Abstract
Mitochondrial biogenesis requires coordinated expression of genes encoding mitochondrial proteins, which in Saccharomyces cerevisiae is achieved in part via post-transcriptional control by the Pumilio RNA-binding domain protein Puf3. Puf3 binds to the 3′-UTR of many messenger RNAs (mRNAs) that encode mitochondrial proteins, regulating their turnover, translation, and/or mitochondrial targeting. Puf3 hyperphosphorylation correlates with increased mitochondrial biogenesis; however, the kinase responsible for Puf3 phosphorylation is unclear. Here, we show that the casein kinase I protein Hrr25 negatively regulates Puf3 by mediating its phosphorylation. An hrr25 mutation results in reduced phosphorylation of Puf3 in vivo and a puf3 deletion mutation reverses growth defects of hrr25 mutant cells grown on medium with a nonfermentable carbon source. We show that Hrr25 directly phosphorylates Puf3, and that the interaction between Puf3 and Hrr25 is mediated through the N-terminal domain of Puf3 and the kinase domain of Hrr25. We further found that an hrr25 mutation reduces GFP expression from GFP reporter constructs carrying the 3′-UTR of Puf3 targets. Downregulation of GFP expression due to an hrr25 mutation can be reversed either by puf3Δ or by mutations to the Puf3-binding sites in the 3′-UTR of the GFP reporter constructs. Together, our data indicate that Hrr25 is a positive regulator of mitochondrial biogenesis by phosphorylating Puf3 and inhibiting its function in downregulating target mRNAs encoding mitochondrial proteins.
Keywords: Hrr25, Puf3, protein phosphorylation, post-transcriptional regulation, mitochondrial biogenesis
THE maintenance of mitochondrial functions during cell growth and development depends on contributions from the nuclear genome, which encodes the majority of mitochondrial proteins, and the mitochondrial genome, which encodes a small but essential number of mitochondrial proteins that are mostly components of the mitochondrial respiratory complexes (Poyton and McEwen 1996; Foury et al. 1998; Calvo and Mootha 2010). The prominent function of mitochondria is ATP synthesis. The heteromeric Hap2/3/4/5 transcription factor is an important part of yeast’s metabolic remodeling when cells switch from glycolysis to respiratory metabolism to produce ATP (Forsburg and Guarente 1989; Olesen and Guarente 1990; Rosenkrantz et al. 1994; McNabb et al. 1995; Blom et al. 2000; Buschlen et al. 2003). The Hap2/3/5 trimer binds to CCAAT sequence elements in the promoter of target genes and requires Hap4 to provide the transcriptional activation domain activity (Olesen et al. 1987; Forsburg and Guarente 1989; Olesen and Guarente 1990; McNabb et al. 1995; McNabb and Pinto 2005). A heme-activated protein, Hap1, also contributes to mitochondrial respiratory metabolism by activating the expression of genes under aerobic conditions, including those encoding components of complexes III and IV of the electron transport chain (Hickman and Winston 2007; Kundaje et al. 2008).
Puf3, a Pumilio RNA-binding domain protein, acts as a post-transcriptional regulator of mitochondrial biogenesis by binding to the 3′-UTR of many mitochondrial protein-encoding messenger RNAs (mRNAs) (Olivas and Parker 2000; Gerber et al. 2004; Jackson et al. 2004; Houshmandi and Olivas 2005; García-Rodríguez et al. 2007; Saint-Georges et al. 2008; Zhu et al. 2009; Lee et al. 2010; Chatenay-Lapointe and Shadel 2011; Freeberg et al. 2013; Miller et al. 2014; Kershaw et al. 2015; Lapointe et al. 2015; Wilinski et al. 2017). PUF (Pumilio and FBF) proteins typically bind to mRNA targets through a concave face formed typically by eight α-helical repeats (Edwards et al. 2001; Wang et al. 2002; Zhu et al. 2009). The Puf3-binding motif is the sequence UGUAHAUA (H is A, U, or C), with a C often found at the −2 position (Gerber et al. 2004; Houshmandi and Olivas 2005; Zhu et al. 2009). PUF proteins can regulate the stability and/or translation of target mRNAs, often leading to their decay or translational inhibition (Miller and Olivas 2011; Quenault et al. 2011; Wang et al. 2018). Due to its role in promoting target mRNA deadenylation and decay, Puf3 is considered to be a negative regulator of mitochondrial biogenesis under glucose repression conditions (Olivas and Parker 2000; Gerber et al. 2004; Jackson et al. 2004; Foat et al. 2005; Lee et al. 2010; Chatenay-Lapointe and Shadel 2011; Gupta et al. 2014; Miller et al. 2014). When cells switch from fermentative to respiratory growth, the negative regulatory role of Puf3 is reduced or maybe even converted to a positive regulatory role by promoting the translation of Puf3-bound mRNAs (Lee and Tu 2015; Lapointe et al. 2018). Puf3 also promotes mitochondrial biogenesis by localizing mitochondrial protein-encoding transcripts to the mitochondrial outer membrane for cotranslational import into mitochondria (García-Rodríguez et al. 2007; Saint-Georges et al. 2008; Eliyahu et al. 2010; Gadir et al. 2011; Quenault et al. 2011). Consistent with the proposed latter role, puf3 mutant cells show mild growth defects on nonfermentable carbon sources (Gerber et al. 2004; Jiang et al. 2010; Lee and Tu 2015). Puf3 targets are not limited to mRNAs encoding mitochondrial proteins, suggesting that Puf3 may have other cellular roles (Gerber et al. 2004; Freeberg et al. 2013; Kershaw et al. 2015; Lapointe et al. 2015; Wilinski et al. 2017).
PUF proteins are found in most, if not all, eukaryotes (Gerber et al. 2006; Galgano et al. 2008; Morris et al. 2008; Stumpf et al. 2008; Jiang et al. 2010; Tam et al. 2010; Hogan et al. 2015; Wilinski et al. 2017). The number of PUF protein-encoding genes varies in each species (Wang et al. 2018). The Saccharomyces cerevisiae genome encodes six PUF proteins, Puf1–6 (Olivas and Parker 2000; Gu et al. 2004), which associate with different set of functionally related mRNAs (Olivas and Parker 2000; Gerber et al. 2004; Gu et al. 2004; Freeberg et al. 2013; Miller et al. 2014; Lapointe et al. 2017). Despite the structural conservation of PUF proteins, their functions have diverged. For example, the Puf3 homologs in S. cerevisiae, Drosophila melanogaster, and Homo sapiens interact with nearly identical RNA sequence motifs, but their target mRNAs encode proteins with different functions (Gerber et al. 2004, 2006; Galgano et al. 2008). Functional diversification of PUF proteins is not limited to major lineages of eukaryotic organisms during evolution (Hogan et al. 2015). Among 80 fungal species analyzed, only Puf3 homologs from the species in the Saccharomycotina subphylum and Arthrobotrys oligospora, an early diverging Pezizomycotina species, bind a common set of 176 target mRNAs encoding mitochondrial proteins (Gasch et al. 2004; Jiang et al. 2010; Hogan et al. 2015; Wilinski et al. 2017). Although they have been shown or predicted to share a common binding motif with Puf3 of S. cerevisiae, Puf3 homologs in Leotiomyceta species (Pezizomycotina species excluding A. oligospora and Tuber melanosporum) have lost association with mRNAs orthologous to most Saccharomycotina Puf3 targets. Instead, they are predicted to bind a set of 409 orthologous genes, 113 of which encode mitochondrial proteins, with remarkable enrichment of subunits of complex I of the electron transport chain (Hogan et al. 2015). More than 150 mRNAs in Leotiomyceta species orthologous to the Saccharomycotina Puf3 targets have been shown to acquire new sets of binding motifs, which are recognized by Leotiomyceta Puf4 (Hogan et al. 2015). These findings have led to the proposal that rewiring and reprogramming of PUF protein targets is employed to coordinate the expression of genes with related functions during evolution (Keene 2007).
A lot of progress has been made in the identification of mRNA targets of Puf3 orthologs in the budding yeast and other species, but little is known about how Puf3 activity is regulated. Lee and Tu reported that Puf3 phosphorylation is increased when cells switch from fermentation growth to respiratory growth (Lee and Tu 2015). Their data suggest that increased Puf3 phosphorylation leads to a switch from its function as a negative regulator under fermentation conditions to other functions under respiratory growth conditions. Puf3 has an N-terminal domain that contains most of its phosphorylation sites and a C-terminal region that contains the RNA-binding domain (Olivas and Parker 2000; Lee and Tu 2015). Lee and Tu reported that two nutrient-responsive kinases, Sch9 and PKA, and the PP2A-related phosphatase Sit4 might mediate Puf3 phosphorylation. However, it is not clear whether the observed effects are direct or indirect. It is also not clear whether mutations in SCH9, PKA, and SIT4 affect Puf3 activity. Here, we show that casein kinase I protein Hrr25 is the primary kinase that phosphorylates Puf3. There are four different casein kinase I isoforms in S. cerevisiae, Yck1, Yck2, Yck3, and Hrr25. Yck1 and Yck2 play redundant roles in cell morphogenesis, amino acid sensing, and glucose-sensing pathways (Robinson et al. 1993; Moriya and Johnston 2004; Liu et al. 2008). Yck3 is a vacuolar membrane-localized kinase that mediates vacuolar membrane fusion (LaGrassa and Ungermann 2005; Zick and Wickner 2012; Lawrence et al. 2014). Hrr25 is implicated in multiple cellular processes, including vesicular trafficking, ribosome biogenesis, autophagy, transcriptional regulation, meiosis, endocytosis, microtubule assembly and spindle positioning, and the DNA damage response (Hoekstra et al. 1991; Petronczki et al. 2006; Schäfer et al. 2006; Ray et al. 2008; Mehlgarten et al. 2009; Corbett and Harrison 2012; Sarkar et al. 2013; Mochida et al. 2014; Pfaffenwimmer et al. 2014; Tanaka et al. 2014; Abdel-Fattah et al. 2015; Ghalei et al. 2015; Nakatogawa 2015; Peng et al. 2015a,b; Davis et al. 2016; Argüello-Miranda et al. 2017; Collins et al. 2017; Zhang et al. 2018; Zientara-Rytter et al. 2018; Nemec et al. 2019). The kinase domains of these four casein kinase I isoforms are highly similar, while the sequences outside the kinase domain are not conserved (Wang et al. 1996; Gross and Anderson 1998). The different functions of these four casein kinase I proteins can be partially attributed to the sequences outside the kinase domain, which target them to different cellular locations (Vancura et al. 1994; Babu et al. 2002, 2004; Sun et al. 2004; Peng et al. 2015a,b; Zhang et al. 2016). Although Yck1, Yck2, and Yck3 all contain a C-terminal dicysteine motif for palmitoylation, Yck1 and Yck2 localize to the plasma membrane, while Yck3 is targeted to the vacuoles. Consistent with its multiple cellular functions, Hrr25 is localized to different cellular locations, including endocytic sites, bud necks, P-bodies, spindle pole bodies, and the nucleus (Peng et al. 2015a,b; Zhang et al. 2016). Hrr25 has an N-terminal kinase domain, a middle region, and a C-terminal P/Q-rich region (Dhillon and Hoekstra 1994; Peng et al. 2015a,b; Ye et al. 2016; Zhang et al. 2016, 2018). The middle region has been proposed or shown to be required for Hrr25’s localization to P-bodies, spindle pole bodies, endocytic sites, and meiosis I centromeres. We show that the kinase domain of Hrr25 alone is both necessary and sufficient for an interaction with the N-terminal domain of Puf3. We found that hrr25 mutant cells were respiratory-deficient, which could be reversed by puf3 mutations. Our data suggest that Hrr25 inhibits the function of Puf3 as a negative regulator of mitochondrial biogenesis by phosphorylating it.
