Germ granules, also known as P granules in Caenorhabditis elegans, are phase-separated cellular bodies that are frequently found at the perinuclear region of germ cell nuclei in various animals. However...
Keywords: Germ granule, RNA helicase, phase separation, VASA, P granule, ATP hydrolysis
Abstract
P granules are phase-separated liquid droplets that play important roles in the maintenance of germ cell fate in Caenorhabditis elegans. Both the localization and formation of P granules are highly dynamic, but mechanisms that regulate such processes remain poorly understood. Here, we show evidence that the VASA-like germline RNA helicase GLH-1 couples distinct steps of its ATPase hydrolysis cycle to control the formation and disassembly of P granules. In addition, we found that the phenylalanine-glycine-glycine repeats in GLH-1 promote its localization at the perinucleus. Proteomic analyses of the GLH-1 complex with a GLH-1 mutation that interferes with P granule disassembly revealed transient interactions of GLH-1 with several Argonautes and RNA-binding proteins. Finally, we found that defects in recruiting the P granule component PRG-1 to perinuclear foci in the adult germline correlate with the fertility defects observed in various GLH-1 mutants. Together, our results highlight the versatile roles of an RNA helicase in controlling the formation of liquid droplets in space and time.
Germ granules, also known as P granules in Caenorhabditis elegans, are phase-separated liquid droplets that are found in the germ cells of all animals (Eddy 1975; Brangwynne et al. 2009) . In C. elegans, P granules have been shown to function in maintaining a germ cell fate (Updike et al. 2014). Both the formation and localization of P granules are dynamic, but tightly regulated, during germ line development. P granules are first assembled in the cytoplasms of one-cell embryos, where they are asymmetrically sorted to those cells of the germ line lineage. Starting at the four-cell stage, P granules start to anchor to the nuclear periphery of the germ cell precursor, where they stay throughout most of germ cell development (Updike and Strome 2010). RNA-binding proteins such as GLH-1, PGL-1/PGL-3, and MEG-3/MEG-4 have been reported to play important roles in the formation of P granules (Updike and Strome 2009; Hanazawa et al. 2011; Wang et al. 2014). Recent studies have shown that the gradients of RNA-bound PGL-3 and MEG-3/MEG-4 are responsible for the formation of PGL-1/3 granules at the posterior of one-cell embryos (Saha et al. 2016; Smith et al. 2016). In addition, PGL-3 and MEG-3, can form liquid condensates in an RNA-dependent manner in vitro. However, GLH-1 is not able to form liquid condensates in vitro, nor does it form granules when expressed in nongermline cells in vivo (Hanazawa et al. 2011; Updike et al. 2011; Alberti et al. 2018). Nonetheless, glh-1 is required for the formation of PGL-1/3 granules in vivo (Spike et al. 2008; Updike and Strome 2009). Together, these observations raise the interesting question: how does GLH-1 regulate P granule formation?
GLH-1 is one of the VASA helicase homologs in C. elegans and was the first constitutive component of P granules identified (Roussell and Bennett 1993; Gruidl et al. 1996). Subsequently, three additional GLH family members—GLH-2, GLH-3, and GLH-4—were reported (Kuznicki et al. 2000). VASA mutants exhibit fertility defects in all animals tested so far (Gustafson and Wessel 2010). Similarly in C. elegans, depletion of or deletions in glh genes lead to reduced broods and sterile progeny (Kuznicki et al. 2000; Spike et al. 2008), and the loss of both GLH-1 and GLH-4 results in complete sterility. All the GLHs belong to the DEAD box family of RNA helicases and have the requisite RNA helicase motifs. GLH-1, 2, and 4 also possess distinct N-terminal phenylalanine-glycine-glycine (FGG) repeats. It was proposed that FGG repeats may facilitate the interaction of GLH-1 with the FG hydrogel formed at nuclear pores (Frey et al. 2006; Updike et al. 2011). In support of this, knockdown of many nuclear pore factors leads to loss of perinuclear germ granules (Updike and Strome 2009; Voronina and Seydoux 2010). In addition, a GFP fusion protein that contains multimeric FGG repeats fused to the N-terminus of GFP leads to localization of 10% of the GFP granules at the nuclear periphery (Updike et al. 2011). However, direct evidence that the FGGs in GLH-1 are required for its localization to the nuclear periphery remains missing. In addition, it is unclear whether the ATPase/RNA helicase activity and/or the RNA-binding activity of GLH-1 is required for its role in P granule formation.
Here, we show that GLH-1 couples distinct steps of its ATP hydrolysis cycle to regulate the formation and disassembly of P granules. In addition, the FGG repeats in GLH-1 are required for its localization at the nuclear periphery and for the fertility that is associated with proper perinuclear localization of P granule components. Proteomic analyses of the glh-1 mutant that exhibits defects in P granule disassembly revealed transient interactions between GLH-1 with Argonautes and the polyA-binding protein PAB-1. Together, our analyses show that GLH-1 RNA helicase is a multifunctional protein that regulates the dynamics and localization of P granules.
Materials and Methods
C. elegans strains
Animals were grown on standard nematode growth media (NGM) plates seeded with OP50 Escherichia coli at 20° or temperatures where indicated. The strains used in this study are listed in Supplemental Material, Table S1. Some strains were provided by the Caenorhabditis Genetics Center. See File S1 for information regarding the glh-1 and glh-4 gene-edited alleles created for this study.
RNA interference
RNA interference (RNAi) experiments were performed as described previously (Timmons and Fire 1998). L1 hermaphrodites were fed with E. coli HT115 (DE3) strains expressing the appropriate double-stranded RNA at 20° for 72 hr prior to scoring for phenotypes. RNAi bacterial strains were obtained from the C. elegans RNAi Collection (Ahringer; Source BioScience). The HT115 (DE3) strain expressing the empty vector L4440 was used as the control. HT115 (DE3) bacterial cultures were grown in LB broth with 100 μg/ml ampicillin overnight at 37°. Fresh cultures were seeded on NGM plates containing 100 μg/ml ampicillin and 1 mM IPTG, and incubated at room temperature for 24 hr before use.
Clustered regularly interspaced short palindromic repeats/Cas9 gene editing
Single guide RNAs (sgRNAs) were designed with the online tool sgRNA Scorer 2.0 (https://crispr.med.harvard.edu/) or manually picked by searching for the target sequences consisting of N20(NGG) closest to the double strand break cutting sites desired. Clustered regularly interspaced short palindromic repeats (CRISPR) experiments were conducted by co-CRISPR or Cas9 ribonucleoprotein (RNP) strategies (Kim et al. 2014; Dokshin et al. 2018).
For the co-CRISPR strategy, DNA mixtures were introduced into the germline of C. elegans young adults by microinjection. Final concentrations of plasmids in the injection mixtures were as follows: pCCM935 unc-22 sgRNA 50 ng/μl, pDD162 Cas9+sgRNA 50 ng/μl, pRF4 rol-6dm 30 ng/μl, and single-stranded DNA (ssDNA) donor oligo 50 ng/μl (or plasmid-based donor 50 ng/μl). F1 unc-22 twitchers or rol-6 rollers were picked, followed by genotyping of desired mutations. When applicable, transgenic strains were outcrossed to remove unc-22 mutations.
For the RNP strategy, we followed the protocol described in Dokshin et al. (2018). Final concentrations of injection components were as follows: Cas9 250 ng/μl, transactivating CRISPR RNA 100 ng/μl, and CRISPR RNA 56 ng/μl were mixed and incubated at 37° for 10 min, then ssDNA donor oligo 110 ng/μl and pRF4 rol-6dm 50 ng/μl were added, and injection mixtures were introduced into the germline of C. elegans young adults by microinjection. The F1 progeny from plates containing rollers were picked and genotyped to identify the desired mutations.
Plasmid construction
Cas9/sgRNA constructs:
We used the online tool sgRNA Scorer 2.0 (https://crispr.med.harvard.edu/) to design sgRNAs. The sgRNAs were cloned into pDD162 (Dickinson et al. 2013) by overlapping PCR using pDD162 as the PCR template and the appropriate primers (see sgRNA sequences used in this study in Table S2). Overlapping PCR products were inserted into pDD162 linearized with SpeI/BsrBI digestion by the seamless ligation cloning extract (SLiCE) method (Zhang and Glotzer 2015).
Donor constructs:
To generate the flag::mCherry::glh-1 donor construct, 800 bp upstream and 500 bp downstream of glh-1, and flag::mCherry coding sequences were amplified by PCR using N2 genomic DNA or plasmids containing flag::mCherry as templates, and the primers WJC049 and LB18, LB20 and WJC137, WJC110 and WJC111, and WJC136 and LB14.
To generate the glh-1 FGGΔ donor construct, 900 bp upstream and 500 bp downstream of glh-1 FGGΔ, and FGGΔ flanking sequences were amplified by PCR using N2 genomic DNA as the template, and the primers WJC049 and WJC050, and WJC051 and WJC052 (see Table S2 for PCR primer sequences). To generate the flag::prg-1 donor construct, 500 bp upstream and downstream of the prg-1 sequence were amplified by primers LB05 and LB27, LB20 and LB25, and LB26 and LB10, respectively. PCR fragments were inserted into pUC19 linearized with HindIII/KpnI digestion by SLiCE. Silent mutations were introduced in guide RNA-targeting sites by site-directed mutagenesis in donor constructs using Phusion High-Fidelity DNA Polymerases (Thermo Fisher Scientific).
