Abstract
Patients with novel coronavirus disease 2019 (COVID-19) experience various degrees of liver function abnormalities. Liver injury requires extensive work-up and continuous surveillance and can be multifactorial and heterogeneous in nature. In the context of COVID-19, clinicians will have to determine whether liver injury is related to an underlying liver disease, drugs used for the treatment of COVID-19, direct effect of the virus, or a complicated disease course. Recent studies proposed several theories on potential mechanisms of liver injury in these patients. This review summarizes current evidence related to hepatobiliary complications in COVID-19, provides an overview of the available case series and critically elucidates the proposed mechanisms and provides recommendations for clinicians.
Keywords: SARS-CoV2, COVID-19, liver injury, liver function test, cholangiocytes, lymphopenia, cytokine storm
Key points
Altered liver function tests are reported in up to half of the patients with COVID-19 infection.
Disease severity, pre-existing liver disease and older age present a risk for liver injury.
Drug-induced liver injury is an important consideration in patients with COVID-19.
Hepatotoxic antiviral medications require careful monitoring of adverse effects.
SARS-CoV-2 may directly bind to ACE2 positive cholangiocytes and can cause hepatic injury.
Activation of the immune system and ‘cytokine storm’ may contribute to an immune-mediated process of hepatic injury in COVID-19.
The control of cytokine dysregulation at an early stage could be beneficial to curb the disease progression.
Introduction
In the current pandemic coronavirus disease (COVID-19), almost every country in the world has now registered COVID-19 cases, and the confirmed cases have exceeded one million to date. While initial clinical studies, especially from China, the USA and Italy, have highlighted the dominant clinical symptoms including fever, cough, fatigue and shortness of breath, the later research unveiled shreds of evidence on the extrapulmonary manifestations of the disease. These reports highlighted that beyond severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a complicated course of the disease or even viral infection itself can lead to involvement of other organs and multi-organ failure. The liver is the primary organ for detoxification and metabolism, and maintaining an optimal function is imperative to engage all available therapeutic modalities in the treatment of COVID-19. Abnormal liver function requires clinical evaluation, continuous surveillance and, potentially, specific therapy. To support clinical decision making and optimize the outcome in the treatment of COVID-19, it will be crucial to clearly understand the possible mechanisms involved in liver injury. The current review summarizes the pathophysiology and potentially specific role of COVID-19 in liver disease based on the available data and case series published, ahead of print and non-peer-reviewed preprints as of 2 April. The search strategy is detailed in the Supplementary Material online.
Pathophysiological basis of liver injury in patients with COVID-19
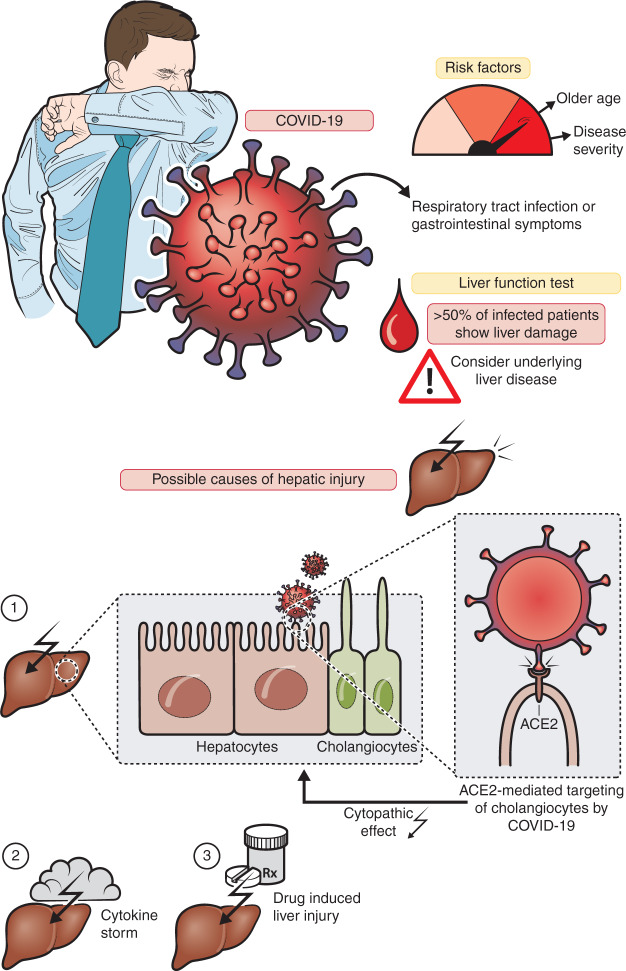
Emerging data from small clinical case studies have proposed that liver injury in COVID-19 is frequently seen, but the extent and underlying mechanisms remain undetermined. Figure 1 summarizes the pathophysiological findings, which are discussed below.
