Mesbahi et al. find that environmental factors, including diet, starvation, and population density can differentially influence the penetrance of collagen mutant phenotypes. Factors that decrease the penetrance of rolling in dominant...
Keywords: Caenorhabditis elegans, collagen, cuticle, density, diet, elt-3, gene–environment, rol-6, sqt-3, tbx-2
Abstract
The nematode Caenorhabditis elegans is protected from the environment by the cuticle, an extracellular collagen-based matrix that encloses the animal. Over 170 cuticular collagens are predicted in the C. elegans genome, but the role of each individual collagen is unclear. Stage-specific specialization of the cuticle explains the need for some collagens; however, the large number of collagens suggests that specialization of the cuticle may also occur in response to other environmental triggers. Missense mutations in many collagen genes can disrupt cuticle morphology, producing a helically twisted body causing the animal to move in a stereotypical pattern described as rolling. We find that environmental factors, including diet, early developmental arrest, and population density can differentially influence the penetrance of rolling in these mutants. These effects are in part due to changes in collagen gene expression that are mediated by the GATA family transcription factor ELT-3. We propose a model by which ELT-3 regulates collagen gene expression in response to environmental stimuli to promote the assembly of a cuticle specialized to a given environment.
THE ability to respond to environmental cues is required for animals to survive in changing environments and allows an animal flexibility in the environments it inhabits. In the wild, Caenorhabditis elegans is exposed to frequent changes in environment and has developed strategies to cope with pathogens, temperature, osmolarity, and intermittent availability of oxygen or food. For C. elegans, the first barrier against environmental insults is the cuticle, which acts as a physical barrier to protect the animal from pathogens, desiccation, and other stresses (Cassada and Russell 1975; Gravato-Nobre et al. 2016; Xiong et al. 2017). The cuticle is a multilayered, flexible, collagen-rich exoskeleton synthesized by underlying hypodermal cells (Cox et al. 1981). During development, it is shed at each molt to allow for growth and is resynthesized five times during the life of the animal. Although not all larval stages (referred to as L) have been examined in detail, L1, L4, and adult cuticles differ in morphology and collagen composition (Cox et al. 1981; Cox and Hirsh 1985). In the dauer state, an alternate larval stage specialized for long-term survival and resistance to desiccation, the cuticle is thicker and structurally distinct from cuticles at all other stages (Popham and Webster 1978; Cox et al. 1981; Peixoto and De Souza 1994). Stage-specific cuticles are generated through transcriptional regulation of collagen genes. This regulation ensures that the appropriate collagens are expressed at the correct time and allows specialization of the cuticle for each larval stage (Cox et al. 1981; Cox and Hirsh 1985; Jackson et al. 2014; Teuscher et al. 2019).
Modulation of collagen expression may also be used to promote resistance to specific stresses. In fact, the expression of several collagens is modulated in response to exposure to different bacterial and fungal species (Coolon et al. 2009; Engelmann et al. 2011), and environmental stresses, such as high-salt conditions (Dodd et al. 2018). The regulation of collagen gene expression may be required to produce changes to the cuticle that promote increased survival in specific environments. Consistent with this idea, interventions that extend life span and reduce insulin-like signaling influence the expression of many collagen genes, suggesting that the ability to remodel the cuticle may contribute to life span extension (Ewald et al. 2015).
The C. elegans genome contains 181 predicted collagen genes, 173 of which are considered cuticular collagens (Teuscher et al. 2019). C. elegans collagens have been broadly classified into five groups based on the position of conserved cysteines (Johnstone 1994; Page and Johnstone 2007). Nematode collagens are most similar to fibril-associated collagens with interrupted triple helices, but differ from vertebrate collagens in that they are generally smaller and less complex. These collagens assemble into trimeric collagen fibrils that are assembled before secretion. Collagen proteins are characterized by several conserved cysteine residues and by the presence of Gly-X-Y domains, where X and Y are enriched for proline and hydroxyproline. The Gly-X-Y domains are required for the formation of triple-helical structures, while the cysteines form intermolecular disulfide bridges. Both homo- and heterotrimeric complexes have been described for vertebrate collagens, and C. elegans collagens are likely to be assembled in a similar manner.
Mutations in 21 cuticular collagen genes have been isolated from unbiased genetic screens (Cox et al. 1980; Page and Johnstone 2007). These mutations result in changes in body size and morphology, producing dumpy (Dpy) or long (Lon) animals, blistered animals, and rolling animals. The rolling movement observed in these mutants is the result of a twisted cuticle that causes the animal to rotate around its long axis. Mutations in several collagens, including sqt-1, dpy-17, lon-3, rol-6, dpy-5, and bli-1, produce observable phenotypes, and different mutations in the same gene can produce distinct phenotypes. For example, different mutations in sqt-1 can produce Dpy, Lon, or rolling animals (Kusch and Edgar 1986). Mutations that result in rolling are often dominant mutations, whereas null alleles of these genes do not produce rollers, suggesting that the rolling phenotype results from the incorporation of aberrant collagen molecules into the cuticle. For example, while dominant mutations in rol-6 produce rollers, null alleles are superficially wild type (Kramer et al. 1990).
Mutations in rol-6, sqt-3, dpy-10, and sqt-1 that produce a rolling phenotype have been isolated. These mutants are classified as right or left rollers depending on the direction of the roll (Cox et al. 1980). Mutations in sqt-3 and dpy-10 produce left rollers, whereas rol-6 and sqt-1 mutations produce right rollers (Cox et al. 1980). Intriguingly, rolling in these mutants can be suppressed by loss or knockdown of other collagens (Kramer et al. 1990; Nyström et al. 2002). For instance, rolling in dominant rol-6 mutants can be suppressed by loss of sqt-3 or sqt-1 (Kramer et al. 1990; Kramer and Johnson 1993). Similarly, rolling in sqt-3 mutants can be suppressed by knockdown of rol-6 (Cai et al. 2011). One proposed explanation for these findings is that these collagens form heteromeric complexes or a common substructure and reducing levels of specific collagens may reduce the incorporation of aberrant collagen proteins in the cuticle (Johnstone 1994). This model is consistent with the finding that the expression of collagen genes capable of mutating to produce roller phenotypes is highly coordinated, with their expression occurring in a short window before the molt (Hendriks et al. 2014).
The dominant rol-6 (su1006) allele is commonly used as a co-injection marker when generating transgenic animals because it produces a readily observable phenotype with little toxicity. The penetrance of rolling in these strains is often incomplete, is temperature sensitive, and differs between strains. Further, we observed that the penetrance within a strain is not robust but is sensitive to growth conditions. Based on these observations, we hypothesized that the cuticle is altered in response to environmental stimuli. Indeed, we find that the penetrance of rolling in mutants of the collagens rol-6 and sqt-3 is differentially affected by bacterial diet, metabolic disruption, starvation, and population density. We identify collagens whose expression is modulated in response to these environmental factors, and identify the GATA family transcription factor (TF) ELT-3 as a regulator of these genes. The effect of environmental factors on the penetrance of rolling is reversed in elt-3 mutants, consistent with elt-3 playing a critical role in environmental-response pathways that ultimately function to tailor the cuticle to specific environments.
