Abstract
Introduction
Capsule endoscopy is an important modality for monitoring of Crohn’s disease. Recently, a novel panenteric capsule, PillCam Crohn’s (Medtronic, USA), was approved for use. No quantitative index of inflammation for this method is currently available. This sub-study of a prospective randomized controlled Comprehensive individUalized pRoactive ThErapy of Crohn’s Disease trial (CURE-CD) which aimed to compare the correlation and reliability of the novel PillCam Crohn’s score with the existing small bowel capsule Lewis inflammatory score.
Methods
The study cohort included Crohn’s disease patients in remission who were evaluated with PillCam Crohn’s. Each result was independently reviewed by two experienced readers. Inflammation was scored in all studies using Lewis inflammatory score and PillCam Crohn’s score (comprised of a sum of scores for most common and most severe lesions multiplied by percentage of segmental involvement + stricture score).
Results
Fifty-four PillCam Crohn’s studies from 41 patients were included. The median Lewis inflammatory score was 225 for both readers. The median PillCam Crohn’s score was six (0–14) and four (3–15) for readers 1 and 2, respectively. There was a high inter-rater reliability coefficient between the two readers for Lewis inflammatory and PillCam Crohn’s score (0.9, p < 0.0001 for both). The correlation between PillCam Crohn’s score and fecal calprotectin was stronger than for Lewis inflammatory score (r = 0.32 and 0.54 respectively, p = 0.001 for both).
Conclusions
The novel panenteric capsule score correlates well with the Lewis inflammatory score, has excellent reliability, and may be potentially more accurate in estimation of the panenteric inflammatory burden.
Keywords: Crohn’s disease, PillCam Crohn’s, treat to target, monitoring Crohn’s disease, capsule endoscopy
Key points
Summarize the established knowledge on this subject
1. Capsule endoscopy is one of the prime modalities to monitor Crohn’s disease.
2. PillCam Crohn’s is a novel panenteric capsule endoscope.
3. No quantitative score for PillCam Crohn’s has been described.
What are the significant and/or new findings of this study?
1. We describe a novel quantitative score for Crohn’s capsule.
2. The score has excellent reproducibility and is well-correlated with the Lewis score and fecal calprotectin.
Introduction
Capsule endoscopy (CE) is one of the prime modalities for monitoring of Crohn’s disease (CD). The main indications of CE in established CD include disease classification, monitoring for mucosal healing, and evaluation of unexplained symptoms and anemia.1–5 In the recent European Crohn's and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] guidelines, CE is recommended along with intestinal ultrasound and magnetic resonance enterography for initial evaluation and follow-up of established CD.2 In a recent study, quantitative assessment of small bowel inflammation on CE was the most accurate predictor of relapse within two years in CD patients in remission.3 The Lewis score (LS) is a quantitative index of inflammation for CE, embedded in the software of the Medtronic capsules, that incorporates parameters of mucosal ulceration, edema, and stenosis.6,7
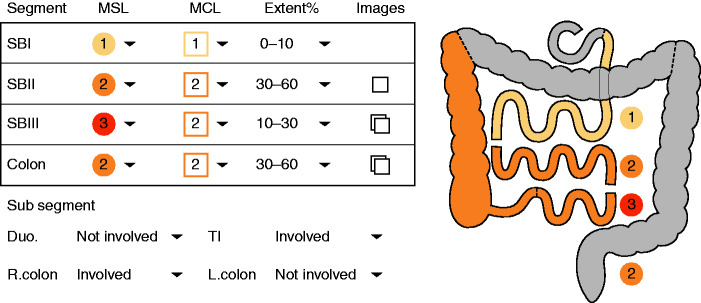
Patients with CD are frequently required to undergo multiple diagnostic evaluations including ileo-colonoscopies, cross-sectional imaging, and CE. Thus, the concept of a “one-stop-shop” modality to evaluate the entire digestive tract is appealing to both patients and physicians. In recent years, several studies have described the use of the double-headed colon capsule for the evaluation of both small and large bowel in CD.8–10 Recently, a novel PillCam Crohn’s (PCC) (Medtronic, Dublin, Ireland) was issued. PCC combines a long-lasting (up to 14 h) battery with two adjustable frame-rate wide-angle cameras and novel software (Rapid 9) that allows for streamlined and efficient reading of CE images for both the small bowel and the colon11 (see Figures 1 and 2). However, currently, no validated quantitative endoscopic index exists for PCC.