Materials and Methods
Strains, plasmids, growth media, growth conditions, and yeast transformation
Yeast strains and plasmids used in this study are listed in Table 1 and Table 2, respectively. Yeast strains were grown at 30° in YPD (1% Bacto-yeast extract, 2% Bacto-peptone, and 2% glucose), YPL [1% Bacto-yeast extract, 2% Bacto-peptone, and 3.7% DL-lactic acid (w/w, 85%), adjusted to pH 5.3 using NaOH], YPEG (1% Bacto-yeast extract, 2% Bacto-peptone, 2% ethanol, and 3% glycerol), YNBcasD and YNBcas5D (0.67% yeast nitrogen base, 1% casamino acids, and 2% or 5% dextrose as indicated), YNBcasR (0.67% yeast nitrogen base, 1% casamino acids, and 2% raffinose), minimal dextrose medium (SD) (0.67% yeast nitrogen base and 2% glucose), complete supplement mixture medium (CSM) (0.67% yeast nitrogen base, 2% glucose, and 0.6 g/liter CSM minus histidine, leucine, and tryptophan), YNBcasE (0.67% yeast nitrogen base, 1% casamino acids, and 2% ethanol), YNBcasL [0.67% yeast nitrogen base, 1% casamino acids, and 3.7% DL-Lactic acid (w/w, 85%), adjusted to pH 5.3 using NaOH], or YNBcasEG (0.67% yeast nitrogen base, 1% casamino acids, 2% ethanol, and 3% glycerol) as indicated. Amino acids and uracil were added to growth medium at standard concentrations to cover auxotrophic requirements if required (Amberg et al. 2005). For yeast two-hybrid analysis involving the HIS3 reporter gene under the control of Gal4, 3-amino-1,2,4-triazole was added to the CSM medium. Agar was added at a final concentration of 2% for solid growth medium. For transformation, yeast cells were freshly grown in YPD media and transformed with plasmids using the high-efficiency method (Gietz et al. 1992).
Table 1. Yeast strains used in this study.
| Strain | Genotype | Source | Application |
|---|---|---|---|
| BY4741 | MATa ura3 leu2 his3 met15 | Figure 1, Figure 2, Figure 6, Figure 7, and Figure S4 | |
| ZLY4467 | BY4741 hrr25Δ::kanMX4 [pRS415-HRR25(E52D)] | Collins et al. (2017) | Figure 1, Figure 2, Figure 6, Figure 7, and Figure S4 |
| ZLY4578 | BY4741 hrr25Δ::kanMX4 puf3Δ::kanMX4 [pRS415-HRR25(E52D)] | This study | Figure 1, Figure 3, Figure 6, and Figure 7 |
| ZLY4562 | BY4741 puf3Δ::kanMX4 | Yeast genome deletion project | Figure 3, Figure 4A, and Figure 7 |
| ZLY4578 | BY4741 puf3Δ::kanMX4 hrr25Δ::kanMX4 | This study | Figure 4A and Figure S1 |
| ZLY5754 | MATa ura3 leu2 his3 met15 hrr25Δ::kanMX4 pRS416-HRR25-myc | This study | Figure S1 |
| AH109 | MATa ura3-52 his3-200 trp1-901 leu2-3,112 gal4Δ gal80Δ URA3::MEL1UAS-MEL1TATA -lacZ GAL2UAS-GAL2TATA-ADE2 LYS2::GAL1UAS-GAL1TATA-HIS3 | Clontech Laboratories | Figure 4, B and C |
| Y187 | MATα ura3-52 his3-200 ade2-101 trp1-901 leu2-3,112 gal4Δ gal80Δ met – URA3::GAL1UAS-GAL1TATA -lacZ | ||
| YL28 | MATa PUF3-FLAG-KanMX6. Prototrophic CEN PAK background | Lee and Tu (2015) | Figure 3A |
| YL632 | MATa PUF3(24A)-FLAG-NatNT2. CEN PAK background | Lee and Tu (2015) | Figure 3A |
| ZLY2630 | BY4741 aco1Δ::kanMX4 | This study | Figure 7 and Figure S4 |
| ZLY3206 | MATα met rho0 | Laboratory stock | Figure 7 and Figure S4 |
Table 2. Plasmids used in this study.
| Plasmid | Description | Reference | Application |
|---|---|---|---|
| pZL1939 | pRS416-PUF3-HA, expressing PUF3 with a C-terminal 3xHA tag under the control of the endogenous promoter. | This study | Figure 1, Figure 2C, Figure 3, Figure 4A, and Figure S1 |
| pZL3340 | pRS416-TEF2p-PUF3-HA, expressing PUF3 with a C-terminal 3xHA tag under the control of strong TEF2 promoter. | This study | Figure 2, A and B |
| pZL3338 | pRS415-HRR25-myc, expressing HRR25 from the endogenous promoter with a 3xMyc epitope tag at the C-terminus. | Collins et al. (2017) | Figure 4A and Figure S1 |
| pZL3542 | pRS424-ADH1-GBD. | Collins et al. (2017) | Figure 4, B and C |
| pZL3557 | pRS424-ADH1-GBD-HRR25. HRR25 ORF was cloned into the BamHI and SalI sites of pZL3542. | Collins et al. (2017) | Figure 4, B and C |
| pZL3740 | pRS424-ADH1-GBD-HRR25(K38A). The kinase-dead mutant allele of HRR25, hrr25(K38A), was cloned into pZL3542. | This study | Figure 4B |
| pZL3736 | pRS424-ADH1-GBD-HRR25(KD). The DNA sequence encoding the Hrr25 kinase domain was cloned into pZL3542. | This study | Figure 4C |
| pZL3744 | pRS424-ADH1-GBD-HRR25ΔC. The DNA sequence encoding a C-terminal truncation construct of Hrr25 was cloned into pZL3542. | This study | Figure 4C |
| pZL3742 | pRS424-ADH1-GBD-HRR25ΔM. The DNA sequence encoding a middle region truncation construct of Hrr25 was cloned into pZL3542. | This study | Figure 4C |
| pMB218 | pRS424-ADH1-GBD-HRR25Μ. The DNA sequence encoding the middle region of Hrr25 was cloned into pZL3542. | This study | Figure S2 |
| pMB220 | pRS424-ADH1-GBD-HRR25C. The DNA sequence encoding the C-terminal region of Hrr25 was cloned into pZL3542. | This study | Figure S2 |
| pZL3805 | pRS424-ADH1-GBD-HRR25(M+C). The DNA sequence encoding the middle region and the C-terminal domain of Hrr25 was cloned into pZL3542. | This study | Figure S2 |
| pZL3539 | pRS415-TEF-GAD. | This study | Figure 4, B and C, and Figure S2 |
| pMB212 | pRS415-TEF-GAD-PUF3. The PUF3 ORF was cloned into the BamHI and XhoI sites of pZL3539. | This study | Figure 4B and Figure S2 |
| pMB214 | pRS415-TEF-GAD-PUF3N. A DNA sequence encoding the N-terminal domain (amino acid residues 1–533) of Puf3 was cloned into pZL3539. | This study | Figure 4, B and C, and Figure S2 |
| pMB216 | pRS415-TEF-GAD-PUF3C. A DNA sequence encoding the C-terminal domain (amino acid residues 511–879) of Puf3 was cloned into pZL3539. | This study | Figure 4, B and C, and Figure S2 |
| pAB101 | pET21a-HRR25ΔC, expressing a C-terminal truncation construct of Hrr25 with a C-terminal 6xHis tag. | This study | Figure 5, A and B |
| pAB104 | pET21a-HRR25(K38A)ΔC, expressing a C-terminal truncation construct of Hrr25(K38A) with a C-terminal 6xHis tag. | This study | Figure 5A |
| pZL3359 | pET24a-PUF3-HA, expressing Puf3 with a C-terminal 3xHA tag and a 6xHis tag. | This study | Figure 5, A and B |
| pZL3419 | pET24a-PUF3N-HA, expressing the N-terminal domain of Puf3 with a C-terminal 3xHA tag and a 6xHis tag. | This study | Figure 5, A and B |
| pZL3421 | pET24a-PUF3C-HA, expressing the C-terminal domain of Puf3 with a C-terminal 3xHA tag and a 6xHis tag. | This study | Figure 5, A and B |
| pZL1760 | pET24a-MKS1, expressing Mks1 with a C-terminal His6 tag in E. coli. | Liu et al. (2003) | Figure 5C |
| pZL971 | pET24a-RTG2, expressing Rtg2 with a C-terminal His6 tag in E. coli. The RTG2 ORF was cloned into the SacI and XhoI sites of pET24a. | This study | Figure 5C |
| pZL4262 | pRS416-PUF3-HA, expressing PUF3 with a C-terminal 3xHA tag. The genomic DNA of CEN PAK background strain YL27 (wild-type PUF3) was used to amplify the PUF3 DNA fragment for cloning into a pRS416-HA plasmid. | This study | Figure 5D |
| pZL4264 | pRS416-puf3 (24A)-HA, expressing puf3(24A) with a C-terminal 3xHA tag. The genomic DNA of the CEN PAK background strain YL27 (wild-type PUF3) was used to amplify the PUF3 DNA fragment for cloning into a pRS416-HA plasmid. | This study | Figure 5D |
| pZL4257 | pET24a-PUF3-HA, expressing Puf3 with a C-terminal 3xHA tag and a 6xHis tag. The PUF3 coding sequence was amplified using yeast YL28 genomic DNA and cloned into pET24a. | This study | Figure 5E |
| pZL4258 | pET24a-puf3 (24A)-HA, expressing a Puf3(24A) mutant with a C-terminal 3xHA tag and a 6xHis tag. The puf3(24A) coding sequence was amplified using yeast YL632 genomic DNA and cloned into pET24a. | This study | Figure 5E |
| pZL3507 | pRS416-ADH1-GFP-CYC1ter, expressing GFP under the control of ADH1 promoter and the 3′-UTR of CYC1. | This study | Figure 6 |
| pZL3407 | pRS416-ADH1-GFP-CYT2ter | This study | Figure 6 |
| pZL3510 | pRS416-ADH1-GFP-CYT2ter (Puf3 site mutant) | This study | Figure 6 |
| pZL3416 | pRS416-ADH1-GFP-PET123ter | This study | Figure 6 |
| pZL3512 | pRS416-ADH1-GFP-PET123ter (Puf3 site 1 mutant) | This study | Figure 6 |
| pZL3519 | pRS416-ADH1-GFP-PET123ter (Puf3 site 1,2,3 triple mutant) | This study | Figure 6 |
| pZL4248 | pRS416-MKS1-GFP-CYC1ter, expressing GFP under the control of the MKS1 promoter and the 3′-UTR of CYC1. | This study | Figure S3 |
| pZL4250 | pRS416-MKS1-GFP-CYT2ter | This study | Figure S3 |
| pZL4252 | pRS416-MKS1-GFP-CYT2ter (Puf3 site mutant) | This study | Figure S3 |
| pZL4253 | pRS416-MKS1-GFP-PET123ter | This study | Figure S3 |
| pZL4255 | pRS416-MKS1-GFP-PET123ter (Puf3 site 1 mutant) | This study | Figure S3 |
| pZL4256 | pRS416-MKS1-GFP-PET123ter (Puf3 site 1,2,3 triple mutant) | This study | Figure S3 |
Yeast cell extract preparation, phosphatase treatment, Ponceau staining, and western blotting
Total cellular proteins were prepared by treating yeast cell pellets with 7.5% β-mercaptoethanol and 1.85 N NaOH solution, and precipitated with trichloroacetic acid as described previously (Yaffe and Schatz 1984). For phosphatase treatment, trichloroacetic acid-precipitated total cellular proteins were neutralized with unbuffered Tris and incubated with 400 units of λ protein phosphatase (New England Biolabs, Beverly, MA) in a final volume of 20 μl at 30° for 90 min. When indicated, phosphatase inhibitors (1 μM sodium orthovanadate, 10 mM β-glycerol phosphate, 10 mM sodium pyrophosphate, and 10 mM NaF) were added to inhibit protein phosphatase activity. Protein samples were resuspended in SDS-PAGE sample buffer with 100 mM dithiothreitol and boiled for 3 min before being separated by SDS-PAGE. Prestained protein ladder (broad range, 10–230 kDa, P7710S, New England Biolabs) was used in all protein gels. Proteins were transferred to a nitrocellulose membrane for immunoblotting. The following antibodies were used in this study: anti-myc, clone 9E10; anti-HA, clone 3F10; Tetra·His antibody (# 3467; QIAGEN, Valencia, CA); Cox2, mouse monoclonal antibody 4B12-A5 (Molecular Probes, Eugene, OR); Cox3, mouse monoclonal antibody DA5 (Molecular Probes); porin/Por1, clone 16G9E6BC4 (Fisher Scientific, Pittsburgh, PA); aconitase (Aco1), rabbit polyclonal antibodies against recombinant yeast aconitase; Ilv5, rabbit polyclonal antibodies against recombinant yeast Ilv5; and Pgk1, rabbit polyclonal antibodies against recombinant yeast phosphoglycerate kinase. HRP-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA) were used to probe primary antibodies. When indicated, membranes were stained in 0.1% Ponceau S in 0.1% acetic acid for 10 min and images of Ponceau-stained membranes were taken before immunoblotting. Chemiluminescence images of western blots were captured using the Bio-Rad ChemiDoc MP imaging system ∼(Hercules, CA) and processed using Bio-Rad Image Lab software. Protein bands on western blots were quantified using the same software.