Materials and Methods
C. elegans propagation
C. elegans were propagated by standard methods (Stiernagle 2006). Soy peptone was used in NGM. The following strains used were obtained from the Caenorhabditis Genetics Center: NR222 rde-1(ne219); kzIs9 [pKK1260(lin-26p::nls::GFP) + pKK1253(lin-26p::rde-1) + pRF6(rol-6(su1006)], VP303 rde-1(ne219); kbIs7[nhx-2p::rde-1 + rol-6(su1006)](Espelt et al. 2005), FK181 ksIs2[pdaf-7::GFP + rol-6(su1006)] (Murakami et al. 2001), MS438 irIs25[elt-2::NLS::GFP::lacZ + rol-6(su1006)], CB187 rol-6(e187) (Kramer and Johnson 1993), BE22 rol-1(sc22) (Cox et al. 1980), BE148 rol-9(sc148) (Bergmann et al. 1998), and BE8 sqt-3(sc8), elt-3(gk121) (C. elegans Deletion Mutant Consortium 2012). LMN40 was generated by outcrossing VP303 to N2 to remove the rde-1(ne219) mutation. elt-3(gk121) was crossed to ERT60 to generate elt-3 mutants with the rol-6(su1006) transgene.
Scoring rolling animals
Rolling was scored in adult animals for all strains. Plates were tapped to promote movement and animals rolling in a circle or rolling on their longitudinal axis were counted as rollers. Three replicate plates were scored for each condition. For most assays, 50–100 eggs were added to each prepared plate and animals were scored as adults. Density experiments were plated as indicated in Figure 1 and Figure 2. NR222 animals were scored at 25° because they did not produce appreciable numbers of rollers at 20°; all other animals were scored and maintained at 20°. Significance of rolling effects was measured with one-way ANOVA and Dunnett’s multiple comparison test in GraphPad Prism.
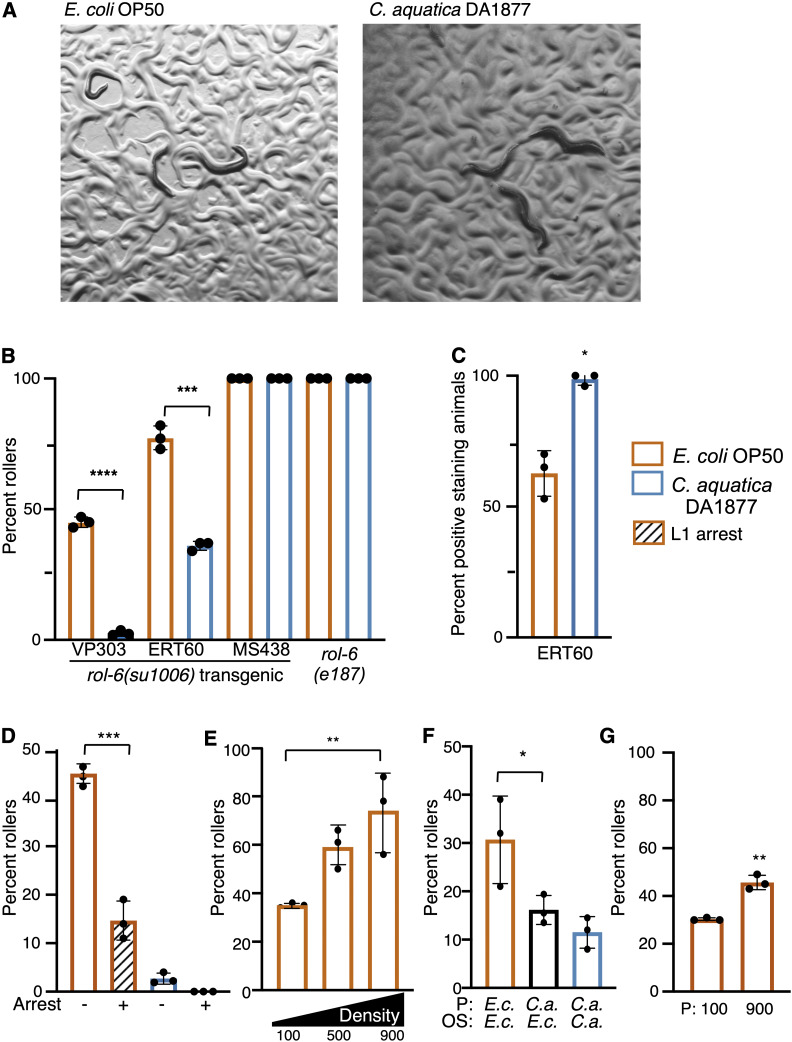
Figure 1.
Environment influences rolling in animals carrying rol-6 mutations. (A) rol-6(su1006)T (VP303) animals exposed to E. coli OP50 and C. aquatica DA1877. (B) Percent of population rolling when grown on C. aquatica or E. coli OP50 in three transgenic rol-6(su1006)T lines and in the mutant rol-6(e187). (C) Hoechst staining of rol-6(su1006)T animals (ERT60) exposed to E. coli or C. aquatica. (D) Percent of VP303 adults rolling following L1 arrest (+) or developed from eggs (−). (E) Percent of VP303 population rolling when developed at indicated densities. Density indicated is number of animals per 60 mm dish. (F) Maternal exposure to C. aquatica reduces rolling in the next generation. Offspring (OS) from E. coli (E.c.) or C. aquatica (C.a.) fed parents (P) were scored (VP303). (G) Intergenerational effects of population density in rol-6(su1006)T animals (VP303). Parental generation (P) was grown at high (900/60mm) or low density (100/60mm). Assays were done in triplicate; each dot represents a population of 50 animals. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison test for B, D and F; one-way ANOVA with Tukey’s multiple comparison test for E; t-test for C and G. Asterisks indicate P-values (* P <0.05, ** P <0.005, *** P <0.0005, **** P <0.00005).
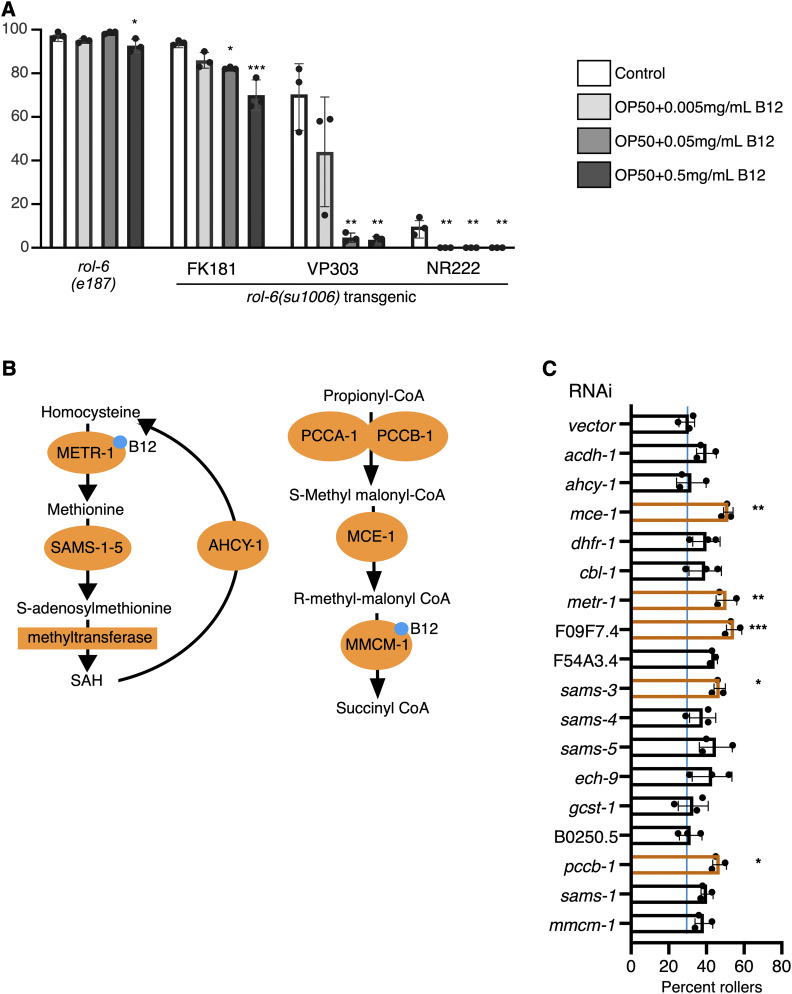
Figure 2.
