Abstract
Background
Exercise improves symptoms of irritable bowel syndrome, but few data are available about functional dyspepsia. We compared the prevalence and frequency of different types of exercise between individuals with functional dyspepsia and general population controls.
Methods
A mailed survey was returned by 3160 people randomly obtained from the Australian electoral register. The survey included questions to identify the Rome III diagnosis for functional dyspepsia. Exercise was classified by the presence (yes or no) and the frequency (number of times) spent walking, and engaging in moderate and vigorous exercise, over the last 2 weeks based on the National Health Survey. Controls did not meet criteria for functional dyspepsia. Potential confounders included the presence of irritable bowel syndrome, smoking, body mass index, age and gender.
Results
A total of 14.8% (95% confidence interval (CI) 13.6%, 16.1%) subjects had functional dyspepsia. They reported significantly less walking (57% versus 63%, P = 0.04) and lower frequency of exercising, in terms of walking (P = 0.008) and engaging in moderate (P = 0.03) and vigorous activity (P = 0.02), compared with controls. The association remained significant for moderate exercise, independent of age, gender, body mass index and smoking, and excluding overlap with irritable bowel syndrome (odds ratio (OR) = 0.94 (95% CI 0.88, 0.99), P = 0.02). Postprandial distress syndrome was associated with less-vigorous exercise adjusting for confounders (OR = 0.65 (95% CI 0.42, 1.0), P = 0.05), but not epigastric pain syndrome.
Conclusion
Functional dyspepsia is associated with lower exercise levels, but the causality still needs to be determined.
Keywords: Epidemiology, functional dyspepsia, lifestyle, physical activity, irritable bowel syndrome
Key Summary
1. Summarize the established knowledge on this subject
Lifestyle factors may be important in functional gastrointestinal disorders.
Increasing physical activity has been associated with possible improvement of irritable bowel syndrome, but it is unknown if exercise is associated with functional dyspepsia.
2. What are the significant and/or new findings of this study?
Functional dyspepsia is associated with lower exercise levels.
Reduced activity was significantly associated with functional dyspepsia, independent of age, gender, body mass index and smoking, and excluding overlap with irritable bowel syndrome .
Prescribing exercise may have therapeutic benefits in functional dyspepsia, but this needs to be confirmed in a randomized clinical trial.
Background
More than 1 in 10 people from Western populations suffer with chronic unexplained fullness after meals, inability to finish a normal-sized meal, and/or epigastric pain or burning, a symptom complex referred to as functional dyspepsia (FD).1–3 FD lacks an established pathophysiological basis1 and, as a result, current treatments are generally unsatisfactory, resulting in substantial economic4 and personal costs.5
Potentially modifiable lifestyle factors could provide a cost-effective and viable intervention option for patients suffering with this condition. For example, smoking has been associated with a 50% increased risk of FD for current smokers versus those who have never smoked,6 although the results need confirmation. On the other hand, the role of exercise in the pathogenesis of FD symptoms is largely unknown. While very strenuous exercise such as marathon running can have detrimental effects on gut health, including the slowing of transit, increased intestinal permeability and even endotoxemia,7 moderate exercise has general health benefits. For example, the duodenal microbiome has reduced diversity in FD, and regular exercise can potentially modify the intestinal microbiome, improving diversity and enhancing the number of probably beneficial bacteria.8,9 Further, small intestinal immune activation has been observed in FD with increased circulating small intestinal homing T cells and duodenal eosinophilia,10,11 and moderate exercise positively modulates the intestinal immune system while severe exercise is immunosuppressive.12
In the irritable bowel syndrome (IBS), a similar disorder that overlaps more than expected by chance with FD,13 no association between exercise and IBS was found in a student population.14 We observed in a population-based study that those with IBS-constipation exercised more than those with functional constipation.15 In a recent prospective randomized trial, 43% of IBS patients who exercised three times a week improved compared to 26% of those in a control group,16 but these data failed to reach statistical significance, are yet to be replicated and no such trials have been undertaken in FD.