Puf3 phosphorylation assay in Escherichia coli
A pET24a plasmid encoding full-length Puf3, the N-terminal fragment of Puf3 (Puf3N, amino acid residues 1–533), or the C-terminal fragment of Puf3 (Puf3C, amino acid residues 511–879), and a pET21a plasmid encoding the C-terminal domain (CTD) deletion construct of wild-type Hrr25 or kinase dead mutant of Hrr25 [Hrr25ΔC or Hrr25(K38A)ΔC], were cotransformed into BL21 (DE3) cells. Transformants were induced with 1 mM IPTG at OD600 ∼0.5 and grown overnight at 22°. Bacterial cells were pelleted and boiled in SDS gel-loading buffer for 3 min. Protein samples were separated on a 7.5% SDS-PAGE gel and transferred to a nitrocellulose membrane in a semidry blotter. Tetra·His antibody was used to detect 6xHis-tagged Hrr25 and Puf3 proteins. To prepare protein samples from E. coli for phosphatase treatment, 1 ml bacterial cell culture was added to a 160 μl solution of 7.5% β-mercaptoethanol and 1.85 N NaOH, and incubated on ice for 10 min. The mixture was centrifuged at 21,000 × g for 5 min to get rid of the pellet. The remaining supernatant was added to 84 μl 100% (w/v) trichloroacetic acid and the mixture was incubated on ice for 10 min. Total E. coli cellular proteins were precipitated by centrifugation at 21,000 × g for 5 min. Phosphatase treatment of proteins expressed in E. coli was conducted as described for yeast proteins.
Yeast two-hybrid analysis
DNA fragments encoding full-length Puf3, and the N- and C-terminal fragments of Puf3, were amplified by PCR and cloned into the BamHI and XhoI sites of pZL3539 (pRS415-TEF-GAD), a Gal4 transcriptional activation domain vector (GAD) to generate pRS415-TEF-GAD-PUF3, PUF3N, and PUF3C plasmids. DNA fragments encoding full-length wild-type Hrr25, kinase-dead mutant Hrr25(K38A), and various Hrr25 fragments were cloned into the BamHI and SalI sites of pZL3542 (pRS424-ADH1-GBD), a Gal4 DNA-binding domain vector (GBD), to generate pRS424-ADH1-GBD-HRR25 plasmids. Yeast two-hybrid strains AH109 and Y187 (Clontech Laboratories) were transformed with plasmids expressing GBD and GAD constructs, respectively, and crossed to generate diploid cells coexpressing GBD and GAD fusion constructs. Transformants were streaked on CSM dextrose medium without leucine, tryptophan, and with or without histidine, for the analysis of ADE2 and HIS3 reporter genes under the control of the Gal4-dependent promoter. An inhibitor of His3, 3-amino-1,2,4-triazole, was added to CSM medium when required.
Data availability
The authors affirm that all data necessary to draw conclusions in this article are present within the texts, figures, and tables. Strains, plasmids, and other noncommercial research reagents are available upon request. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988126.
Results
Hrr25 is required for mitochondrial respiratory function
Hrr25 is one of the four casein kinase I protein kinases in yeast that has been implicated in a number of cellular processes including autophagy and DNA damage response. We recently reported that Hrr25 is a negative regulator of Haa1, a transcriptional activator that is important for the cellular adaptation to acetic acid stress response. In that study, we generated a partial loss-of-function mutant allele, hrr25(E52D), that encodes a mutant protein with an aspartic acid residue in place of glutamic acid residue at position 52. We found that hrr25(E52D) mutant cells exhibited strong growth defects on medium with ethanol or lactate as the carbon source (Figure 1). The growth defect of hrr25(E52D) mutant cells on different nonfermentable carbon sources indicates that mitochondrial respiratory function rather than the ability to use different carbon sources is compromised in hrr25(E52D) mutant cells.
Figure 1.
A mutation in HRR25 leads to Puf3-dependent growth defects on nonfermentable carbon sources. Wild-type (WT) (BY4741), and isogenic mutant strains hrr25(E52D) (ZLY4467) and hrr25(E52D) puf3Δ (ZLY4578) carrying empty vector (pRS416) or a centromeric plasmid encoding PUF3 with a C-terminal 3xHA tag (pPUF3-HA, pZL1939), were serially diluted and plated on YNBcasD (0.67% yeast nitrogen base, 1% casamino acids, and 2% dextrose), YNBcasE (0.67% yeast nitrogen base, 1% casamino acids, and 2% ethanol), and YNBcasL (0.67% yeast nitrogen base, 1% casamino acids, and 3.7% DL-Lactic acid (w/w, 85%), adjusted to pH 5.3 using NaOH) plates. Cells were grown at 30° before pictures were taken.
We then looked for the mechanism behind respiratory deficiency in hrr25(E52D) mutant cells. Genome-wide studies have revealed 315 genetic and biochemical interactions between Hrr25 and other proteins (curated data from the Saccharomyces genome database). One interaction is with Puf3, which functions as a global regulator of mitochondrial biogenesis by binding to the 3′-UTR of many mRNAs encoding mitochondrial proteins, often leading to their degradation. To determine whether Puf3 contributes to respiratory deficiency in hrr25(E52D) mutant cells, we generated an hrr25(E52D) puf3Δ double mutant and tested its growth on ethanol and glycerol medium. Figure 1 shows that the hrr25(E52D) puf3Δ double mutant grew on ethanol or lactate medium. Reintroduction of PUF3 with a C-terminal 3xHA tag (PUF3-HA) on a centromeric plasmid into the double mutant restored growth defects, indicating that Puf3 is implicated in respiratory deficiency in hrr25(E52D) mutant cells.
hrr25 mutant cells are hypersensitive to PUF3 overexpression
To confirm the notion that Hrr25 is a negative regulator of Puf3, we wanted to determine whether hrr25 mutant cells were sensitive to PUF3 overexpression. Accordingly, we constructed a centromeric plasmid that overexpresses PUF3 by encoding PUF3 with a C-terminal 3xHA tag under the control of a strong TEF2 promoter (TEF2p-PUF3-HA), and transformed the plasmid into wild-type and hrr25(E52D) mutant strains. Overexpression of PUF3 in wild-type and hrr25(E52D) strains resulted in slow-growing transformant colonies on dextrose selective medium (Figure 2A). Upon transfer to a fresh plate, wild-type transformants carrying the TEF2p-PUF3-HA plasmid could grow into visible colonies, albeit at a slower rate compared to cells transformed with an empty vector (Figure 2B). In contrast, hrr25(E52D) transformants carrying the TEF2p-PUF3-HA plasmid failed to grow into visible colonies, indicating that hrr25(E52D) mutant cells are hypersensitive to PUF3 overexpression.
Figure 2.
hrr25 mutant cells are hypersensitive to PUF3 overexpression. (A) PUF3 overexpression leads to the formation of small colonies on plate medium. Wild-type (WT) (BY4741) and hrr25(E52D) mutant cells (ZLY4467) were transformed with a centromeric plasmid encoding PUF3 under the control of the strong TEF2 promoter (pZL3340), and transformants were selected on YNBcasD (0.67% yeast nitrogen base, 1% casamino acids, and 2% dextrose) medium. Pictures were taken after 5 days growth at 30°. (B) Transformants of WT and hrr25(E52D) mutant cells carrying empty vector or TEF2p-PUF3 overexpression plasmid from panel (A) were streaked on YNBcasD solid medium, and the picture was taken after 3 days of growth at 30°. (C) WT and hrr25(E52D) mutant cells carrying an empty vector or a plasmid encoding PUF3 under the control of the endogenous promoter were serially diluted, and spotted onto solid medium with dextrose or ethanol, and glycerol as carbon sources. Pictures were taken after 2 days of growth at 30° on the dextrose plate and 7 days of growth at 30° on the ethanol and glycerol plate.
Previously, overexpression of PUF3 from a multicopy vector has been reported to cause no growth defects in wild-type cells grown on dextrose medium (García-Rodríguez et al. 2007). However, our data in Figure 2A suggest that overexpression of PUF3 under the control of a strong TEF2 promoter has a strong growth-inhibitory effect. The difference between these results could be due to the extent of PUF3 overexpression. Therefore, to further assess the hrr25(E52D) mutant’s hypersensitivity to PUF3 overexpression, we transformed wild-type and hrr25(E52D) mutant cells with a control vector or a centromeric plasmid encoding PUF3 under the control of the endogenous promoter, and examined their growth phenotype via serial dilution. Figure 2C shows that on dextrose medium, an extra copy of PUF3 had no effect on growth in wild-type cells. However, hrr25(E52D) mutant cells carrying an extra copy of PUF3 in comparison to those carrying a control vector exhibited reduced growth. On ethanol and glycerol medium, hrr25(E52D) mutant cells exhibited strong growth defects as expected, and an extra copy of PUF3 completely inhibited cell growth. Together, our data suggest that Hrr25 is a negative regulator of Puf3. Since Hrr25 is a protein kinase, we hypothesize that Hrr25 negatively regulates Puf3 by phosphorylating it. It is also possible that Hrr25 and Puf3 function in parallel pathways in mediating cell growth.
Puf3 phosphorylation is largely abolished in hrr25(E52D) mutant cells
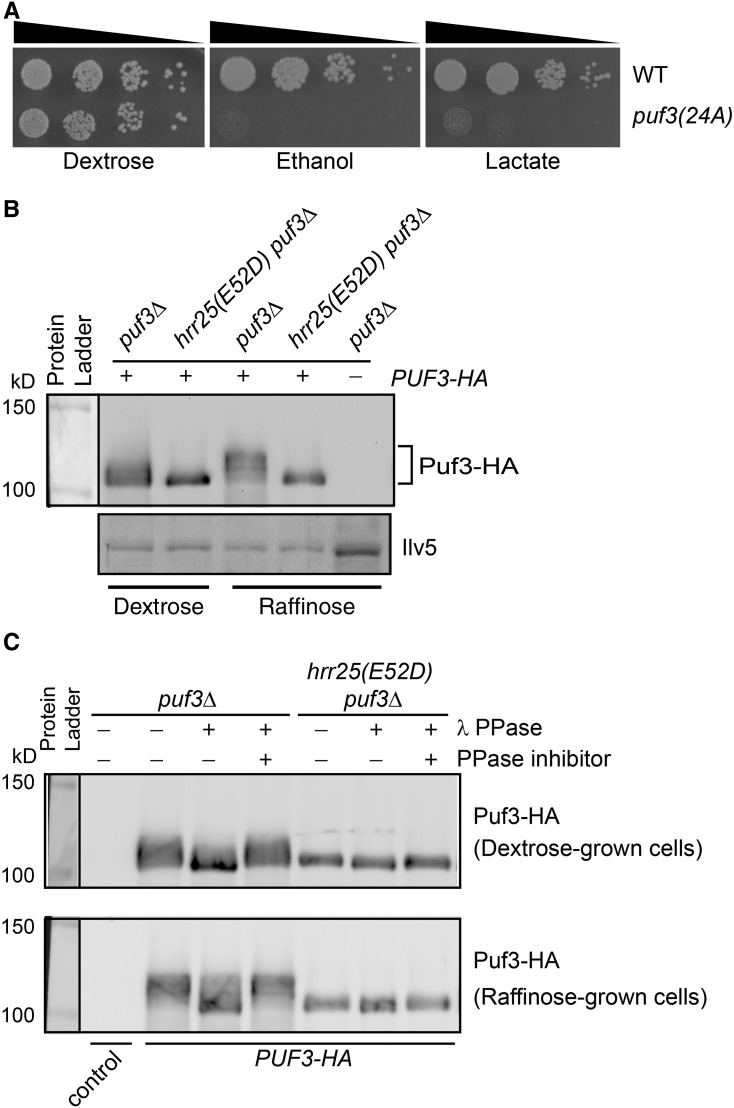
Puf3 is known to be phosphorylated and Puf3 phosphorylation is increased in cells requiring robust mitochondrial respiratory metabolism compared to cells undergoing fermentative growth (Lee and Tu 2015). A puf3(24A) mutant, which has alanine substitutions at 24 serine/threonine phosphorylation sites, exhibited growth defects on medium with ethanol or lactate as the sole carbon source (Figure 3A), consistent with published findings (Lee and Tu 2015). These observations suggest that the kinase(s) responsible for Puf3 phosphorylation is a negative regulator of Puf3 and a positive regulator of mitochondrial respiratory functions. We have shown that hrr25(E52D) mutant cells exhibit Puf3-dependent growth defects on ethanol or lactate medium (Figure 1). Similar growth defects on nonfermentable carbon sources due to an hrr25(E52D) mutation in Figure 1 and a puf3(24A) mutation in Figure 3A suggest that Hrr25 may be the primary kinase responsible for Puf3 phosphorylation. Thus, we examined Puf3 phosphorylation in wild-type vs. hrr25(E52D) mutant cells. Puf3 phosphorylation can be monitored by a mobility shift on western blots (Lee and Tu 2015). Figure 3B shows that Puf3 with a C-terminal 3xHA tag (the molecular weight of unmodified Puf3-HA is 102.3 kDa) migrated diffusely on western blots from cells grown in both dextrose and raffinose medium. Puf3 from raffinose-grown cells showed reduced mobility compared with cells grown in dextrose medium (Figure 3B), which is consistent with the observation by Lee and Tu that Puf3 phosphorylation is increased in cells undergoing glucose starvation (Lee and Tu 2015). Importantly, an hrr25(E52D) mutation largely abolished the diffuse migration pattern of Puf3 by collapsing slower mobility forms into a relatively sharp band of the fastest mobility form of Puf3 (Figure 3B), suggesting that Puf3 is mostly unphosphorylated in hrr25(E52D) mutant cells. Significantly, in hrr25(E52D) mutant cells, raffinose-induced phosphorylation of Puf3 is almost completely abolished, suggesting that Hrr25 is a kinase involved in the phosphorylation of Puf3.