Fluorescence microscopy
GFP- and mCherry-tagged fluorescent proteins were visualized in living nematodes or dissected embryos by mounting young adult animals on 2% agarose pads with M9 buffer (KH2PO4 22 mM, Na2HPO4 42 mM, and NaCl 86 mM) with 10–50 mM levamisole, or mounting one-cell embryos on 2% agarose pads by dissecting gravid hermaphrodites into egg salt buffer (HEPES pH 7.4 5 mM, NaCl 118 mM, KCl 40 mM, MgCl2 3.4 mM, and CaCl2 3.4 mM). Fluorescent images were captured using a Zeiss ([Carl Zeiss, Thornwood, NY) LSM 800 confocal microscope with a 40× objective.
Images were processed and quantified in ImageJ. The quantification of germline granule fluorescence was performed using ImageJ. For every image, a region of interest (ROI) was selected manually and the threshold was set automatically. The area of the whole ROI, the number of puncta within the ROI, and the area (i.e., size) of each punctum were measured. The densities of germline granules were calculated as: the number of puncta within the ROI/the area of the whole ROI. Densities of germline granules were determined for 10–12 ROIs, and the mean and SD were calculated using GraphPad Prism. The size of all puncta in 10–12 ROIs were direct scatter-plotted in the diagram, and the median and interquartile range were calculated using GraphPad Prism.
Fluorescence recovery after photobleaching
Photobleaching and fluorescence recovery of GFP::PRG-1 and mCherry::GLH-1, in germlines or early embryos, were conducted and recorded using a Zeiss LSM 800 confocal microscope with a Plan-Apochromat 63×/1.4 Oil DIC M27 objective, controlled by the ZEN software. The ZEN built-in fluorescence recovery after photobleaching (FRAP) module was used to perform FRAP experiments. Bleaching was performed using 100% laser power in the 488 and 553 channels for GFP::PRG-1 and mCherry::GLH-1, respectively. The region slightly larger than a single granule was selected, and fluorescence intensity in the whole region was bleached to < 20% of initial intensity before fluorescence recovery was allowed. Multiple granules were selected in each imaging field, and three of them were imaged without photobleaching to serve as a normalization control. Images were acquired every 1 sec during a recovery phase of 120 sec after bleaching.
Images were processed and quantified in ImageJ. The total fluorescence intensity was measured for areas containing bleached granules (I), unbleached granules (Inorm), or areas without granules (Ibkg) at each time point. Fluorescence recovery rates were calculated as Fn = (In−Ibkgn)(Inormi/Inormn)/(Ii−Ibkgi), where n stands for time points after photobleaching and i stands for initial phase before photobleaching. Recovery rates for all granules at different time points were calculated and plotted by using GraphPad Prism 6.0.
RNA polymerase II inhibition
The transcriptional inhibitor α-amanitin (50 µg/ml in water, A2263; Sigma [Sigma Chemical], St. Louis, MO) was injected into the gonads of young adults. Injected worms were allowed to develop for 5 hr and then mounted on 2% agarose pads in M9 buffer with 2.5 mM levamisole. Fluorescent images were captured using a Zeiss LSM 800 confocal microscope with a 40× objective. Images were processed and quantified in ImageJ.
Immunoprecipitation
First, 100,000 staged worms were frozen in liquid nitrogen and stored at −80°. Worm pellets were resuspended in equal volumes of immunoprecipitation buffer (20 mM Tris- HCl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 0.5% NP-40, 80 U ml−1 RNaseOUT, and 1 mM dithiothreitol) and protease inhibitor cocktail without EDTA, and homogenized using an MP Fastprep 24 benchtop homogenizer set to 6 meter/sec and run for 3 × 40 sec, keeping the tubes on ice for 5 min between runs. Lysates were clarified by spinning down at 30,000 × g for 15 min. Supernatants were precleared with 100 μl protein G beads and incubated with the indicated antibodies, such as anti-PRG-1 (1:100, gift from C. C. Mello) (Batista et al. 2008), anti-GLH-1 (1:100, made by K. L. Bennett’s laboratory) (Kuznicki et al. 2000), and anti-FLAG (1:100, F1804; Sigma), for 1 hr and bound to protein G beads for 1 hr at 4°. In the RNase experiments, the beads were split into two samples and incubated at 37° with or without 100 U/ml RNAse (New England Biolabs, Beverly, MA). Beads were washed with wash buffer (20 mM Tris-HCl pH 7.5, 200 mM NaCl, 2.5 mM MgCl2, and 0.5% NP-40) six times, and then resuspended in TBS buffer for mass spectrometry or western blotting.
Mass spectrometry
Samples on immunoprecipitated beads were denatured with 8 M urea in 50 mM Tris-HCl, reduced with 5 mM Tris (2-carboxyethyl) phosphine hydrochloride and alkylated with 10 mM chloroacetamide. Next, 50 mM Tris-HCl (pH 8.5) was added to dilute the urea to 2 M, following which 0.5 μg trypsin/LysC mix (Promega, Madison, WI) was added overnight at 37°. The supernatant was collected from all samples and cleaned up with C-18 spin columns (Thermo Fisher Scientific).
A Thermo Fisher Scientific Q Exactive Plus Mass Spectrometer coupled with a Thermo Fisher Scientific Easy-nLC1200 was used for analysis. Digested peptides were loaded onto an Acclaim PepMap C18 trapping column (3 μm particle size, 100 Å pore size, 2 cm length, and 75 μm outer diameter) and eluted on a PepMapTM RSLC C18 column (2 μm particle size, 100 Å pore size, 15 cm length, and 75 μm outer diameter) with a linear gradient from 3 to 35% acetonitrile (in water with 0.1% fluoroacetic acid) developed over 120 min at room temperature at a flow rate of 650 nl/min, and effluent was electrosprayed into the mass spectrometer. A blank was run prior to the sample run to make sure there was no significant signal from solvents or the column.
Raw files generated from the run were analyzed using Thermo Fisher Scientific Proteome Discoverer 2.2. SEQUEST HT was utilized to perform database searches with a few modifications: trypsin digestion, two maximum missed cleavages, precursor mass tolerance of 10 ppm, fragment mass tolerance of 0.8 kDa, a fixed modification of +57.021 kDa on cysteine, and a variable modification of +15.995 kDa on methionine. The spectral false discovery rate was set to ≤ 1%. The FASTA database used was a C. elegans proteome downloaded from Uniprot.
The full list of peptides identified by mass spectrometry can be found at File S2 and File S3.
Western blotting
Lysates were prepared from ∼100 synchronized young adult worms by boiling worms in 1× SDS sample buffer for 10 min. Proteins were separated by standard SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA) using a Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA). Membranes were blocked in TBST (20 mM Tris-HCl pH 7.4, 150 mM NaCl, and 0.05% Tween-20) containing 5% skimmed milk for 2 hr at room temperature, then probed with primary antibodies against PRG-1 (1:1000, gift from C. C. Mello) (Batista et al. 2008), GLH-1 or GLH-4 (1:1000, made by K. L. Bennett’s laboratory) (Kuznicki et al. 2000; Orsborn et al. 2007), and FLAG (1:1000, F1804; Sigma) overnight at 4°, separately. After five washes with TBST buffer, the membranes were incubated with secondary antibodies against the species of the primary antibodies at room temperature for 1 hr and then washed five times with TBST. ECL substrates were used for detection of proteins by a FUJIFILM LAS 4000 luminescent image analyzer.
Ex vivo assay
Gravid young adult hermaphrodites were dissected in ex vivo buffer (10 mM Tris pH 7.5, 300 mM NaCl, and 5 mM EDTA pH 7.5), and gonads were cut by a needle to let the P granules release into the buffer. When needed, 100 U/ml RNaseA/T1 mix (Thermo Fisher Scientific) was added into the ex vivo buffer. Dissected gonads were then mounted on 2% agarose pads made with ex vivo buffer. Images were captured using a Zeiss Axio Scope.A1 compound fluorescent microscope with an EC Plan-Neofluar 40×/0.75 M27 objective and a Retiga R6 CCD (Teledyne QImaging). Images were processed and quantified in ImageJ.
Brood size analysis
Single hermaphrodite L4s (P0s) were picked onto individual freshly seeded NGM plates and allowed to grow for 24 hr at 20 or 25°. P0 adults were transferred to new NGM plates every 24 hr until they no longer laid eggs. All the F1 progeny on each plate were counted. The brood size of each P0 animal is the total sum of F1s for all plates where the P0 animal laid eggs.
Data availability
Strains and plasmids are available upon request. Movie S1–S6 contains supplemental movies. File S1 contains the information of glh-1 and glh-4 alleles created in this study. File S2 and File S3 contain the lists of peptides identified in proteomic analyses of GLH-1 and PRG-1 complexes. Table S1 and Table S2 contain the strains and oligonucleotides used in this study. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11786094.