Figure 1.
Gastro-intestinal (GI) map as produced by Rapid (Medtronic, Dublin, Ireland) for PillCam Crohn’s capsule (PCC). MCL: most common lesion; MSL: most severe lesion; SBI: small bowel first tertile; SBII: small bowel second tertile; SBIII: smll bowel third tertile.
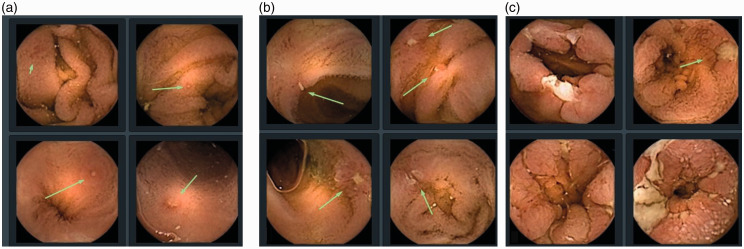
Figure 2.
Severity of inflammatory lesions (source - Pillcam Crohn’s capsule atlas, Rapid Reader version 9, Medtronic, Dublin, Ireland).
(a) mild; (b) moderate; (c) severe.
Thus, in the current study we aimed to create and evaluate a quantitative PCC score to monitor panenteric inflammation in CD.
Methods
This was an ancillary project of the Comprehensive individUalized pRoactive ThErapy of Crohn’s Disease trial (CURE-CD). The CURE-CD trial is a prospective randomized controlled trial of CD patients in remission which aimed to evaluate a PCC-based treat-to-target strategy to prevent clinical relapse. Patient enrolled in the study are prospectively followed by serial PCC, intestinal ultrasound, magnetic resonance enterography (MRE), and fecal calprotectin. The complete protocol of the study is available online (NCT03555058; https://clinicaltrials.gov/ct2/show/NCT03555058).
The study was approved by the Sheba Medical Center Institutional Review Board (IRB) in 2018.
Study population
The study population includes adult CD patients in steroid-free clinical remission (Crohn’s disease activity index (CDAI) of < 150) with a duration of 3–24 months on a stable medication dose (for 60 days for thiopurines, methotrexate, infliximab, and vedolizumab, and 30 days for all other agents).
Ethical approval
The study was approved by Sheba IRB committee on 30 November 2018 (approval number 4945-18-SMC). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s Human Research Committee. Written, informed consent was obtained from each patient included in the study
PCC procedure
Upon enrollment, eligible patients underwent patency capsule examination (Medtronic, Dublin Ireland). If the patency capsule was undetectable or was excreted within 30 h of ingestion, PCC was performed. PCC preparation included a clear liquid diet on the day prior to capsule swallowing and administration of a purgative sulfate-free polyethylene glycol electrolyte lavage (SF-ELS) solution (e.g. PEG, Fortrans, Solution Bohm) divided into two doses: 1.5 l on the evening before the examination and 1.5 l on the morning of the examination day. Following capsule ingestion, and depending on capsule progression through the digestive tract, subjects were required to take an additional volume of laxative in order to enhance capsule propulsion and maintain adequate cleansing of the colon. All subjects received the first dose of the additional laxative which consists of one sachet of PICO-SALAX (10 mg sodium picosulfate) diluted in 75 ml of water upon small bowel detection on real-time viewer. If the capsule remained in the stomach for more than an hour after ingestion, metoclopramide 10 mg Per orum (PO) could be administered as per the investigators decision. If the PCC was not excreted within three hours of ingestion, a second sachet of PICO-SALAX was administered three hours after the first. If towards the end of the CE procedure (two hours after the second boost) the capsule was not excreted, the subjects were asked to use a 10 mg Bisacodyl suppository. All booster doses were followed by intake of one liter of water in the following hour. Clear liquid ingestion was permitted throughout the examination and preparation.