Figure 3.
Puf3 phosphorylation is largely abolished in hrr25(E52D) mutant cells. (A) A Puf3 phosphomutant, puf3(24A), exhibited growth defects on nonfermentable carbon sources. WT (YL28) and isogenic puf3(24A) mutant cells (YL632) were serially diluted and plated on YNBcasD (0.67% yeast nitrogen base, 1% casamino acids, and 2% dextrose), YNBcasE (0.67% yeast nitrogen base, 1% casamino acids, and 2% ethanol), and YNBcasL (0.67% yeast nitrogen base, 1% casamino acids, and 3.7% DL-Lactic acid (w/w, 85%), adjusted to pH 5.3 using NaOH) plates. (B) puf3Δ (ZLY4562) and hrr25(E52D) puf3Δ (ZLY4578) mutant strains carrying a plasmid encoding PUF3-HA (pZL1939) were grown in YNBcas5D (0.67% yeast nitrogen base, 1% casamino acids, and 5% dextrose) or YNBcasR (0.67% yeast nitrogen base, 1% casamino acids, and 2% raffinose) medium as indicated, and Puf3-HA was detected by immunoblotting. The result was representative of three independent experiments. (C) Phosphatase treatment of Puf3-HA expressed in puf3Δ and hrr25(E52D) puf3Δ mutant cells results in protein bands of the same minimal size on western blots. PPase, protein phosphatase; WT, wild-type.
To further confirm that the change in Puf3 mobility in hrr25(E52D) mutant cells on western blots is due to a change in Puf3 phosphorylation, we treated total cellular proteins from puf3Δ and hrr25(E52D) puf3Δ double-mutant cells expressing PUF3-HA with λ protein phosphatase in the absence and presence of phosphatase inhibitors, and analyzed Puf3 migration by immunoblotting. Figure 3C shows that phosphatase treatment of Puf3-HA expressed in puf3Δ cells grown in both dextrose and raffinose medium increased its mobility, which was prevented by phosphatase inhibitors. This result is consistent with previous reports that Puf3 is a hyperphosphorylated protein (Lee and Tu 2015). Phosphatase treatment of Puf3-HA expressed in hrr25(E52D) puf3Δ mutant cells marginally increased its mobility, which was also prevented by phosphatase inhibitors, indicating that Puf3 is largely unphosphorylated in these cells. Importantly, phosphatase treatment led to the appearance of the fast-migrating form of Puf3 in both wild-type and hrr25(E52D) mutant cells, indicating that the Puf3 mobility shift observed in Figure 3B is only due to a change in phosphorylation rather than another type of post-translational modification. Together, our data indicate that Hrr25 is responsible for both basal and induced phosphorylation of Puf3 in vivo.
Hrr25 interacts with Puf3
Our data suggest that Hrr25 is required for Puf3 phosphorylation. Hrr25 may directly or indirectly phosphorylate Puf3. If Hrr25 directly phosphorylates Puf3, a physical connection between these two proteins might be detected. In a large-scale protein–protein interaction study, Hrr25 and Puf3 were reported to interact with each other (Breitkreutz et al. 2010). We wanted to confirm their interaction by using a co-immunoprecipitation assay. Accordingly, we constructed a centromeric plasmid encoding C-terminal 3xMyc-tagged Hrr25 (HRR25-myc). The HRR25-myc construct was determined to be functional based on its ability to rescue the hrr25(E52D) mutant’s growth defects on ethanol and glycerol medium (data not shown). The PUF3-HA construct on a centromeric plasmid was already confirmed to be functional based on its complementation of the puf3Δ mutation in the hrr25(E52D) puf3Δ double-mutant cells grown on ethanol and glycerol medium (Figure 1). The two plasmids were then introduced into an hrr25Δ puf3Δ double mutant to generate a strain expressing both HRR25-myc and PUF3-HA. As a control, the PUF3-HA plasmid was introduced into a puf3Δ mutant strain to generate cells expressing tagged PUF3-HA alone. Transformants were grown in dextrose medium and raffinose medium to test whether their interaction was carbon source-dependent. An anti-myc antibody was utilized to pull down Hrr25-myc from total cellular lysates. Myc- and HA-tagged proteins in the immunoprecipitates were examined by western blotting. Figure 4A shows that more Puf3-HA was recovered in the anti-myc immunoprecipitates from cells coexpressing Hrr25-myc and Puf3-HA than from cells expressing tagged Puf3-HA alone, confirming that Hrr25 interacts with Puf3. We found that the amount of Puf3-HA recovered in the Hrr25-myc immunoprecipitate from raffinose-grown cells was 4.4-fold higher than in dextrose-grown cells. Increased interaction between these two proteins cannot be fully accounted for by a slightly higher level of Puf3-HA in raffinose-grown cells, which is only 30% more than that in dextrose-grown cells. A reverse co-immunoprecipitation experiment resulted in similar results (Supplemental Material, Figure S1). Together, our data not only confirm that Puf3 and Hrr25 interact, but also reveal that there is a stronger interaction between these two proteins when there is increased Hrr25-dependent Puf3 phosphorylation. We would like to note that the interaction between Puf3 and Hrr25 may not be direct. It is possible that their interaction may be mediated by another binding partner.
Figure 4.
An interaction between Hrr25 and Puf3 is mediated through the kinase domain of Hrr25 and the N-terminal domain of Puf3. (A) Hrr25 interacts with Puf3 in a co-immunoprecipitation assay. Yeast strains carrying plasmids encoding PUF3-HA and HRR25-myc as indicated were grown in YNBcas5D (0.67% yeast nitrogen base, 1% casamino acids, and 5% dextrose) and YNBcasR (0.67% yeast nitrogen base, 1% casamino acids, and 2% raffinose) medium to midlogarithmic phase. Cell lysates were prepared and Hrr25-myc was immunoprecipitated with anti-myc antibody and protein G agarose beads. Proteins in the immunoprecipitates (IP pellet) and from the total cell lysates (cell lysate) were separated by SDS-PAGE, and analyzed by immunoblotting. The result was representative of two independent experiments. Protein bands were quantified using Bio-Rad Image Lab software and the numbers (average with SD, n = 2) indicate the relative levels of Puf3-HA in cell lysates (arbitrary units) or the ratio of Puf3-HA to Hrr25-myc in IP pellets. (B) A yeast two-hybrid analysis of an interaction between Hrr25 and Puf3. AH109 cells carrying plasmids encoding the Gal4 DNA-binding domain (GBD) (pZL3542), GBD-Hrr25 (pZL3557), or GBD-Hrr25(K38A) (pZL3740), and Y187 cells carrying plasmids encoding the Gal4 transcriptional activation domain (GAD) (pZL3539), GAD-Puf3 (pMB212), GAD-Puf3N (pMB214), or GAD-Puf3C (pMB216), were crossed and the resulting diploid cells were selected and streaked onto complete supplement mixture medium (CSM) dropout medium with histidine (+histidine), without histidine (−histidine), or without histidine plus 3 mM 3-amino-1,2,4-triazole (3AT) (−histidine + 3 mM 3AT). The result was representative of two independent experiments. (C) The kinase domain of Hrr25 is sufficient for an interaction with the N-terminal domain of Puf3. Yeast two-hybrid strains coexpressing a GBD control (pZL3542) or a GBD fusion protein [GBD-Hrr25, pZL3557; GBD-Hrr25(KD), pZL3736; Hrr25ΔC, pZL3744; or Hrr25ΔM, pZL3742], and a GAD control (pZL3539) or a GAD fusion protein (GAD-Puf3, pMB212; GAD-Puf3N, pMB214; or GAD-Puf3C, pMB216) as indicated, were grown on CSM dropout medium as described for panel (B). The result was representative of two independent experiments.
Puf3 has an N-terminal domain, which contains most of the phosphorylation sites, and a CTD that binds to target mRNAs (Olivas and Parker 2000; Lee and Tu 2015). To determine which domain of Puf3 mediates its interaction with Hrr25, we first conducted a co-immunoprecipitation assay and failed to detect an interaction between Hrr25 and either the N-terminal, or C-terminal, fragment of Puf3. As an alternative approach, we used the yeast two-hybrid assay to examine their interactions. A plasmid encoding a fusion protein of the Gal4 DNA-binding domain and Hrr25 (GBD-Hrr25) was generated and introduced into the yeast two-hybrid strain AH109. Plasmids encoding fusion proteins of the Gal4 activation domain, and the N-terminal or CTD of Puf3, and full-length Puf3 (GAD-Puf3N, GAD-Puf3C, and GAD-Puf3) were created and introduced into the yeast two-hybrid strain Y187. AH109 and Y187 transformants were then crossed to analyze the interaction of GBD- and GAD-fusion proteins, via Gal4-dependent HIS3 and ADE2 reporter genes. When the bait and prey proteins interact, Gal4 activity is reconstituted, which drives the expression of ADE2 and HIS3 reporter genes, and make cells appear less red and able to grow on medium without histidine. Figure 4B shows that yeast cells coexpressing GBD-Hrr25 and GAD-Puf3, or GAD-Puf3N, on CSM medium supplemented with histidine and adenine (CSM + histidine) appeared less red than cells coexpressing GBD-Hrr25 and GAD control, or GAD-Puf3C, indicating that Hrr25 interacts with Puf3 through Puf3′s N-terminal domain. On CSM medium without histidine, yeast cells expressing GBD-Hrr25 and GAD control were able to grow, indicating that GBD-Hrr25 has basal activity, which has been reported previously (Collins et al. 2017). Cells coexpressing GBD-Hrr25 and GAD-Puf3N grew slightly better than cells coexpressing GBD-Hrr25 and GAD-Puf3, or GAD-Puf3C, on CSM medium without histidine, indicating that Hrr25 interacts with the N-terminal domain of Puf3.
The weak interaction between Puf3 and Hrr25 in the yeast two-hybrid analysis could be the result of Puf3 phosphorylation by Hrr25, and subsequent release from Hrr25. We hypothesized that a kinase-dead mutant allele of Hrr25 might increase its interaction with Puf3. To test this possibility, we generated a plasmid encoding a GBD fusion of the K38A mutant of Hrr25, which has lost kinase activity (Murakami et al. 1999; Mehlgarten and Schaffrath 2003). Figure 4B shows that cells coexpressing GBD-Hrr25(K38A) and GAD-Puf3, or GAD-Puf3N, appear whiter on CSM medium supplemented with histidine than cells coexpressing GBD-Hrr25(K38A) and GAD, or GAD-Puf3C, indicating that Hrr25(K38A) interacts with both Puf3 and Puf3N. Cells coexpressing GBD-Hrr25(K38A) and GAD had a higher basal activity on CSM medium without histidine (Figure 4B, middle panel). However, the addition of 3 mM 3-amino-1,2,4 triazole to CSM medium without histidine to inhibit His3 effectively eliminated the growth of yeast cells coexpressing GBD-Hrr25(K38A) and GAD, or GAD-Puf3C, but not the growth of cells coexpressing GBD-Hrr25(K38A) and GAD-Puf3, or GAD-Puf3N. Together, these data indicate that Hrr25 interacts with Puf3 through the N-terminal domain of Puf3.