Results
Evidence that GLH helicases promote P granule assembly through their ATP/RNA binding
To investigate the role of the GLH-1 RNA helicase in P granule assembly, we examined the localization of several P granule-enriched components, including PGL-1, PRG-1, and CSR-1 in glh-1 mutants (Kawasaki et al. 2004; Batista et al. 2008; Claycomb et al. 2009). Consistent with previous reports that the localization of PGL-1 to P granules requires glh-1 (Spike et al. 2008; Updike and Strome 2009), a glh-1 null mutation (uoc1) reduced both the size and number of PGL-1 granules (Figure 1A, top and Figure S1A). In addition, we observed a reduction of granule density of PRG-1 granules and CSR-1 granules in the glh-1 (uoc1) mutant, although the reduction of CSR-1 granule density was less than that of PRG-1 or PGL-1 granules (Figure 1B and Figure S1A). As it was reported that GLH-1 and GLH-4 act redundantly to promote the formation of PGL-1 granules and fertility in C. elegans (Kuznicki et al. 2000; Spike et al. 2008), we found fewer PGL-1 granules in the glh-1glh-4 (uoc5; gk225) double null mutant than in the glh-1 (uoc1) null mutant (Figure S1A). We then examined whether GLH-1 and GLH-4 may also play a redundant role in the formation of PRG-1 or CSR-1 granules as well. While PRG-1 granules looked normal in a glh-4 (uoc10) mutant, we observed few, if any, PRG-1 granules in the glh-1glh-4 (uoc1; uoc6) double mutant (Figure 1B). In addition, significantly fewer GFP::CSR-1 granules were found in the glh-1glh-4 (uoc5; gk225) double mutant than in the glh-1 (uoc1) null mutant (Figure S1A). Together, these observations suggest that GLH-1 and GLH-4 jointly promote the localization of several P granule-enriched components to P granules, including PGL-1, CSR-1, and PRG-1.
Figure 1.
Evidence that germline helicases promote the formation of P granules through their ATP and RNA binding. (A) The domain structure of GLH-1 shows the location of the GLH-1 mutations used in this study (top). The asterisks indicate frameshift deletions that lead to an early stop codon. A model shows how GLH-1 couples its ATP hydrolysis cycle to its RNA binding/release and the proposed effects of the indicated mutations (bottom). (B) GLH-1 and GLH-4 act redundantly to promote the formation of PRG-1 granules. Fluorescent micrographs show the localization of the P granule component GFP::PRG-1 in the indicated strains (left). Image analyses show GFP::PRG-1 granule density and granule size in the indicated strains. Note that uoc1, uoc6, and uoc10 are gene-edited null alleles of the indicated genes. Bar, 10 μm. For the measurements of granule density, the averages and the SDs are indicated. For the measurements of granule size, the median, and 25th and 75th percentiles are indicated. Note that unless specifically labeled, the significance test was measured between wild-type and the indicated strain. *** or ###: P < 0.001. n.s., nonsignificant. (C) The localization of P granule components mCherry::GLH-1 and GFP::PRG-1 is seen in the indicated strains in the adult germline (left) or in the four-cell embryo (right). The arrows indicate the cytoplasmic P granules in the four-cell embryo. Bar, 10 μm. (D) The effects of α-amanitin, an RNA polymerase II inhibitor, on the localization of P granule components mCherry::GLH-1 and GFP::PRG-1 are shown in the adult germline. Images were taken 5 hr after the injection of α-amanitin. Bar, 10 μm.
The GLH homolog VASA and other DEAD box helicases have been shown to act as ATP-dependent RNA clamps, in which ATP binding leads to a closed conformation of the helicase that promotes its RNA binding (Figure 1A bottom) (Theissen et al. 2008; Putnam and Jankowsky 2013; Xiol et al. 2014). As RNA binding by PGL-3 and MEG-3/4 plays a critical role in P granule formation (Saha et al. 2016; Smith et al. 2016), we wondered whether ATP binding and/or RNA binding of a GLH helicase contributes to the localization of itself and other factors at P granules. We created gene-edited worms that express GLH-1 with well-characterized mutations that affect distinct steps in the ATP hydrolysis cycle of DEAD box helicases (Figure 1A). We first examined the effects of a glh-1 DAAD mutation, which changes glutamic acid in the DEAD box to alanine. This mutation abolishes the ability of several DEAD box helicases to bind and hydrolyze ATP in vitro, and thereby reduces RNA-binding activity (Putnam and Jankowsky 2013). In transgenic worms expressing GLH-1 with a DAAD mutation, we observed a dramatic reduction in the size and number of GLH-1 granules in the adult gonad (Figure 1C, left). The size and number of PRG-1 granules were also reduced, but not absent, in the glh-1 DAAD mutant. The presence of these residual PRG-1 granules in glh-1 DAAD mutants could be explained, at least in part, by the redundant roles of glh-1 and glh-4 in PRG-1 granule formation (Figure 1B). Similarly, we found defects in GLH-1 and PRG-1 granules in glh-1 Q368A mutants. This mutation has been shown to abolish ATP binding and thus is required for ATP hydrolysis in in vitro analyses of other DEAD RNA helicases (Putnam and Jankowsky 2013) (Figure S1C). Together, these analyses suggest that the ATP binding and hydrolysis of GLH-1 is required for its localization to the P granule, as well as for its role in promoting the localization of PRG-1 to the P granule.
We then examined early embryos, where repetitive cycles of assembly and disassembly of P granules can be observed (Seydoux 2018). Unlike GLH-1 granules, which are only observed in the germline lineage cell (P2) of the four-cell embryo (Figure 1C), we noticed two types of PRG-1 granules; one group of PRG-1 granules colocalized with GLH-1 granules and were only present in germline lineage cells. Another group of PRG-1 granules, which we referred to as PRG-1 puncta to avoid confusion, were smaller and found in both somatic and germline lineage cells. These PRG-1 puncta are formed independent of glh-1 (Movie S1 and Movie S2). Notably, very few, if any, GLH-1 granules or GLH-1-colocalized PRG-1 granules were found in four-cell embryos of the GLH-1 DAAD mutant (Figure 1C, right). Together, these results suggest that the ATP binding/hydrolysis of GLH-1 promotes the localization of GLH-1 and PRG-1 to P granules, both in the adult germline and in the embryo.
To further examine the role of the RNA binding of GLH-1 in promoting the localization of itself and other factors to P granules, we examined the effects of a mutation in the conserved threonine at motif V of the GLH-1 helicase domain (T663A). This threonine makes direct contact with the RNA backbone and is required for the RNA binding of DEAD/DExH helicases in vitro (Sengoku et al. 2006; Tanaka and Schwer 2006). We observed very few, if any, GLH-1 granules in the glh-1 T663A mutant adult gonad (Figure 1C). In addition, we observed a reduction of granule density and size of PRG-1 granules in glh-1 T663A to a similar degree as that found in the glh-1 null mutant. We reasoned that if RNAs play a key role in the formation of GLH-1 or PRG-1 granules, RNase should destabilize these granules. Indeed, after extruding the adult germline from the body cavity, we observed that nearly all perinuclear PRG-1 granules dissipated in RNase-containing buffer within 2 min, while around 50 and 30% of PRG-1 granules remained visible after 2 and 5 min in control buffer, respectively (Figure S1E). Furthermore, a previous report has demonstrated that P granules are the major sites of messenger RNA (mRNA) export in the germline (Sheth et al. 2010). We reasoned that if binding of newly exported RNAs by GLH-1 is required for localization of GLH-1 and other P granule factors to the P granule, inhibition of RNA transcription should lead to loss of GLH-1 granules and PRG-1 granules. Indeed, a previous study has shown that inhibition of RNA export or RNA transcription leads to loss of perinuclear PGL-1 granules (Sheth et al. 2010). Similarly, we observed that injection of α-amanitin, an RNA polymerase II inhibitor, into the adult germline leads to around a 98% loss of perinuclear GLH-1 granules and around a 85% loss of PRG-1 granules (Figure 1D and Figure S1D). This observation supports the role of newly transcribed RNAs in the maintenance of GLH-1 granules. It also indicates that while some PRG-1 granules are more tolerant to a decrease in RNA transcription than GLH-1 granules, the maintenance of most PRG-1 granules also requires newly transcribed RNAs. Together, our results strongly support a model that the RNA binding of the GLH-1 helicase promotes its localization at P granules and thereby promotes the localization of other P granule components to P granules.