Figure 1.
Clinical characteristics and pathophysiology of liver injury from COVID-19.
ACE2: angiotensin-2 converting enzyme
Direct viral effect on the liver
The liver exerts a crucial function in host defense against microbes and is involved in most systemic infections as it receives both the portal and systemic circulation. Certain viruses exert a direct cytopathic effect on hepatocytes and cholangiocytes although, in most cases, the pathogenesis seems multifactorial. Yang et al. reported that SARS-CoV could cause direct cytopathic liver injury rather than inducing cellular stress from low oxygen supplies or cytokines as seen in sepsis.1 Autopsy studies in patients revealed that SARS-CoV was detectable in 41% of the liver tissue, with a maximum viral load of 1.6 × 106 copies/g of tissue.2 The pathological findings of liver biopsy specimens from SARS patients showed hepatocellular necrosis, mitoses, cellular infiltration and fatty degeneration. In a recent autopsy analysis of liver tissue from a patient with COVID-19, moderate microvesicular steatosis and mild inflammation in the lobular and portal area was observed. However, this pattern of histological injury is not specific for one etiology but can also be observed during sepsis or drug-induced liver injury (DILI).3
The role of cholangiocytes in COVID-19
Similar to SARS-CoV, SARS-CoV-2 uses the angiotensin-2 converting enzyme (ACE2) receptor protein to attack the host system.4 The cell entry receptor, ACE2, is widely expressed across the human body, including the lungs (type II alveolar cells), gastrointestinal tract (esophageal epithelial cells and absorptive enterocytes of ileum and colon), hepatobiliary system (hepatocytes and cholangiocytes), cardiovascular system (myocardial cells), the renal system (proximal tubule cells and urothelial bladder cells) and the pancreas.5 Recent studies have observed that ACE2 expression in the cell clusters of cholangiocytes was significantly higher than that in the hepatocytes population (59.7% vs. 2.6%).6 The authors conclude that SARS-CoV-2 may directly bind to ACE2 positive cholangiocytes, but not hepatocytes, to exert a cytopathic effect. Cholangiocytes are involved in many aspects of liver physiology, including regeneration and adaptive immune response mechanisms, and the disruption of cholangiocyte function can cause hepatobiliary damage. This is supported by cholestatic markers, including gamma-glutamyl transferase (GGT), that can be found in some, but not all, case series of COVID-19.7–9 Notably, a recent review reported unpublished data with GGT elevations in 54% of cases.8 In a human organoid model of liver ductal organoids, permissiveness to SARS-CoV-2 infection was observed. Here viral infection impaired the barrier and bile acid transporting functions of cholangiocytes through dysregulation of genes involved in tight junction formation and bile acid transportation, supporting the susceptibility of cholangiocytes in SARS-CoV-2-related liver injury.10
Activation of the immune system in COVID-19
Dysregulation of the innate immune response can be one aspect of liver injury in COVID-19. Patients with COVID-19 exhibit marked activation of inflammatory markers, including abnormal levels of C-reactive protein (CRP), lymphocytes, neutrophils and cytokines, in particular interleukin-6 (IL-6).8,11–13 These mechanisms may contribute to pulmonary and extrapulmonary injuries12,14 and the control of cytokine dysregulation at an early stage could be beneficial to curb the disease progression.15
Hepatic inflammation involving activation of innate immune cells and the release of cytokines is a well-established driver of liver injury from various causes.16 In some of the available case series of COVID-19, a correlation between lymphopenia and liver injury was observed and CRP ≥20 mg/L and a lymphocyte count <1.1 × 109/L were independent risk factors for liver injury. Notably, lymphopenia in COVID-19 studies was reportedly observed in 63% to 70.3% of patients and those with lower lymphocyte counts more susceptible to fatal outcomes.11
Clinical evidence
Elevated liver function tests (LFTs) in COVID-19
More than 20 publications to date reported abnormal levels of aminotransferases in patients with COVID-19.7--9,11–13,17--19,21,23--27,29–33,35,36 A recent systematic review and meta-analysis on LFT abnormalities provided a pooled elevation of aspartate aminotransferase (AST) in 33.3% and alanine aminotransferase (ALT) in 24.1% of cases.39 Various investigators across different studies reported a correlation between the severity of COVID-19 and the degree of liver dysfunction.8,11,25 In one retrospective study, one patient experienced severe hepatitis with ALT of 7590 U/L and AST of 1445 U/L.17 In a report from Shanghai, 50.7% of patients presented with elevated LFTs at the time of hospitalization. Interestingly, these were more likely to have a moderate-to-high-grade fever when compared with the patients with normal LFT (44% vs. 27.4%; p = 0.035).7 On the other hand, mild and moderate cases experienced only discrete abnormal LFT values. These reports support the concept that the disease severity and an older age predispose to more severe liver injury from COVID-19. Based on these case series, patients with severe COVID-19 and pre-existing liver conditions8 – but also elderly patients11 – should undergo surveillance and individually tailored therapeutic approaches for potential liver injury.