We next used a yeast two-hybrid analysis to identify the domain of Hrr25 that mediates the interaction with Puf3. We generated plasmids encoding fusion proteins between GBD and the Hrr25 kinase domain [Hrr25(KD)], the middle region (Hrr25M), the C-terminal region (Hrr25C), the middle region plus the C-terminal region [Hrr25(M+C)], a middle region truncation protein (Hrr25ΔM), or a C-terminal region truncation protein (Hrr25ΔC). Figure 4C shows that the kinase domain of Hrr25 was sufficient for an interaction with Puf3N. We failed to detect an interaction between full-length Puf3, Puf3N, or Puf3C and Hrr25M, Hrr25C, or Hrr25(M+C) (Figure S2). Similar to the GBD-Hrr25(K38A) construct, GBD-Hrr25ΔM had high basal activity in driving the expression of HIS3 and ADE2 reporter genes. Despite the high basal activity, it was clear that Hrr25(ΔM) interacts with both full-length Puf3 and Puf3N (Figure 4C). Together, our data indicate that the kinase domain of Hrr25 is both necessary and sufficient for the interaction with Puf3.
Hrr25 directly phosphorylates Puf3
We used the E. coli expression system to test whether Hrr25 directly phosphorylates Puf3. The E. coli genome does not encode a typical serine/threonine protein kinase and phosphorylation at serine and threonine residues in E. coli proteins is extremely rare (Macek et al. 2008; Pereira et al. 2011). We coexpressed Hrr25ΔC with full-length Puf3, the N-terminal domain of Puf3 (Puf3N), or the CTD of Puf3 (Puf3C) and analyzed the phosphorylation of Puf3 by immunoblotting. We chose Hrr25ΔC instead of full-length Hrr25 for two reasons: Hrr25ΔC interacts with Puf3 (Figure 4C) and the expression of Hrr25ΔC in E. coli is better than that of full-length Hrr25 (Corbett and Harrison 2012). Figure 5A shows that coexpression of Puf3 and Hrr25ΔC led to slower mobility forms of Puf3 on SDS-PAGE, which was similar to Hrr25-dependent Puf3 modification in yeast cells (compare Figure 5A with Figure 3B). The slower mobility forms of Puf3 are due to phosphorylation because coexpression of a kinase-dead mutant of Hrr25, Hrr25(K38A)ΔC, and Puf3 did not lead to a mobility shift of Puf3 on western blots. It has been reported that the N-terminal domain of Puf3 not only contains the majority of the phosphorylation sites of full-length Puf3 but also is phosphorylated in vivo when expressed as a truncation construct, without the C-terminal RNA-binding domain (Lee and Tu 2015). Consistently, we found that the N-terminal domain of Puf3, Puf3N, could also be hyperphosphorylated by wild-type Hrr25ΔC, but not by the kinase-dead mutant Hrr25(K38A)ΔC (Figure 5A). Although there was no interaction found between Hrr25 and Puf3C using the assays described for Figure 4, the CTD of Puf3 (Puf3C) was modified by Hrr25ΔC, but not by Hrr25(K38A)ΔC, when they were coexpressed in E. coli. Instead of a diffuse migration pattern of full-length Puf3 and Puf3N on western blots due to their hyperphosphorylation by Hrr25, a single, slower mobility form of Puf3C could be detected in E. coli cells coexpressing Hrr25ΔC, but not from cells coexpressing Hrr25(K38A)ΔC. Hrr25ΔC appeared to autophosphorylate itself because there were two slower mobility forms that could be observed for the wild-type Hrr25 construct but not for the kinase-dead Hrr25 (K38A) construct (Figure 5A). This apparent autophosphorylation seems to be not physiologically relevant because we could not detect a similar modification to Hrr25ΔC when it was expressed in yeast cells (data not shown). We further confirmed that the slower mobility forms of Hrr25ΔC and the three Puf3 constructs in Figure 5A were due to phosphorylation, because λ protein phosphatase treatment led to their disappearance and a corresponding increase in the level of the unmodified form (Figure 5B). Together, our data indicate that Hrr25 can directly phosphorylate Puf3 in E. coli.
Figure 5.
Hrr25 phosphorylates Puf3 in E. coli. (A) BL21 (DE3) cells coexpressing C-terminal 6xHis-tagged Hrr25ΔC (pAB101) or Hrr25(K38A)ΔC (pAB104), and C-terminal 6xHis and HA-tagged full-length Puf3 (pZL3359), the N-terminal domain of Puf3 (Puf3N, pZL3419), or the C-terminal domain of Puf3 (Puf3C, pZL3421) were boiled in SDS-PAGE sample buffer and proteins were separated by SDS-PAGE and detected by immunoblotting using antibody against the His-tag. The result was representative of three independent experiments. (B) Phosphatase treatment of recombinant proteins in panel (A) converts the slower mobility forms to the unmodified form. Total cellular proteins from BL21 (DE3) cells coexpressing 6xHis-tagged Hrr25ΔC and 6xHis, and HA-tagged full-length Puf3, Puf3N, or Puf3C, were prepared and subjected to treatment without and with λ protein phosphatase (PPase). Proteins were separated by SDS-PAGE and probed with anti-His tag antibody on western blots. The result was representative of two independent experiments. (C) Mks1 and Rtg2 are minimally or not phosphorylated by Hrr25ΔC in E. coli. BL21 (DE3) cells coexpressing 6XHis-tagged Hrr25ΔC or Hrr25(K38A)ΔC, and C-terminal 6xHis and HA-tagged Puf3N, C-terminal 6xHis-tagged Mks1 (pZL1760), or Rtg2 (pZL971) were treated in SDS-PAGE sample buffer. Proteins were separated by SDS-PAGE and detected by immunoblotting using antibody against the His-tag. The result was representative of two independent experiments. (D) puf3Δ (ZLY4562) and hrr25(E52D) puf3Δ (ZLY4578) mutant strains, carrying a plasmid encoding C-terminal 3xHA-tagged wild-type (WT) Puf3 (pZL4262) or mutant Puf3(24A) (pZL4264), were grown in YNBcas5D (0.67% yeast nitrogen base, 1% casamino acids, and 5% dextrose) or YNBcasR (0.67% yeast nitrogen base, 1% casamino acids, and 2% raffinose) medium, and Puf3-HA and Puf3(24A) were detected by immunoblotting. The result was representative of two independent experiments. (E) BL21 (DE3) cells coexpressing Hrr25ΔC (WT) or Hrr25(K38A)ΔC (K38A), and WT Puf3 (pZL4257) or a Puf3(24A) mutant (24A, pZL4258), were analyzed for Puf3 modification by Hrr25ΔC as described for panel (A). The result was representative of three independent experiments.
Figure 5A shows that all three constructs of Puf3 exhibit Hrr25-dependent phosphorylation; thus, we next asked whether such phosphorylation is specific. We chose to analyze the effect of Hrr25 on Mks1 and Rtg2, which were previously studied in our laboratory (Liu et al. 2003). Mks1, Rtg2, and Puf3N are of similar size (Figure 5C), and contain a comparable number of serine and threonine residues, with 112, 78, and 99 serine/threonine residues, respectively. When they were coexpressed with Hrr25ΔC in E. coli, His6-tagged Mks1 and Rtg2 showed little to no phosphorylation (Figure 5C), suggesting that Puf3 phosphorylation by Hrr25 is relatively specific. We then wanted to examine whether the phosphorylation sites of Puf3 expressed in E. coli and yeast are largely the same. To that end, we took advantage of a Puf3 phosphomutant, Puf3(24A), that shows a minimal level of phosphorylation when expressed in yeast (Figure 5D), consistent with the published findings by Lee and Tu (2015). Puf3(24A)-HA from raffinose-grown yeast cells migrates as a slower and relatively sharp band on western blots compared to dextrose-grown yeast cells (Figure 5D, compare lanes 5 and 7). Importantly, an hrr25(E52D) mutation blocked the formation of the slower mobility form (Figure 5D, compare lanes 7 and 8). In E. coli, when Hrr25∆C was coexpressed with Puf3 (24A), unlike the diffuse migration pattern of wild-type Puf3, Puf3(24A) exhibited a slower and relatively sharp band on western blots (Figure 5E). These data suggest that the Puf3 phosphorylation sites in E. coli largely match those in yeast. Together, these data indicate that Hrr25 can directly phosphorylate Puf3.
An hrr25(E52D) mutation fails to inhibit Puf3
Our data suggest that Hrr25-dependent phosphorylation of Puf3 leads to reduced function of Puf3 as a negative regulator of mRNAs encoding mitochondrial proteins. To test this possibility directly, we generated GFP reporter constructs carrying different 3′-UTRs under the control of the ADH1 promoter (Figure 6A). We first generated a GFP construct carrying a 252-bp terminator sequence of CYC1, which does not contain a Puf3-binding site (Mumberg et al. 1995). We introduced the plasmid into wild-type cells, an hrr25(E52D) mutant, and an hrr25(E52D) puf3Δ double mutant, and the transformants were grown in raffinose medium. Total cellular proteins were prepared and GFP expression was analyzed by western blotting. Figure 6, B and G show that GFP protein expression was increased in hrr25(E52D) mutant cells compared to wild-type. This increase was reduced in the hrr25(E52D) puf3Δ double mutant. The change in GFP expression shown in Figure 6B is probably due to a compensatory increase in the expression of glycolytic genes including ADH1, encoding an alcohol dehydrogenase, in hrr25(E52D) mutant cells, which are respiratory-deficient (Figure 1 and Figure 6C). In hrr25(E52D) puf3Δ cells, the restoration of mitochondrial respiratory function is expected to return GFP expression to a similar level found in wild-type cells. We then generated GFP reporter constructs carrying the 3′-UTR of CYT2 and PET123, both of which are targets of Puf3 (García-Rodríguez et al. 2007; Miller et al. 2014; Kershaw et al. 2015). Figure 6, D, F, and G show that the GFP protein level from constructs with the 3′UTR of CYT2 and PET123 genes was significantly reduced in hrr25(E52D) mutant cells compared to the wild-type. The GFP protein level is restored to wild-type level in hrr25(E52D) puf3Δ double-mutant cells, indicating that reduced expression of GFP from the constructs carrying CYT2 and PET123 terminators in hrr25(E52D) mutant cells is Puf3-dependent. These results suggest that the hrr25(E52D) mutation fails to inhibit Puf3′s function in promoting the degradation of target mRNAs.
Figure 6.
An hrr25 mutation reduces the expression of GFP reporter genes carrying 3′-UTRs targeted by Puf3. (A) A diagrammatic representation of GFP reporter constructs used in this study. (B) Western blot analysis of GFP expression from the ADH1p-GFP-CYC1 3′-UTR reporter construct (pZL3507) in wild-type (WT, BY4741), hrr25(E52D) (ZLY4467), and hrr25(E52D) puf3Δ (ZLY4578) mutant cells. The CYC1 3′-UTR is not a target of Puf3. The result was representative of three independent experiments. (C) A model to account for an increase in the promoter activity of ADH1 in respiratory-deficient hrr25 mutant cells. Mito. repiration, mitochondrial respiration. (D) Western blot analysis of GFP expression from ADH1p-GFP constructs carrying the WT 3′-UTR of CYT2 (pZL3407) or a Puf3-binding site mutant allele (pZL3510). The result was representative of three independent experiments. The DNA sequence downstream of the stop codon of GFP from WT and Puf3-binding site mutant constructs is given beneath the gel picture. The numbers indicate the positions of nucleotides downstream of the stop codon. (E) Three Puf3-binding motifs are present at or downstream of the stop codon of the PET123 gene. (F) Western blot analysis of GFP expression from ADH1p-GFP constructs carrying the WT 3′-UTR of PET123 (pZL3416), a single site 1 mutant allele, or a triple Puf3-binding site mutant allele (pZL3512 and pZL3519). The result was representative of three independent experiments. (G) Quantification of the ratio of the GFP signals to the Pgk1 control on western blots. The ratio of GFP/Pgk1 was set as 1 in WT cells. The data are presented as mean ± SD, n = 3. The means of the results were compared by Student’s t-test, and asterisks indicate significant difference in the means of two groups of data (*P ≤ 0.05 and ** P ≤ 0.01).