Few data exist on whether people with FD engage in healthy physical activity, despite exercise being widely recommended as part of the first-line treatment for FD by some experts.17 One survey of 15,000 adults in Japan conducted over the internet did find a significantly lower proportion of people with Rome III FD exercised frequently compared with those without FD; however, these observations need confirmation in the West and selection bias is a concern with internet survey research.18
We aimed to determine if people from a general population with FD exercise less than controls. We hypothesized that lower exercise levels may be an unrecognized risk factor for FD.
Methods
Participants
Our sample of 3260 adults was randomly obtained from the Australian electoral register with minimal selection bias observed, as described in detail previously.15
Measures
The measures used in this study have been previously described.15 FD and IBS were diagnosed based upon responses to the valid Rome III questionnaire.19,20
In this study, Rome III was used to classify FD by the presence of at least one of the following upper gastrointestinal symptoms including postprandial fullness, early satiation, epigastric pain and burning.20 FD subgroups were as follows: postprandial distress syndrome (PDS) (presence of postprandial fullness and/or early satiation at least 1 day a week in the last 3 months) and epigastric pain syndrome (EPS) (presence of epigastric pain or discomfort at least 1 day per week during the last 3 months). No investigations were performed to confirm the diagnosis of FD.
To meet criteria for Rome III IBS, abdominal pain or discomfort had to be associated with two or more of the following symptoms: pain relieved by a bowel movement, onset of pain related to a change in frequency of stool or a change in the appearance of stool on at least 3 days for at least 3 months over the prior 6-month period.
Lifestyle factors
Questions from the validated National Health Survey21 were used to assess exercise and smoking status. Exercise levels over the last 2 weeks were grouped by the prevalence (yes or no) as well as frequency with respect to the number of occasions and duration of time spent walking (e.g. walked for sport, recreation or fitness), and engaging in moderate (any exercise that caused a moderate increase in heart rate or breathing) and vigorous exercise (any exercise that caused a large increase in heart rate or breathing). Smoking status included never, past or current smoker. Body mass index (BMI) was also calculated. The association of exercise with IBS-constipation in this cohort has been previously reported.15
Procedure
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a prior approval by the Hunter New England Human Ethics Committee on 25 May 2011. An information sheet was sent to participants but a consent form was not required for this postal study. Return of the survey provided implicit consent. Details on the procedure for this study have been reported elsewhere.15 Participants were not compensated for partaking in the study.
Statistical analyses
Unconditional logistic regression was conducted to differentiate subjects who met Rome III criteria for FD, including FD subgroups from those who did not in terms of the presence and frequency of exercise (Table 1). Cases were FD with or without comorbid IBS, whereas controls did not qualify for FD or IBS. Multivariate logistic modelling was performed to assess the extent to which the presence or frequency of exercise discriminates individuals having FD from those who do not while controlling for smoking, BMI, age and gender.
