, Zhang W, Xie W, Tang X, Zhang J. Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Cell Res 2019; 378:198–205 [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Wu H, Lei J, Yi C, Xu W, Lan W, Yang F, Liu C. HIF1α deletion facilitates adipose stem cells to repair renal fibrosis in diabetic mice. In Vitro Cell Dev Biol Anim 2018; 54:272–86 [DOI] [PubMed] [Google Scholar]
- 42.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15:49–63 [DOI] [PubMed] [Google Scholar]
- 43.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. Bax crystal structures reveal how BH3 domains activate bax and nucleate its oligomerization to induce apoptosis. Cell 2013; 152:519–31 [DOI] [PubMed] [Google Scholar]
- 44.Lee YJ, Lee C. Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway. Virus Res 2018; 253:112–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y, Doi K, Maehara N, Kusunoki S, Takahata A, Noiri E, Suzuki Y, Yahagi N, Nishiyama A, Gunaratnam L, Takano T, Miyazaki T. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med 2016; 22:183–93 [DOI] [PubMed] [Google Scholar]
- 46.Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol 2017; 12:208–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue T, Maekawa H, Inagi R. Organelle crosstalk in the kidney. Kidney Int 2019; 95:1318–25 [DOI] [PubMed] [Google Scholar]
- 48.Li B, Leung JCK, Chan LYY, Yiu WH, Li Y, Lok SWY, Liu WH, Chan KW, Tse HF, Lai KN, Tang S. Amelioration of endoplasmic reticulum stress by mesenchymal stem cells via hepatocyte growth factor/c-Met signaling in obesity-associated kidney injury. Stem Cells Transl Med 2019; 8:898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum – new mechanisms in kidney disease. Nat Rev Nephrol 2014; 10:369–78 [DOI] [PubMed] [Google Scholar]
- 50.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 2015; 4:208–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 2007; 72:1073–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. ] TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 2014; 124:4989–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen BL, Sheu ML, Tsai KS, Lan KC, Guan SS, Wu CT, Chen LP, Hung KY, Huang JW, Chiang CK, Liu SH. CCAAT-Enhancer-Binding protein homologous protein deficiency attenuates oxidative stress and renal Ischemia-Reperfusion injury. Antioxid Redox Signal 2015; 23:1233–45 [DOI] [PubMed] [Google Scholar]
- 54.Song IH, Jung KJ, Lee TJ, Kim JY, Sung EG, Bae YC, Park YH. Mesenchymal stem cells attenuate adriamycin-induced nephropathy by diminishing oxidative stress and inflammation via downregulation of the NF-kB. Nephrology 2018; 23:483–92 [DOI] [PubMed] [Google Scholar]
- 55.Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW, Zhang YY, Zhang DY, Wei GH. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology 2018; 3:728–36 [DOI] [PubMed] [Google Scholar]
- 56.Xie LB, Chen X, Chen B, Wang XD, Jiang R, Lu YP. Protective effect of bone marrow mesenchymal stem cells modified with klotho on renal ischemia-reperfusion injury. Ren Fail 2019; 41:175–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou D, Tan RJ, Lin L, Zhou L, Liu Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int 2013; 84:509–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 2013; 31:117–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen WC, Chou YH, Huang HP, Sheen JF, Hung SC, Chen HF. Induced pluripotent stem cell-derived endothelial progenitor cells attenuate ischemic acute kidney injury and cardiac dysfunction. Stem Cell Res Ther 2018; 9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang JB, Wang XQ, Lu GL, Huang HS, Xu SY. Adipose-derived mesenchymal stem cells therapy for acute kidney injury induced by ischemia-reperfusion in a rat model. Clin Exp Pharmacol Physiol 2017; 44:1232–40 [DOI] [PubMed] [Google Scholar]
- 61.Zhang R, Yin L, Zhang B, Shi H, Sun Y, Ji C, Chen J, Wu P, Zhang L, Xu W, Qian H. Resveratrol improves human umbilical cord-derived mesenchymal stem cells repair for cisplatin-induced acute kidney injury. Cell Death Dis 2018; 9:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiao X, Cai J, Yu X, Ding X. Paracrine activation of the Wnt/β-Catenin pathway by bone marrow stem cell attenuates cisplatin-induced kidney injury. Cell Physiol Biochem 2017; 44:1980–94 [DOI] [PubMed] [Google Scholar]
- 63.Tian SF, Jiang ZZ, Liu YM, Niu X, Hu B, Guo SC, Wang NS, Wang Y. Human urine-derived stem cells contribute to the repair of ischemic acute kidney injury in rats. Mol Med Rep 2017; 6:5541–8 [DOI] [PubMed] [Google Scholar]