The patients were followed by PCC every six months. If no colonic disease was detected on the index PCC, subsequent examinations were performed without colonic cleansing.
To enhance patients' safety, in this particular study setting, patients in whom a severe stricture was disclosed on PCC after patency capsule (PC) passage were excluded from enrollment and further capsule examinations. In the absence of consensus scale definition for severity of intestinal strictures, a severe stricture was pragmatically defined for the purpose of this study as luminal narrowing not trespassed by the capsule within 30 min of its first visualization.
Endoscopic activity scoring
All studies were read using the Rapid PillCam Reader v.9.0 (Medtronic, Dublin, Ireland). The small bowel was scored using the LS using the automated calculator embedded in the software. Each small bowel tertile was scored individually. In addition, the left and the right colon were manually scored the similar operators as those used for the small bowel LS (right and left colon). The small bowel LS was derived of the score of the tertile with the most significant disease involvement + stricture score. Cumulative small bowel LS (CSB-LS) was calculated as the summary of individual small bowel tertile scores + stricture score, and the cumulative panenteric LS (CPE-LS) as the summary of small bowel and colonic segments + stricture score (Table 1). Colonic and panenteric LS were not calculated if studies were performed without colonic preparation or if the capsule did not reach the colon.
Table 1.
Pillcam Crohn’s disease capsule score.
| A. Most common lesion (MCL) |
| 0 = none |
| 1 = mild |
| 2 = moderate |
| 3 = severe |
| B. Most severe lesion (MSL) |
| 0 = none |
| 1 = mild |
| 2 = moderate |
| 3 = severe |
| C. Extent of disease |
| 0 = none |
| 1 = 10–30% |
| 2 = 30–60% |
| 3 = 60–100% |
| D. Stricture |
| 0 = None |
| 1 = One traversed |
| 2 = >1 traversed |
| 3 = Retention |
| ––––––––––––––––––––––– |
| Segmental score = ((A+B)×C)+D |
| Small bowel PCC (PCCS-SB) = SB1+SB2+SB3 |
| Panenteric PCC (PCCS) = SB1+SB2+SB3+RC+LC |
LC: left colon; PCC: PillCam Crohn's capsule; PCCS: panenteric Crohn's capsule score; RC: right colon; SB: small bowel.
Panenteric Crohn's capsule score (PCCS) was calculated using the reporting system embedded in the Rapid PillCam Reader v.9.0. The reporting system uses the following operators for each bowel segment (small bowel – three tertiles (SB1-3), left colon (LC), and right colon (RC)) (Table 1): most common lesion (graded by severity as 1–3), most severe lesion (graded by severity as 1–3), approximated disease extent. Examples of lesion severity and extent appear on Figures 1 and 2. The score was calculated for each segment, in addition to the entire PCCS small bowel (PCCS-SB) (a summation of three small bowel segmental scores, with an additional stricture score) and the PCCS (a summation of small bowel and colonic scores + stricture score). The detailed calculation of the scores appears in Table 1.
Bowel cleansing was evaluated as described in Table 2.
Table 2.
Cleanliness score for capsule endoscopy.
| Poor – inadequate- precluding a complete examination (large amounts of fecal residue). |
| Fair – inadequate- but examination completed (enough feces or turbid fluid present to prevent a reliable exam). |
| Good – adequate with few liquids (small amounts of feces or turbid fluid not interfering with exam). |
| Excellent – no more than small bits of adherent feces. |
Statistical analysis
Descriptive statistics were presented as means ±standard deviations for continuous variables and percentages for categorical variables. Categorical variables were analyzed by chi square/Fisher’s exact test and continuous variables-by Student t-test/Mann Whitney test as appropriate. We performed a Pearson correlation analysis for correlation capsule scores with each other and biomarker levels. For agreement between readers, Cohen’s kappa was and interclass correlation (ICC) were performed. Correlation r values < 0.3 were considered as weak-to-low correlation, 0.3–0.49 as low-to-moderate, 0.5–0.69 as moderate, and ≥0.7 as strong correlation.12 The Wilcoxon signed-rank test was performed to compare the individual scores between timepoints. A univariate linear regression was performed to identify the Pillcam Crohn's capsule endoscope (PCCE) values that correspond to the segmental LS cut-off values of 135, 350, and 790. A two-tailed p value < 0.05 was considered statistically significant. The analysis was performed using IBM SPSS statistic (Version 22.0) (Armonk, New York, USA).