A recently published article by Bangash et al. argues after careful review of seven relevant studies that elevated ALT and AST may not necessarily be of hepatic origin alone. The authors have given a timely reminder that it is common for other respiratory viruses to create similar LFT elevations28 and thus more prospective data related to the clinical relevance COVID-19 and liver injury is required.
The previous pathogenic coronaviruses, such as SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV), were also reported to manifest with elevated levels of ALT and AST.39 More generally, non-hepatotropic viral infections may affect the liver and induce hepatitis or fulminant acute liver failure. However, in the majority of cases, recovery from viral illness is often sufficient to resolve liver injury.40
Like in SARS-CoV and MERS-CoV infections, abnormal levels of albumin and lactate dehydrogenase (LDH) were also reported in SARS-CoV-2 infection, with the maximum of 98% and 76% of the patients affected as reported in the study by Chen et al.17 It is important to remember that LDH and AST elevation could be from muscle damage and not necessarily reflect liver injury.
DILI
The current armory of therapeutic agents explored against SARS-CoV-2 includes several antiviral agents, supportive therapy and trials of alternative medicines in many regions of the world. Given the fact that the liver is involved in the metabolism of many drugs, including nucleoside analogs and protease inhibitors that are currently used to treat COVID-19, hepatotoxicity from these drugs can arise.
A recent randomized controlled trial of lopinavir and ritonavir in severe COVID-19 reported that elevated levels of AST, ALT and total bilirubin occurred as adverse effects in a few patients.41 Another case series from Wuhan reported that 55.4% of patients experienced liver injuries after treatment with lopinavir and ritonavir.26 Fan et al. published a retrospective study on COVID-19 and observed that the utilization rate of this drug combination was significantly higher in patients with abnormal LFTs compared with patients without LFT elevations (56.1% vs. 25%, p = 0.009). In this study, 47.3% of the discharged patients showed elevated LFTs at baseline, and 23.7% developed abnormalities during hospitalization, suggesting emerging liver injury from drugs or during the course of the infection. Importantly, LFT elevation during the hospital stay was associated with prolonged length of hospitalization.7
Chloroquine, an old drug with a potential of repositioning for new treatment indications, has recently been tried in patients infected with SARS-CoV-2. After a profound success in inhibiting viral replication in vitro, concurrent clinical trials (>20) on chloroquine conducted at 10 hospitals across China have demonstrated superior efficacy in viral control.42 The pharmacodynamic activity of this drug in COVID-19 may involve the arresting of cytokine storms or the activation of CD8+ cells or by preventing endocytosis-mediated uptake of the virus.43 Importantly, hepatotoxicity related to chloroquine or hydroxychloroquine has rarely been reported.
In severe cases of COVID-19 with cytokine release, tocilizumab, an IL-6 antagonist, which is humanized IgG1 monoclonal antibody to the IL-6 receptor, has been used as a potential therapy for SARS-CoV-2. In previous clinical trials for other indications tocilizumab was reported to cause mild elevations of LFTs which were usually transient and commonly resolved within 2–6 weeks from exposure.37
Remdesivir is an experimental antiviral nucleotide analog with broad activity against coronaviruses44 that is currently being trialed for SARS-Cov-2 infection. Safety data from ongoing studies will guide on its use in patients, but so far no reports of liver toxicity have emerged.