Metabolic disruption in the hypodermis influences rolling. (A) Response of indicated animals to increasing concentrations of vitamin B12. Vitamin B12 reduced rolling in rol-6(su1006)T animals. Significance refers to comparisons to controls of the same strain. (B) B12-dependent metabolic pathways; enzymes that use vitamin B12 (blue dot) as a cofactor are indicated. (C) RNAi-mediated knockdown of metabolic enzymes in B12-dependent pathways. RNAi was induced by feeding in a single generation (NR222 at 25°). Percent rollers in a population is shown; each dot represents a population of >50 animals. Bars represent mean ± SD. Statistical analysis by one-way ANOVA with Tukey’s multiple comparison test. Asterisks indicate P values (* P <0.05, ** P <0.005, *** P <0.0005).
Hoechst staining
Hoechst staining was performed as described previously (Moribe et al. 2004). Briefly, adult animals were collected in M9 buffer and washed three times with M9 to remove bacteria before addition of 1 μg/ml Hoechst 33258 (Sigma, St. Louis, MO). Animals were placed on a rocker and incubated at room temperature for 20 min. Following incubation, animals were washed twice with M9 buffer, treated with 1 mM levamisole, and examined on a Nikon Eclipse II microscope.
L1 arrest and starvation
Eggs were collected by hypochlorite bleaching, pelleted by centrifugation, and washed three times in M9 buffer. Collected eggs were incubated in M9 buffer for 24 hr with rocking to allow for hatching, synchronization, and starvation. Then, 50–100 L1s were added to prepared plates. Animals were scored in the adult stage.
Density
Eggs were plated at indicated density from a single pool of eggs. Additional Escherichia coli OP50 was added daily to ensure worms did not starve. An overnight culture of E. coli OP50 was pelleted by centrifugation and 1 ml of the concentrated bacterial suspension was added to each plate. For plates with >200 worms, a quarter of each plate was counted.
nCounter measurements
N2 eggs were prepared by hypochlorite treatment, plates were seeded, and animals developed at 20°. Animals were collected when the population was a mix of late L4 and early adult animals, washed three times in M9 and frozen at −80° in TRIzol (Sigma) before RNA preparation. RNA was extracted according to manufacturer’s protocol and further purified using New England Biolabs RNA columns. nCounter probes (NanoString Technologies) were designed by NanoString, synthesized by Integrated DNA Technologies and assays were performed by Mobix, McMaster University, Hamilton, Ontario, Canada. nSolver software was used for analysis. The geometric mean of positive control probes was used for in-lanes normalization. Counts were normalized across samples using the geometric mean of pmp-3, Y45F10D.4, and cdc-42 counts (Hoogewijs et al. 2008). Statistically significant changes were identified by t-test using nSolver software. Probes used are listed in Supplemental Material, Table S3. Additional probes that were used but not reported were excluded because counts were close to, or below, negative threshold levels in multiple samples (as determined by negative probe counts).
Vitamin B12 treatment
Methylcobalamin (vitamin B12) (Sigma) was diluted in water to 5 mg/ml, filter sterilized, and stored at −20° until use. Stocks were diluted in sterile water and 40 μl of diluted B12 solution was added to the surface of a 60 mm plate after growth of the E. coli OP50 lawn at doses indicated in Figure 1 and allowed to dry. Worms were added to plates immediately.
RNA interference
The mmcm-1 clone was obtained from the ORFeome RNAi library (Rual et al. 2004). RNA interference (RNAi) clones for dpy-13, lon-3, and sqt-1 were generated by PCR, inserted into L4440, and transformed into E. coli HT115. Small, unique regions were selected to reduce off-target effects (Table S3). All other RNAi clones were obtained from the Ahringer RNAi library (Kamath et al. 2003). RNAi clones were inoculated into LB supplemented with 60 micrograms/ml ampicillin. We used 50 μl of overnight culture to inoculate 3 ml of LB supplemented with ampicillin and grown for 6 hr at 37° before being used to seed RNAi plates. RNAi plates were made by adding 1mM IPTG and 25 μg/ml carbenicillin to NGM made with soy peptone. RNAi cultures were seeded and grown overnight at 37°. Positive acting clones were sequenced to verify their identity. Three replicates were counted for each knockdown.
Data availability
Strains and plasmids are available upon request. The authors confirm that all data necessary for confirming the conclusions presented are within the article, figures, and tables. Figure S1 contains a summary of chromatin immunoprecipitation sequencing (ChIP-seq) data for ELT-3 binding to collagen gene intergenic regions. Table S1 contains a summary of previously published collagen gene expression in response to bacterial and fungal exposures. Table S2 contains a list of TFs that are hypodermally enriched and were knocked down by RNAi. Table S3 contains NanoString probes used and RNAi target sequences. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11946360
Results
Environment influences the penetrance of the rolling phenotype
To examine environmental influence on rolling, we first tested the effect of diet on the penetrance of rolling in strains carrying a dominant rol-6(su1006) transgene [hereafter referred to as rol-6(su1006)T]. When rol-6(su1006)T strains were fed a diet of Comamonas aquatica DA1877, the penetrance of rolling was dramatically decreased compared to animals fed E. coli OP50 (Figure 1, A and B). This suppression was observed in several integrated transgenic strains with incomplete penetrance of the rolling phenotype, but not in strains with complete, or nearly complete, penetrance, including the MS438 {irIs25 [elt-2::NLS::GFP::lacZ + rol-6(su1006)]} transgenic strain and the endogenous mutant allele, rol-6(e178) (Figure 1B). To further examine the effects of C. aquatica on the cuticle, we measured cuticle permeability by staining animals with Hoechst dye 33258. Compared to animals fed E. coli OP50, exposure to C. aquatica increased permeability of the dye, resulting in a larger number of positive staining animals (Figure 1C). Nuclear staining in rol-6(su1006)T animals was predominantly in the head and tail and generally produced weakly staining nuclei. Collectively, these data suggest that C. aquatica exposure alters the cuticle in rol-6(su1006)T animals.
To extend these findings, we examined the influence of two common environmental variables, starvation and population density, on the penetrance of rolling. To test the effect of starvation on rolling, we allowed L1 rol-6(su1006)T animals to undergo starvation-induced arrest and examined rolling in the adult. Relative to animals that had not undergone arrest, the penetrance of rolling was reduced in animals that had previously undergone L1 arrest (Figure 1D). Next, we tested the effects of increased population density on rolling. Although starvation and high population density both represent unfavorable conditions for the worm (both promote dauer formation), and both could be viewed as conditions signaling limited resources, high population density produced the opposite effect on rolling as the L1 arrest. When population density was high (900 animals per 60-mm dish), the incidence of rolling was approximately twofold higher than that of low-density populations (100 animals per 60-mm dish) (Figure 1E). Together, these observations suggest that many different environmental cues stimulate changes to the cuticle and that the integration of these cues may allow the animal to adapt to different environmental challenges.
Environmental factors induce intergenerational effects
We asked whether environmental effects could be carried over to subsequent generations by testing whether parental exposure to C. aquatica could suppress rolling in the next generation. Eggs were collected by hypochlorite bleaching from animals grown on C. aquatica or E. coli OP50, and offspring were transferred to E. coli OP50. As adults, the offspring of C. aquatica fed parents displayed reduced rolling relative to offspring of E. coli OP50-fed parents (Figure 1F). Similarly, we tested the effects of population density on rolling in the next generation. Eggs were collected from either high-density (900 animals per 60-mm dish) or low-density (100 animals per 60-mm dish) populations and rolling was examined when the offspring became adults. Similar to what we observed with diet, parental population density influenced the penetrance of rolling, with more densely growing populations producing increased penetrance of rolling in the next generation (Figure 1G).