- 64.Geng X, Hong Q, Wang W, Zheng W, Li O, Cai G, Chen, Wu D. Biological membrane-packed mesenchymal stem cells treat acute kidney disease by ameliorating mitochondrial-related apoptosis. Sci Rep 2017; 7:41136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of notch, Wnt and hedgehog. Nat Rev Nephrol 2016; 12:426–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vescovo G, Castellani C, Fedrigo M, Virzì GM, Vescovo GM, Tavano R, Pozzobon M, Angelini A. Stem cells transplantation positively modulates the heart-kidney cross talk in cardiorenal syndrome type II. Int J Cardiol 2019; 275:136–44 [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Lu X, Lou Y, Cai Y, Cui W, Wang J, Nie P, Chen L, Li B, Luo P. Xenogeneic transplantation of human Placenta-Derived mesenchymal stem cells alleviates renal injury and reduces inflammation in a mouse model of lupus nephritis. Biomed Res Int 2019; 2019:9370919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 2006; 176:3108–14 [DOI] [PubMed] [Google Scholar]
- 70.Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J, Zhou X. Reciprocal expression of IL-35 and IL-10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep 2017; 21:1853–69 [DOI] [PubMed] [Google Scholar]
- 71.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 2018; 14:493–507 [DOI] [PubMed] [Google Scholar]
- 72.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 2016; 6:38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ 2018; 25:1209–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mittal M, Tiruppathi C, Nepal S, Zhao YY, Grzych D, Soni D, Prockop DJ, Malik AB. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc Natl Acad Sci U S A 2016; 113:E8151–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing L, Song E, Yu CY, Jia XB, Ma J, Sui MS, Wang MA, Gao X. Bone Marrow-derived mesenchymal stem cells attenuate tubulointerstitial injury through multiple mechanisms in UUO model. J Cell Biochem 2018; 120:9737–46 [DOI] [PubMed] [Google Scholar]
- 76.Rota C, Morigi M, Cerullo D, Introna M, Colpani O, Corna D, Capelli C, Rabelink TJ, Leuning DG, Rottoli D, Benigni A, Zoja C, Remuzzi G. Therapeutic potential of stromal cells of non-renal or renal origin in experimental chronic kidney disease. Stem Cell Res Ther 2018; 9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L, Li L, Liu F, Chen B, Guo G, Wang C, Yang L, Cheng J, Lu Y. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med 2018; 41:2629–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamza AH, Al-Bishri WM, Damiati LA, Ahmed HH. Mesenchymal stem cells: a future experimental exploration for recession of diabetic nephropathy. Ren Fail 2017; 39:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He X, Zhang Y, Zhu A, Zeng K, Zhang X, Gong L, Peng Y, Lai K, Qu S. Suppression of interleukin 17 contributes to the immunomodulatory effects of adipose-derived stem cells in a murine model of systemic lupus erythematosus. Immunol Res 2016; 64:1157–67 [DOI] [PubMed] [Google Scholar]
- 80.Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles 2013; 2:21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taguchi A, Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 2017; 21:730–46 [DOI] [PubMed] [Google Scholar]
- 82.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, Wolvetang EJ, McMahon AP, Holm TM, Davidson AA. Simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 2018; 11:470–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Combes AN, Zappia L, Er PX, Oshlack A, Little MH. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med 2019; 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miner JH. The glomerular basement membrane. Exp Cell Res 2012; 318:973–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boreström C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslöw A, Svensson A, Söderberg M, Reznichenko A, Nyström J, Patrakka J, Hicks R, Maresca M, Valastro B, Collén A. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int 2018; 94:1099–110 [DOI] [PubMed] [Google Scholar]