Table 1.
Prevalence and frequency of walking, and moderate and vigorous exercise in functional dyspepsia, postprandial distress syndrome, epigastric pain syndrome and irritable bowel syndrome.
| Prevalence % (95% CI) |
FD (n = 468) |
Controls (n = 2693) |
FD (n = 468) univariate OR (95% CI), P-value |
FD alone (excluding IBS), n = 252, controlling for age, gender, lifetime smoking and BMI, univariate OR (95% CI), P-value |
PDS alone (excluding IBS), n = 204, controlling for age, gender, lifetime smoking and BMI, univariate OR (95% CI), P-value |
EPS alone (excluding IBS), n = 81, controlling for age, gender, lifetime smoking and BMI, univariate OR (95% CI), P-value |
IBS alone (excluding FD), n = 294, controlling for age, gender, lifetime smoking and BMI, univariate OR (95% CI), P-value |
|---|---|---|---|---|---|---|---|
| Walking | 57 (52, 61) | 63 (61, 65) | 0.77 (0.63, 0.94), P = 0.01 |
0.78 (0.59, 1.05), P = 0.1 |
0.74 (0.54, 1.02), P = 0.07 |
0.80 (0.49, 1.30), P = 0.4 |
1.38 (1.04, 1.84) P = 0.03 |
| Moderate exercise | 58 (53, 62) | 57 (55, 59) | 1.04 (0.85, 1.28), P = 0.7 |
0.95 (0.71, 1.26), P = 0.7 |
0.76 (0.55, 1.04), P = 0.08 |
1.32 (0.81, 2.19), P = 0.3 |
1.17 (0.90, 1.53), P = 0.3 |
| Vigorous exercise | 19 (16, 23) | 22 (21, 24) | 0.83 (0.64, 1.07), P = 0.2 |
0.74 (0.51, 1.08), P = 0.1 |
0.65 (0.42, 1.00), P = 0.05 |
1.17 (0.66, 2.08), P = 0.6 |
1.23 (0.91, 1.68), P = 0.2 |
| Frequency Mean (SD) | |||||||
| Walking | 6.2 (4.2) | 7.0 (4.4) | 0.96 (0.93, 0.99), P = 0.008 |
0.99 (0.95, 1.02), P = 0.5 |
0.98 (0.93, 1.03), P = 0.4 |
0.96 (0.89, 1.04), P = 0.3 |
0.98 (0.94, 1.0) P = 0.4 |
| Moderate exercise | 5.2 (3.6) | 5.8 (3.8) | 0.96 (0.92, 1.0), P = 0.03 |
0.94 (0.88, 0.99), P = 0.02 |
0.95 (0.89, 1.02), P = 0.1 |
0.92 (0.84, 1.0), P = 0.08 |
1.0 (0.96, 1.05), P = 0.9 |
| Vigorous exercise | 3.4 (2.2) | 4.1 (2.9) | 0.90 (0.81, 0.99), P = 0.02 |
0.99 (0.87, 1.12), P = 0.8 |
1.03 (0.89, 1.19), P = 0.7 |
0.95 (0.79, 1.14), P = 0.6 |
1.06 (0.97, 1.16), P = 0.2 |
P < 0.05. BMI: body mass index; CI: confidence interval; EPS: epigastric pain syndrome; FD: functional dyspepsia; IBS: irritable bowel syndrome; OR: odds ratio; PDS: postprandial distress syndrome.
Results
Sample characteristics
The final sample of 3260 people (response rate 38%) consisted of slightly more females (53.8%) compared with males, with a mean age of 54.3 years (SD of 15.6 years), and just more than one-half (54.6%) had obtained a greater than high school education. Specific details of the sample characteristics have been previously reported.15
Prevalence of FD
We observed that n = 468 (14.8% (95% confidence interval (CI) 13.6, 16.1%)) subjects met Rome III criteria for FD. Of these, 252 had FD without IBS, 204 had PDS and 81 had EPS (33 had both).
Prevalence of IBS
We observed that n = 294 people met Rome III criteria for IBS without FD (10.9% (95% CI 9.8, 12.2%)).
Prevalence of lifestyle factors
Lifetime smoking rates were not significantly different between FD (50%) and controls (54%, P = 0.07). Those with FD (mean = 28.5, SD = 6.5) had a significantly higher BMI than controls (mean = 27.8, SD = 6.2) (P = 0.01).
Univariate associations with exercise
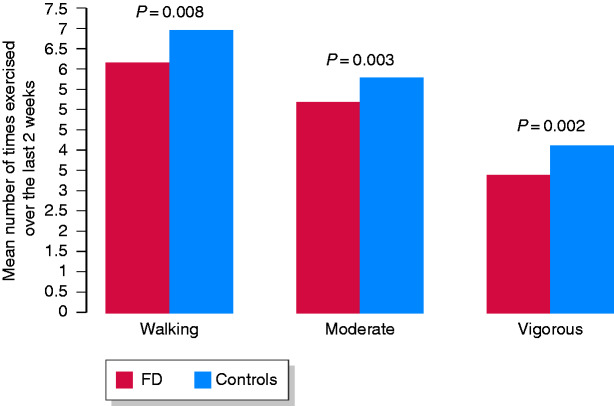
Univariately, significantly fewer people with FD walked. They also engaged in walking and lower moderate or vigorous exercise activity over a 2-week period compared with controls (Table 1 and Figure 1).
Figure 1.
Frequency of different types of exercise over the last 2 weeks among those who exercised in community functional dyspepsia versus controls.
FD; functional dyspepsia.