Results
Patient characteristics
Forty-one patients CD patients in remission were enrolled and underwent 54 PCC examinations (41 at baseline, 9 at 6 months, and 4 at 12 months). The clinical characteristics of the included patients are described in detail in Table 3. Two patients were excluded from the study after the initial PCC due to small bowel strictures.
Table 3.
Clinical and demographic characteristics of the included patients.
| n | % | |
|---|---|---|
| Gender | ||
| Female | 14 | 34.1% |
| Male | 27 | 65.9% |
| Age, years (median, IQR) | 25 | 21–39 |
| Current smoking | 4 | 9.8% |
| Disease duration, years (median, IQR) | 36 | 18–78 |
| Disease location | ||
| Small bowel | 31 | 76.0% |
| Colon | 1 | 2.4% |
| Small bowel and colon | 9 | 22.0% |
| Disease phenotype | ||
| Inflammatory | 24 | 58.5% |
| Stenotic | 11 | 26.8% |
| Penetrating | 6 | 14.6% |
| Perianal disease | 4 | 10.0% |
| Previous surgery | 9 | 23.7% |
| Current medications | ||
| None | 14 | 34.1% |
| 5-ASA | 3 | 7.3% |
| Thiopurines | 4 | 9.8% |
| Adalimumab | 16 | 39.0% |
| Infliximab | 5 | 12.2% |
| Vedolizumab | 2 | 4.9% |
IQR: interquartile range.
PCCE characteristics
Fifty-two (96.3%) of the capsules reached the cecum. Both of the capsules that did not reach the cecum while photographing were eventually excreted by the patient and did not result in capsule retention. Overall, the colon was assessed on 40/54 (74%) PCCs. The rectum or toilet were reached in 38/41 (92.6%) of the capsules while photographing (95%), in one patient PCC stopped photographing in the left colon and in two patients in the terminal ileum. In an additional patient, the colon was not reviewed due to poor preparation
Out of 13 follow-up PCCs (6 and 12 months), the colon was reviewed in 2/14 patients, as in 11/13 patients no colonic disease was detected on the baseline PCC.
Quality of preparation
The quality of small bowel preparation was deemed good or excellent in 50/54 PCCs (94.4%). The quality of the preparation did not preclude capsule reading in any of the capsules. Out of 40 PCCs in which the colon was reviewed, the preparation was good or excellent in 30 (75%), fair in nine (22.5%), and poor (colon images could not be read) in one (2.5%).
Capsule activity scores
The median values of the endoscopic scores for both readers appear in Table 4.
Table 4.
Correlations of capsule inflammatory scores.