Metabolic disruption influences rolling penetrance
C. aquatica and E. coli OP50 differ in the ability to produce vitamin B12; C. aquatica produces vitamin B12, while E. coli does not (Watson et al. 2014). To determine whether vitamin B12 was responsible for the suppression of rolling in animals fed C. aquatica, we added B12 to the E. coli OP50 diet and measured rolling. Addition of vitamin B12 produced a dose-dependent decrease in rolling in rol-6(su1006)T strains with moderate- to low-penetrance rolling (VP303, NR222, and FK181) (Figure 2A). However, similar to what was observed with diet, rolling in rol-6(e187) mutants was not suppressed by vitamin B12.
Vitamin B12 acts as a cofactor for two enzymes, 5-methyltetrahydrofolate-homocysteine methyltransferase (METR-1), functioning in 1-carbon metabolism, and methyl malonyl CoA mutase (MMCM-1), which converts methylmalonyl CoA to succinyl CoA (Figure 2B). To determine which activity was required for the observed suppression of rolling, we knocked down enzymes in both pathways and measured the effect on rolling behavior. Knockdown of metr-1 produced the most dramatic effects; however, knockdown of enzymes in both pathways increased the penetrance of rolling in rol-6(su1006)T animals (Figure 2C), consistent with the finding that addition of vitamin B12 reduces rolling. These findings demonstrate that metabolic imbalances also have the potential to promote cuticle remodeling.
Environmental effects on rolling penetrance are collagen specific
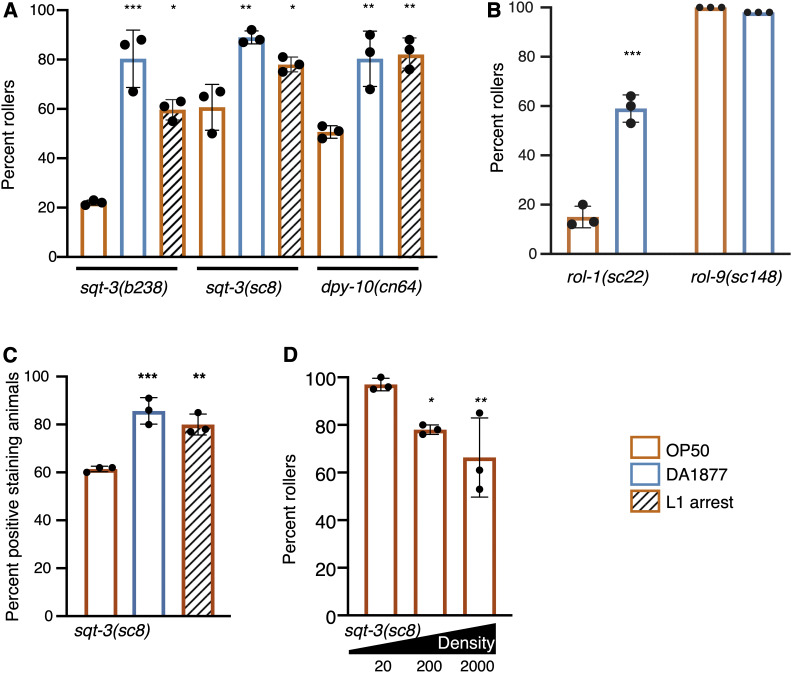
Conceptually, suppression of rolling in rol-6(su1006)T animals could be mediated in two ways, through changes in gene expression that reduce the contribution of ROL-6 to the cuticle or by generating a robust cuticle more tolerant of the aberrant ROL-6 protein. If the latter were true, conditions that suppress rolling in rol-6(su1006)T animals might also suppress rolling resulting from mutation in other collagen genes. To test this hypothesis, we exposed animals carrying rolling-inducing alleles of sqt-3, dpy-10, rol-1, and rol-9 to conditions that suppress rolling in rol-6(su1006)T animals. Similar to what we observed with the rol-6(e187) mutants, rolling was not influenced by diet in the highly penetrant rol-9(sc148) mutants. In contrast to what we observed with rol-6(su1006)T, exposure to C. aquatica dramatically increased rolling in sqt-3(be3), sqt-3(b238), dpy-10(cn64), and rol-1(sc2) mutants (Figure 3, A and B). Similarly, L1 arrest increased the penetrance of rolling in adult sqt-3(sc8), sqt-3(b238), and dpy-10(cn64) mutants (Figure 3A). All together, we noted a dichotomy between rol-6(su1006)T animals and other collagen mutants: conditions that reduced rolling in rol-6(su1006)T animals increased rolling in the other mutants. Although diet and arrest had opposing effects in sqt-3 and rol-6 mutants, sqt-3(sc8) mutant animals, like rol-6(su1006)T, displayed increased Hoechst staining when exposed to C. aquatica (Figure 3C). These data suggest that exposure to a C. aquatica diet or L1 arrest does not produce a more robust cuticle resistant to aberrant collagen proteins, but rather causes a change in the composition or structure of the cuticle that differentially affects sqt-3 and rol-6 mutants.
Figure 3.
Environmental conditions affect the penetrance of rolling in many different mutants. (A) Rolling in increased in sqt-3 and dpy-10 mutant adults that previously underwent L1 arrest or are fed C. aquatica (DA1877). (B) Rolling in increased in response to C. aquatica in rol-1, but not rol-9(sc148) mutants that exhibit complete penetrance independent of diet. (C) Percent sqt-3(sc8) animals with Hoechst-stained nuclei. (D) Rolling is decreased as population density increases in sqt-3(sc8) mutants. Numbers indicate size of population in a 60-mm dish. Assays were done in triplicate; each dot represents a population of 50 animals. Bars represent mean ± SD. Statistical analysis by one-way ANOVA with Tukey’s multiple comparison test for A, C, and D; t-test for B. Asterisks indicate P values (* P <0.05, ** P <0.005, *** P <0.0005).
Given the differential effects on L1 arrest and diet on rolling in rol-6(su1006)T and sqt-3(sc8) animals, we tested the effects of population density on rolling in sqt-3(sc8) mutants. We asked whether density would affect sqt-3(sc8) in the same way it affected rol-6(su1006)T animals or whether the effect on penetrance would again be reversed. Similar to the other environmental exposures, high population density produced the opposite effect in sqt-3(sc8) mutants as in the rol-6(su1006)T animals (Figure 3D). These data are consistent with the idea that in response to environment, C. elegans alters its cuticle, relying more heavily on different collagens under different environmental conditions. For example, the post-L1-arrest adult cuticle may have a decreased dependence on ROL-6 and an increased dependence on SQT-3 and DPY-10, explaining why arrest decreases rolling in rol-6 mutants but increases rolling in sqt-3 and dpy-10 mutants.
Collagen gene expression is regulated in response to environment
The finding that three very different environmental conditions produce opposing effects on rolling in sqt-3 and rol-6 mutants may indicate that a central regulatory mechanism dictates these effects in all three environments. One possible mechanism of this regulation is transcriptional regulation of collagen gene expression. We used available gene expression data to examine collagen gene expression in response to different bacterial diets or exposure to bacterial and fungal pathogens (Coolon et al. 2009; Engelmann et al. 2011; MacNeil et al. 2013). Collagen gene expression was altered in response to bacterial exposure and in fact, of 173 collagens, only 23 were not affected by any of these bacterial or fungal exposures. From this set, we noted that both rol-6 and sqt-3 are regulated in response to specific bacterial exposures (Table S1). With this idea in mind, we selected a subset of collagens to measure gene expression in response to a diet of C. aquatica and to L1 arrest. We included a subset that were responsive to diet, some that have previously reported genetic interactions with sqt-3 or rol-6 and others, including col-141 and col-142, for which regulatory mechanisms had been described.