The CYT2 3′-UTR carries one Puf3-binding motif, CCTGTAAATA (Jackson et al. 2004). We generated an ADH1p-GFP-CYT2 3′-UTR mutant construct by mutating the Puf3-binding site to ACACACAATA. Figure 6, D and G show that the mutation in the Puf3-binding site in the CYT2 3′-UTR abolished the hrr25(E52D) mutant phenotype, since the profile of GFP protein levels was similar to that of the GFP control construct carrying the 3′-UTR of the CYC1 gene (compare Figure 6, B and D).
The PET123 3′-UTR has been reported to contain two Puf3 motifs (Miller et al. 2014), and we found a third Puf3-binding site that overlaps with the stop codon of the PET123 gene (Figure 6E). We introduced mutations into the three Puf3-binding sites of the ADH1p-GFP-PET123 3′-UTR construct and generated a site 1 single mutant and a sites 1, 2, and 3 triple mutant. Western blot analysis of the GFP protein level showed that the site 1 mutation partially and the site 1,2,3 triple mutation fully suppressed the hrr25(E52D) mutant phenotype (Figure 6, F and G). Our data suggest that the Puf3-binding sites in the PET123 3′-UTR have an additive effect in conferring Puf3-dependent downregulation of PET123 mRNA. The use of the ADH1 promoter in the ADH1p-GFP constructs complicated the interpretation of data due to increased transcriptional regulation of GFP expression in response to the hrr25(E52D) mutation. However, in the same hrr25(E52D) mutant background, mutations to the Puf3-binding sites in the 3′-UTR of the GFP reporter constructs increased the protein level of GFP. These results strongly suggest that reduced GFP levels from the GFP-CYT2 3′-UTR and GFP-PET123 3′-UTR constructs in hrr25(E52D) mutant cells are due to a failure to inhibit Puf3′s function in promoting the degradation of target mRNAs. We conducted the same experiments using GFP constructs under the control of a different promoter, the MKS1 promoter, and similar results were obtained (Figure S3). Mks1 is a negative regulator in the mitochondria-to-nucleus retrograde signaling pathway (Liu and Butow 2006). Similar to the ADH1 promoter, the activity of the MKS1 promoter also seemed to be increased due to an hrr25(E52D) mutation. The effects of the hrr25(E52D) mutation on reducing the GFP protein levels from GFP constructs carrying the 3′-UTRs of Puf3 targets were more evident when the MKS1 promoter was used (compare Figures 6 and Figure S3), which is probably due to the fact that the activity of the MKS1 promoter is much lower than that of ADH1 (Liu et al. 2003, 2005). A lower level of GFP expression under the control of the MKS1 promoter might reduce any saturation effect associated with a higher level of GFP expression under the control of the ADH1 promoter.
Expression of mitochondrial proteins is reduced in hrr25(E52D) mutant cells
Our data are consistent with the notion that Hrr25 is required to inhibit Puf3 through phosphorylation to increase the respiratory metabolism. Puf3 is known to bind to hundreds of nuclear gene transcripts encoding mitochondrial proteins. Due to the global effect of Puf3 in the downregulation of mitochondrial proteins, we hypothesized that mitochondrial biogenesis is reduced in hrr25(E52D) mutant cells. To test this possibility, we chose to determine the protein level of Aco1 (a mitochondrial matrix protein), porin (Por1, a mitochondrial outer membrane protein), and Cox2 and Cox3 (two mitochondrial inner membrane proteins and components of cytochrome C oxidase), in wild-type cells, an hrr25(E52D) mutant, an hrr25(E52D) puf3Δ double mutant, and a puf3Δ mutant. Aco1 and Por1 are encoded by ACO1 and POR1 in the nuclear genome respectively, while COX2 and COX3 are encoded in the mitochondrial genome. The coding sequence of POR1 and the two 500-bp flanking regions do not contain a Puf3 consensus binding site, and POR1 has not been recovered as a Puf3 target in six genome-wide RNA-pull-down analyses (Gerber et al. 2004; Hogan et al. 2008; Freeberg et al. 2013; Kershaw et al. 2015; Lapointe et al. 2017; Wilinski et al. 2017). ACO1 and its upstream and downstream 500-bp flanking regions contain a single Puf3-binding consensus site in the coding region, and ACO1 has been reported to be a potential Puf3 target in three out of the six studies. We also included an aco1Δ mutant strain as a negative control for Aco1 detection on western blots. Since they lose mitochondrial DNA and become rho0 cells, aco1 mutant cells can also be used as the negative control for Cox2 and Cox3 detection on western blots (Lin et al. 2008; Farooq et al. 2013). Cells were grown in raffinose medium and total cellular proteins were prepared and separated by SDS-PAGE. Figure 7, A and B show that the hrr25(E52D) mutation led to a small but reproducible reduction in the protein levels of Aco1 and Por1, and a dramatic reduction in the protein levels of Cox2 and Cox3. Reduced expression of these four proteins due to an hrr25(E52D) mutation is completely reversed by puf3Δ. In the puf3Δ mutant cells, the expression of Aco1 and Por1 was not affected, while the protein levels of Cox2 and Cox3 were slightly reduced. Mutations in genes such as ACO1 can lead to mitochondrial DNA loss (Farooq et al. 2013). A reduction in the protein level of Cox2 and Cox3 could be due to increased mitochondrial DNA loss or mutations in hrr25(E52D) mutant cells. To test this possibility, we crossed the wild-type strain, an hrr25(E52D) mutant, and an aco1Δ mutant with a rho0 strain carrying wild-type HRR25 and ACO1. The rho0 strain has lost mitochondrial DNA and is unable to grow on medium with lactate as the sole carbon source (Figure 7C). The resulting diploid cells were serially diluted and spotted on lactate medium. Figure 7C shows that the two diploid strains, wild-type crossed with rho0 and the hrr25(E52D) mutant crossed with rho0, had the same growth fitness on lactate medium, indicating that the mitochondrial DNA in hrr25(E52D) mutant cells is fully functional. In contrast, the diploid strain from the mating between rho0 and an aco1Δ mutant was unable to grow on lactate medium due to loss of mitochondrial DNA in aco1Δ cells (Farooq et al. 2013). To determine whether an hrr25(E52D) mutation affects the frequency of mitochondrial petite mutant formation, we grew hrr25(E52D) mutant cells in dextrose medium and 112 colonies were randomly picked and mated with the rho0 strain. The resultant 112 diploid strains were selected and tested on growth medium with ethanol and glycerol as the carbon source, and all were found to be able to grow, indicating that the hrr25(E52D) mutation does not lead to an increased formation of respiratory-deficient rho0 or mit− cells (Figure S4B). As a control, wild-type and aco1 mutant cells were also tested using this method to determine the frequency of petite mutant formation. Three out of 50 diploid cells from the crossing between wild-type and the rho0 strain were found to be mitochondrial petite (Figure S4A), consistent with a low frequency of spontaneous mitochondrial DNA loss in otherwise wild-type yeast cells. As expected, 50 out of 50 diploid cells from the crossing between the aco1 mutant and the rho0 strain were determined to be mitochondrial petite (Figure S4C). Together, our data suggest that reduced expression of mitochondrial proteins in hrr25(E52D) mutant cells is due to Puf3-dependent downregulation of mitochondrial biogenesis.
Figure 7.
Expression of mitochondrial proteins is reduced in hrr25(E52D) mutant cells. (A) Wild-type (WT) and isogenic mutant strains as indicated were grown in YNBcasR (0.67% yeast nitrogen base, 1% casamino acids, and 2% raffinose) medium to midlogarithmic phase. Total cellular proteins were prepared and separated by SDS-PAGE. Aconitase (Aco1), Porin (Por1), Cox2, Cox3, and Pgk1 were detected by immunoblotting. “*” denotes proteins that cross-react with the antibody. The results are representative of two independent experiments. (B) Quantification of the ratios of the amounts of Aco1, Por1, Cox2, or Cox3 to Pgk1 control on western blots. The data are presented as mean ± SD, n = 2. The means of the results were compared by Student’s t-test and P-values were given for selected sample pairs. (C) Mitochondrial DNA is functional in hrr25(E52D) mutant cells. WT (BY4741), an hrr25(E52D) mutant (ZLY4467), an aco1Δ mutant (ZLY2630), a rho0 strain of the opposite mating type (ZLY3206), and the diploid strains as indicated were serially diluted and spotted on YPD (dextrose) and YPL (lactate) plates.
Discussion
Puf3 is a post-transcriptional regulator of nuclear genes encoding mitochondrial proteins. It is known to be phosphorylated in response to glucose derepression. Here, we report that casein kinase I protein Hrr25 is required for both Puf3 phosphorylation and respiratory growth. We propose that Hrr25 promotes mitochondrial biogenesis by phosphorylating Puf3 and inhibiting Puf3’s function in facilitating the degradation of mRNAs encoding mitochondrial proteins. Hrr25 has been reported to be involved in multiple cellular pathways, including autophagy, Golgi trafficking, chromosome segregation during meiosis, and endocytosis. Here, we expanded the role of Hrr25 in gene expression by showing its regulation of Puf3. Our conclusion that Puf3 inactivation occurs via Hrr25-dependent phosphorylation is consistent with published results showing that a puf3 phosphomutant, puf3 (24A), with most if not all serine and threonine phosphorylation sites mutated to alanine, exhibits severe growth defects on nonfermentable carbon source, which is also a phenotype of hrr25 mutant cells. The similar phenotypes of an hrr25 mutant and the puf3 (24A) mutant can be construed to be due to mutations in a trans-acting factor and cis-acting elements, respectively: the inability to phosphorylate Puf3 in hrr25 mutant cells is expected to result in the same phenotype as exhibited by the puf3 (24A) mutant cells, in which Puf3 cannot be multiply phosphorylated.
Our conclusion that Hrr25 is required for mitochondrial biogenesis by inhibiting Puf3 is supported by two important observations: (1) A puf3Δ mutation is able to rescue respiratory-growth defects caused by an hrr25(E52D) mutation and (2) an hrr25(E52D) mutation leads to a reduction in the protein level of GFP from GFP reporter genes carrying 3′-UTRs that are targets of Puf3. In the second observation, the downregulation can be reversed by either a puf3Δ mutation or by mutations in the Puf3-binding site in the 3′-UTR of GFP reporter constructs. It is still unknown how Puf3 phosphorylation results in its reduced activity in promoting decay of its bound mRNAs. It is possible that Puf3 phosphorylation may inhibit its interaction with its targets. However, this is less likely because it has been shown that Puf3 is able to bind to its targets independent of cell culture conditions (Miller et al. 2014; Lee and Tu 2015). Another possibility is that hyperphosphorylated Puf3 can still bind to its targets, but is unable to recruit other factors that are required for the turnover of its targets. Future work will be directed toward the elucidation of the mechanism of Puf3 inactivation through phosphorylation.
It has been proposed that nutrient-responsive protein kinases are implicated in the regulation of Puf3 phosphorylation (Foat et al. 2005; Lee and Tu 2015). Mutation in SCH9 and genes encoding the catalytic subunits of PKA show reduced phosphorylation of Puf3. However, glucose derepression still leads to significant phosphorylation of Puf3 in these mutants (Lee and Tu 2015). Inhibition of TOR (target of rapamycin) kinase due to rapamycin treatment has been reported to increase the stability of Puf3 targets, presumably by reducing Puf3’s ability to degrade them (Foat et al. 2005). It is unlikely that TOR kinase is the primary kinase that phosphorylates Puf3. If TOR kinase phosphorylates Puf3, rapamycin treatment would reduce Puf3 phosphorylation, which is expected to reduce the stability of Puf3 targets. Foat et al. (2005) have proposed that Puf3 is downstream of the TOR signaling pathway. Based on the data presented herein and by Lee and Tu, we propose that TOR regulation of Puf3 is likely to be indirect. In this report, Puf3 phosphorylation is reduced to a level that is almost unnoticeable on western blots from hrr25 mutant cells grown in both glucose-rich and glucose-limiting growth media. Importantly, Puf3 does not show increased phosphorylation in hrr25 mutant cells grown in raffinose medium compared to dextrose (Figure 3), which is different from what was observed in sch9 and PKA mutant cells (Lee and Tu 2015). Together, we propose that Hrr25 is an important kinase that is responsible for Puf3 phosphorylation and its inactivation. Our data do not exclude the possibility that other protein kinases may mediate Puf3 phosphorylation. Puf3 has also been reported to positively regulate mitochondrial biogenesis by promoting the cotranslational import of mitochondrial proteins through Puf3’s association with the mitochondrial outer membrane. Future work is needed to uncover the regulatory mechanism of Puf3 and characterize Puf3’s dual role in the regulation of mitochondrial biogenesis.