Evidence that GLH-1 couples RNA release to the disassembly of P granule factors from P granules
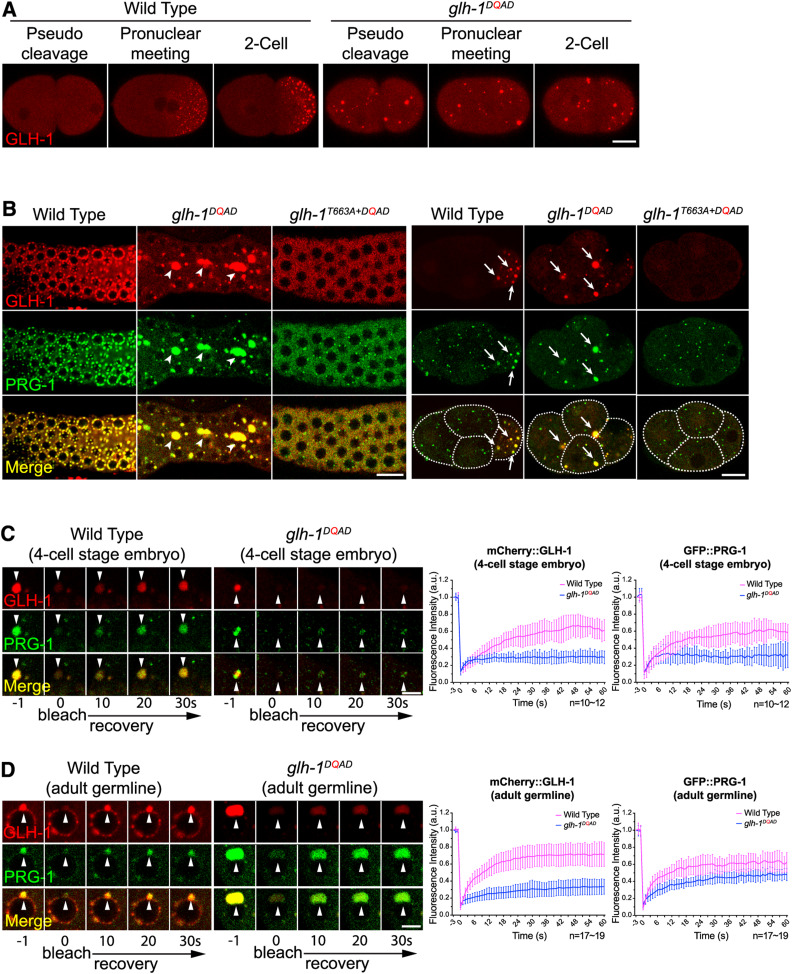
Interestingly, a recent study revealed that a DQAD mutation in the DEAD motif of the VASA helicase inhibits its release of RNA and ATP hydrolysis products in vivo and in vitro (Xiol et al. 2014) (Figure 1B). Since RNA binding promotes the formation of GLH-1 and PRG-1 granules, we wondered whether inhibition of RNA release would lead to stabilization of these granules. As many P granule components are first disassembled in the mature oocyte/newly fertilized embryo before they are reassembled prior to the first cell division (Figure 2A, Movie S3, and Movie S4), we first examined the localization of GLH-1 during these processes. Strikingly, unlike wild-type embryos, in which GLH-1 granules are disassembled at the pseudocleavage stage of the early embryo, many GLH-1 granules remain clearly visible in a glh-1 DQAD mutant (Figure 2A). Time-lapse images of newly fertilized embryos in the glh-1 DQAD mutant clearly revealed that some GLH-1 and PRG-1 granules never undergo the disassembly process seen in wild-type embryos (Movie S5 and Movie S6). In addition, we reasoned that since the asymmetric sorting of P granules in the one-cell embryo relies on a faster assembly and a slower disassembly of P granules in the embryo’s posterior vs. anterior (Wang et al. 2014), the inhibition of global P granule disassembly would exhibit defects in P granule sorting. Indeed, unlike in wild-type animals, the GLH-1 and PRG-1 granules of glh-1 DQAD mutants failed to be exclusively sorted to the germline lineage, but rather were distributed to cells of both somatic and germline lineages in early embryos (Figure 2A, right, Figure 2B, right, Movie S4, and Movie S6). We next examined whether these stabilized granules are less dynamic. If so, we expected the exchange of factors between granules and cytoplasmic pools to decrease. Indeed, using a FRAP assay, we observed that while the wild-type GLH-1 and PRG-1 granules exhibit significant recovery of fluorescence signals within 20 sec after photobleaching in wild-type embryos, their recovery is reduced in the glh-1 DQAD mutant (Figure 2C). We noticed that PRG-1 exhibits a slightly faster and greater recovery than GLH-1 in the glh-1 DQAD mutant, likely due to the function of other GLH helicases, such as GLH-4 in regulating PRG-1 dynamics. Together, these results demonstrated that the disassembly of GLH-1 and PRG-1 granules is defective in glh-1 DQAD mutants, which supports a model that GLH-1 couples RNA release to promote the release of itself and PRG-1 from P granules.
Figure 2.
Evidence that germline helicases couple RNA release to promote the turnover of P granule components. (A) The disassembly and the asymmetric sorting of mCherry::GLH-1 granules are defective in early embryos of the glh-1 DQAD mutant. Fluorescent micrographs show the localization of mCherry::GLH-1 at the specified stages of newly fertilized embryos in the indicated strains. Bar, 10 μm. (B) The localization of mCherry::GLH-1 and GFP::PRG-1 in the indicated strains is seen in the adult germline (left) or in the four-cell embryo (right). The arrowheads indicate that many of the enlarged aggregates of P granules in the adult germline do not associate with germline nuclei. The arrows indicate that in embryos, P granules are properly sorted to the cells of the germline lineage (P cell) in wild-type but are improperly sorted to cells of the somatic lineage in the glh-1 DQAD mutant. Bar, 10 μm. (C) Fluorescence recovery after photobleaching (FRAP) analyses indicate that the dynamics of P granule components, including mCherry::GLH-1 and GFP::PRG-1, are both reduced in the four-cell embryos of glh-1 DQAD mutant worms (left). The arrowheads indicate the P granules that are photobleached. Bar, 5 μm. The quantification of FRAP analyses of images showing the average fluorescence signals of mCherry::GLH-1 and GFP::PRG-1 at the indicated times (seconds) after photobleaching (right). Error bars indicated the SDs of fluorescence intensities. (D) FRAP analyses indicate reduced dynamics of P granule components, including GLH-1 and PRG-1, in the adult germlines of glh-1 DQAD mutant worms. Bar, 5 μm. The quantification of FRAP analyses of images shows the average fluorescence signals of mCherry::GLH-1 and GFP::PRG-1 at the indicated times (seconds) after photobleaching (right). Error bars indicate the SD of fluorescence intensities.
While perinuclear P granules in the adult germline do not undergo cycles of assembly and disassembly, they are liquid-like assemblies and are actively exchanging their granules’ components with cytoplasmic pools (Sheth et al. 2010). Remarkably, many P granule components—including GLH-1, PRG-1, CSR-1, and PGL-1—all form enlarged granules in the adult germline of glh-1 DQAD mutants (Figure 2B, left and Figure S2, A and B). Notably, the majority of these enlarged granules found in the DQAD mutants have lost their perinuclear localization in the germ cells of the adult germline. Similar to the embryo, we found that these enlarged GLH-1 and PRG-1 granules in the glh-1 DQAD mutant also exhibit slower recovery than those granules in wild-type animals (Figure 2D). These results suggest that GLH-1 and PRG-1 granules are less dynamic in the glh-1 DQAD mutant. Since these enlarged PRG-1 granules in glh-1 DQAD worms were less dynamic we expected them to be more stable when they were diluted in an ex vivo buffer. Indeed, unlike the wild-type PRG-1 granules, which mostly dissipated into solution after 2 min after extruding the adult germline from the body cavity, the enlarged PRG-1 granules in the glh-1 DQAD mutant remain similar in their size 5 min after the same procedure (Figure S2C). Together, these results show that both GLH-1 and PRG-1 granules in glh-1 DQAD mutants are less dynamic.
We reasoned that if enlarged GLH-1 and PRG-1 granules are caused by defects in RNA release from GLH-1, disruption of GLH-1 RNA binding should interfere with aggregation. Indeed, in glh-1 mutants carrying both the T663A and DQAD mutations, we no longer observed enlarged granules, but rather saw a few tiny GLH-1 or PRG-1 granules, as seen in glh-1 T663A mutants alone (Figure 2B). This observation suggests that RNA binding is required for the GLH-1 DQAD mutant to form enlarged P granules. Furthermore, if these enlarged, less-dynamic GLH-1 and PRG-1 granules are caused by defects in the RNA release of GLH-1, their maintenance should be less sensitive to the loss of newly transcribed RNAs, as more GLH-1 DQAD would remain bound to its associated RNAs. Indeed, unlike wild-type P granules, the enlarged GLH-1 and PRG-1 granules in the GLH-1 DQAD mutant did not shrink in size after the injection of RNA polymerase II inhibitor (Figure S1D and Figure S2D). Together, these results are consistent with the model that RNA release from GLH-1 is coupled to the disassembly of GLH-1 and PRG-1 granules in the embryo, and is coupled to the turnover of GLH-1 and PRG-1 from P granules in the adult germline.