- 86.Krause M, Rak-Raszewska A, Naillat F, Saarela U, Schmidt C, Ronkainen VP, Bart G, Ylä-Herttuala S, Vainio SJ. Exosomes as secondary inductive signals involved in kidney organogenesis. J Extracell Vesicles 2018; 7:1422675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 2019; 16:255–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 2019; 25:373–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep 2018; 10:751–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Martí E, Zalvidea D, Trepat X, Roca-Cusachs P, Gavaldà-Navarro A, Cozzuto L, Campistol JM, Izpisúa Belmonte JC, Hurtado Del Pozo C, Montserrat N. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater 2019; 18:397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015; 526:564–8 [DOI] [PubMed] [Google Scholar]
- 92.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater 2017; 16:1112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, Nishinakamura R. Organoids from nephrotic Disease-Derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep 2018; 11:727–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, Little MH. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun 2018; 9:5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harder JL, Menon R, Otto EA, Zhou J, Eddy S, Wys NL, O’Connor C, Luo J, Nair V, Cebrian C, Spence JR, Bitzer M, Troyanskaya OG, Hodgin JB, Wiggins RC, Freedman BS, Kretzler M, European Renal cDNA Bank (ERCB); Nephrotic Syndrome Study Network (NEPTUNE). Organoid single cell profiling identifies a transcriptional signature of glomerular disease. JCI Insight 2019; 4:122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rahman MS, Wruck W, Spitzhorn LS, Nguyen L, Bohndorf M, Martins S, Asar F, Ncube A, Erichsen L, Graffmann N, Adjaye J. The FGF, TGFβ and WNT axis modulate self-renewal of human SIX2+ urine derived renal progenitor cells. Sci Rep 2020; 10:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogers SA, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int 1998; 54:27–37 [DOI] [PubMed] [Google Scholar]
- 98.Marshall D, Clancy M, Bottomley M, Symonds K, Brenchley PE, Bravery CA. Transplantation of metanephroi to sites within the abdominal cavity. Transplant Proc 2005; 37:194–7 [DOI] [PubMed] [Google Scholar]
- 99.Nam SA, Seo E, Kim JW, Kim HW, Kim HL, Kim K, Kim TM, Ju JH, Gomez IG, Uchimura K, Humphreys BD, Yang CW, Lee JY, Kim J, Cho DW, Freedman BS, Kim YK. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med 2019; 51:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang X, Chen W, Ren G, Zhao L, Guo J, Gong D, Zeng C, Hu W, Liu Z. Autologous hematopoietic stem cell transplantation for refractory lupus nephritis. Clin J Am Soc Nephrol 2019; 14:719–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sidiqi MH, Aljama MA, Buadi FK, Warsame RM, Lacy MQ, Dispenzieri A, Dingli D, Gonsalves WI, Kumar S, Kapoor P, Kourelis T, Hogan WJ, Gertz MA. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol 2018; 36:1323–9 [DOI] [PubMed] [Google Scholar]
- 102.Yu XJ, Zhang X, Li DY, Wang SX, Zhou FD, Zhao MH. Renal pathologic spectrum and clinical outcome of monoclonal gammopathy of renal significance: a large retrospective case series study from a single institute in China. Nephrology 2020; 25:202–11 [DOI] [PubMed] [Google Scholar]
- 103.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy 2018; 20:660–9 [DOI] [PubMed] [Google Scholar]
- 104.Lee MS, Lee FY, Chen YL, Sung PH, Chiang HJ, Chen KH, Huang TH, Chen YL, Chiang JY, Yin TC, Chang HW, Yip HK. Investigated the safety of intra-renal arterial transfusion of autologous CD34+ cells and time courses of creatinine levels, endothelial dysfunction biomarkers and micro-RNAs in chronic kidney disease patients-phase I clinical trial. Oncotarget 2017; 8:17750–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zou X, Gu D, Zhang G, Zhong L, Cheng Z, Liu G, Zhu Y. NK cell regulatory property is involved in the protective role of MSC-Derived extracellular vesicles in renal ischemic reperfusion injury. Hum Gene Ther 2016; 27:926–35 [DOI] [PubMed] [Google Scholar]
- 106.Zhong L, Liao G, Wang X, Li L, Zhang J, Chen Y, Liu J, Liu S, Wei L, Zhang W, Lu Y. Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp Biol Med 2018; 243:1233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016; 6:34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng J, Lu C, Dai Q, Sheng J, Xu M. SIRT3 facilitates amniotic fluid stem cells to repair diabetic nephropathy through protecting mitochondrial homeostasis by modulation of mitophagy. Cell Physiol Biochem 2018; 46:1508–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM915901 Supplemental Material for Stem cell-based treatment of kidney diseases by Binbin Pan and Guoping Fan in Experimental Biology and Medicine