Hrr25 plays a number of distinct cellular roles in the budding yeast. The identification of Puf3 as a new target of Hrr25 expands the role of this important kinase in the regulation of gene expression. Hrr25 has been reported to phosphorylate the CTD of the largest subunit of RNA polymerase II (Nemec et al. 2019). The CTD consists of YSPTSPS heptad repeats, and Thr4 can be phosphorylated by Hrr25 and other kinases. Nemec et al. (2019) characterized the role of Hrr25 in the phosphorylation of Thr4 of the CTD and found that Hrr25 associates with the CTD of actively transcribing Pol II across most genes. They also found that Hrr25 plays a role in the transcription termination of a specific class of noncoding small nucleolar RNA genes. Hrr25 has been previously reported to be a negative regulator of calcineurin signaling by phosphorylating the zinc-finger transcription factor Crz1 (Kafadar et al. 2003). We reported that Hrr25 is a negative regulator of the transcriptional activator Haa1, which is important in cellular adaptation to acetic acid stress (Collins et al. 2017). Hrr25 has also been reported to regulate Cth2, an RNA-binding protein that plays an important role in iron hemostasis (Romero et al. 2018). Phosphorylation of Cth2 by Hrr25 leads to Cth2’s recognition by the SCFGrr1 E3 ubiquitin ligase and degradation via the 26S proteasome. Therefore, Hrr25 plays important roles at different steps of gene expression: the regulation of pathway-specific transcription factors and the general transcription factor RNA polymerase II, transcription termination, and post-transcriptional regulation of transcript stability and/or translation by regulating the activity of RNA-binding proteins. Future work will be directed toward uncovering the mechanism behind the functional versatility of Hrr25 and may reveal possible coordinated regulation of these cellular processes by Hrr25.
Acknowledgments
We thank Amita Bhattarai, Morgan Collins, Rui Silva, Anna E. Stebbins, and Flavio Antonio de Oliveira Simoes for technical support and the W. M. Keck Foundation for the Keck Facility. This study was supported by a grant from the National Institutes of Health (1R15 GM-121998-01).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988126.
Communicating editor: E. Tran
Literature cited
- Abdel-Fattah W., Jablonowski D., Di Santo R., Thuring K. L., Scheidt V. et al. , 2015. Phosphorylation of Elp1 by Hrr25 is required for elongator-dependent tRNA modification in yeast. PLoS Genet. 11: e1004931 10.1371/journal.pgen.1004931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D. C., Burke D. J., and Strathern J. N., 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. [Google Scholar]
- Argüello-Miranda O., Zagoriy I., Mengoli V., Rojas J., Jonak K. et al. , 2017. Casein kinase 1 coordinates cohesin cleavage, gametogenesis, and exit from M phase in meiosis II. Dev. Cell 40: 37–52. 10.1016/j.devcel.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Babu P., Bryan J. D., Panek H. R., Jordan S. L., Forbrich B. M. et al. , 2002. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J. Cell Sci. 115: 4957–4968. 10.1242/jcs.00203 [DOI] [PubMed] [Google Scholar]
- Babu P., Deschenes R. J., and Robinson L. C., 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279: 27138–27147. 10.1074/jbc.M403071200 [DOI] [PubMed] [Google Scholar]
- Blom J., De Mattos M. J., and Grivell L. A., 2000. Redirection of the respiro-fermentative flux distribution in Saccharomyces cerevisiae by overexpression of the transcription factor Hap4p. Appl. Environ. Microbiol. 66: 1970–1973. 10.1128/AEM.66.5.1970-1973.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz A., Choi H., Sharom J. R., Boucher L., Neduva V. et al. , 2010. A global protein kinase and phosphatase interaction network in yeast. Science 328: 1043–1046. 10.1126/science.1176495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschlen S., Amillet J. M., Guiard B., Fournier A., Marcireau C. et al. , 2003. The S. Cerevisiae HAP complex, a key regulator of mitochondrial function, coordinates nuclear and mitochondrial gene expression. Comp. Funct. Genomics 4: 37–46. 10.1002/cfg.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo S. E., and Mootha V. K., 2010. The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 11: 25–44. 10.1146/annurev-genom-082509-141720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenay-Lapointe M., and Shadel G. S., 2011. Repression of mitochondrial translation, respiration and a metabolic cycle-regulated gene, SLF1, by the yeast Pumilio-family protein Puf3p. PLoS One 6: e20441 10.1371/journal.pone.0020441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J.M., Hong E. L., Amundsen C., Balakrishnan R., Binkley G., . 2012. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Res. Jan;40 (Database issue):D700-5. https://www.yeastgenome.org/reference/S000147515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. E., Black J. J., and Liu Z., 2017. Casein kinase I isoform Hrr25 is a negative regulator of Haa1 in the weak acid stress response pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 83: e00672-17. 10.1128/AEM.00672-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. D., and Harrison S. C., 2012. Molecular architecture of the yeast monopolin complex. Cell Rep. 1: 583–589 [corrigenda: Cell Rep. 17: 929 (2016)]. 10.1016/j.celrep.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Wang J., Zhu M., Stahmer K., Lakshminarayan R. et al. , 2016. Sec24 phosphorylation regulates autophagosome abundance during nutrient deprivation. Elife 5: e21167. 10.7554/eLife.21167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N., and Hoekstra M. F., 1994. Characterization of two protein kinases from Schizosaccharomyces pombe involved in the regulation of DNA repair. EMBO J. 13: 2777–2788. 10.1002/j.1460-2075.1994.tb06571.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T. A., Pyle S. E., Wharton R. P., and Aggarwal A. K., 2001. Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell 105: 281–289. 10.1016/S0092-8674(01)00318-X [DOI] [PubMed] [Google Scholar]
- Eliyahu E., Pnueli L., Melamed D., Scherrer T., Gerber A. P. et al. , 2010. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. Mol. Cell. Biol. 30: 284–294. 10.1128/MCB.00651-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M. A., Pracheil T. M., Dong Z., Xiao F., and Liu Z., 2013. Mitochondrial DNA instability in cells lacking aconitase correlates with iron citrate toxicity. Oxid. Med. Cell. Longev. 2013: 493536 10.1155/2013/493536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foat B. C., Houshmandi S. S., Olivas W. M., and Bussemaker H. J., 2005. Profiling condition-specific, genome-wide regulation of mRNA stability in yeast. Proc. Natl. Acad. Sci. USA 102: 17675–17680. 10.1073/pnas.0503803102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., and Guarente L., 1989. Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev. 3: 1166–1178. 10.1101/gad.3.8.1166 [DOI] [PubMed] [Google Scholar]
- Foury F., Roganti T., Lecrenier N., and Purnelle B., 1998. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 440: 325–331. 10.1016/S0014-5793(98)01467-7 [DOI] [PubMed] [Google Scholar]
- Freeberg M. A., Han T., Moresco J. J., Kong A., Yang Y. C. et al. , 2013. Pervasive and dynamic protein binding sites of the mRNA transcriptome in Saccharomyces cerevisiae. Genome Biol. 14: R13 10.1186/gb-2013-14-2-r13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadir N., Haim-Vilmovsky L., Kraut-Cohen J., and Gerst J. E., 2011. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 17: 1551–1565. 10.1261/rna.2621111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgano A., Forrer M., Jaskiewicz L., Kanitz A., Zavolan M. et al. , 2008. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One 3: e3164 10.1371/journal.pone.0003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez L. J., Gay A. C., and Pon L. A., 2007. Puf3p, a Pumilio family RNA binding protein, localizes to mitochondria and regulates mitochondrial biogenesis and motility in budding yeast. J. Cell Biol. 176: 197–207. 10.1083/jcb.200606054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A. P., Moses A. M., Chiang D. Y., Fraser H. B., Berardini M. et al. , 2004. Conservation and evolution of cis-regulatory systems in ascomycete fungi. PLoS Biol. 2: e398 10.1371/journal.pbio.0020398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. P., Herschlag D., and Brown P. O., 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2: E79 10.1371/journal.pbio.0020079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. P., Luschnig S., Krasnow M. A., Brown P. O., and Herschlag D., 2006. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103: 4487–4492. 10.1073/pnas.0509260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalei H., Schaub F. X., Doherty J. R., Noguchi Y., Roush W. R. et al. , 2015. Hrr25/CK1δ-directed release of Ltv1 from pre-40S ribosomes is necessary for ribosome assembly and cell growth. J. Cell Biol. 208: 745–759. 10.1083/jcb.201409056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., and Schiestl R. H., 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425 10.1093/nar/20.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. D., and Anderson R. A., 1998. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell. Signal. 10: 699–711. 10.1016/S0898-6568(98)00042-4 [DOI] [PubMed] [Google Scholar]
- Gu W., Deng Y., Zenklusen D., and Singer R. H., 2004. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 18: 1452–1465. 10.1101/gad.1189004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta I., Clauder-Munster S., Klaus B., Jarvelin A. I., Aiyar R. S. et al. , 2014. Alternative polyadenylation diversifies post-transcriptional regulation by selective RNA-protein interactions. Mol. Syst. Biol. 10: 719 10.1002/msb.135068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman M. J., and Winston F., 2007. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell. Biol. 27: 7414–7424. 10.1128/MCB.00887-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G. et al. , 1991. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science 253: 1031–1034. 10.1126/science.1887218 [DOI] [PubMed] [Google Scholar]
- Hogan D. J., Riordan D. P., Gerber A. P., Herschlag D., and Brown P. O., 2008. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 6: e255 10.1371/journal.pbio.0060255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan G. J., Brown P. O., and Herschlag D., 2015. Evolutionary conservation and diversification of puf RNA binding proteins and their mRNA targets. PLoS Biol. 13: e1002307 10.1371/journal.pbio.1002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshmandi S. S., and Olivas W. M., 2005. Yeast Puf3 mutants reveal the complexity of Puf-RNA binding and identify a loop required for regulation of mRNA decay. RNA 11: 1655–1666. 10.1261/rna.2168505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. S. Jr., Houshmandi S. S., Lopez Leban F., and Olivas W. M., 2004. Recruitment of the Puf3 protein to its mRNA target for regulation of mRNA decay in yeast. RNA 10: 1625–1636. 10.1261/rna.7270204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Guan W., and Gu Z., 2010. Tinkering evolution of post-transcriptional RNA regulons: puf3p in fungi as an example. PLoS Genet. 6: e1001030 10.1371/journal.pgen.1001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafadar K. A., Zhu H., Snyder M., and Cyert M. S., 2003. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 17: 2698–2708. 10.1101/gad.1140603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., 2007. RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8: 533–543. 10.1038/nrg2111 [DOI] [PubMed] [Google Scholar]
- Kershaw C. J., Costello J. L., Talavera D., Rowe W., Castelli L. M. et al. , 2015. Integrated multi-omics analyses reveal the pleiotropic nature of the control of gene expression by Puf3p. Sci. Rep. 5: 15518 10.1038/srep15518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundaje A., Xin X., Lan C., Lianoglou S., Zhou M. et al. , 2008. A predictive model of the oxygen and heme regulatory network in yeast. PLoS Comput. Biol. 4: e1000224 10.1371/journal.pcbi.1000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrassa T. J., and Ungermann C., 2005. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J. Cell Biol. 168: 401–414. 10.1083/jcb.200407141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe C. P., Wilinski D., Saunders H. A., and Wickens M., 2015. Protein-RNA networks revealed through covalent RNA marks. Nat. Methods 12: 1163–1170. 10.1038/nmeth.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe C. P., Preston M. A., Wilinski D., Saunders H. A. J., Campbell Z. T. et al. , 2017. Architecture and dynamics of overlapped RNA regulatory networks. RNA 23: 1636–1647. 10.1261/rna.062687.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe C. P., Stefely J. A., Jochem A., Hutchins P. D., Wilson G. M. et al. , 2018. Multi-omics reveal specific targets of the RNA-binding protein Puf3p and its orchestration of mitochondrial biogenesis. Cell Syst 6: 125–135.e6. 10.1016/j.cels.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G., Brown C. C., Flood B. A., Karunakaran S., Cabrera M. et al. , 2014. Dynamic association of the PI3P-interacting Mon1-Ccz1 GEF with vacuoles is controlled through its phosphorylation by the type 1 casein kinase Yck3. Mol. Biol. Cell 25: 1608–1619. 10.1091/mbc.e13-08-0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. D., and Tu B. P., 2015. Glucose-Regulated phosphorylation of the PUF protein Puf3 regulates the translational fate of its bound mRNAs and association with RNA granules. Cell Rep. 11: 1638–1650. 10.1016/j.celrep.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Ohn T., Chiang Y. C., Quigley G., Yao G. et al. , 2010. PUF3 acceleration of deadenylation in vivo can operate independently of CCR4 activity, possibly involving effects on the PAB1-mRNP structure. J. Mol. Biol. 399: 562–575. 10.1016/j.jmb.2010.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. P., Hakala K. W., Weintraub S. T., and McAlister-Henn L., 2008. Suppression of metabolic defects of yeast isocitrate dehydrogenase and aconitase mutants by loss of citrate synthase. Arch. Biochem. Biophys. 474: 205–212. 10.1016/j.abb.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., and Butow R. A., 2006. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40: 159–185. 10.1146/annurev.genet.40.110405.090613 [DOI] [PubMed] [Google Scholar]