Multivariate model
FD
In a multivariate model excluding those with IBS that controlled for age, gender, smoking and BMI, a lower frequency of engaging in moderate exercise (odds ratio (OR) = 0.94 (95% CI 0.88, 0.99), P = 0.02) were independently associated with FD (Table 1).
FD subgroups
A lower prevalence of engaging (yes/no) in vigorous exercise (OR = 0.65 (95% CI 0.42, 1.00), P = 0.05) was borderline significantly associated with PDS-FD after controlling for confounders. Neither the prevalence nor frequency of exercising was significantly associated with EPS-FD (Table 1).
IBS
In a multivariate model excluding those with FD that controlled for age, gender, smoking and BMI, a higher prevalence of walking (OR = 1.38 (95% CI 1.04, 1.84) P = 0.03) was independently associated with IBS (Table 1).
Discussion
This study provides, for the first time, population-based data on the association between exercise and FD in a Western population. Our results suggest that FD, including PDS but not EPS, is associated with lower exercise levels and that this is not driven by age, gender, smoking, BMI or IBS.
We found that 57% of people with FD engaged in walking over the prior 2 weeks compared with 62% of those people without FD, a difference that was significant in the univariate analysis. People with FD also reported exercising on average fewer times over the prior 2 weeks in terms of walking, and engaging less in moderate and vigorous activity compared with those without FD. This remained significant for moderate exercise, independent of age, gender, BMI, smoking and IBS. These results are consistent with the findings from a recent internet survey of 15,000 adults in Japan, which reported that a significantly lower proportion of people with Rome III FD exercised frequently compared with those without FD.18 However, only a broad single item was used to measure exercise in the Japanese study, and the prevalence of background Helicobacter pylori infection and gastric cancer is much higher in Japan than Australia,18 where H. pylori infection rates are between 15–31%.22 The advantage in the current study was that both the presence and frequency of three levels of physical activity were assessed, and potential confounders were carefully considered.
Whether the relationship between exercise and FD is causal remains to be determined, and cannot be ascertained by this cross-sectional investigation. Physical activity has been shown to increase circulating endogenous endorphins, which it has been hypothesized may reduce symptom severity.23 Endorphins interact with the receptors in the brain to reduce the perception of pain.24 Exercise can have a positive effect on mood,25 which is notably disturbed in a significant proportion of people with functional gastrointestinal disorders including FD.26 Some research has also found that low-intensity exercise, compared with moderate-intensity exercise, is associated with faster gastric emptying in healthy individuals.27 It is conceivable that reduced exercise may be a consequence of FD symptoms, but we suspect that lower exercise rates may alter the upper intestinal microbiome, which has been implicated in the pathogenesis of FD7,28 along with low-grade duodenal inflammation, especially duodenal eosinophilia, in PDS.11 Prospective longitudinal studies are needed to determine the causal role of exercise in the development of FD symptoms, if any.
The strengths of the study included that the sample was randomly obtained from the Australian electoral register, which legally requires all persons aged ≥ 18 years to be registered. FD was assessed using a valid questionnaire based on the Rome III criteria.19 The prevalence of FD in the sample is very consistent with other data on prevalence in Australia and globally.29–30 The response rate in this study was similar to that found in other large community-based studies in Australia and overseas,31 and only minimal selection bias in those who responded to the survey versus those who did not was found. Limitations of the current study include exercise measured via self-report, albeit with a standardized measure. We did not ask about other potential confounders including comorbid medical conditions that may also affect the ability to engage in physical activity, and we did not endoscopically analyse subjects to confirm the diagnosis of FD, but other data suggest that the probability of finding an organic explanation in the population setting if an upper endoscopy is done would be low.30 Medications and sleep disturbances were also not able to be assessed in this study.
Although the proportion of IBS individuals who reported walking was higher than healthy controls, the effect size was small and only reached statistical significance due to the very large sample size, which yielded high statistical power for subtle effect sizes. A recent meta-analysis has suggested that exercise could be an effective treatment for IBS patients, but further rigorous studies are needed to determine the true benefits of exercise on IBS.32 This also applies to FD, as at present there are no randomized controlled trials assessing the effectiveness of exercise on reducing FD symptoms. Based on the current results, the clinical practice of advising patients with FD to increase physical activity appears warranted, but future randomized controlled trials are awaited with great interest.
