Abstract
Objective
To investigate the influence of cochlear implant (CI) use on subjective benefits in quality of life in cases of asymmetric hearing loss (AHL).
Study Design
Prospective clinical trial.
Setting
Tertiary academic center.
Subjects and Methods
Subjects included CI recipients with AHL (n = 20), defined as moderate-to-profound hearing loss in the affected ear and mild-to-moderate hearing loss in the contralateral ear. Quality of life was assessed with the Speech, Spatial, and Qualities of Hearing Scale (SSQ) pragmatic subscales, which assess binaural benefits. Subjective benefit on the pragmatic subscales was compared to word recognition in quiet and spatial hearing abilities (ie, masked sentence recognition and localization).
Results
Subjects demonstrated an early, significant improvement (P < .01) in abilities with the CI as compared to preoperative abilities on the SSQ pragmatic subscales by the 1-month interval. Perceived abilities were either maintained or continued to improve over the study period. There were no significant correlations between results on the Speech in Quiet subscale and word recognition in quiet, the Speech in Speech Contexts subscale and masked sentence recognition, or the Localization subscale and sound field localization.
Conclusions
CI recipients with AHL report a significant improvement in quality of life as measured by the SSQ pragmatic subscales over preoperative abilities. Reported improvements are observed as early as 1 month postactivation, which likely reflect the binaural benefits of listening with bimodal stimulation (CI and contralateral hearing aid). The SSQ pragmatic subscales may provide a more in-depth insight into CI recipient experience as compared to behavioral sound field measures alone.
Keywords: cochlear implant, asymmetric hearing loss, single sided deafness, quality of life, binaural hearing
Patients with asymmetric hearing loss (AHL), defined as moderate-to-profound hearing loss in the poorer ear and mild-to-moderate hearing loss in the better ear, experience significant impairment in daily activities as well as reduced quality of life compared to people with bilaterally normal hearing.1-6 Benefits of binaural stimulation include head shadow effects, binaural squelch, and binaural summation.7-9 These binaural benefits are important for understanding speech in noise as well as complex listening environments. Patients with AHL may report a poorer quality of life due to an inability to benefit from binaural cues as a result of the severity of the hearing loss in the poorer-hearing ear.2,3,6 Some of these limitations can be demonstrated with behavioral sound field measures, including decreased speech recognition in noise and sound source identification (also known as localization).2,3 There may be additional negative consequences of AHL on real-world experiences that are not revealed with traditional, behavioral sound field measures.
Traditional management options for patients with AHL include no treatment, use of contralateral routing of the signal (CROS) hearing aids, and use of bone conduction hearing aids (BCHAs).10 Subjects report that perceived binaural hearing abilities do not improve significantly with traditional treatment options, such as CROS hearing aids and BCHAs, as compared to the unaided condition.5 Both subjective report and behavioral performance in the sound field on tasks of speech recognition and spatial hearing demonstrate the limitations of traditional treatment options.5,11,12 This is likely due to the fact that neither hearing aids nor BCHAs provide ear-specific stimulation, precluding or severely limiting true binaural hearing.
Cochlear implantation is an alternative to traditional treatment options for patients with AHL or unilateral hearing loss (UHL), with initial investigations demonstrating the effectiveness of cochlear implant (CI) use on measures of subjective benefit, speech recognition in quiet and noise, and localization.2,3,5,13-16 For instance, Arndt et al5 demonstrated subjective improvement in perceived binaural hearing abilities with CI use in subjects with UHL and significantly improved speech recognition in noise due to the head shadow effect. Subjects did not experience a significant difference in speech recognition in noise with CI use in the binaural summation or binaural squelch conditions.5 Buss et al15 reported UHL subjects experienced improved word recognition in quiet with the CI alone and improved spatial hearing (ie, masked sentence recognition and localization) with the CI plus the normal-hearing ear as compared to preoperative performance. Subjective benefit was assessed via questionnaires (ie, Abbreviated Profile of Hearing Aid Benefit [APHAB]17; Speech, Spatial, and Qualities of Hearing [SSQ]18), and responses were compared to performance on behavioral sound field measures.14,16-18 A significant correlation between the subjective report and masked sentence recognition was observed at the 12-month interval; however, a significant correlation was not seen for sound localization.14 In addition, there was no significant correlation between subjective report and either behavioral measure before surgery.14 These findings suggest there may be a discrepancy between traditional sound field measures and a patient’s perception of difficulty with hearing, both before and after cochlear implantation. This, in turn, suggests that some quality-of-life measures may reveal aspects of binaural hearing benefit not quantified with traditional, behavioral sound field measures.