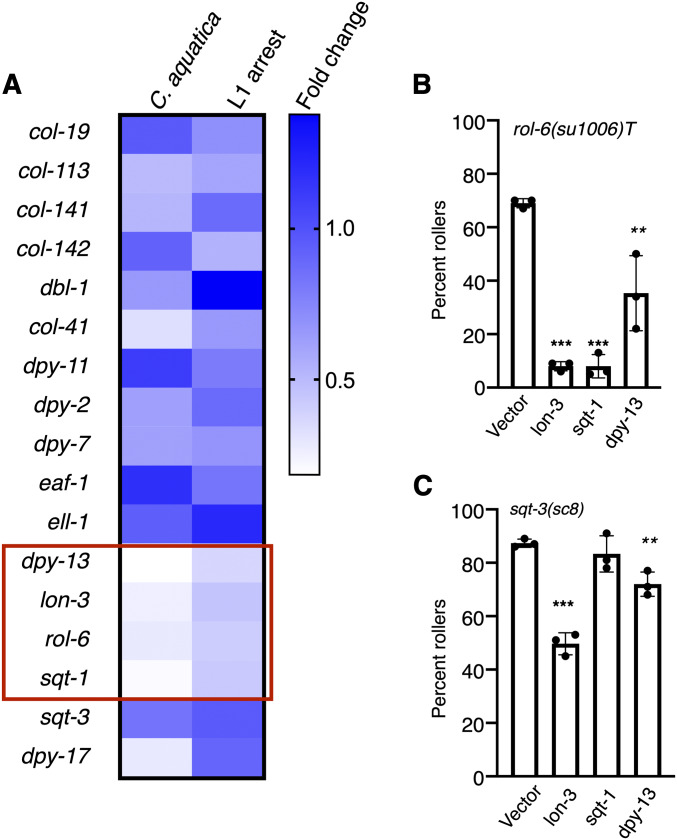
Using nCounter assays, we measured messenger RNA expression of collagens, and known regulators of collagen expression in wild-type adults exposed to C. aquatica or L1 arrest and compared these to control animals fed E. coli OP50 (Figure 4A). Because exposure to C. aquatica and L1 arrest had similar effects in all strains tested, we focused on collagens whose expression changed, relative to animals fed E. coli OP50, in response to both conditions. Strikingly, expression of dpy-13, lon-3, rol-6, and sqt-1 was decreased in both conditions relative to control animals grown on E. coli OP50 (Figure 4A). Suppression of rolling in rol-6(su1006)T animals could be explained by downregulation of rol-6, which would also decrease expression of the transgene. Further, rolling in rol-6 neomorphic alleles is suppressed by a lon-3 null allele and by knockdown of sqt-1 or dpy-13 (Nyström et al. 2002; Cai et al. 2011). We validated this finding in the rol-6(su1006)T animals, and found that, indeed, knockdown of lon-3, sqt-1, or dpy-13 suppressed rolling (Figure 4B). Decreased expression of these genes in response to C. aquatica or L1 arrest could explain the decreased penetrance of rolling observed in rol-6(su1006)T animals. However, knockdown of lon-3 and dpy-13 also reduces rolling in sqt-3(sc8) mutants (Figure 4C), and therefore do not explain the enhancement of rolling in sqt-3 mutants. Our analysis of collagen gene expression was not exhaustive, and increased or decreased expression of another collagen that exacerbates rolling in sqt-3(sc8) animals, but not rol-6 (su1006)T animals, may explain the differential effects observed in these mutants.
Figure 4.
Environment influences expression of cuticular collagens. Expression of collagens and regulators was measured in wild-type animals (N2) using nCounter technology. Of the collagen genes tested, only dpy-13, lon-3, rol-6, and sqt-1 (red box) produced statistically significant different changes in gene expression of ≥1.5-fold (P < 0.05) in animals fed C. aquatica and in animals having experienced L1 arrest, relative to nonarrested animals fed E. coli OP50. Relative expression is shown, and counts were normalized to expression in animals fed E. coli OP50. (B) Knockdown of several collagen genes by feeding decreases rolling in rol-6(su1006)T animals (ERT60) and sqt-3(sc8) mutants. RNAi knockdown by feeding was used in a single generation. Each dot represents a population of ∼50 animals. Statistical analysis by one-way ANOVA with Tukey’s multiple comparison test. Asterisks indicate P values (** P <0.005, **** P <0.0005).
ELT-3 regulates environmentally induced changes in collagen gene expression
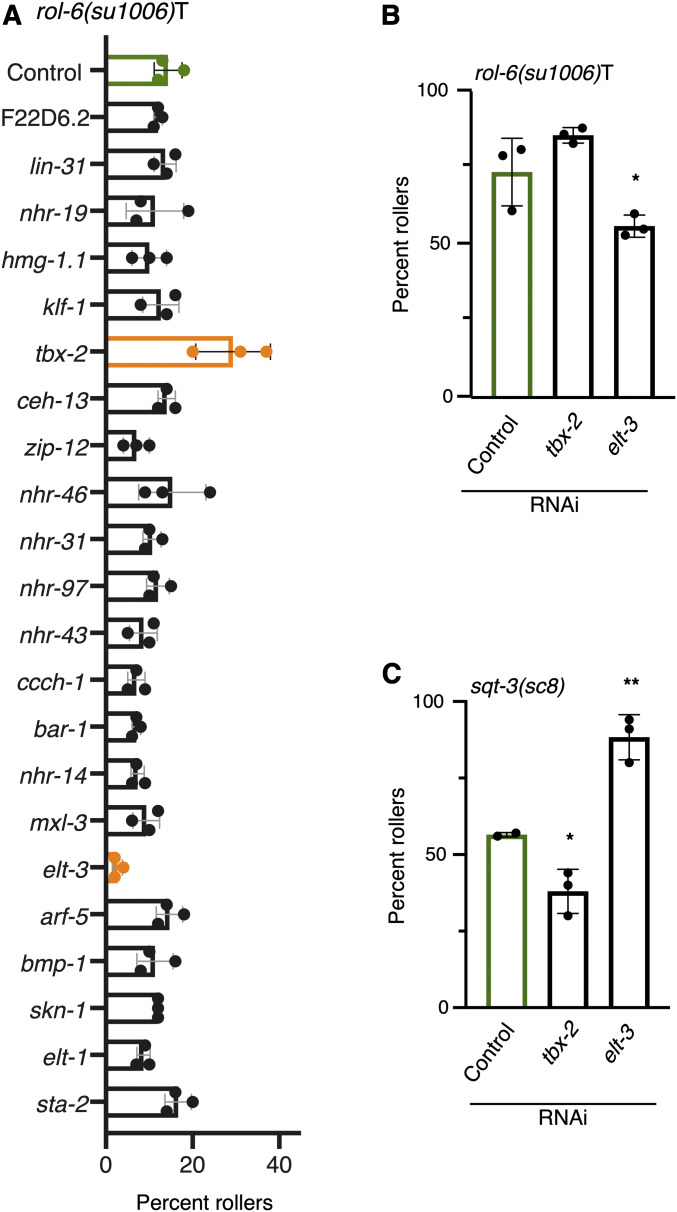
A decrease in the expression of dpy-13, lon-3, sqt-1, and rol-6 in response to two different conditions, L1 arrest or exposure to C. aquatica, suggests that these collagens may be targets of the same TF. Because conditions that increase rolling in sqt-3(sc8) mutants decrease rolling in rol-6(su1006)T, and vice versa, we searched for a TF whose loss would have opposing effects on rolling in these two genetic backgrounds. Using a cell-type-specific data set to identify TFs enriched in the hypodermis (Kaletsky et al. 2018) (Table S2), we knocked down each TF for which an RNAi clone was available and examined the penetrance of rolling in rol-6(su1006)T animals (Figure 5A). Two knockdowns produced significant changes in rolling: elt-3, which reduced rolling, and tbx-2, which increased rolling (Figure 5A). We tested both knockdowns with a second rol-6(su1006)T strain (ERT60) and observed similar results (Figure 5B). To further extend our findings, we tested the effects of knocking down these two TFs on rolling in sqt-3(sc8) mutants. Knocking down these two genes produced opposite effects in sqt-3(sc8) and rol-6(su1006)T backgrounds, similar to what we observed with diet, density, and L1 arrest; tbx-2 knockdown decreased rolling, whereas elt-3 knockdown increased rolling (Figure 5C).