The GLH-1 complex in the DQAD mutant enriches Argonautes and the polyA-binding protein PAB-1
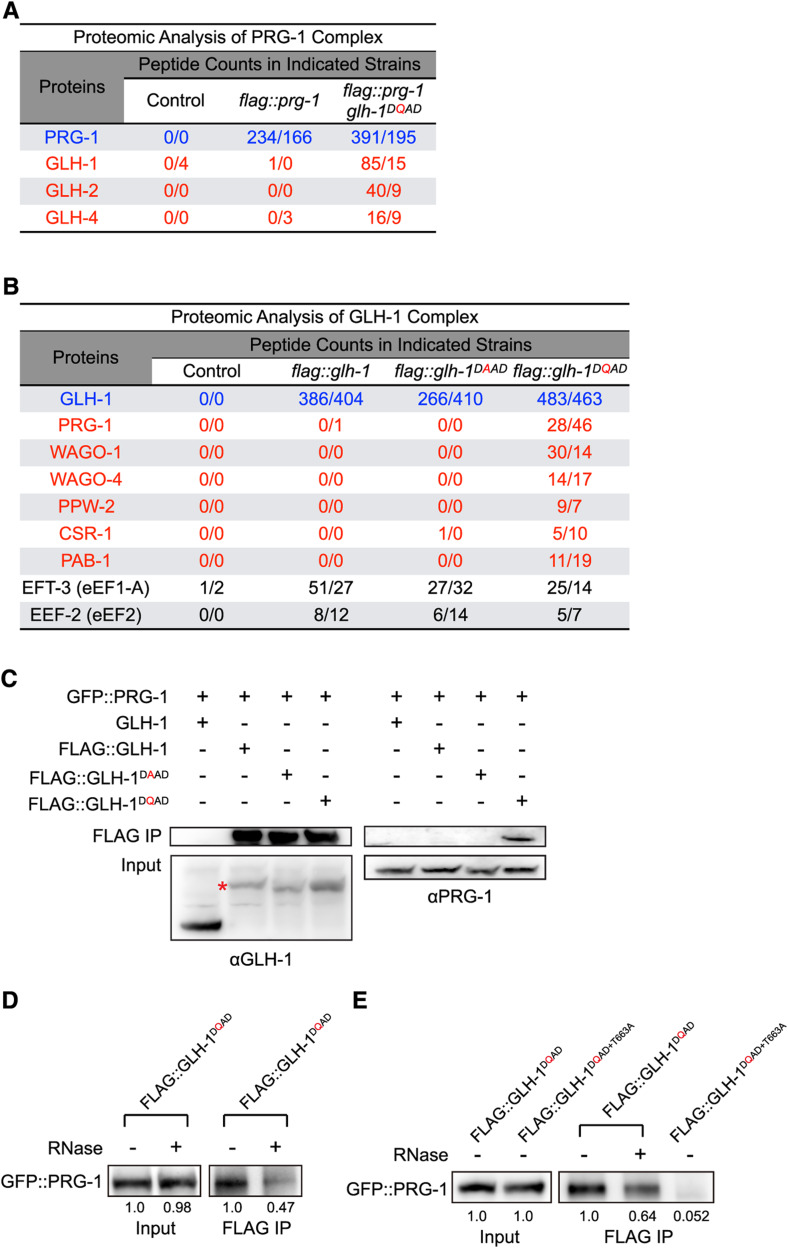
We reasoned that since the GLH-1 DQAD mutation significantly reduces the disassembly of P granule factors, it offered an opportunity to isolate the components of P granules that otherwise may be too unstable to capture. Indeed, a previous study has found that the DQAD mutation in VASA leads to the stabilization of transient interactions between VASA and its interactors, including the PIWI Argonautes and factors required for PIWI-interacting RNA amplification in insect culture cells (Xiol et al. 2014). Therefore, we performed proteomic analyses to examine the components of the GLH-1 and PIWI PRG-1 complexes in various GLH-1 mutant backgrounds (File S2 and File S3). In the wild-type GLH-1 background, we identified few GLH-1 and GLH-4 peptides in PRG-1 immunoprecipitated complexes (Figure 3A), implying that the interactions between PRG-1 and GLH1-1/GLH-4 are unstable. However, we identified significantly more peptides from FG-containing GLHs (GLH-1, GLH-2, and GLH-4) in the PRG-1 complex in the glh-1 DQAD mutant background than in wild-type animals (Figure 3A), suggesting that the interactions between PRG-1 and these GLHs are stabilized in the GLH-1 DQAD mutant. Similarly, in GLH-1 immunoprecipitated complexes, we identified significantly more peptides of several P granule-enriched factors, including the Argonaute proteins PRG-1, WAGO-1, WAGO-4, CSR-1, and the polyA-binding protein PAB-1 in the glh-1 DQAD mutant background than in wild-type animals (Batista et al. 2008; Claycomb et al. 2009; Gu et al. 2009; Ko et al. 2013) (Figure 3B). The presence of PRG-1 in the GLH-1 complex in the DQAD mutant background was further confirmed by western blot analyses (Figure 3C). Not all the factors in the GLH-1 complex were enriched in the DQAD background. For example, similar amounts of peptides of the translation factors EFT-3 and EEF-2 were found in various GLH backgrounds, including in wild-type animals, and DAAD and DQAD mutants (Figure 3B). Since the release of RNA from GLH-1 is expected to be defective in the DQAD background, these results imply that the interactions between GLH-1 and Argonautes/PAB-1 are strengthened through RNAs. Indeed, we observed that the interaction of GLH-1 and PRG-1 in the DQAD mutant was reduced by RNase treatments of immunoprecipitated complexes (Figure 3, D and E). In addition, the interaction between GLH-1 and PRG-1 was completely abolished in glh-1 mutants carrying both the T663A and DQAD mutations, in which RNA binding of glh-1 is expected to be compromised due to the T663A mutation (Figure 3E). These results suggest that the interaction between GLH-1 and PRG-1 is at least partially mediated through RNAs.
Figure 3.
GLH-1 forms a complex that accumulates Argonautes and other RNA-binding proteins in the glh-1 DQAD mutant background. (A) Proteomic analysis of the PRG-1 complex is shown for the indicated strains: wild-type glh-1 and the glh-1 DQAD mutant. The numbers of peptides of the indicated proteins identified in two independent experiments are shown. The proteins whose PRG-1 interactions are stabilized in the glh-1 DQAD mutant are highlighted in red. (B) A proteomic analysis of the GLH-1 complex is given for the indicated glh-1 mutant backgrounds. The proteins whose GLH-1 interactions are stabilized in the glh-1 DQAD mutant are highlighted in red. The numbers of peptides of the indicated proteins identified in two independent experiments are shown. (C) Western blot analyses show the interaction between FLAG::GLH-1 and GFP::PRG-1 is stabilized in the glh-1 DQAD strain. The red asterisk indicates the FLAG::GLH-1. (D) Western blot analyses show that the interaction between FLAG::GLH-1 and GFP::PRG-1 in the glh-1 DQAD mutant is reduced after the RNAse treatment of the purified complex. The number indicates the relative signal intensity. (E) Western blot analyses show that the interaction between FLAG::GLH-1 and GFP::PRG-1 is abolished in the glh-1 DQAD, T663A double mutant and is reduced after the RNAse treatment of the purified complex. The number indicates the relative signal intensity. IP, immunoprecipitation.
While we cannot rule out the possibility that GLH-1 or PRG-1 complexes in DQAD mutants may represent abnormal aggregates, several lines of evidence support that these complexes capture transient, biologically relevant interactions that occur in wild-type animals. First, many components enriched in the PRG-1 or GLH-1 complexes in DQAD mutants are known to be localized to P granules, including the Argonaute proteins, GLHs, and PAB-1 (Ko et al. 2013). Second, we were able to detect the interaction between PRG-1 and GLH-4 in the wild-type background (Figure S3A). Third, factors identified in the GLH-1 DQAD complex are known to regulate P granule assembly. For example, knockdown of pab-1 reduces the formation of PGL-1 granules, while knockdown of csr-1 leads to enlarged PGL-1 granules (Updike and Strome 2009; Ko et al. 2013). In pab-1 knockdown experiments, we also observed fewer GLH-1 and PRG-1 granules (Figure S3B). In summary, our proteomic analyses show that the GLH-1 complex in the DQAD mutant enriches several RNA-binding proteins, including Argonautes and PAB-1.