Declaration of conflicting interests
NAK has nothing to disclose.
MPJ: consultancies with GI Therapies (abdominal stimulation in constipation) and SFI (prokinetics).
MMW has received grant research support from Prometheus Laboratories Inc. (IBS Diagnostic) and Commonwealth Diagnostics International (biomarkers for Functional Gastrointestinal Disorders (FGIDs))
GH has received unrestricted educational support from Bayer Ptd, Ltd and the Falk Foundation; research support provided via the Princess Alexandra Hospital, Brisbane by GI Therapies Pty Ltd, Takeda Development Center Asia, Pty Ltd, Eli Lilly Australia Pty Ltd, F.Hoffmann-La Roche Ltd, MedImmune Ltd Celgene Pty Ltd, Celgene International II Sarl, Gilead Sciences Pty Ltd, Quintiles Pty Ltd, Vital Food Processors Ltd, Datapharm Australia Pty Ltd Commonwealth Laboratories, Pty Ltd, Prometheus Laboratories, Falk GmbH and Co Kg, Nestle Pty Ltd and Mylan; patent holder ‘A biopsy device to take aseptic biopsies’ (US 20150320407 A1).
NJT has received personal fees from Allergans PLC (GI Development Programs), personal fees from Viscera Labs (IBS), personal fees from IM Health Sciences (FD), personal fees from Napo Pharmaceutical (IBS), personal fees from Outpost Medicine (IBS), personal fees from Progenity Inc. San Diego (capsule SIBO), personal fees from Allakos (gastric eosinophilic disease), personal fees from Samsung Bioepis (IBD), personal fees from Synergy (IBS), personal fees from Takeda (gastroparesis), personal fees from Theravance (gastroparesis), grants and personal fees from Viscera USA (IBS), grants from Commonwealth Diagnostics (International) Inc. (IBS), non-financial support from HVN National Science Challenge NZ (IBS), grants and personal fees from GI therapies (constipation), personal fees from Cadila Pharmaceuticals (CME), personal fees from Planet Innovation (Gas capsule), personal fees from Danone (Probiotic), personal fees from Pfizer (IBS), from Dr Reddy’s Laboratories (webinar), personal fees from Arlyx (IBS), personal fees from Sanofi (Probiotic), outside the submitted work; in addition, Dr Talley has a patent ‘Biomarkers of IBS’ licensed, a patent ‘Licensing Questionnaires Talley Bowel Disease Questionnaires’ licensed to Mayo/Talley, a patent ‘Nestec European Patent’ licensed, a patent Singapore Provisional Patent ‘Microbiota Modulation Of BDNF Tissue Repair Pathway’ issued, and a patent ‘Nepean Dyspepsia Index’ licensed to Talley copyright; committees: Australian Medical Council (Council member), Australian Telehealth Integration Programme, MBS Review Taskforce, NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors; boards: GESA Board Member, Sax Institute, Committees of the Presidents of Medical Colleges; community groups: Advisory Board, International Foundation for Functional GI Disorders; miscellaneous: Avant Foundation (judging of research grants); editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, and Sungkyunkwan University School of Medicine (South Korea).
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a prior approval by the Hunter New England Human Ethics Committee on 25 May 2011.
Funding
Funding was provided by Janssen as an Investigator-Initiated Study.
Informed consent
Return of the survey provided implicit consent.