In regards to subjective benefit, the majority of prior investigations of the effectiveness of CI in cases of UHL and AHL used the SSQ questionnaire, which assesses perceived abilities on 3 subscales: Speech Hearing, Spatial Hearing, and Qualities of Hearing.18 Subject responses on the SSQ can provide additional insight into their perceived abilities when scored on the pragmatic subscales.19 The pragmatic subscales under each traditional subscale are listed in Table 1 . Recent reports have used the pragmatic subscales to assess binaural benefits in subjects with UHL and AHL.4,14,19 These studies have found correlations between scores on these pragmatic subscales and asymmetry of hearing thresholds, as well as an improvement seen when stimulating the poorer ear either with a hearing aid (HA) or CI.
Table 1.
Speech, Spatial, and Qualities of Hearing (SSQ) Pragmatic Subscales as Defined by Gatehouse and Akeroyd.19
| Speech Hearing |
| □ Speech in Quiet |
| □ Speech in Noise |
| □ Speech in Speech Contexts |
| □ Multiple Speech-Stream Processing and Switching |
| Spatial Hearing |
| □ Localization |
| □ Distance and Movement |
| Qualities of Hearing |
| □ Sound Quality and Naturalness |
| □ Identification of Sounds and Objects |
| □ Segregation of Sounds and Objects |
| □ Listening Effort |
The main objective of the present study was to determine if CI use in subjects with AHL provides subjective benefits, as reflected in scores on the SSQ pragmatic subscales. A secondary objective was to compare the subjective responses on specific pragmatic subscales to behavioral performance on sound field measures (ie, word recognition in quiet, masked sentence recognition, and localization). We hypothesized that there would be early, significant improvements in subjective benefit with CI use.
Methods
A prospective clinical trial was performed at a single institution investigating the effectiveness of CI use in the poorer-hearing ear for subjects with AHL. The study procedures were approved as part of an Investigational Device Exemption by the Food and Drug Administration and the University of North Carolina’s Institutional Review Board. Subjects provided informed consent and underwent cochlear implantation as part of the clinical trial. The study procedures included subjective questionnaires and tasks of word recognition in quiet, masked sentence recognition, and localization.
Candidacy criteria for the ear-to-be implanted included an unaided pure-tone average (PTA; 500, 1000, and 2000 Hz) of ≥70 dB hearing level (HL) and poor aided word recognition, defined as ≤60% correct on consonant-nucleus-consonant (CNC) words in quiet.20 Candidacy criteria for the contralateral ear included an unaided PTA between 35 and 55 dB HL and aided word recognition of ≥80% correct. A value of 120 dB HL was entered into the PTA calculation when no response to the stimulus was provided. Subjects completed at least a 1-month trial with an alternative treatment option for AHL, including conventional HAs, Bi-CROS HAs, or BCHAs, with limited benefit.
Subjects underwent cochlear implantation with a MED-EL Synchrony Standard electrode array (MED-EL Corporation, Innsbruck, Austria). The electrode array was inserted via a round window approach, and an intraoperative x-ray was obtained to confirm array placement. Initial activation of the CI device occurred 2 to 4 weeks after implantation.
Subjects were evaluated preoperatively and at 1 month and 12 months postactivation. Subjects completed the SSQ questionnaire at each interval, typically before speech recognition and spatial hearing assessment. Tasks of word recognition in quiet, masked sentence recognition, and localization were completed in a sound booth in an unaided condition at the preoperative interval and in the bimodal condition (CI plus contralateral HA) at the postactivation intervals, as described previously.15 Briefly, word recognition in quiet in the poorer-hearing ear was assessed with CNC words. Subjects listened with a HA at the preoperative interval and with their CI at the postactivation intervals. Recorded materials were presented at 60 dB sound pressure level (SPL) with the subject seated 1 m from the speaker and masking presented to the contralateral ear. For masked sentence recognition and localization, subjects listened with the HA in the contralateral ear at the preoperative interval and in the bimodal condition at the postactivation intervals. Masked sentence recognition in spatially separated noise was assessed with AzBio sentences in a 10-talker babble; the masker was at 60 dB SPL, and the target was at 0 dB signal-to-noise ratio (SNR). The target sentence was presented from the front speaker and the masker was presented 90° contralateral to the CI ear. Results for CNC words and AzBio sentences were scored as the percentage of correctly repeated words. For localization, subjects were seated in the middle of a 180° arc of 11 speakers. A 200-ms noise burst was presented from a randomly selected speaker at 52, 62, or 72 dB SPL, and the subject reported the speaker number of the perceived sound source. Localization results are reported as the root mean squared (RMS) error, a metric that quantifies the difference between the perceived vs actual sound source location.