The FGG repeats in GLH-1 promote perinuclear P granule localization
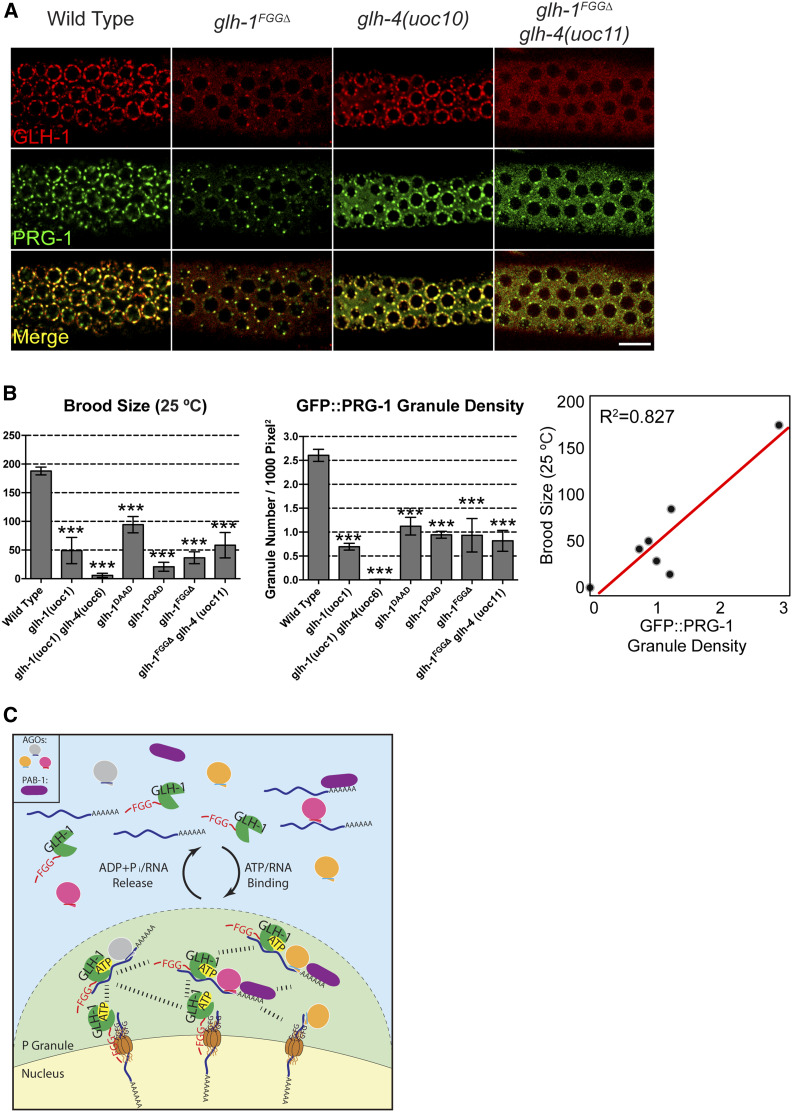
Our results suggest that P granule dynamics can be controlled by GLH-1, but the mechanism by which P granules localize to the nuclear periphery remains unknown. Germ (P) granules are frequently found at the nuclear periphery in various animals (Eddy 1975) and previous electron microscopy analyses suggest that P granules localize to the cytoplasmic face in proximity to clusters of nuclear pores in C. elegans (Pitt et al. 2000). As mentioned in the introduction, an intriguing model has been proposed that FGG repeats of the GLHs interact with the FG meshwork of nuclear pore proteins and thus help anchor germ granules to nuclear pore clusters (Updike et al. 2011). However, by expressing FGG repeats of GLH-1 in intestinal cells, the authors reported that these FGG repeats are not sufficient but they can promote the perinuclear localization of PGL-1 when ectopically expressed (Updike et al. 2011). To directly test the role of the GLH-1 FGG repeats in its perinuclear localization and the localization of other P granule factors, we examined the localization of GLH-1 and PRG-1 in worms expressing a GLH-1 mutant protein with its N-terminus deleted, which removes all of its FGG repeats (glh-1 FGGΔ) (Figure 1B). Unlike the glh-1 DAAD mutant, in which the formation of cytoplasmic PRG-1 granules was severely compromised in early embryos (Figure 1C), no defect in the formation of cytoplasmic GLH-1 or PRG-1 granules was found in the glh-1 FGGΔ mutant, nor in glh-1 FGGΔ in the glh-4 (uoc11) null background (Figure S4A). These results suggest that the FGG repeats of GLH-1 are not required for the formation of cytoplasmic GLH-1 or PRG-1 granules in embryos. It also suggested that this glh-1 FGGΔ did not affect the ATP hydrolysis or RNA binding of GLH-1. However, in the adult germline, the number of perinuclear GLH-1 granules was reduced to 50 and 15% of wild-type in glh-1 FGGΔ and in glh-1 FGGΔ glh-4 (uoc11) double mutants, respectively (Figure 4A and Figure S4B). The number of perinuclear PRG-1 granules were also reduced to 40 and 35% of wild-type in glh-1 FGGΔ and in glh-1 FGGΔ glh-4 double mutants, respectively. In addition, the defects in forming perinuclear GLH-1 and PRG-1 granules in the glh-1 FGGΔ mutant were exacerbated when worms were grown at a higher temperature of 25° (Figure S4C). Overall, we noticed that the defects in perinuclear localization in glh-1 FGGΔ are more dramatic for GLH-1 itself than for PRG-1, suggesting that additional GLHs or other unknown factors may promote the formation of some residual PRG-1-containing granules at perinuclear foci in glh-1 FGGΔ mutants. In addition, defects of PRG-1 perinuclear localization were less severe in the glh-1 FGGΔ mutant than in the glh-1 (uoc1) null mutant (Figure 1A and Figure 4A), suggesting that while the FGG repeats of GLH-1 contribute to the perinuclear localization of PRG-1, other domains of GLH-1 also contribute to the localization of PRG-1 at P granules. Taken together, these observations indicate that the FGG repeats of GLH-1 promote the localization of GLH-1 and PRG-1 at perinuclear granules, perhaps through anchoring GLH-1 itself to the FG hydrogel formed at nuclear pores.
Figure 4.
The N-terminal FGG repeats in GLH-1 promote the nuclear anchoring of P granule factors that correlate with fertility. (A) The localization of P granule components GLH-1 and PRG-1 in the adult germline of the indicated strains is seen. Note the strains were grown at their normal growth temperature, 20°. Bar, 10 μm. (B) The brood sizes of worms of the indicated strains are given. Note the strains were grown at an elevated temperature, 25°, which exacerbates their fertility defects (left). The PRG-1 granule density of the indicated strains grown at 20° is shown (middle). The SEM is provided. ***P < 0.001 was determined using one-way ANOVA with the Bonferroni multiple comparison test comparing indicated mutants to wild-type animals. The correlation between brood size and PRG-1 granule density is seen (right). (C) A proposed model shows how GLH-1 controls the dynamics and perinuclear localization of P granules. In this model, the FGG repeats of GLH-1 tether GLH-1 to nuclear pores. GLH-1 then binds newly exported RNAs and facilitates multivalent interactions between the RNAs and RNA-binding proteins to promote P granule formation. As a consequence, GLH-1 couples its RNA binding and release to promote the exchange of P granule factors at the nuclear periphery.
Defects in perinuclear P granules correlate with fertility defects
As mentioned in the introduction, P granules are known to play a critical role in germ cell maintenance; various GLH mutants or GLH RNAi-treated worms exhibit fertility defects, in particular when worms are grown at an elevated temperature (Kuznicki et al. 2000; Spike et al. 2008; Updike et al. 2014). We examined whether the defects in fertility correlate with the degree of PRG-1 granule defects in various glh mutants, including glh-1 DAAD, DQAD, FGGΔ, glh-1 FGGΔ glh-4 (uoc11), and the glh-1glh-4 (uoc5; gk225) double mutant. Overall, we found a positive correlation (R = 0.83) between the number of perinuclear PRG-1 granules in the adult germline and the brood size of worms grown at the elevated temperature of 25° (Figure 4B). On the contrary, a previous study failed to identify a correlation between the degree of cytoplasmic P granule defects in embryos and infertility between several meg mutants (Wang et al. 2014). Notably, in GLH-1 FGGΔ mutant worms, which had normal cytoplasmic GLH-1/PRG-1 granules in embryos but reduced perinuclear PRG-1 and GLH-1 granules in the adult germline, we saw a significant reduction in brood size when grown at the elevated temperature. Together, our observations suggest a critical role of germline perinuclear P granules in germ cell development.