Patients with pre-existing liver disease
Scare data has been published for COVID-19 infection in patients with pre-existing liver disease. Experience from previous episodes of coronavirus infection can guide on the extent of hepatic involvement and on the management of patients with pre-existing liver disease. In SARS, the highest mortality rates were observed in the elderly and adults with underlying liver disease.45 Therefore, it has to be expected that the patients with COVID-19 are also more vulnerable to hepatic injury.9 In a case series from the Zhejiang province, a prevalence of 11% of underlying liver disease was reported. About half of them experienced symptoms for more than 10 days after the illness onset.21 In another study from Wuhan, 9% of patients had the underlying liver disease of cirrhosis or hepatitis.23 Li et al., who investigated risk factors involved with hepatic injury, stated that two patients had presented with alcoholic liver disease at baseline. One of them had a moderate elevation of ALT (120 U/L) within a week of hospitalization, while the other showed no such abnormalities.11 In the initial cohort described from China, 2.7% exhibited hepatitis B virus infection with no mention of worsening outcomes.26 Therefore, the association of the pre-existing liver conditions with disease prognosis and outcomes in COVID-19 will have to be evaluated by comprehensive data registries which recently started enrolling patients (e.g. COVID-Hep Registry and SECURE-Cirrhosis Registry).
Liver transplant recipients
Management of post liver transplant recipients during the COVID-19 pandemic presents a special challenge for clinicians because of the limited data available and the crucial need to continue immunosuppressive drugs in these patients, which puts them at risk for more severe courses of COVID-19 infection and possible prolonged viral shedding. Case reports from China did not reveal an increased mortality in organ transplant recipients. Qin et al. reported the first case of SARS-CoV-2 infection in a patient with hepatocellular carcinoma who underwent liver transplantation.29 Lowering immunosuppression to the most acceptable level appears reasonable in infected liver transplant patients, in particular, in the setting of lymphopenia or clinical worsening of infection.46
In addition clinicians have to be aware of drug–drug interactions in the transplant setting. In particular immunosuppressive drugs and ritonavir-boosted antiviral therapies exhibit relevant interactions through CYP34A which lead to increased levels of calcineurin and mTOR inhibitors. Accordingly, chloroquine-based regimes or remdesivir (compassionate use program only) appear to be safe, while boosted protease inhibitors should be avoided (see Table 2). Additionally, preventive strategies in those vulnerable patients include early and prolonged screening with polymerase chain reaction-based testing for patients with early symptoms, a contact history or infection. Personal protective equipment in high risk settings can help to protect this vulnerable patient group.
Table 2.
Drug–drug Interactions of experimental COVID-19 agents and immunosuppressive therapy.
|
Combination |
Potential risk of interactions | Recommendations | |
|---|---|---|---|
| Immunosuppressants | COVID-19 therapy | ||
| Calcineurin inhibitor (tacrolimus or ciclosporin) | Atazanavir or lopinavir/ritonavir or chloroquine or hydrocholoquine | Potentially increased exposure of immunosuppressant | Dose adjustment or close monitoring |
| Sirolimus | Atazanavir or lopinavir/ritonavir | Potentially increased exposure of immunosuppressant | Avoid coadministration |
| Sirolimus | Chloroquine or hydrocholoquine | Potentially increased exposure of immunosuppressant | Dose adjustment or close. monitoring |
| Tacrolimus or ciclosporin or sirolimus | Tocilizumab | Potentially decreased exposure of immunosuppressant | Interaction of weak intensity; additional action/monitoring or dose adjustment unlikely required |
| Mycophenolate | Lopinavir/ritonavir | Potentially increased or decreased exposure of mycophenolate | Dose adjustment or close monitoring |
| Basiliximab | Tocilizumab | Enhanced immunosuppressive effect | Avoid coadministration |
| Azathioprine | Ribavarin | Myelotoxicity due to accumulation of 6-methylthioinosine monophosphate | Dose adjustment or close monitoring |
| Azathioprine | Tocilizumab or interferon-β | Additive hematological toxicity | Caution required; close monitoring of hematological parameters |
Modified from Liverpool Drug interactions Group (5 April 2020; https://www.hep-druginteractions.org/).