Data Analysis
Repeated-measures analysis of variance (ANOVA) was used to assess the change in subjective benefit for the Speech Hearing, Spatial Hearing, and Qualities of Hearing pragmatic subscales over the study period using the SPSS statistical software (version 26; SPSS, Inc, an IBM Company, Chicago, Illinois). The preoperative, 1-month, and 12-month intervals were selected to review performance with early CI use (ie, preoperative to 1 month) and over the postactivation period (ie, 1-12 months). The Greenhouse-Geisser correction factor was applied when Mauchly’s test of sphericity indicated nonsphericity. One subject did not complete the 12-month interval. Group mean values were used to replace missing data in the repeated-measures ANOVA. The pattern of results reported below was confirmed with a linear mixed model, with missing data and a random intercept for subject with the R statistical software (version 3.6.1).21
Bivariate Pearson correlations were used to assess the association between behavioral sound field results (ie, word recognition in quiet, masked sentence recognition, and localization) and responses on the pragmatic subscales at each interval using SPSS. Specifically, CNC scores were compared to responses on the Speech in Quiet subscale, AzBio sentences in spatially separated noise scores were compared to the Speech in Speech Contexts subscale, and RMS error was compared to the Localization subscale. A rationalized arcsine transform was applied to the percent correct speech recognition scores (CNC words and AzBio sentences) to normalize error variance22: rationalized arcsine units (RAUs) are nearly identical to percent correct over the range of 20% to 80% correct. A paired-samples t test was used to compare subject performance between the preoperative and 12-month intervals on these same measures using SPSS.
Results
Demographics
Twenty adult subjects (11 female) received a CI and participated in the study procedures. Most subjects reported sudden onset of their hearing loss in the affected ear (n = 14) and an unknown etiology for the hearing loss (n = 15). Suspected etiologies for the remaining subjects included Ménière’s disease (n = 3), viral infection (n = 1), and noise-induced hearing loss (n = 1). In the affected ear, the mean PTA was 88 dB HL (range, 70-120 dB HL), with a mean aided CNC score of 8% (range, 0%-30%). In the contralateral ear, the mean PTA was 38 dB HL (range, 35-50 dB HL), with a mean aided CNC score of 87% (range, 80%-100%). The mean age at implantation was 70 years (range, 52-79 years).
Speech Hearing Pragmatic Subscales
Results for the 4 Speech Hearing pragmatic subscales at the preoperative, 1-month, and 12-month intervals are plotted in Figure 1 . A higher value indicates better perceived abilities. There was a significant main effect of interval (F(2, 38) = 15.34, P < .001, η2 = 0.45). Responses differed significantly between the Speech Hearing pragmatic subscales (F(1.8, 33.7) = 112.1, P < .001, η2 = 0.86), with higher levels of perceived ability noted on the Speech in Quiet subscale. The interaction between interval and subscale was not significant (F(6, 114) = 0.93, P = .479, η2 = 0.05), indicating a similar pattern of improvement over time in perceived abilities across subscales. Review of the simple main effects revealed significant differences in responses for all subscales between the preoperative and 1-month intervals (P≤ .027), but not between the 1-month and 12-month interval (P≥ .49). This indicates that subjects report an early, significant improvement in perceived abilities for speech understanding in different auditory environments—from quiet to multiple talkers—that is stable through the 12-month interval.
Figure 1.
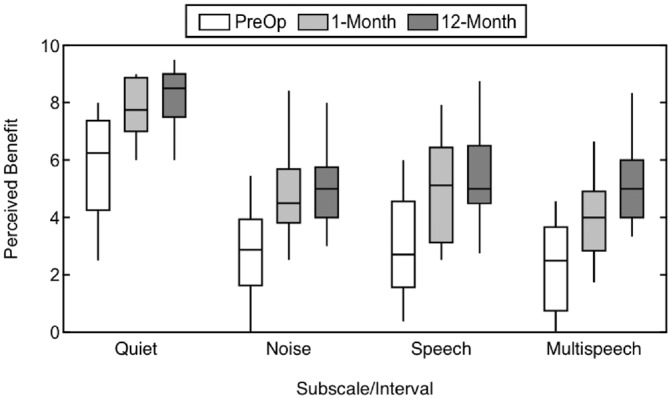
Subjective benefit over time measured with the Speech Hearing pragmatic subscales, including Speech in Quiet, Speech in Noise, Speech in Speech Contexts, and Multiple Speech Stream Processing and Switching. Higher values indicate better perceived ability.