Discussion
The function of GLH-1 ATPase and RNA binding in regulating the dynamics of P granules
In this study, we have shown that the RNA helicase GLH-1 plays multiple roles in controlling the dynamics and localization of P granules, the liquid droplets found in the germlines of all animals. We found distinct P granule defects associated with specific mutations that are known to affect the binding or release of ATP/RNA in other DEAD box helicases. Specifically, we found that in glh-1 DAAD, Q368A, or T663A mutants—mutations expected to interfere with ATP or RNA binding (Figure 1B)—the localization of GLH-1 and PRG-1 to P granules was compromised both in adult germlines and in embryos. On the contrary, in the glh-1 DQAD mutant—a mutation expected to interfere with the release of ATP hydrolyzed products and RNAs from GLH-1—GLH-1 and PRG-1 granules became less mobile (Figure 2C and Figure 2D). In the glh-1 DQAD mutant embryo, GLH-1 and PRG-1 granules failed to undergo disassembly and exhibited defects in their sorting to the germline lineage (Figure 2A and Figure 2B). In the glh-1 DQAD adult germline, enlarged GLH-1 and PRG-1 granules were present in the cytoplasm, while the formation of perinuclear GLH-1 and PRG-1 granules was compromised. Other P granule factors, including CSR-1 and PGL-1, also formed large granules in the glh-1 DQAD adult germline. The formation of these enlarged GLH-1 and PRG-1 granules was compromised in the glh-1 DQAD, T663A double mutant, suggesting that the RNA-binding activity of GLH-1 is a prerequisite for the formation of these large aggregates (Figure 2B). An independent study also observed similar defects of GLH-1 localization in several glh-1 mutants described in this study, such as in the glh-1 DAAD, DQAD, and T663A mutants (Marnik et al. 2019). Together, these observations suggest a simple model that the binding, and release, of RNAs from GLH-1 allow for the influx and efflux of components into and out of P granules, respectively (Figure 4C). In this model, the RNA binding of GLH-1 facilitates multivalent interactions between RNAs, GLH-1, and other RNA-binding proteins to promote the enrichment of P granule factors at the perinucleus (Figure 4C). This model provides an explanation for the previous seemingly opposite effects of mutations in the RNA helicase VASA on germ (P) granules reported in insect cells: VASA DQAD mutation leads to enlarged VASA granules and PIWI (Ago3) granules in insect cell culture, while mutation of the conserved threonine of motif V leads to loss of VASA granules in Drosophila (Xiol et al. 2014; Dehghani and Lasko 2015). Together, these observations suggest the VASA-like helicases share a conserved role in regulating the dynamics of germ (P) granules in diverse animals. Importantly, DEAD box RNA helicases have also been reported to play a role in regulating the phase separation of other cellular bodies. For example, the DQAD mutation in the yeast DHH1 helicase also leads to enlarged processing bodies, while mutations affecting the RNA binding of DHH1 lead to loss of processing bodies in yeast (Mugler et al. 2016). Together, our study provides an example of how RNA helicases control the formation and localization of phase-separated bodies, and strengthens the emerging theme of DEAD box helicases as a key regulator of phase separation in cells (Hondele et al. 2019).
The redundancy of the GLHs in regulating the dynamics of P granules
While GLH-1 is found to be involved in the localization of PGL-1 granules, some PGL-1 granules remain present in the glh-1 mutant (Spike et al. 2008). Similarly, we observed residual PRG-1, PGL-1, and CSR-1 granules in the glh-1 null mutant. As mentioned in the introduction, a previous study showed that GLH-1 and GLH-4 play a redundant role for regulating the localization of PGL-1 granules (Spike et al. 2008). Our analyses of the localization of P granule factors further support the redundancy of GLH-1 and GLH-4 in P granule regulation, where the localization of PRG-1, PGL-1, and CSR-1 are all severely disrupted in glh-1glh4 (uoc5; gk225) double null mutants, although some granules can still be found. We suspect GLH-2 also plays a redundant role with GLH-1 and GLH-4, as our proteomic analyses revealed the presence of all three FGG-containing GLHs—GLH-1, GLH-2, and GLH-4—in PRG-1 complexes of the glh-1 DQAD mutant. It is unknown why GLH-1 seems to play the more major role in the localization of most P granules factors examined. One possible explanation is that GLH-1 is the most highly expressed gene among the three FGG-containing GLHs, where the expression levels of GLH-1, GLH-2, and GLH-4 are 50, 15, and 12 reads per kilobase million, respectively (McMurchy et al. 2017).
Are there GLH-independent mechanisms for P granule localization? Electron microscopy images showed that in glh-1 glh-2 double knockdown experiments electron-dense perinuclear foci remain, despite the fibrillar-like perinuclear signals seen in wild-type worms being absent (Schisa et al. 2001). They further showed that knockdown of both glh-1 and glh-2 led to loss of PGL-1 granules but not RNA granules. These experiments demonstrated that RNA, and likely other factors, can still localize to perinuclear foci independent of GLH-1 and GLH-2. Interestingly, it was shown that the mutator foci, another group of perinuclear granules frequently associated with P granules, are enriched for small RNA biogenesis factors, and can be formed independent of GLH1 and GLH-4 (Phillips et al. 2012). It will be important in the future to determine whether those residual RNA granules and electron-dense signals found in glh-1glh-2 knockdown experiments represent P granules or other perinuclear structures, such as mutator foci. It would be important to characterize the localization of P granule components in a glh-1glh-2glh-4 triple null mutant in the future. This will allow scientists to determine whether there are GLH-independent pathways for P granule formation.
The FGG repeats of GLH-1 promote its localization at perinuclear foci
Germ (P) granules are frequently found at the perinuclear region in various animals. The mechanisms by which P granules associate with the nucleus were not clear. A previous study has shown that the FGG repeats of GLH-1 are not sufficient but they can promote the association of PGL-1 with the nuclear envelope when ectopically expressed (Updike et al. 2011). In this study, we found that while the FGG repeats of GLH-1 are not required for the formation of cytoplasmic GLH-1 and PRG-1 granules in the embryo, they are critical for their perinuclear localization in the adult germline. In the GLH-1 FGGΔ mutant, the localization of GLH-1 and PRG-1 to the nuclear periphery are both compromised. In contrast to our findings, a recent study reported that deletion of the FGG repeats of glh-1 has minimal effects on GLH-1 perinuclear localization (Marnik et al. 2019). We note that our FGG deletion removed all the FGG repeats and two zinc finger motifs, while their deletion only removed FGG repeats before those zinc finger motifs, leaving three additional FGG repeats untouched. Considering their evidence that deletion of all four zinc finger motifs has no effect on GLH localization (Marnik et al. 2019), we think the FGG repeats indeed promote GLH-1 perinuclear localization, but such defects can only be observed when all the FGG repeats are removed. Together, our analyses further strengthen the role of FGG repeats in GLH-1’s perinuclear association and are consistent with the model that the FGG repeats in GLH-1 interact with the hydrogels formed by FGG-containing nucleoporins to anchor GLH-1 to the nuclear pores (Figure 4C). Notably, most VASA proteins in other animals do not have FGG repeats, but rather have arginine-glycine-glycine (RGG) repeats. This raises questions as to how RGG-containing VASA proteins localize to perinuclear foci. These arginine residues of the VASA RGG repeats have been reported to be methylated, and the arginine methylation of RGG repeats of the Aub Argonaute is essential for its localization to germ granules in the fruit fly (Kirino et al. 2010a,b; Webster et al. 2015). Therefore, it is possible that various N-terminal repeats in VASA helicases have evolved to use different strategies to promote perinuclear localization of germ granules in animals.
A potential role for the P granule as a site for mRNA surveillance that promotes germ cell development
A previous study has demonstrated that perinuclear P granules are major sites of mRNA export, raising the intriguing hypothesis that P granules may facilitate mRNA surveillance (Sheth et al. 2010). Several observations further support this model. First, consistent with a previous study showing the loss of GLH-2 and PGL-1 granules upon inhibiting RNA polymerase II transcription using α-amanitin (Sheth et al. 2010), we found that injection of α-amanitin into the adult germline leads to loss of GLH-1 granules and a partial loss of PRG-1 granules in wild-type animals. This observation supports a model that GLHs associate with newly exported mRNAs to promote the perinuclear localization of GLHs and other P granule factors. Second, our proteomic analyses showed that the interactions between GLH-1 and Argonautes are stabilized in the GLH-1 DQAD mutant, where RNA release is expected to be inhibited (Xiol et al. 2014), implying that their interactions are strengthened by RNAs and raising the possibility that GLH-1 may facilitate the binding of Argonautes to GLH-bound mRNAs. Intriguingly, a genetic screen for factors required for RNAi inheritance has identified a glh-1 mutant defective in transmitting RNAi over generations, suggesting a link between germline small RNA pathways and GLH-1 (Spracklin et al. 2017). Third, the degree of fertility defects in various GLH mutants correlates with the severity of defects in forming perinuclear PRG-1 granules in the adult germline, but not the defects in forming cytoplasmic PRG-1 granules in embryos, suggesting critical roles for perinuclear localization of P granule factors in promoting germ cell viability. The functional importance of the localization of various RNA-binding proteins at perinuclear P granules in various RNA surveillance pathways will be an important question to investigate in the future.
Acknowledgments
We thank Dr. Jonathan Staley and Dr. Edwin Ferguson for the critical comments on the manuscript, and members of the Lee laboratory for helpful discussions. Some strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD-010440). This work is supported in part by NIH predoctoral training grant T32 GM-07197 to J.S.B., National Science Foundation Research Experiences for Undergraduates grant DBI-1659490 to S.S., the National Natural Science Foundation of China (grants 31771500 and 31922019), the program for Huazhong University of Science and Technology Academic Frontier Youth Team (grant 2018QYTD11) to D.Z., and NIH grants R00-108866 and R01 GM-132457 to H.-C.L. The authors declare that no competing interests exist.
Author contributions: conceptualization and design, H.-C.L. and D.Z.; investigation, W.C., Y, H., C.F.L., J.S.B., S.S., and D.Z.; resources, W.C., Y.H., X.S., K.B., and D.Z.; writing, H.-C.L. and D.Z.; reviewing and editing, H.-C.L., D.Z., W.C., Y.H., J.S.B., and K.B.; funding acquisition, H.-C.L. and D.Z.; and supervision, H.-C.L., E.M., and D.Z.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11786094.