|
Correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | FCP | LS R1 | LS R2 | CSB-LS R1 | CSB- LS R2 | CPE-LS R1 | CPE-LS R2 | PCCS-SB R1 | PCCS-SB R2 | PCCS R2 | PCCS R1 | |
| Median (IQR) | 2.3(0–5.6) | 72(30–236) | 225(135–600) | 225(0–981) | 450(225–921) | 267(0–1200) | 225(135–675) | 225(0–608) | 6(6–15) | 4(0–12) | 4(0–14) | 6(3–15) |
| CRP | ||||||||||||
| r | 1.00 | 0.47 | 0.40 | 0.29 | 0.36 | 0.24 | 0.28 | 0.30 | 0.37 | 0.22 | 0.36 | 0.47 |
| p | 0.00 | 0.00 | 0.04 | 0.01 | 0.09 | 0.05 | 0.04 | 0.01 | 0.13 | 0.01 | 0.00 | |
| FCP | ||||||||||||
| r | 0.47 | 1.00 | 0.38 | 0.32 | 0.39 | 0.35 | 0.33 | 0.40 | 0.50 | 0.45 | 0.54 | 0.55 |
| p | 0.00 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| LS R1 | ||||||||||||
| r | 0.40 | 0.38 | 1.00 | 0.93 | 0.95 | 0.88 | 0.84 | 0.85 | 0.69 | 0.71 | 0.72 | 0.61 |
| p | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| LS R2 | ||||||||||||
| r | 0.29 | 0.32 | 0.93 | 1.00 | 0.93 | 0.94 | 0.84 | 0.88 | 0.77 | 0.80 | 0.78 | 0.66 |
| p | 0.04 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| CSB-LS R1 | ||||||||||||
| r | 0.36 | 0.39 | 0.95 | 0.93 | 1.00 | 0.95 | 0.84 | 0.84 | 0.81 | 0.83 | 0.84 | 0.72 |
| p | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| CSB- LS R2 | ||||||||||||
| r | 0.24 | 0.35 | 0.88 | 0.94 | 0.95 | 1.00 | 0.77 | 0.83 | 0.83 | 0.87 | 0.85 | 0.70 |
| p | 0.09 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| CPE-LS R1 | ||||||||||||
| r | 0.28 | 0.33 | 0.84 | 0.84 | 0.84 | 0.77 | 1.00 | 0.95 | 0.56 | 0.49 | 0.64 | 0.66 |
| p | 0.05 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| CPE-LS R2 | ||||||||||||
| r | 0.30 | 0.40 | 0.85 | 0.88 | 0.84 | 0.83 | 0.95 | 1.00 | 0.68 | 0.59 | 0.74 | 0.76 |
| p | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PCCS-SB R1 | ||||||||||||
| r | 0.37 | 0.50 | 0.69 | 0.77 | 0.81 | 0.83 | 0.56 | 0.68 | 1.00 | 0.90 | 0.92 | 0.91 |
| p | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PCCS-SB R2 | ||||||||||||
| r | 0.22 | 0.45 | 0.71 | 0.80 | 0.83 | 0.87 | 0.49 | 0.59 | 0.90 | 1.00 | 0.94 | 0.75 |
| p | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PCCS R2 | ||||||||||||
| r | 0.36 | 0.54 | 0.72 | 0.78 | 0.84 | 0.85 | 0.64 | 0.74 | 0.92 | 0.94 | 1.00 | 0.90 |
| p | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PCCS R1 | ||||||||||||
| r | 0.47 | 0.55 | 0.61 | 0.66 | 0.72 | 0.70 | 0.66 | 0.76 | 0.91 | 0.75 | 0.90 | 1.00 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
CPE-LS: cumulative panenteric Lewis score; CRP: C-reactive protein; CSB-LS: cumulative Lewis score; FCP: fecal calprotectin; LS: Lewis score; PCCS: panenteric Crohn’s capsule score; R1/2: reader 1/2; SB: small bowel.
The correlation between readers was excellent for LS (r = 0.93), CSB-LS (r = 0.94), CPE-LS (r = 0.95), PCCS-SB and PCCS (r = 0.9 for both), p < 0.001 for al correlations. There was a strong correlation between CSB-LS and PCC-SB for both readers (r = 0.8 for reader 1 and r = 0.82 for reader 2, p < 0.001 for both), a moderate correlation between LS and PCC-SB for reader 1 and strong for reader 2 (r = 0.69 and 0.80, p < 0.001, respectively) . For panenteric scores, the correlation between PCCS and CPE-LS was moderate (r = 0.66, p < 0.0001) for reader 1 and strong (r = 0.74, p < 0.0001) for reader 2.
The agreement between the readers was strong (>0.9 for all scores) (Table 5). There was no significant difference between capsule scores obtained at baseline and after 6 months for patients who had >1 capsule endoscopy performed (Supplementary Material Table).
Table 5.