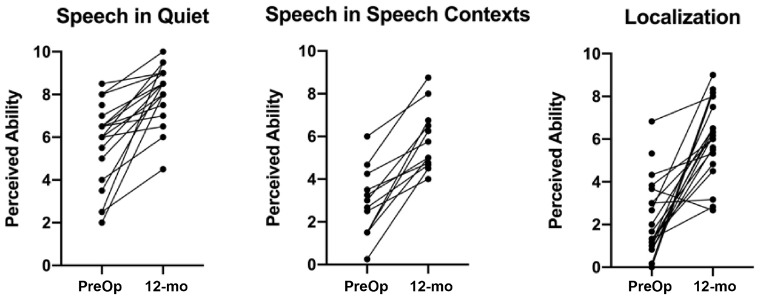
Figure 2 shows individual changes from the preoperative to the 12-month interval for the Speech in Quiet and Speech in Speech Context pragmatic subscales. These individual data mirror the group means, with improvement in perceived ability seen for all subjects. Table 2 shows group means for the Speech Hearing pragmatic subscale responses at the 12-month interval compared with responses from listeners with bilateral normal hearing4 and CI recipients with UHL.16 Subjects with AHL did not reach the perceived ability levels of either those with normal hearing or UHL, although it should again be noted that there was significant improvement from the preoperative timepoint in this cohort.
Figure 2.
Individual changes from preoperative to 12-month interval for Speech in Quiet, Speech in Speech Contexts, and Localization pragmatic subscales. Higher values indicate better perceived ability.
Table 2.
Responses from Listeners with Normal Hearing,4 CI Recipients with UHL,16 and Current CI Recipients with AHL on the Speech, Spatial, and Qualities of Hearing Pragmatic Subscales.
| Pragmatic Subscale | Speech in Quiet | Speech in Noise | Speech in Speech Contexts | Multiple Speech-Stream Processing and Switching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL |
| Range | 7.5-10 | 7.5-10 | 4.5-10 | 6.8-10 | 4.8-8.5 | 2.3-8.8 | 5.5-10 | 5.0-9.3 | 1.5-8.8 | 4.7-10 | 3.3-9.3 | 2.3-8.3 |
| Mean | 9.7 | 8.8 | 8.1 | 8.5 | 6.5 | 5.1 | 8.4 | 7.2 | 5.4 | 8.1 | 6.1 | 5.0 |
| SD | 0.6 | 0.7 | 1.4 | 1.0 | 1.1 | 1.7 | 1.5 | 1.0 | 1.9 | 1.4 | 1.8 | 1.6 |
| Pragmatic Subscale | Localization | Distance and Movement | ||||||||||
| Cohort | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL | ||||||
| Range | 6.7-10 | 2,7-9.2 | 2.7.-9.0 | 7.0-10 | 2.8-9.7 | 2.7-8.6 | ||||||
| Mean | 8.8 | 6.5 | 5.9 | 8.7 | 6.5 | 5.9 | ||||||
| SD | 1.0 | 1.8 | 1.8 | 0.9 | 2.2 | 1.7 | ||||||
| Pragmatic Subscale | Sound Quality and Naturalness | Identification of Sound and Objects | Segregation of Sounds | Listening Effort | ||||||||
| Cohort | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL | Normal Hearers | CI with UHL | CI with AHL |
| Range | 7.8-10 | 4.0-9.8 | 3.2-9.8 | 7.0-10 | 6.0-10 | 5.8-9.4 | 7.3-10 | 5.0-10 | 4.0-9.7 | 6.7-10 | 2.0-9.7 | 1.0-9.0 |
| Mean | 9.5 | 7.7 | 7.3 | 9.2 | 8.5 | 7.6 | 9.1 | 8.4 | 7.4 | 8.9 | 5.5 | 4.6 |
| SD | 0.6 | 1.5 | 1.8 | 0.8 | 1.2 | 1.3 | 0.9 | 1.6 | 1.7 | 1.0 | 1.9 | 2.3 |
Abbreviations: CI, cochlear implant; UHL, unilateral hearing loss.