Summary and clinical recommendations
Liver function abnormalities – predominantly AST elevation – in COVID-19 appear to be frequent but not severe in most cases. Direct viral hepatotoxicity, DILI, ‘bystander effects’ during a systemic viral infection and potentially sepsis, or exacerbation of an underlying liver disease have to be considered. Ex vivo studies offer that SARS-CoV-2 can selectively target the liver, in particular cholangiocytes through ACE2, and thus hepatobiliary injury appears plausible. Irrespective of the mechanisms involved in the hepatic injury of patients with COVID-19, activation of the immune-mediated pathway seems to be critical. Special high risk populations require close monitoring. These include the elderly population, patients with end-stage liver disease and liver transplant recipients. Symptomatic treatment with acetaminophen and avoidance of non-steroidal anti-inflammatory drugs in cirrhosis is recommended. Cautious use of antiviral agents in patients with decompensated liver disease and drug–drug interactions in post liver transplant patients has to be considered. As emphasized by a recent position paper of the European Study of Liver Disease, elective procedures and routine tests should be postponed according to the risk–benefit at the given time. On the other hand, emergency medical care needs to be done with appropriate measures to prevent infection.46
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620924157 for Liver injury in COVID-19: The current evidence by Saleh A Alqahtani and Jörn M Schattenberg in United European Gastroenterology Journal
Declaration of conflicting interests
SA has nothing to declare. JMS has acted independently of this study as a consultant to Boehringer Ingelheim, Galmed, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Novartis, Roche, Siemens Healthineers, and has received research funding from Gilead Sciences.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Table 1.
Abnormalities in hepatobiliary function and inflammatory markers along with the proposed theories of hepatic injury in COVID-19.
| Ref. no. | Author (et al.) | Publication type | Publication date | Study type | No. of patients with COVID-19 | Pre-existing liver diseases | Hepatobiliary function markers | Inflammatory markers (and other relevant blood tests) | Proposed possible theories of hepatic injury |
|---|---|---|---|---|---|---|---|---|---|
| 12 | Huang C | Published | 24 Jan 2020 | Prospective case series | 41 | Chronic liver disease in one patient | AST (max., 48.0 U/L) increased in 37%, more in the ICU group | 73% had LDH >245 U/L (max., 408 U/L)37% had LYM ≥1.0 × 109/L (max., 1.1 × 109/L) | Overall disease exacerbation: Cytokine storm |
| 17 | Chen N | Published | 30 Jan 2020 | Retrospective case series | 99 | No histories of hepatic diseases reported | ALT, AST, and TIBIL increased in 28%, 35%, and 18% of patients | CRP, ESR, IL-6, and LDH elevated in 86%, 85%, 52%, and 76% of patientsALB and LYM reduced in 98% and 35% cases, respectively | Overall disease exacerbation: Damage to T lymphocytes |
| 22 | Wang D | Published | 7 Feb 2020 | Retrospective case series | 138 | Chronic liver disease in 2.9% of patients | No significant liver abnormalities | LYM (Median: 0.8 × 109/L) reduced in 70.3% cases, and LDH (Median: 261 U/L) increased in 39.9% of patients | Overall disease exacerbation: Cytokine storm induced by virus invasionSustained inflammatory response |
| 26 | Cai Q | Preprint | 19 Feb 2020 | Retrospective case series | 298 | 2.7% had liver disease (details unspecified) Severe cases were associated with underlying diseases | 14.8% experienced liver injury (ALT (max., 59.5 U/L) and AST (max., 65 U/L): 8.7 %, respectively) | CRP (max., 47.13 mg/dL) increased in 70% casesIL-6 (max., 28.72 ng/L) increased in 76% of patientsESR (max., 50 mm/h) increased in 60.9%LYM (min, 0.91 × 109/L) reduced in 38.3% | Overall disease exacerbation: Inflammatory factor storm |