Interclass correlations between two readers for endoscopic capsule scores.
| ICCs | 95% CI | p | ICCa | 95% CI | p | |
|---|---|---|---|---|---|---|
| LS | 0.94 | 0.89–0.96 | <0.001 | 0.96 | 0.95–0.98 | <0.001 |
| PCCS-SB | 0.9 | 0.84–0.93 | <0.001 | 0.95 | 0.91–0.97 | <0.001 |
| PCCS | 0.9 | 0.84–0.94 | <0.001 | 0.95 | 0.91–0.97 | <0.001 |
CI: confidence interval; ICCa: interclass correlation, average measures; ICCs: interclass correlation, single measures; LS: Lewis score; PCCS: panenteric Crohn's capsule score; SB: small bowel.
The correlation between endoscopic scores and C-reactive protein (CRP) was low or low-to moderate for all scores (see Table 5) with the strongest correlation obtained by PCCS for reader 1 (r = 0.47, p = 0.001). For fecal calprotectin (FCP), the correlation was stronger and statistically significant for all scores, with the best correlation obtained for PCCS (r = 0.55 and 0.54 for readers 1 and 2 respectively, p < 0.001 for both comparisons).
Agreement for clinically relevant cut-offs
For LS < 135, there was a moderate agreement between readers (k = 0.58). For LS > 135 and LS > 350, the agreement was strong (k = 0.88 and 0.86, respectively, p < 0.001 for both comparisons).
We performed a univariate linear regression to calculate the PCCS-SB values that parallel the clinically relevant cut-off values of 135, 350, and 790. The regression was calculated for reader 2, as the correction between the scores was stronger for reader 2 (r = 0.8, r2 = 0.64, p < 0,001). The obtained regression equation was PCCS-SB = 2.6 + (0.007) × LS. PCCS values were rounded off. Thus, the calculation of the cut-off values is LS 135 = 4, LS 350 = 5, LS 790 = 8.
Discussion
The current study is a validation of a quantitative activity score for the novel PIllCam Crohn’s CE by two independent experienced capsule readers. The reliability of the PCCs was excellent, and the correlation with fecal calprotectin is superior to that of the LS. CE is essential for both initial diagnosis and monitoring of established CD. The main indications for the use of CE in established CD include disease classification,13 monitoring for mucosal healing,14 evaluation of unexplained symptoms,15–17 and anemia.1,2,4 Panenteric capsule endoscopy provides an efficient, patient-friendly, option for comprehensive monitoring of the small bowel and the colon without the need for additional endoscopic procedures. Several recent publications have described the use of a colonic capsule while disengaging its two-hour sleep mode (PillCam Colon Capsule 2) for the same purpose,8,10,18–20 however PillCam Crohn’s capsule represents a further evolution of this technology. The major upgrade introduced in the PCCE is the completely redesigned reading system that was adjusted to facilitate and streamline the reading of CD images. The features of the new system include a new mode to describe and assess disease severity and extent as well as options to report and compare patient treatment over time and review prior studies (Figures 1 and 2). In a recently published pilot study that included 41 patients, all performed procedure were successful and with no retention, with 83% reaching the toilet while photographing.11
Several quantitative inflammatory scores for CE have been developed over the years. The most frequently utilized is the LS6 that is embedded in the Rapid Reader software. LS addresses the parameters of mucosal appearance and ulceration (both longitudinal and circumferential extent) in each of the three small bowel segments (Figure). The LS is derived from the worst segmental score augmented by stricture score. Several important cut-off values were identified for LS. LS < 135 signifies normal colonic mucosa, while LS > 790 is deemed to represent moderate-to-severe inflammation.6 In clinical remission, patients with LS > 350 have a 10-fold risk of relapse in comparison to those with a lower LS.3 There are several limitations to the LS. Primarily, the score is not cumulative. In addition, the numerical value assigned to strictures is very high and it easily surpasses any value derived from inflammatory findings such as ulcers. The correlation of LS with fecal calprotectin is moderate at best as demonstrated in several studies.4,21,22 Interestingly, a modified “cumulative” variation of the score (a summation of segmental scores) does not perform differently from the “classic” LS for correlation with FCP.5,23 An additional CE score (capsule endoscopy Crohn’s disease activity index-CECDAI)24 has also been developed; there is a strong correlation between CECDAI and LS and a similar correlation with FCP levels.25
To date, no quantitative activity index was available for the PCC. In the current study, we developed a score that is based on the descriptive operators defined by the PCC reading software with a very simple and intuitive calculation (as described in the Methods section). The reliability of the score was excellent, and there was a significant correlation with the LS. The stronger correlation with FCP may be explained by the panenteric and cumulative nature of the score. Thus the PCC score may possibly be a more accurate surrogate of the true panenteric inflammatory burden.