Communicating editor: B. Goldstein
Literature Cited
- Alberti S., Saha S., Woodruff J. B., Franzmann T. M., Wang J. et al. , 2018. A user’s guide for phase separation assays with purified proteins. J. Mol. Biol. 430: 4806–4820. 10.1016/j.jmb.2018.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista P. J., Ruby J. G., Claycomb J. M., Chiang R., Fahlgren N. et al. , 2008. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell 31: 67–78. 10.1016/j.molcel.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C. et al. , 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. TL - 324. Science 324: 1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- Claycomb J. M., Batista P. J., Pang K. M., Gu W., Vasale J. J. et al. , 2009. The Argonaute CSR-1 and its 22g-RNA cofactors are required for holocentric chromosome segregation. Cell 139: 123–134. 10.1016/j.cell.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani M., and Lasko P., 2015. In vivo mapping of the functional regions of the DEAD-box helicase Vasa. Biol. Open 4: 450–462. 10.1242/bio.201410579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., and Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. 10.1038/nmeth.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokshin G. A., Ghanta K. S., Piscopo K. M., and Mello C. C., 2018. Robust genome editing with short single-stranded and long, partially single-stranded DNA donors in Caenorhabditis elegans. Genetics 210: 781–787. 10.1534/genetics.118.301532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy E. M., 1975. Germ plasm and the differentiation of the germ cell line. Int. Rev. Cytol. 43: 229–280. 10.1016/S0074-7696(08)60070-4 [DOI] [PubMed] [Google Scholar]
- Frey S., Richter R. P., and Gorlich D., 2006. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314: 815–817. 10.1126/science.1132516 [DOI] [PubMed] [Google Scholar]
- Gruidl M. E., Smith P. A., Kuznicki K. A., McCrone J. S., Kirchner J. et al. , 1996. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93: 13837–13842. 10.1073/pnas.93.24.13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Shirayama M., Conte D., Vasale J., Batista P. J. et al. , 2009. Distinct Argonaute-mediated 22g-RNA pathways direct genome surveillance in the C. elegans germline. Mol. Cell 36: 231–244. 10.1016/j.molcel.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson E. A., and Wessel G. M., 2010. Vasa genes: emerging roles in the germ line and in multipotent cells. Bioessays 32: 626–637. 10.1002/bies.201000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M., Yonetani M., and Sugimoto A., 2011. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol. 192: 929–937. 10.1083/jcb.201010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondele M., Sachdev R., Heinrich S., Wang J., Vallotton P. et al. , 2019. DEAD-box ATPases are global regulators of phase-separated organelles. Nature 573: 144–148. 10.1038/s41586-019-1502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki I., Amiri A., Fan Y., Meyer N., Dunkelbarger S. et al. , 2004. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167: 645–661. 10.1534/genetics.103.023093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ishidate T., Ghanta K. S., Seth M., Conte D. et al. , 2014. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 197: 1069–1080. 10.1534/genetics.114.166389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y., Vourekas A., Kim N., de Lima Alves F., Rappsilber J. et al. , 2010a Arginine methylation of vasa protein is conserved across phyla. J. Biol. Chem. 285: 8148–8154. 10.1074/jbc.M109.089821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y., Vourekas A., Sayed N., de Lima Alves F., Thomson T. et al. , 2010b Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. RNA 16: 70–78. 10.1261/rna.1869710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S., Kawasaki I., and Shim Y.-H., 2013. PAB-1, a Caenorhabditis elegans poly(A)-binding protein, regulates mRNA metabolism in germline by interacting with CGH-1 and CAR-1. PLoS One 8: e84798 10.1371/journal.pone.0084798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki K. A., Smith P. A., Leung-Chiu W. M., Estevez A. O., Scott H. C. et al. , 2000. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development 127: 2907–2916. [DOI] [PubMed] [Google Scholar]
- Marnik E. A., Fuqua J. H., Sharp C. S., Rochester J. D., Xu E. L. et al. , 2019. Germline maintenance through the multifaceted activities of GLH/vasa in Caenorhabditis elegans P granules. Genetics 213: 923–939. 10.1534/genetics.119.302670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchy A. N., Stempor P., Gaarenstroom T., Wysolmerski B., Dong Y. et al. , 2017. A team of heterochromatin factors collaborates with small RNA pathways to combat repetitive elements and germline stress. Elife 6: e21666. . 10.7554/eLife.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler C. F., Hondele M., Heinrich S., Sachdev R., Vallotton P. et al. , 2016. ATPase activity of the DEAD-box protein Dhh1 controls processing body formation. Elife 5: e18746. 10.7554/eLife.18746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsborn A. M., Li W., McEwen T. J., Mizuno T., Kuzmin E. et al. , 2007. GLH-1, the C. elegans P granule protein, is controlled by the JNK KGB-1 and by the COP9 subunit CSN-5. Development 134: 3383–3392. 10.1242/dev.005181 [DOI] [PubMed] [Google Scholar]
- Phillips C. M., Montgomery T. A., Breen P. C., and Ruvkun G., 2012. MUT-16 promotes formation of perinuclear Mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 26: 1433–1444. 10.1101/gad.193904.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. N., Schisa J. A., and Priess J. R., 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219: 315–333. 10.1006/dbio.2000.9607 [DOI] [PubMed] [Google Scholar]
- Putnam A. A., and Jankowsky E., 2013. DEAD-box helicases as integrators of RNA, nucleotide and protein binding. Biochim. Biophys. Acta 1829: 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussell D. L., and Bennett K. L., 1993. glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc. Natl. Acad. Sci. USA 90: 9300–9304. 10.1073/pnas.90.20.9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Weber C. A., Nousch M., Adame-Arana O., Hoege C. et al. , 2016. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166: 1572–1584.e16. 10.1016/j.cell.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J. A., Pitt J. N., and Priess J. R., 2001. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Sengoku T., Nureki O., Nakamura A., Kobayashi S., and Yokoyama S., 2006. Structural basis for RNA unwinding by the DEAD-box protein Drosophila vasa. Cell 125: 287–300. 10.1016/j.cell.2006.01.054 [DOI] [PubMed] [Google Scholar]
- Seydoux G., 2018. The P granules of C. elegans: a genetic model for the study of RNA–protein condensates. J. Mol. Biol. 430: 4702–4710. 10.1016/j.jmb.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Pitt J., Dennis S., and Priess J. R., 2010. Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development 137: 1305–1314. 10.1242/dev.044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Calidas D., Schmidt H., Lu T., Rasoloson D. et al. , 2016. Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3. Elife 5: e21337. 10.7554/eLife.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike C., Meyer N., Racen E., Orsborn A., Kirchner J. et al. , 2008. Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins. Genetics 178: 1973–1987. 10.1534/genetics.107.083469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spracklin G., Fields B., Wan G., Becker D., Wallig A. et al. , 2017. The RNAi inheritance machinery of Caenorhabditis elegans. Genetics 206: 1403–1416. 10.1534/genetics.116.198812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., and Schwer B., 2006. Mutations in PRP43 that uncouple RNA-dependent NTPase activity and pre-mRNA splicing function †. Biochemistry 45: 6510–6521. 10.1021/bi052656g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen B., Karow A. R., Kohler J., Gubaev A., and Klostermeier D., 2008. Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proc. Natl. Acad. Sci. USA 105: 548–553. 10.1073/pnas.0705488105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., and Fire A., 1998. Specific interference by ingested dsRNA. Nature 395: 854 10.1038/27579 [DOI] [PubMed] [Google Scholar]
- Updike D., and Strome S., 2010. P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31: 53–60. 10.2164/jandrol.109.008292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., and Strome S., 2009. A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans. Genetics 183: 1397–1419. 10.1534/genetics.109.110171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., Hachey S. J., Kreher J., and Strome S., 2011. P granules extend the nuclear pore complex environment in the C. elegans germ line. J. Cell Biol. 192: 939–948. 10.1083/jcb.201010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., Knutson A. K., Egelhofer T. A., Campbell A. C., and Strome S., 2014. Germ-granule components prevent somatic development in the C. elegans germline. Curr. Biol. 24: 970–975. 10.1016/j.cub.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E., and Seydoux G., 2010. The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules. Development 137: 1441–1450. 10.1242/dev.047654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Smith J., Chen B.-C., Schmidt H., Rasoloson D. et al. , 2014. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife 3: e04591 10.7554/eLife.04591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Li S., Hur J. K., Perkins E. M., Patel D. J. et al. , 2015. Aub and Ago3 are recruited to nuage through two mechanisms to form a ping-pong complex assembled by Krimper. Mol. Cell 59: 564–575. 10.1016/j.molcel.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiol J., Spinelli P., Laussmann M. A., Homolka D., Yang Z. et al. , 2014. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell 157: 1698–1711. 10.1016/j.cell.2014.05.018 [DOI] [PubMed] [Google Scholar]
- Zhang D., and Glotzer M., 2015. The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. Elife 4 10.7554/eLife.08898 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Movie S1–S6 contains supplemental movies. File S1 contains the information of glh-1 and glh-4 alleles created in this study. File S2 and File S3 contain the lists of peptides identified in proteomic analyses of GLH-1 and PRG-1 complexes. Table S1 and Table S2 contain the strains and oligonucleotides used in this study. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11786094.