| 21 | Xu XW | Published | 19 Feb 2020 | Retrospective case series | 62 | 11% had underlying liver disease (details unspecified) About half of them experienced symptoms for more than 10 days after illness onset | AST (max., 32 U/L) increased in 16% of patients | 42% showed LYM reduction27% had LDH >245 U/LQuinolones and β-lactams administered if CRP >30mg/L | None described |
| 18 | Yang X | Ahead of Print | 24 Feb 2020 | Retrospective case series | 52 | No histories of hepatic diseases reported | 29% had liver dysfunction (no specifics given) | LYM reduced in 85% cases | None described |
| 23 | Shi H | Published | 24 Feb 2020 | Retrospective case series | 81 | Hepatitis or liver cirrhosis in 9% of cases | AST (>40 U/L) increased in 53% of patients, lower in asymptomatic patients | LYM (≥1.0 × 109/L) increased in 67%.CRP (Mean: 6.9 mg/L) was lower in asymptomatic patients | None described |
| 27 | Cao W | Preprint | 25 Feb 2020 | Retrospective case series | 128 | None described | ALT (Mean: 43.87 IU/L) and AST (Mean: 44.13 IU/L) increased in severe cases | LYM (Mean: 0.67 × 109/L) reduced and CRP (Mean: 37.92 mg/L) increased in severe cases | None described |
| 19 | Zhang B | Preprint | 27 Feb 2020 | Retrospective case series with the data of non-survivors | 82 | Liver disease in 2.4% cases | ALT (>40 U/L), AST (>40 U/L), and TBIL (>20.5 mmol/L) increased in 30.6%, 61.1%, and 30.6% cases | LYM (<1.0 × 109/L), ALB (<40 g/L) and CD8+ cells (<220 × 109/L) reduced in 89.2%, 77.8%, and 98.3% cases, respectivelyLDH (>250 U/L) and CRP (>10 U/L) elevated in 93.2% and 100% of patients, respectively | Overall disease exacerbation: Viral invasion of organsInflammatory factor elicitationPerturbation of immune system |
| 25 | Guan WJ | Ahead of print | 28 Feb 2020 | Retrospective case series | 1099 | Hepatitis B in 2.1% of patients | Increase of AST (>40 U/L) in 22.2%, ALT (>40 U/L) in 21.3%, and TBIL (>17.1µmol/L) in 10.5% | Elevation of CRP (≥10 mg/L) and LDH (≥250 U/L) in 60.7% and 41.0%, respectively | None described |
| 7 | Fan Z | Preprint | 28 Feb 2020 | Retrospective case series | 148 | None described | 50.7% of patients had liver function abnormalities at admission21.6%, 18.2%, 17.6%, 6.1%, and 4.1% patients had elevated AST, ALT, GGT, TBIL, and ALP, respectively. | 35.1% showed LDH elevationCD4+ and CD8+ T cells decreased and CRP increased in abnormal liver function group | Drug-induced inflammatory factor stormViral infection of the liver |
| 8 | Zhang C | Published | 4 Mar 2020 | Review with a description of unpublished case series | 56 | 3.6% of patients had pre-existing liver diseases (details unspecified) | 28.6% of cases had abnormal liver functionsOne fatal case with evaluated liver injury (ref. no. 3) | None described | Direct viral infection of liver cellsDrug-induced immune dysfunction |
| 24 | Huang Y | Preprint | 5 Mar 2020 | Retrospective case series with the data of non-survivors | 36 | No histories of hepatic diseases reported | ALT, AST and TBIL increased in 13.3%, 58.1%, and 12.9% of patients, respectively | LYM and ALB decreased in 70.6% and 80.6% cases, respectively. LDH and IL-6 increased in all patients. CRP increased in 96.97% of cases | None described |
| 11 | Li L | Preprint | 10 Mar 2020 | Retrospective case series | 85 | Hepatitis B, alcoholic liver disease, and fatty liver disease (n=2 in each category) | 24.7% had ALT elevation at admission | CRP ≥20mg/L and LYM count <1.1 × 109/L were independent risk factors for hepatic injury. ALB (Mean: 33.4 g/L) in ALT elevated group was significantly lower | Inflammatory cytokine stormDeterioration of the disease with a dynamic process |
| 13 | Cui Y | Ahead of print | 17 Mar 2020 | Case report of an infant | 1 | A healthy infant with no medical history | ALT (84 IU/L), AST (100 IU/L) and TBIL (33.7 µmol/L) elevated | CD8+ T cells (2208 cells/µL) and LYM count (5.22 × 109/L) elevated | Overall disease exacerbation: An initial increase in T helper 2 cell response, followed by suppression of inflammatory responses |