Our study has several limitations. Primarily, the study cohort was comprised of patients in clinical remission, with a relatively low degree of mucosal inflammation. Furthermore, the proportion of patients with colonic disease was quite low. The number of subjects with repeated PCC examinations was quite low. Nevertheless, the main purpose of our study was to describe and evaluate the reliability of the novel PCC index; its performance for repeated evaluations will need to be evaluated in further detail in subsequent studies.
An additional limitation stems from the different methods of segmentation used by the small bowel capsules and the PCC. LS, designed for small bowel capsules, splits the small bowel into three equal segments using transit time. PCC software, on the other hand, approximates anatomical segmentation. The created tertiles do not necessarily overlap, potentially creating a measurable difference in individual tertile scores obtained by two systems. However we believe that this is a very minor limitation for all practical purposes, and this is confirmed by strong correlations of the total scores reported in this study.
One important limitation of the PillCam Crohn’s capsule is the need for vigorous colonic preparation, similar to the regimen used for colonic capsules.11 Although the small bowel capsule is a very use-friendly modality,26 extensive preparation used for the PCC may potentially hamper the patient’s willingness to undergo and repeat the procedure. In our study, colonic preparation was poor and precluded reading the images in only one patient. Moreover, in the current study we did not require colonic preparation in patients without colonic involvement on the initial examination; the main reason for this decision was the attempt to improve the patient experience. However, in the future we should consider evaluating a simpler and less rigorous cleansing protocol for PCCE.
Despite the aforementioned limitations, the PCC score proposed by our group provides a reliable, accurate, and user-friendly quantitative index for panenteric CE. Its usability, responsiveness to change, and predictive accuracy merits further prospective evaluation.
Supplemental Material
Supplemental material, UEG913368 Supplemental Material for A novel PillCam Crohn’s capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn’s disease by Rami Eliakim, Doron Yablecovitch, Adi Lahat, Bella Ungar, Eyal Shachar, Dan Carter, Limor Selinger, Sandra Neuman, Shomron Ben-Horin and Uri Kopylov in United European Gastroenterology Journal
Author contribution
RE and UK reviewed the capsules. RE, SBH, UK, AL, SN, LS were involved in study design. All authors reviewed the manuscript and contributed valuable scientific input.
Declaration of conflicting interests
RE received consultant and speaker fees from Janssen, Abbvie, Takeda, and Medtronic. SB-H received consulting and advisory board fees and research support from AbbVie, Janssen, Takeda, and CellTrion and consulting and speaker fees from Pfizer, GlaxoSmithKline, and MSD. UK received speaker and consultant fees from Abbvie, Janssen, Medtronic, and Takeda, and research support from Takeda, Medtronic, and Janssen. BU received consultancy and lecture fees from Jannsen Abbvie and Takeda. The remaining authors did not have any conflicts of interest to declare.
Ethics approval
The study was approved by Sheba Medical Center Ethics Review board.
Funding
This work was supported by a generous grant from the Leona M. and Harry B. Helmsley Charitable Trust.