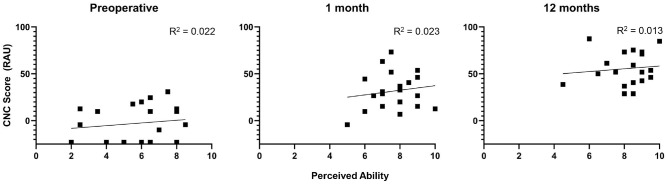
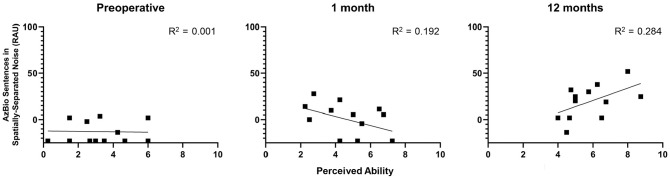
The next analysis evaluated whether the perceived benefit reported on the Speech Hearing pragmatic subscales was reflective of the speech recognition performance measured behaviorally. First, the responses on the Speech in Quiet subscale were compared to the CNC scores in the affected ear obtained at the preoperative, 1-month, and 12-month intervals. Figure 3 plots the CNC scores using transformed percentages against perceived ability on the Speech in Quiet subscale for each interval. There was no significant association between CNC scores and the responses on the Speech in Quiet subscale (P≥ .520) for any of the intervals. Next, the responses on the Speech in Speech Context subscale were compared to scores for the AzBio sentences in spatially separated noise. Thirteen subjects were assessed at 0 dB SNR with the AzBio sentences at the preoperative and postactivation intervals. Figure 4 plots performance on AzBio sentences using transformed percentages against perceived ability on the Speech in Speech Context subscale for each interval. There was no significant association between masked sentence recognition and perceived benefit on the Speech in Speech Context subscales (P≥ .130) for the preoperative or 1-month intervals. This correlation approached significance at the 12-month interval (r = .53, P = .075). This should be interpreted with caution, however, as the analyses did not include a correction for multiple comparisons.
Figure 3.
Comparison of responses on Speech in Quiet subscale and consonant-nucleus-consonant (CNC) scores. Results are reported in rationalized arcsine units (RAUs), with higher values indicating better performance.
Figure 4.
Comparison of subjective responses on the Speech in Speech Context subscale and scores for AzBio sentences in spatially separated noise. Results are reported in rationalized arcsine units (RAUs), with higher values indicating better performance.
Spatial Hearing Pragmatic Subscales
Figure 5 plots the responses on the 2 Spatial Hearing pragmatic subscales at the preoperative, 1-month, and 12-month intervals. Similar to the Speech Hearing pragmatic subscales, there was a significant main effect of interval (F(2, 38) = 27.67, P < .001, η2 = 0.59). There was no significant effect of subscale (F(1, 19) = 0.06, P = .804, η2 = 0.003), indicating similar responses for the Localization subscale and the Distance and Movement subscale. There was also no significant interaction between interval and subscale (F(2, 38) = 2.57, P = .090, η2 = 0.12). Review of the simple main effects revealed significant differences in the responses between the preoperative and 1-month intervals (P≤ .001), indicating subjects reported early improvements in perceived abilities on questions related to sound source identification. Subjects continued to report a significant improvement on the Distance and Movement subscale between the 1-month and 12-month intervals (P = .016). Responses did not differ significantly between the 1-month and 12-month intervals on the Localization subscale (P = .122).
Figure 5.
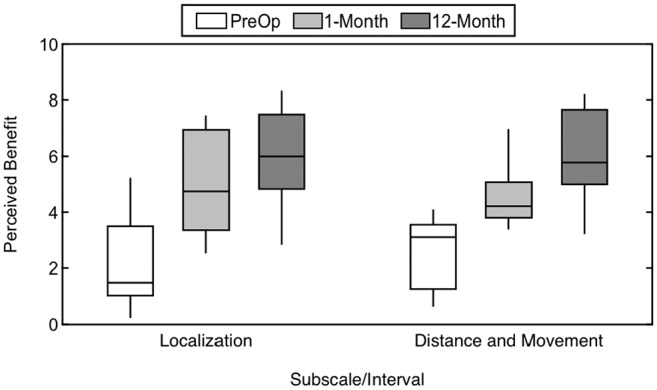
Subjective benefit over time as measured with the Spatial Hearing pragmatic subscales, including Localization and Distance and Movement. A higher value indicates better perceived ability.