Figure 5.
Identification of transcription factors mediating effects on rolling behavior. (A) RNAi mediated knockdown of individual transcription factors in rol-6(su1006)T animals. Significant differences (P <0.05) in rolling relative to control are shown in orange. Knockdown of tbx-2 and elt-3 significantly increased and decreased (respectively) rolling in LMN40 rol-6(su1006)T animals. (B) Knockdown of tbx-2 and elt-3 also significantly increased, and decreased (respectively) rolling in ERT60 rol-6(su1006)T animals. (C) Knockdown of tbx-2 and elt-3 produce the opposite effect on rolling in sqt-3(sc8) mutants than in rol-6(su1006)T animals. Statistical analysis with one-way ANOVA and Dunnett’s multiple comparison test. Asterisks indicate P-values (* P <0.05, ** P <0.005).
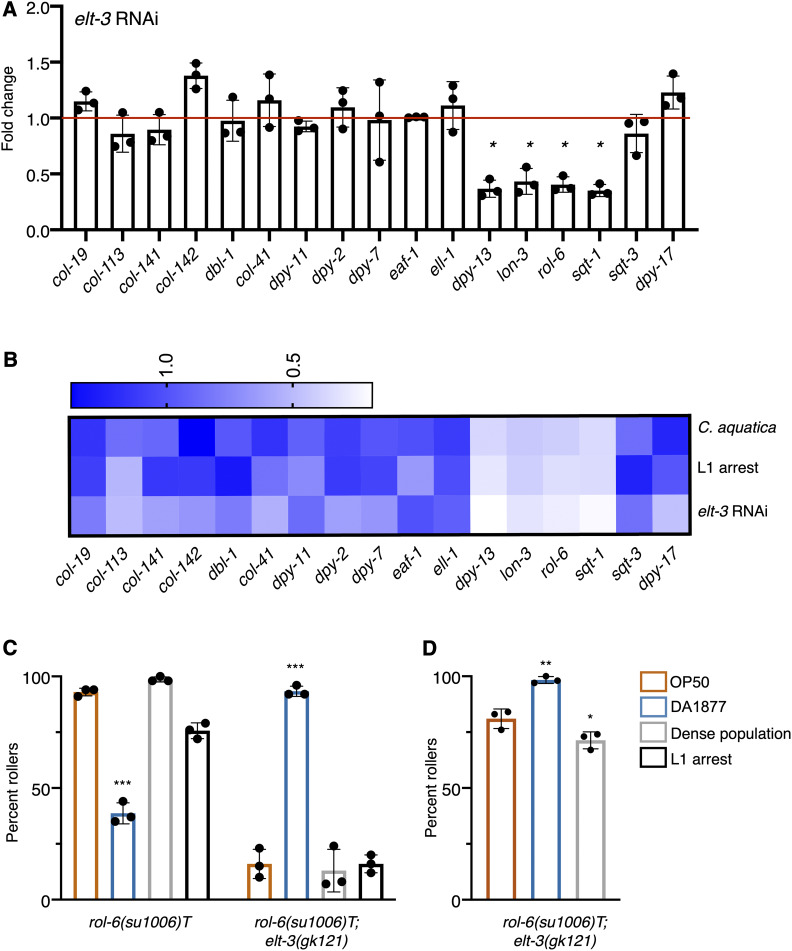
To address whether ELT-3 directly regulates collagen gene expression, we took advantage of available ChIP-seq data (Gerstein et al. 2010) and examined the intergenic regions of rol-6, lon-3, dpy-13, and sqt-1 for the presence of ELT-3 binding sites. All four collagen genes have ELT-3 ChIP peaks in their intergenic regions (Figure S1A), suggesting that ELT-3 may act as a direct transcriptional regulator of these collagens. In contrast, of the other collagens we measured, five out of 10, (including sqt-3) had significant ELT-3 ChIP peaks in their intergenic regions (Figure S1B). To determine if ELT-3 was responsible for the downregulation of rol-6, sqt-1, lon-3, and dpy-13, we measured the expression of these collagens following elt-3 knockdown. Indeed, expression of all four genes was decreased when elt-3 was knocked down (Figure 6A). These changes in gene expression are similar to what we observed in response to the C. aquatica diet or to L1 arrest (Figure 6B), suggesting that ELT-3 mediates changes in collagen gene expression in response to environmental stimuli.
Figure 6.
ELT-3 is a regulator of collagen gene expression. (A) elt-3 was knocked down in wild-type (N2) animals and messenger RNA expression of indicated collagens and collagen regulators was measured using nCounter assay. Expression is shown as fold change relative to treatment with empty vector control (red line). Each dot indicates a single replicate, bars represent the mean, and error bars are SD. * P <0.05 (t-test). (B) Comparison of mean expression values for indicated treatments. L1 arrest and C. aquatica exposure are relative to animals grown on E. coli OP50. elt-3 RNAi is shown relative to empty vector control in E. coli HT115. (C) Analysis of rolling in ERT60 rol-6(su1006)T and elt-3(gk121); rol-6(su1006)T animals under well-fed conditions. (D) Rolling in response to indicated exposures in elt-3(gk121); rol-6(su1006)T in conditions that generated higher penetrance of rolling (populations that were routinely prepared from egg preps and allowed to starve before collecting eggs produced increased numbers of rollers). Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison test. Asterisks indicate P-values (* P <0.05, ** P <0.005, *** P <0.0005).
To determine if elt-3 was required for the environmental responses that influence rolling, we introduced the elt-3(gk121) mutation into rol-6(su1006)T animals (using ERT60) and examined the effect of diet, L1 arrest, and population density on rolling in these animals. Surprisingly, loss of elt-3 did not inhibit the response to environment and its subsequent effect on rolling, but rather it reversed the effect of these factors on rolling. The C. aquatica diet increased rolling, while density decreased rolling (Figure 6, C and D). These effects would be consistent with a model where ELT-3 transcriptional targets are activated in response to E. coli OP50 or increased population density, but repressed when animals are fed C. aquatica. Our data suggest that ELT-3 plays a role in both activities.
Discussion
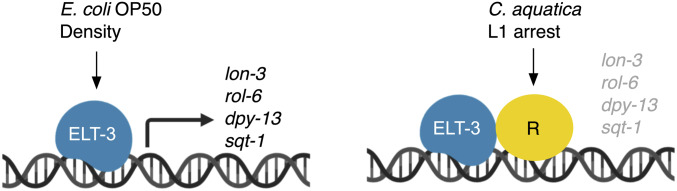
The cuticle serves as a physical barrier that protects C. elegans from the outside environment. In different environments and in response to different stresses, there is likely an advantage to altering the composition of this collagen-rich structure. Our data support a model by which the GATA TF ELT-3 mediates environmentally induced changes in collagen gene expression that ultimately modify the cuticle. The effect of different environmental factors on rolling is reversed in elt-3 mutants, suggesting that ELT-3 may function in both activating and repressing these genes. ELT-3 could accomplish this by functioning as an activator when animals are fed E. coli OP50 but functioning with a repressor protein when animals are fed C. aquatica (Figure 7). In this scenario, when ELT-3 is lost, expression of elt-3 responsive genes would be decreased in normally activating conditions and increased in normally repressing conditions, generating the observed reversal of effects on rolling. Alternatively, ELT-3 may compete for binding to the same site as a repressor protein and the balance of the two proteins could dictate collagen levels.