- Liu Z., Sekito T., Spirek M., Thornton J., and Butow R. A., 2003. Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol. Cell 12: 401–411. 10.1016/S1097-2765(03)00285-5 [DOI] [PubMed] [Google Scholar]
- Liu Z., Spirek M., Thornton J., and Butow R. A., 2005. A novel degron-mediated degradation of the RTG pathway regulator, Mks1p, by SCFGrr1. Mol. Biol. Cell 16: 4893–4904. 10.1091/mbc.e05-06-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Thornton J., Spirek M., and Butow R. A., 2008. Activation of the SPS amino acid-sensing pathway in Saccharomyces cerevisiae correlates with the phosphorylation state of a sensor component, Ptr3. Mol. Cell. Biol. 28: 551–563. 10.1128/MCB.00929-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macek B., Gnad F., Soufi B., Kumar C., Olsen J. V. et al. , 2008. Phosphoproteome analysis of E. coli reveals evolutionary conservation of bacterial Ser/Thr/Tyr phosphorylation. Mol. Cell. Proteomics 7: 299–307. 10.1074/mcp.M700311-MCP200 [DOI] [PubMed] [Google Scholar]
- McNabb D. S., and Pinto I., 2005. Assembly of the hap2p/hap3p/hap4p/hap5p-DNA complex in Saccharomyces cerevisiae. Eukaryot. Cell 4: 1829–1839. 10.1128/EC.4.11.1829-1839.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb D. S., Xing Y., and Guarente L., 1995. Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes Dev. 9: 47–58. 10.1101/gad.9.1.47 [DOI] [PubMed] [Google Scholar]
- Mehlgarten C., and Schaffrath R., 2003. Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactiszymocin in budding yeast. Mol. Genet. Genomics 269: 188–196. 10.1007/s00438-003-0807-5 [DOI] [PubMed] [Google Scholar]
- Mehlgarten C., Jablonowski D., Breunig K. D., Stark M. J., and Schaffrath R., 2009. Elongator function depends on antagonistic regulation by casein kinase Hrr25 and protein phosphatase Sit4. Mol. Microbiol. 73: 869–881. 10.1111/j.1365-2958.2009.06811.x [DOI] [PubMed] [Google Scholar]
- Miller M. A., and Olivas W. M., 2011. Roles of Puf proteins in mRNA degradation and translation. Wiley Interdiscip. Rev. RNA 2: 471–492. 10.1002/wrna.69 [DOI] [PubMed] [Google Scholar]
- Miller M. A., Russo J., Fischer A. D., Lopez Leban F. A., and Olivas W. M., 2014. Carbon source-dependent alteration of Puf3p activity mediates rapid changes in the stabilities of mRNAs involved in mitochondrial function. Nucleic Acids Res. 42: 3954–3970. 10.1093/nar/gkt1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K., Ohsumi Y., and Nakatogawa H., 2014. Hrr25 phosphorylates the autophagic receptor Atg34 to promote vacuolar transport of α-mannosidase under nitrogen starvation conditions. FEBS Lett. 588: 3862–3869. 10.1016/j.febslet.2014.09.032 [DOI] [PubMed] [Google Scholar]
- Moriya H., and Johnston M., 2004. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101: 1572–1577. 10.1073/pnas.0305901101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. R., Mukherjee N., and Keene J. D., 2008. Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol. Cell. Biol. 28: 4093–4103. 10.1128/MCB.00155-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Muller R., and Funk M., 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156: 119–122. 10.1016/0378-1119(95)00037-7 [DOI] [PubMed] [Google Scholar]
- Murakami A., Kimura K., and Nakano A., 1999. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. Implication of negative regulation by the Hrr25 kinase in the vesicle budding from the endoplasmic reticulum. J. Biol. Chem. 274: 3804–3810. 10.1074/jbc.274.6.3804 [DOI] [PubMed] [Google Scholar]
- Nakatogawa H., 2015. Hrr25: an emerging major player in selective autophagy regulation in Saccharomyces cerevisiae. Autophagy 11: 432–433. 10.1080/15548627.2015.1017195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec C. M., Singh A. K., Ali A., Tseng S. C., Syal K. et al. , 2019. Noncanonical CTD kinases regulate RNA polymerase II in a gene-class-specific manner. Nat. Chem. Biol. 15: 123–131. 10.1038/s41589-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. T., and Guarente L., 1990. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 4: 1714–1729. 10.1101/gad.4.10.1714 [DOI] [PubMed] [Google Scholar]
- Olesen J., Hahn S., and Guarente L., 1987. Yeast HAP2 and HAP3 activators both bind to the CYC1 upstream activation site, UAS2, in an interdependent manner. Cell 51: 953–961. 10.1016/0092-8674(87)90582-4 [DOI] [PubMed] [Google Scholar]
- Olivas W., and Parker R., 2000. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 19: 6602–6611. 10.1093/emboj/19.23.6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Grassart A., Lu R., Wong C. C., Yates J. III et al. , 2015a Casein kinase 1 promotes initiation of clathrin-mediated endocytosis. Dev. Cell 32: 231–240. 10.1016/j.devcel.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Moritz M., Han X., Giddings T. H., Lyon A. et al. , 2015b Interaction of CK1δ with γTuSC ensures proper microtubule assembly and spindle positioning. Mol. Biol. Cell 26: 2505–2518. 10.1091/mbc.E14-12-1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S. F., Goss L., and Dworkin J., 2011. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol. Mol. Biol. Rev. 75: 192–212. 10.1128/MMBR.00042-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M., Matos J., Mori S., Gregan J., Bogdanova A. et al. , 2006. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell 126: 1049–1064. 10.1016/j.cell.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Pfaffenwimmer T., Reiter W., Brach T., Nogellova V., Papinski D. et al. , 2014. Hrr25 kinase promotes selective autophagy by phosphorylating the cargo receptor Atg19. EMBO Rep. 15: 862–870. 10.15252/embr.201438932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., and McEwen J. E., 1996. Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem. 65: 563–607. 10.1146/annurev.bi.65.070196.003023 [DOI] [PubMed] [Google Scholar]
- Quenault T., Lithgow T., and Traven A., 2011. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol. 21: 104–112. 10.1016/j.tcb.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Ray P., Basu U., Ray A., Majumdar R., Deng H. et al. , 2008. The Saccharomyces cerevisiae 60 S ribosome biogenesis factor Tif6p is regulated by Hrr25p-mediated phosphorylation. J. Biol. Chem. 283: 9681–9691. 10.1074/jbc.M710294200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. C., Menold M. M., Garrett S., and Culbertson M. R., 1993. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol. Cell. Biol. 13: 2870–2881. 10.1128/MCB.13.5.2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A. M., Martínez-Pastor M., Du G., Solé C., Carlos M. et al. , 2018. Phosphorylation and proteasome recognition of the mRNA-binding protein Cth2 facilitates yeast adaptation to iron deficiency. mBio 9: e01694-18. 10.1128/mBio.01694-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrantz M., Kell C. S., Pennell E. A., and Devenish L. J., 1994. The HAP2,3,4 transcriptional activator is required for derepression of the yeast citrate synthase gene, CIT1. Mol. Microbiol. 13: 119–131. 10.1111/j.1365-2958.1994.tb00407.x [DOI] [PubMed] [Google Scholar]
- Saint-Georges Y., Garcia M., Delaveau T., Jourdren L., Le Crom S. et al. , 2008. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One 3: e2293 10.1371/journal.pone.0002293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Shenoy R. T., Dalgaard J. Z., Newnham L., Hoffmann E. et al. , 2013. Monopolin subunit Csm1 associates with MIND complex to establish monopolar attachment of sister kinetochores at meiosis I. PLoS Genet. 9: e1003610 10.1371/journal.pgen.1003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T., Maco B., Petfalski E., Tollervey D., Böttcher B. et al. , 2006. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 441: 651–655. 10.1038/nature04840 [DOI] [PubMed] [Google Scholar]
- Stumpf C. R., Kimble J., and Wickens M., 2008. A Caenorhabditis elegans PUF protein family with distinct RNA binding specificity. RNA 14: 1550–1557. 10.1261/rna.1095908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Chen L., Cao W., Roth A. F., and Davis N. G., 2004. The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal. Mol. Biol. Cell 15: 1397–1406. 10.1091/mbc.e03-09-0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P. P., Barrette-Ng I. H., Simon D. M., Tam M. W., Ang A. L. et al. , 2010. The Puf family of RNA-binding proteins in plants: phylogeny, structural modeling, activity and subcellular localization. BMC Plant Biol. 10: 44 10.1186/1471-2229-10-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C., Tan L. J., Mochida K., Kirisako H., Koizumi M. et al. , 2014. Hrr25 triggers selective autophagy-related pathways by phosphorylating receptor proteins. J. Cell Biol. 207: 91–105. 10.1083/jcb.201402128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancura A., Sessler A., Leichus B., and Kuret J., 1994. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J. Biol. Chem. 269: 19271–19278. [PubMed] [Google Scholar]
- Wang X., Hoekstra M. F., DeMaggio A. J., Dhillon N., Vancura A. et al. , 1996. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol. Cell. Biol. 16: 5375–5385. 10.1128/MCB.16.10.5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., McLachlan J., Zamore P. D., and Hall T. M., 2002. Modular recognition of RNA by a human pumilio-homology domain. Cell 110: 501–512. 10.1016/S0092-8674(02)00873-5 [DOI] [PubMed] [Google Scholar]
- Wang M., Ogé L., Perez-Garcia M. D., Hamama L., and Sakr S., 2018. The PUF protein family: overview on PUF RNA targets, biological functions, and post transcriptional regulation. Int. J. Mol. Sci. 19 10.3390/ijms19020410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilinski D., Buter N., Klocko A. D., Lapointe C. P., Selker E. U. et al. , 2017. Recurrent rewiring and emergence of RNA regulatory networks. Proc. Natl. Acad. Sci. USA 114: E2816–E2825. 10.1073/pnas.1617777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., and Schatz G., 1984. Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81: 4819–4823. 10.1073/pnas.81.15.4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Ur S. N., Su T. Y., and Corbett K. D., 2016. Structure of the Saccharomyces cerevisiae Hrr25:Mam1 monopolin subcomplex reveals a novel kinase regulator. EMBO J. 35: 2139–2151. 10.15252/embj.201694082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Shi Q., Varia S. N., Xing S., Klett B. M. et al. , 2016. The activity-dependent regulation of protein kinase stability by the localization to P-bodies. Genetics 203: 1191–1202. 10.1534/genetics.116.187419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Butler A. M., Shi Q., Xing S., and Herman P. K., 2018. P-body localization of the hrr25/casein kinase 1 protein kinase is required for the completion of meiosis. Mol. Cell. Biol. 38: e00678-17. 10.1128/MCB.00678-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Stumpf C. R., Krahn J. M., Wickens M., and Hall T. M., 2009. A 5′ cytosine binding pocket in Puf3p specifies regulation of mitochondrial mRNAs. Proc. Natl. Acad. Sci. USA 106: 20192–20197. 10.1073/pnas.0812079106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M., and Wickner W., 2012. Phosphorylation of the effector complex HOPS by the vacuolar kinase Yck3p confers Rab nucleotide specificity for vacuole docking and fusion. Mol. Biol. Cell 23: 3429–3437. 10.1091/mbc.e12-04-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientara-Rytter K., Ozeki K., Nazarko T. Y., and Subramani S., 2018. Pex3 and Atg37 compete to regulate the interaction between the pexophagy receptor, Atg30, and the Hrr25 kinase. Autophagy 14: 368–384. 10.1080/15548627.2017.1413521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors affirm that all data necessary to draw conclusions in this article are present within the texts, figures, and tables. Strains, plasmids, and other noncommercial research reagents are available upon request. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988126.