Individual changes from the preoperative to the 12-month interval for the Localization subscale are shown in Figure 2 . This shows improvement seen in nearly all subjects, with 1 subject showing a slight decline in perceived ability. Table 2 compares responses at the 12-month interval with published data from listeners with normal hearing4 and CI recipients with UHL.16 As with the Speech Hearing pragmatic subscales, perceived ability of subjects with AHL in the present study did not reach the level seen for the other cohorts.
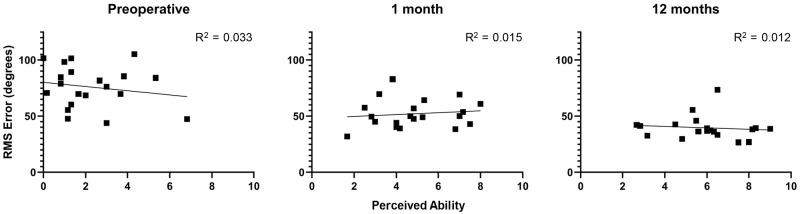
The responses on the Localization subscale were compared to the results on the localization task to assess the relationship between subjective responses and behavioral results. Figure 6 plots RMS localization error as a function of the perceived ability on the Localization subscale, with results shown separately for the preoperative, 1-month, and 12-month intervals. A lower value on RMS error indicates better localization. There was not a significant association between RMS error and perceived benefit on the Localization subscale (P≥ .44) at any interval.
Figure 6.
Comparison of responses on the Localization subscale and sound field localization. Results are reported as root mean squared (RMS) error, with lower values indicating better sound source localization.
Qualities of Hearing Pragmatic Subscales
Figure 7 plots the responses on the 4 Qualities of Hearing pragmatic subscales. Over the study period, there were significant main effects of interval (F(2, 38) = 13.21, P < .001, η2 = 0.41) and of subscale (F(2.3, 42.7) = 36.35, P < .001, η2 = 0.66), as well as a significant interaction of interval and subscale (F(6, 114) = 3.33, P = .005, η2 = 0.15). Review of the simple main effects of subscale by interval revealed a significant difference between the preoperative and 1-month intervals for the Segregation of Sounds and Objects and Listening Effort subscales (P≤ .048) but not for the other subscales (P≥ .097). The difference between the preoperative and 1-month responses was particularly evident for the Listening Effort subscale, which is an aspect of hearing not routinely measured clinically. Responses for each subscale did not change significantly between the 1-month and 12-month intervals (P≥ .120).
Figure 7.
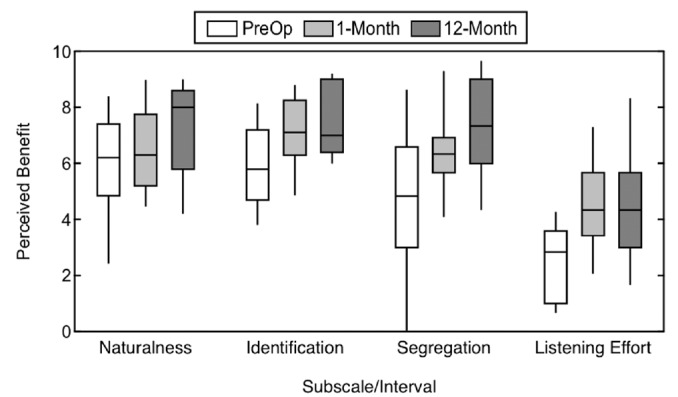
Subjective benefit over time as measured with the Qualities of Hearing pragmatic subscales, including Sound Quality and Naturalness, Identification of Sounds and Objects, Segregation of Sounds, and Listening Effort. Higher values indicate better perceived ability.
Table 2 compares responses from listeners with bilaterally normal hearing,4 CI recipients with UHL,16 and the present sample of CI recipients with AHL on the Qualities of Hearing pragmatic subscales. As with the Speech Hearing and Spatial Hearing subscales, subjects with AHL did not reach the perceived ability in those seen with either normal hearing or UHL.
Discussion
Early, significant improvements in perceived abilities were reported by CI recipients with AHL, which corroborates the significant improvements in quality of life reported in previous studies with similar cohorts.2,3,14,23 Significant benefits in perceived ability were observed as early as the 1-month interval for all 3 subscales. These results indicate that CI recipients with AHL may recognize benefits with the CI within the initial weeks of listening experience, even while acclimating to bimodal stimulation. Interestingly, the significant improvements on the majority of pragmatic subscales were relatively stable over the postactivation period, while the responses on the Distance and Movement pragmatic subscale continued to improve over time.