ORCID iDs
Michael Jones https://orcid.org/0000-0003-0565-4938
References
- 1.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2016; 374: 896. [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol 2006; 12: 2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. American J Gastroenterol 2002; 97: 2290–2299. [DOI] [PubMed] [Google Scholar]
- 4.Lacy BE, Weiser KT, Kennedy AT, et al. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther 2013; 38: 170–177. [DOI] [PubMed] [Google Scholar]
- 5.Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol 2000; 95: 67–71. [DOI] [PubMed] [Google Scholar]
- 6.Ohlsson B. The role of smoking and alcohol behaviour in management of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol 2017; 31: 545–552. [DOI] [PubMed] [Google Scholar]
- 7.Costa RJ, Snipe RM, Kitic CM, et al. Systematic review: exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment Pharmacol Ther 2017; 46: 246–265. [DOI] [PubMed] [Google Scholar]
- 8.Zhong L, Shanahan ER, Raj A, et al. Dyspepsia and the microbiome: time to focus on the small intestine. Gut 2017; 66: 1168–1169. [DOI] [PubMed] [Google Scholar]
- 9.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017; 2017: 3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol 2011; 106: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol 2007; 5: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 12.Valdés-Ramos R, Martínez-Carrillo BE, Aranda-González II, et al. Diet, exercise and gut mucosal immunity. Proc Nutr Soc 2010; 69: 644–650. [DOI] [PubMed] [Google Scholar]
- 13.von Wulffen M, Talley NJ, Hammer J, et al. Overlap of irritable bowel syndrome and functional dyspepsia in the clinical setting: prevalence and risk factors. Dig Dis Sci 2019; 64: 480–486. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Jin F, Chi B, et al. Gender differences in irritable bowel syndrome among medical students at Inner Mongolia Medical University, China: a cross-sectional study. Psychol Health Med 2016; 21: 964–974. [DOI] [PubMed] [Google Scholar]
- 15.Koloski NA, Jones M, Young M, et al. Differentiation of functional constipation and constipation predominant irritable bowel syndrome based on Rome III criteria: a population-based study. Aliment Pharmacol Ther 2015; 41: 856–866. [DOI] [PubMed] [Google Scholar]
- 16.Johannesson E, Ringström G, Abrahamsson H, et al. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J Gastroenterol 2015; 21: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mönkemüller K, Malfertheiner P. Drug treatment of functional dyspepsia. World J Gastroenterol 2006; 12: 2694–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miwa H. Life style in persons with functional gastrointestinal disorders–large-scale internet survey of lifestyle in Japan. Neurogastroenterol Motil 2012; 24: 464–471. [DOI] [PubMed] [Google Scholar]
- 19.Rome Foundation. Guidelines–Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointest Liver Dis 2006; 15: 307–312. [PubMed] [Google Scholar]
- 20.Whitehead WE, Palsson OS, Thiwan SIM, et al. Development and validation of the Rome III diagnostic questionnaire In: Drossman DA, Corazziari E, Delvaux M, eds. Rome III: The functional gastrointestinal disorders, 3rd ed McLean, VA: Degnon Associates, 2006; 835–853. [Google Scholar]
- 21.National Health Survey: Users’ Guide -- Electronic Publication, 2007. –08. https://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/CC0FB5A08570984ECA25762E0017CF2B/$File/4363055001_2007-08.pdf (accessed 23 March 2020)
- 22.Mitchell H, Katelaris P. Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection. Med J Aust 2016; 204: 376–380. [DOI] [PubMed] [Google Scholar]
- 23.Harber VJ, Sutton JR. Endorphins and exercise. Sports Med 1984. ; 1: 154–171. [DOI] [PubMed] [Google Scholar]
- 24.Sprouse-Blum AS, Smith G, Sugai D, et al. Understanding endorphins and their importance in pain management. Hawaii Med J 2010; 69: 70. [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung RR. The acute effects of exercise on mood state. J Psychom Res 1996; 40: 123–141. [DOI] [PubMed] [Google Scholar]
- 26.Koloski NA, Jones M, Kalantar J, et al. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 2012; 61: 1284–1290. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki J, Suzuki H, Masaoka T, et al. Influence of regular exercise on gastric emptying in healthy men: a pilot study. J Clin Biochem Nutr 2016; 59: 130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang SS, Jeraldo PR, Kurti A, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener 2014; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther 2016; 44: 592–600. [DOI] [PubMed] [Google Scholar]
- 30.Aro P, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology 2009; 137: 94–100. [DOI] [PubMed] [Google Scholar]
- 31.Cook JV, Dickinson HO, Eccles MP. Response rates in postal surveys of healthcare professionals between 1996 and 2005: an observational study. BMC Health Serv Res 2009; 9: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil 2018; 19: e13461. [DOI] [PubMed] [Google Scholar]