| 9 | Xu L | Ahead of print | 14 Mar 2020 | Review with a comment of unpublished data | Not described | Not described | GGT increased in severe cases (values not reported) | Not described | Direct virus-induced cytopathic effectsOvershooting of inflammatory responsesViral hepatitis Drug-induced |
| 28 | Bangash MN | Ahead of Print | 20 Mar 2020 | Review | – | – | – | – | Virally induced cytotoxic T cellsInduction of a dysregulated innate immune response |
| 29 | Qin J | Ahead ofprint | 27 Mar 2020 | Case report | 1 | Hepatitis BHCC | ALT (80 U/L) and AST (132 U/L) declined post-liver transplantation but elevated gradually | LYM reduced to 0.64 × 109/L | None described |
| 30 | Xie H | Ahead of print | 2 Apr 2020 | Retrospective case series | 79 | Patients with previous liver diseases were excluded | 31.6%, 35.4% and 5.1% of patients had elevated ALT, AST and TBIL, respectively. Median (range) values were 36.5 (17.5–71.5) U/L, 34.5 (25.3-–55.3) U/L and 12.7 (8.1–15.4) mmol/L, respectively. | CRP (max., 79.6 µmol/L) and ESR (max., 58 mm/h) increased; while LYM reduced (min., 0.9 × 109/L) | Overall disease exacerbation: Disease severity |
| 31 | Zhang Y | Ahead of print | 2 Apr 2020 | Retrospective case series | 115 | Two patients had chronic Hepatitis B (excluded) | ALT and AST increased in 9.57% and 14.78% patients, respectively on admission. TBIL elevation was rarely observed. Mean levels higher in severe cases. | 54.78% had reduced ALB, significantly lower in severe cases. 57.39% had increased CRP, higher in severe cases (80.75±69.18). LDH level (Mean±SD:346.10±257.26) significantly elevated in severe cases | Dysfunction of immune system |
| 32 | Ding Q | Ahead of print | 20 Mar 2020 | Prospective case series | Five patients who had both COVID-19 and influenza infection | Two patients had Hepatitis B | ALT and AST increased in 40% (each) of cases, respectivelyAcute liver injury occurred in 60% of patients | CRP increased in 80%, while LYM reduced in 80% of cases. | None described |
| 33 | Zhao D | Ahead of print | 12 Mar 2020 | Prospective case series | 19 | Hepatitis B in one patient | Liver function damage is more frequent in COVID-19 than other pneumonia patients. 27.78% (each) cases had elevated ALT and AST, respectively, while GGT increased in 44.4% | LDH increased in 31.58% of cases | Overall disease exacerbation: Dysregulation of immune system |
| 34 | Feng G | Published | 30 Mar 2020 | Review article | – | – | – | – | Hypoxia-reperfusion dysfunctionSystemic inflammation responseDrug-induced Viral infection of the liverACE2 positive cholangiocytes |
| 35 | Chen H | Published | 12 Feb 2020 | Retrospective case series | 9 | None described | 33% each had increased ALT and AST, respectively. One had ALT reaching 2093 U/L and AST reaching 1263 U/L | 56% patients had LYM (<1.0 × 109 cells/L) reduction75% of cases had elevated CRP (>10 mg/L) | None described |
| 36 | Pan F | Ahead of print | 13 Feb 2020 | Retrospective case series | 21 | None described | Elevated levels of ALT (max., 107 U/L) and AST (max., 95 U/L) were observed | Elevated CRP (max., 88.6 mg/L), LDH (max., 377 U/L) and ESR (max., 93 s) were reported | None described |
ALB: albumin; ALP, Alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; GGT: gamma-glutamyl transferase; HCC: hepatocellular carcinoma; ICU: intensive care unit; IL-6: interleukin-6; LDH: lactate dehydrogenase; LYM: lymphocytes; max.: maximum; min.: minimum; TBIL: total bilirubin
ORCID iD
Jörn M Schattenberg https://orcid.org/0000-0002-4224-4703
References
- 1.Yang Z, Xu M, Yi JQ, et al. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int 2005; 4: 60–63. [PubMed] [Google Scholar]
- 2.Farcas GA, Poutanen SM, Mazzulli T, et al. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis 2005; 191: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420--422. [DOI] [PMC free article] [PubMed]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271--280.e8. [DOI] [PMC free article] [PubMed]
- 5.Liu F, Long X, Zou W, et al. Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. medrxiv Preprint 3 March 2020: 2020.2002. 2028.20029181. DOI: 10.1101/2020.02.28.20029181.