The pragmatic subscales in the Spatial Hearing and Qualities of Hearing categories assess abilities in auditory scene analysis and cognitive abilities that are not necessarily evaluated in other questionnaires or sound field measures.24 Pragmatic subscales such as Listening Effort and Sound Quality and Naturalness reflect abilities and experiences that are not currently evaluated clinically. The continued improvement in these categories over time would be missed with conventional testing. Subjective measures like the SSQ could therefore provide important information to supplement speech perception and spatial hearing assessment, with a goal of providing a comprehensive understanding of the patient’s listening experience.
The responses on the Speech in Quiet subscale and Speech in Speech Context subscale did not significantly correlate with CNC scores in quiet and AzBio sentence scores in spatially separated noise, nor did the responses on the Localization subscale significantly correlate with RMS error on the localization task. Behavioral assessment in the sound booth provides a limited view of the CI recipient’s ability in a controlled setting. While the controlled nature of behavioral testing in the sound booth supports comparisons of performance over time, this may not reflect the CI recipient’s functional hearing ability in the real world.
The present cohort reported significantly improved abilities on questions related to binaural hearing within the initial weeks of bimodal listening. These findings are similar to those previously reported for a group of CI recipients with UHL14; however, there were some differences. On the Speech Hearing pragmatic subscales, the UHL cohort reported continued improvements between the 1-month and 12-month postactivation intervals, whereas the AHL cohort did not report gains above and beyond those observed at the 1-month interval. Some notable differences between the cohorts include the obvious discrepancy in hearing thresholds of the contralateral ear (ie, normal to near-normal hearing vs mild-to-moderate hearing loss) but also increased age at implantation of the AHL cohort. The average age at implantation was 70 years for the AHL cohort and 50 years for the UHL cohort. Increased age at implantation is associated with decreased speech recognition even when hearing thresholds are consistent across groups, possibly due to a change in cognitive ability and auditory processing abilities.24,25 Consistent with this hypothesis is the finding that speech recognition has been reported to be worse in older as compared to younger CI recipients meeting the traditional candidacy criteria for cochlear implantation of bilateral moderate-to-profound hearing loss.26,27 It is also possible that the AHL cohort may need a longer duration of listening experience with bimodal stimulation before additional subjective benefits are observed. Ongoing work is assessing the long-term outcomes (ie, >12 months) of CI recipients with UHL and AHL on measures of speech recognition, spatial hearing, and subjective benefit, as well as the potential covariates—such as advanced age at implantation.
Conclusion
Cochlear implant recipients with AHL report early, significant improvement in quality of life as measured by pragmatic subscales of the SSQ, revealing aspects of bimodal hearing benefit that are not observed with traditional clinical test measures. The significant improvement in perceived abilities is demonstrated with early CI use and is either maintained or continues to improve over the first year of listening experience. The SSQ pragmatic subscales provide greater insight of CI recipient experience as compared to traditional sound field measures alone and may be beneficial to include in the assessment of CI recipients with UHL or AHL. Results reported here add to the growing evidence of cochlear implantation as an effective treatment option for patients with AHL.
Author Contributions
Nicholas J. Thompson, analysis, interpretation, drafting, final approval, accountable for aspects; Margaret T. Dillon, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; Emily Buss, conception, design, analysis, interpretation, drafting, final approval, accountable for aspects; Meredith A. Rooth, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; English R. King, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; Andrea L. Bucker, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; Sarah A. McCarthy, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; Ellen J. Deres, conception, design, acquisition, analysis, interpretation, drafting, final approval, accountable for aspects; Brendan P. O’Connell, conception, design, analysis, interpretation, drafting, final approval, accountable for aspects; Harold C. Pillsbury III, conception, design, analysis, interpretation, drafting, final approval, accountable for aspects; Kevin D. Brown, conception, design, analysis, interpretation, drafting, final approval, accountable for aspects.
Disclosures
Competing interests: Margaret T. Dillon, supported by a research grant from MED-EL Corporation; Meredith A. Rooth, supported by a research grant from MED-EL Corporation; English R. King, serves on the MED-EL Audiology Advisory Board; Brendan P. O’Connell, consultant for Advanced-Bionics Corporation; Harold C. Pillsbury III, consultant for MED-EL Corporation; Kevin D. Brown, serves on the MED-EL Surgical Advisory Board.