Figure 7.
Expression of cuticular collagen genes is controlled by ELT-3. ELT-3 promotes expression of dpy-13, lon-3, rol-6, and sqt-1 when animals are fed E. coli OP50 but contributes to the repression of these genes when animals are fed C. aquatica or following L1 arrest. This may occur through the recruitment of a repressor protein whose activity or recruitment to the promoter requires ELT-3.
The cuticle is replaced at each larval stage during development. Our findings that early life exposure, or exposure in the previous generation, can influence the assembly of the adult cuticle suggests that C. elegans use prior experience to tailor the cuticle later in life. This is consistent with the observation that the penetrance of rolling in sqt-2(sc64) mutants is influenced by developmental events, sqt-2(sc64) mutants derived from L3 animals are Sqt (homozygotes are Dpy and heterozygotes are Rol), whereas homozygotes derived from dauers are rollers (Cox et al. 1980). Changes in the presentation and penetrance of these defects may similarly occur through the modification of the composition of the cuticle, resulting in the aberrant SQT-2 protein having a greater effect in animals that have undergone dauer arrest. The idea that C. elegans modulates collagen expression in response to environment is also consistent with the finding that in the L2 stage preceding dauer, rol-6 expression is not observed, whereas it is observed in L2 animals not undergoing dauer (Park and Kramer 1994).
How SQT-3 and ROL-6 proteins interact in the cuticle is unclear. While a balance between ROL-6 dependence and SQT-3 dependence is an attractive hypothesis based on the environmental effects observed in this study, it is unlikely that these proteins substitute for one another. SQT-3 is predicted to encode a transmembrane collagen, while ROL-6 is predicted to encode a globular collagen (Teuscher et al. 2019). Further, redundancy in the function of these two proteins would not be consistent with the fact that knockdown of sqt-3 can suppress rolling in rol-6 mutants (Cai et al. 2011). One proposed explanation for this suppression is that SQT-3 and ROL-6 are part of the same structure, along with other suppressing collagens, and that in the absence of one of these proteins, the incorporation of the aberrant collagen in the cuticle is reduced. However, the effects that we observe in these two mutants in response to different environments cannot be explained by this simple model.
We observed decreased expression of dpy-13, rol-6, lon-3, and sqt-1 in conditions that suppress rolling in rol-6(su1006)T animals but enhance rolling in sqt-3(sc8) animals. While decreased expression of these genes is consistent with suppression of rol-6(su1006)T, it does not explain the increased penetrance of rolling in sqt-3 mutants. Our analysis of collagen gene expression was not comprehensive and it seems likely that the downregulation of these four collagen genes is matched with the upregulation of other compensatory collagen genes that fill these roles. Additional changes in the expression of collagens, collagen-interacting proteins, collagen-modifying enzymes, or factors involved in assembly or secretion of collagens likely explain increased rolling in sqt-3 mutants. We did not observe increased expression of sqt-3, which may have provided the most obvious explanation for the effects observed. However, the timing or duration of expression, or changes to the SQT-3 protein or its export from the endoplasmic reticulum, could increase the penetrance of rolling in sqt-3 mutants and may explain the observed effects.
ELT-3 has been implicated in both stress response and damage response in the cuticle (Pujol et al. 2008; Hu et al. 2017; Dodd et al. 2018). Disruption of annular furrows in the cuticle increases accumulation of osmolytes and activates specific stress-response pathways that require ELT-3 (Dodd et al. 2018). ELT-3 regulation of collagens may also play a role in recovery from cuticle damage and pathogen resistance and, as such, inability to regulate collagen gene expression in response to stress may contribute to the observed decreased resistance of elt-3 mutants to the fungus Drechmeria coniospora, a pathogen that adheres to the C. elegans cuticle (Pujol et al. 2008). ELT-3 may promote the production of a cuticle that is more resistant to infection by this pathogen in much the same way col-92 overexpression increases resistance to Bacillus thuringiensis infection (Iatsenko et al. 2013). In addition to the regulation of rol-1, sqt-1, lon-3, and dpy-13 described here, ELT-3 is also reported to regulate the expression of col-144 (Budovskaya et al. 2008), col-41 (Yin et al. 2015), col-34, and dpy-7 (Yin et al. 2015), suggesting that these additional collagens could also be modulated in response to environmental factors.
Modifying the cuticle may be most easily accomplished by transcriptional coregulation of collagens that interact to ensure proper assembly of cuticle structures. In response to the E. coli diet, ELT-3 would therefore function to generate a cuticle richer in ROL-6, SQT-1, LON-3, and DPY-13 collagens. Transcriptional co-regulation of additional groups of collagens likely functions to generate cuticles optimized to specific developmental stages or environmental conditions. For example, NHR-23 regulates expression of dpy-2, dpy-3, dpy-7, dpy-8, dpy-10, and dpy-5 (Kouns et al. 2011), and LIN-29 is required for the expression of L4-specific collagens col-38, col-49, col-63, and col-138 (Abete-Luzi and Eisenmann 2018). These TFs function to ensure the production of stage-specific cuticles; however, other factors may ensure the production of environment-specific cuticles. Additional transcriptional regulators of collagen gene expression have been identified, including the TFs EAF-1 and ELL-1, which regulate expression of dpy-3, dpy-13, and sqt-3 (Cai et al. 2011), and BAR-1, which regulates bli-1, col-38, col-49, and col-71 expression (Jackson et al. 2014). Similarly, TGF-β signaling regulates expression of col-141 and col-142 (Madaan et al. 2018). Intriguingly, TGF-β signaling has been implicated in environmental response, raising the possibility that modification of the cuticle may also be integrated into these responses.
How vitamin B12 promotes changes to the cuticle is not clear. However, the appearance of Dpy animals when B12 is depleted, although infrequent, suggests that B12 normally contributes to cuticle development (Bito et al. 2013). One possible explanation for the connection between vitamin B12 and ELT-3 is oxidative stress. Depletion of vitamin B12 increases oxidative stress in C. elegans (Bito et al. 2017), and although the E. coli OP50 diet is not devoid of vitamin B12, the low levels of vitamin B12 in the E. coli diet relative to the C. aquatica diet may result in increased stimulation of detoxification responses. ELT-3 has been identified as a mediator of these responses (Hu et al. 2017), which may suggest the link between vitamin B12 availability and ELT-3 activation is stress response. If true, ELT-3 would couple the induction of detoxification response with changes in the cuticle. Based on our observations with Hoechst staining, an E. coli OP50 diet, where ELT-3 targets are more highly expressed, results in a less permeable cuticle. Decreasing the permeability of the cuticle may provide additional protection from conditions that activate detoxification responses.
Our model suggests an unknown repressor protein participates in the environmentally induced regulation of cuticular collagens. One candidate for this repressor is TBX-2, identified in our RNAi screen as having an activity opposite to that of ELT-3. TBX-2 is the sole C. elegans homolog of Tbx2 subfamily of T-box factors. In humans, TBX2 can act as a repressor or an activator; however, in C. elegans, it has only been described as a repressor. Intriguingly, in mammalian cells, TBX2 is proposed to act as a regulator of the collagen COL1A2. Stable expression of TBX2 reduces expression of COL1A2 in fibroblast cell lines (Teng et al. 2007). However, in osteoblast cells, TBX2 expression has the opposite effect on COL1A2 expression, promoting increased expression (Chen et al. 2001). COL1A2 is also regulated by a GATA factor, GATA4 (Wang et al. 2005; Antoniv et al. 2005), suggesting the potential for conservation of the regulation of collagens by TBX-2 and ELT-3 in mammalian cells.