- 6.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv Preprint 4 February 2020: 2020.2002.2003.931766. DOI: 10.1101/2020.02. 03.931766.
- 7.Fan Z, Chen L, Li J, et al. Clinical Features of COVID-19 related liver damage. medRxiv Preprint 28 February 2020: 2020.2002.2026.20026971. DOI: 10.1101/2020.02.26.20026971.
- 8.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol 2020; 5: 428--430. [DOI] [PMC free article] [PubMed]
- 9.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int Epub ahead of print 14 March 2020. DOI: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed]
- 10.Zhao B, Ni C, Gao R, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver organoids. biorxiv Preprint 17 March 2020: 2020.2003.2016.990317. DOI: 10.1101/2020.03.16. 990317. [DOI] [PMC free article] [PubMed]
- 11.Li L, Li S, Xu M, et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv Preprint 10 March 2020: 2020.2002. 2028.20028514. DOI: 10.1101/2020.02.28.20028514.
- 12.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, Tian M, Huang D, et al. A 55-day-old female infant infected with COVID 19: Presenting with pneumonia, liver injury, and heart damage. J Infect Dis Epub ahead of print 17 March 2020. DOI: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed]
- 14.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: The perspectives on immune responses. Cell Death Differ 2020; 27: 1451--1454. [DOI] [PMC free article] [PubMed]
- 15.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald B, Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology 2016; 151: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. Epub ahead of print 24 February 2020. DOI: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 19.Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv Preprint 27 February 2020: 2020.2002.2026.20028191. DOI: 10.1101/2020.02.26.20028191.
- 20.Yang Y, Lu Q, Liu M, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv Preprint 21 February 2020: 2020.2002. 2010.20021675. DOI: 10.1101/2020.02.10.20021675.
- 21.Xu X-W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ 2020; 368: m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Diss 2020; 20: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Yang R, Xu Y, et al. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv Preprint 5 March 2020: 2020.2002.2027. 20029009. DOI: 10.1101/2020.02.27.20029009.
- 25.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. Epub ahead of print 28 February 2020. DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 26.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. medRxiv Preprint 19 February 2020: 2020.2002. 2017.20024018. DOI: 10.1101/2020.02.17.20024018. [DOI] [PubMed]
- 27.Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv Preprint 25 February 2020: 2020.2002.2023.20026963. DOI: 10.1101/2020.02.23.20026963.
- 28.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol 2020. Epub ahead of print 20 March 2020. DOI: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed]
- 29.Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology 2020. Epub ahead of print 27 March 2020. DOI: 10.1002/hep.31257. [DOI] [PubMed]
- 30.Xie H, Zhao J, Lian N, et al. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury. A retrospective study. Liver Int 2020. Epub ahead of print 2 April 2020. DOI: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed]
- 31.Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int 2020. Epub ahead of print 2 April 2020. DOI: 10.1111/liv.14455. [DOI] [PubMed]
- 32.Ding Q, Lu P, Fan Y, et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol 2020. Epub ahead of print 20 March 2020. DOI: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed]
- 33.Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis 2020. Epub ahead of print 12 March 2020. DOI: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed]
- 34.Feng G, Zheng KI, Yan Q-Q, et al. COVID-19 and liver dysfunction: Current insights and emergent therapeutic strategies. J Clin Transl Hepatol 2020; 8: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology Epub ahead of print 13 February 2020; DOI: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Tocilizumab. [Updated 18 June 2015]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548243/ [Accessed: 2 April 2020]. [PubMed]
- 38.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020: 101623. [DOI] [PMC free article] [PubMed]
- 39.Wang JT, Sheng WH, Fang CT, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 2004; 10: 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol 2006; 168: 1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med Epub ahead of print 18 Mar 2020. DOI: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 42.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14: 72–73. [DOI] [PubMed] [Google Scholar]
- 43.Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol 2020; 15: 247--249. [DOI] [PMC free article] [PubMed]
- 44.De Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020; 117: 6771–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003; 361: 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep 2020. DOI: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620924157 for Liver injury in COVID-19: The current evidence by Saleh A Alqahtani and Jörn M Schattenberg in United European Gastroenterology Journal