Sponsorships: None.
Funding source: We acknowledge the regulatory assistance of the North Carolina Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through grant award number UL1TR002489. The clinical trial was supported by a research grant from MED-EL Corporation.
Footnotes
This article was presented at the AAO-HNSF Annual Meeting & OTO Experience; September 15, 2019; New Orleans, Louisiana.
References
- 1. Noble W, Gatehouse S. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int J Audiol. 2004;43:100-114. [DOI] [PubMed] [Google Scholar]
- 2. Sladen DP, Carlson ML, Dowling BP, et al. Cochlear implantation in adults with asymmetric hearing loss: speech recognition in quiet and in noise, and health related quality of life. Otol Neurotol. 2018;39:576-581. [DOI] [PubMed] [Google Scholar]
- 3. Ketterer MC, Knopke S, Haussler SM, et al. Asymmetric hearing loss and the benefit of cochlear implantation regarding speech perception, tinnitus burden and psychological comorbidities: a prospective follow-up study. Eur Arch Otorhinolaryngol. 2018;275:2683-2693. [DOI] [PubMed] [Google Scholar]
- 4. Dwyer NY, Firszt JB, Reeder RM. Effects of unilateral input and mode of hearing in the better ear: self-reported performance using the speech, spatial and qualities of hearing scale. Ear Hear. 2014;35:126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32:39-47. [DOI] [PubMed] [Google Scholar]
- 6. Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol. 2010;119:772-781. [PubMed] [Google Scholar]
- 7. Bronkhorst AW, Plomp R. The effect of head-induced interaural time and level differences on speech intelligibility in noise. J Acoust Soc Am. 1988;83:1508-1516. [DOI] [PubMed] [Google Scholar]
- 8. Blauert J. Binaural localization. Scand Audiol Suppl. 1982;15:7-26. [PubMed] [Google Scholar]
- 9. Brown KD, Balkany TJ. Benefits of bilateral cochlear implantation: a review. Curr Opin Otolaryngol Head Neck Surg. 2007;15:315-318. [DOI] [PubMed] [Google Scholar]
- 10. Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136:141-153. [DOI] [PubMed] [Google Scholar]
- 11. Agterberg MJH, Snik AFM, Van de Goor RMG, et al. Sound-localization performance of patients with single-sided deafness is not improved when listening with a bone-conduction device. Hear Res. 2019;372:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mertens G, De Bodt M, Van de Heyning P. Evaluation of long-term cochlear implant use in subjects with acquired unilateral profound hearing loss: focus on binaural auditory outcomes. Ear Hear. 2017;38:117-125. [DOI] [PubMed] [Google Scholar]
- 13. Arndt S, Laszig R, Aschendorff A, et al. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017;65:98-108. [DOI] [PubMed] [Google Scholar]
- 14. Dillon MT, Buss E, Rooth MA, et al. Effect of cochlear implantation on quality of life in adults with unilateral hearing loss. Audiol Neurootol. 2018;22:259-271. [DOI] [PubMed] [Google Scholar]
- 15. Buss E, Dillon MT, Rooth MA, et al. Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear. 2018;22:2331216518771173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dillon MT, Buss E, Anderson ML, et al. Cochlear implantation in cases of unilateral hearing loss: initial localization abilities. Ear Hear. 2017;38:611-619. [DOI] [PubMed] [Google Scholar]
- 17. Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear. 1995;16:176-186. [DOI] [PubMed] [Google Scholar]
- 18. Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gatehouse S, Akeroyd M. Two-eared listening in dynamic situations. Int J Audiol. 2006;45(suppl 1):S120-S124. [DOI] [PubMed] [Google Scholar]
- 20. Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62-70. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 1999. [Google Scholar]
- 22. Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28:455-462. [DOI] [PubMed] [Google Scholar]
- 23. van Loon MC, Smits C, Smit CF, et al. Cochlear implantation in adults with asymmetric hearing loss: benefits of bimodal stimulation. Otol Neurotol. 2017;38:e100-e106. [DOI] [PubMed] [Google Scholar]
- 24. Fullgrabe C, Moore BC, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2014;6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eddins AC, Ozmeral EJ, Eddins DA. How aging impacts the encoding of binaural cues and the perception of auditory space. Hear Res. 2018;369:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25:298-301. [DOI] [PubMed] [Google Scholar]
- 27. Waltzman SB, Fisher SG, Niparko JK, et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 1995;165:15-18. [PubMed] [Google Scholar]