Informed consent
All the patients signed an informed consent form.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 2019; 13: 273–284. [DOI] [PubMed] [Google Scholar]
- 2.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Horin S, Lahat A, Amitai MM, et al. Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn’s disease flare: A prospective cohort study. Lancet Gastroenterol Hepatol 2019; 4: 519–528. [DOI] [PubMed] [Google Scholar]
- 4.Kopylov U, Koulaouzidis A, Klang E, et al. Monitoring of small bowel Crohn’s disease. Expert Rev Gastroenterol Hepatol 2017; 11: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 5.Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: Systematic review and meta-analysis. Dig Liver Dis 2017; 49: 854–863. [DOI] [PubMed] [Google Scholar]
- 6.Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008; 27: 146–154. [DOI] [PubMed] [Google Scholar]
- 7.Cotter J, Dias de Castro F, Magalhaes J, et al. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy 2015; 47: 330–335. [DOI] [PubMed] [Google Scholar]
- 8.D’Haens G, Lowenberg M, Samaan MA, et al. Safety and feasibility of using the second-generation PillCam colon capsule to assess active colonic Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1480-6.e3. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho PB, Rosa B, Cotter J. Mucosal healing in Crohn’s disease – are we reaching as far as possible with capsule endoscopy? J Crohns Colitis 2014; 8: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 10.Boal Carvalho P, Rosa B, Dias de Castro F, et al. PillCam COLON 2 in Crohn’s disease: A new concept of pan-enteric mucosal healing assessment. World J Gastroenterol 2015; 21: 7233–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliakim R, Spada C, Lapidus A, et al. Evaluation of a new pan-enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease – feasibility study. Endosc Int Open 2018; 6: e1235–e1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton B and Peat J. Medical statistics: A guide to SPSS, data analysis and critical appraisal. 2nd ed. Chichester: Wiley Publishing, 2014. [Google Scholar]
- 13.Greener T, Klang E, Yablecovitch D, et al. The impact of magnetic resonance enterography and capsule endoscopy on the re-classification of disease in patients with known Crohn’s disease: A prospective Israeli IBD Research Nucleus (IIRN) study. J Crohns Colitis 2016; 10: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall BJ, Holleran GE, Smith SM, et al. A prospective 12-week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. Eur J Gastroenterol Hepatol 2014; 26: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 15.Kopylov U, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease. World J Gastroenterol 2014; 20: 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopylov U, Ben-Horin S, Seidman EG, et al. Video capsule endoscopy of the small bowel for monitoring of Crohn’s disease. Inflamm Bowel Dis 2015; 21: 2726–2735. [DOI] [PubMed] [Google Scholar]
- 17.Kopylov U, Nemeth A, Koulaouzidis A, et al. Small bowel capsule endoscopy in the management of established Crohn’s disease: Clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis 2015; 21: 93–100. [DOI] [PubMed] [Google Scholar]
- 18.Oliva S, Aloi M, Viola F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2019; 17: 2060–2067.e1. [DOI] [PubMed] [Google Scholar]
- 19.Melmed GY, Dubinsky MC, Rubin DT, et al. Utility of video capsule endoscopy for longitudinal monitoring of Crohn’s disease activity in the small bowel: A prospective study. Gastrointest Endosc 2018; 88: 947–955.e2. [DOI] [PubMed] [Google Scholar]
- 20.Hall B, Holleran G, McNamara D. PillCam COLON 2((c)) as a pan-enteroscopic test in Crohn’s disease. World J Gastrointest Endosc 2015; 7: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2016; 28: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 22.Koulaouzidis A, Sipponen T, Nemeth A, et al. Association between fecal calprotectin levels and small-bowel inflammation score in capsule endoscopy: A multicenter retrospective study. Dig Dis Sci 2016; 61: 2033–2040. [DOI] [PubMed] [Google Scholar]
- 23.Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 24.Gal E, Geller A, Fraser G, et al. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig Dis Sci 2008; 53: 1933–1937. [DOI] [PubMed] [Google Scholar]
- 25.Yablecovitch D, Lahat A, Neuman S, et al. The Lewis score or the capsule endoscopy Crohn’s disease activity index: Which one is better for the assessment of small bowel inflammation in established Crohn’s disease? Therap Adv Gastroenterol 2018; 11: 1756283X17747780. doi: 10.1177/1756283X17747780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahat A, Kopylov U, Amitai MM, et al. Magnetic resonance enterography or video capsule endoscopy – what do Crohn’s disease patients prefer? Patient Prefer Adherence 2016; 10: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG913368 Supplemental Material for A novel PillCam Crohn’s capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn’s disease by Rami Eliakim, Doron Yablecovitch, Adi Lahat, Bella Ungar, Eyal Shachar, Dan Carter, Limor Selinger, Sandra Neuman, Shomron Ben-Horin and Uri Kopylov in United European Gastroenterology Journal