We found that diet, population density, early-life starvation, and parental environment affect cuticle formation. Transcriptional regulation of cuticular collagens provides one mechanism by which C. elegans adapts to the surrounding environment. Although we identified ELT-3 as one factor involved in this regulation, other regulators of collagen gene expression likely exist and function to tailor the cuticle to specific environments or specific stresses.
Acknowledgments
We thank S. Taubert and T. Kubiseski for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (grant P40 OD0104440). We gratefully acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (grant RGPIN-2016-06339).
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11946360.
Present address: Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, M5G 1L7.
Communicating editor: B. Grant
Literature Cited
- Abete-Luzi P., and D. M. Eisenmann, 2018 Regulation of C. elegans L4 cuticle collagen genes by the heterochronic protein LIN-29. Genesis 56: e23106. 10.1002/dvg.23106 [DOI] [PMC free article] [PubMed]
- Antoniv T. T., Tanaka S., Sudan B., De Val S., Liu K. et al. , 2005. Identification of a repressor in the first intron of the human alpha2(I) collagen gene (COL1A2). J. Biol. Chem. 280: 35417–35423. 10.1074/jbc.M502681200 [DOI] [PubMed] [Google Scholar]
- Bergmann D. C., Crew J. R., Kramer J. M., and Wood W. B., 1998. Cuticle chirality and body handedness in Caenorhabditis elegans. Dev. Genet. 23: 164–174. [DOI] [PubMed] [Google Scholar]
- Bito T., Matsunaga Y., Yabuta Y., Kawano T., and Watanabe F., 2013. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio 3: 112–117. 10.1016/j.fob.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito T., Misaki T., Yabuta Y., Ishikawa T., Kawano T. et al. , 2017. Vitamin B deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 11: 21–29. 10.1016/j.redox.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y. V., Wu K., Southworth L. K., Jiang M., Tedesco P. et al. , 2008. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell 134: 291–303. 10.1016/j.cell.2008.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Phong B. L., Fisher A. L., and Wang Z., 2011. Regulation of fertility, survival, and cuticle collagen function by the Caenorhabditis elegans eaf-1 and ell-1 genes. J. Biol. Chem. 286: 35915–35921. 10.1074/jbc.M111.270454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada R. C., and Russell R. L., 1975. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326–342. 10.1016/0012-1606(75)90109-8 [DOI] [PubMed] [Google Scholar]
- C. elegans Deletion Mutant Consortium , 2012. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 (Bethesda) 2: 1415–1425. 10.1534/g3.112.003830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhong Q., Wang J., Cameron R. S., Borke J. L. et al. , 2001. Microarray analysis of Tbx2-directed gene expression: a possible role in osteogenesis. Mol. Cell. Endocrinol. 177: 43–54. 10.1016/S0303-7207(01)00456-7 [DOI] [PubMed] [Google Scholar]
- Coolon J. D., Jones K. L., Todd T. C., Carr B. C., and Herman M. A., 2009. Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. PLoS Genet. 5: e1000503 10.1371/journal.pgen.1000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., and Hirsh D., 1985. Stage-specific patterns of collagen gene expression during development of Caenorhabditis elegans. Mol. Cell. Biol. 5: 363–372. 10.1128/MCB.5.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Laufer J. S., Kusch M., and Edgar R. S., 1980. Genetic and phenotypic characterization of roller mutants of CAENORHABDITIS ELEGANS. Genetics 95: 317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Staprans S., and Edgar R. S., 1981. The cuticle of Caenorhabditis elegans. II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev. Biol. 86: 456–470. 10.1016/0012-1606(81)90204-9 [DOI] [PubMed] [Google Scholar]
- Dodd W., Tang L., Lone J.-C., Wimberly K., Wu C.-W. et al. , 2018. A damage sensor associated with the cuticle coordinates three core environmental stress responses in Caenorhabditis elegans. Genetics 208: 1467–1482. 10.1534/genetics.118.300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann I., Griffon A., Tichit L., Montañana-Sanchis F., Wang G. et al. , 2011. A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS One 6: e19055 10.1371/journal.pone.0019055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelt M. V., Estevez A. Y., Yin X., and Strange K., 2005. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J. Gen. Physiol. 126: 379–392. 10.1085/jgp.200509355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald C. Y., Landis J. N., Porter Abate J., Murphy C. T., and Blackwell T. K., 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519: 97–101. 10.1038/nature14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein M. B., Lu Z. J., Van Nostrand E. L., Cheng C., Arshinoff B. I. et al. , 2010. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330: 1775–1787. 10.1126/science.1196914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravato-Nobre M. J., Vaz F., Filipe S., Chalmers R., and Hodgkin J., 2016. The invertebrate lysozyme effector ILYS-3 is systemically activated in response to danger signals and confers antimicrobial protection in C. elegans. PLoS Pathog. 12: e1005826 10.1371/journal.ppat.1005826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks G.-J., Gaidatzis D., Aeschimann F., and Großhans H., 2014. Extensive oscillatory gene expression during C. elegans larval development. Mol. Cell 53: 380–392. 10.1016/j.molcel.2013.12.013 [DOI] [PubMed] [Google Scholar]
- Hoogewijs D., Houthoofd K., Matthijssens F., Vandesompele J., and Vanfleteren J. R., 2008. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol. Biol. 9: 9 10.1186/1471-2199-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., D’Amora D. R., MacNeil L. T., Walhout A. J. M., and Kubiseski T. J., 2017. The oxidative stress response in Caenorhabditis elegans requires the GATA transcription factor ELT-3 and SKN-1/Nrf2. Genetics 206: 1909–1922. 10.1534/genetics.116.198788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatsenko I., Sinha A., Rödelsperger C., and Sommer R. J., 2013. New role for DCR-1/dicer in Caenorhabditis elegans innate immunity against the highly virulent bacterium Bacillus thuringiensis DB27. Infect. Immun. 81: 3942–3957. 10.1128/IAI.00700-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. M., Abete-Luzi P., Krause M. W., and Eisenmann D. M., 2014. Use of an activated beta-catenin to identify Wnt pathway target genes in Caenorhabditis elegans, including a subset of collagen genes expressed in late larval development. G3 (Bethesda) 4: 733–747. 10.1534/g3.113.009522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone I. L., 1994. The cuticle of the nematode Caenorhabditis elegans: a complex collagen structure. BioEssays 16: 171–178. 10.1002/bies.950160307 [DOI] [PubMed] [Google Scholar]
- Kaletsky R., Yao V., Williams A., Runnels A. M., Tadych A. et al. , 2018. Transcriptome analysis of adult Caenorhabditis elegans cells reveals tissue-specific gene and isoform expression. PLoS Genet. 14: e1007559 10.1371/journal.pgen.1007559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R. et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- Kouns N. A., Nakielna J., Behensky F., Krause M. W., Kostrouch Z. et al. , 2011. NHR-23 dependent collagen and hedgehog-related genes required for molting. Biochem. Biophys. Res. Commun. 413: 515–520. 10.1016/j.bbrc.2011.08.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., and Johnson J. J., 1993. Analysis of mutations in the sqt-1 and rol-6 collagen genes of Caenorhabditis elegans. Genetics 135: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., and Johnson J. J., 1990. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol. Cell. Biol. 10: 2081–2089. 10.1128/MCB.10.5.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch M., and Edgar R. S., 1986. Genetic studies of unusual loci that affect body shape of the nematode Caenorhabditis elegans and may code for cuticle structural proteins. Genetics 113: 621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil L. T., Watson E., Arda H. E., Zhu L. J., and Walhout A. J. M., 2013. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell 153: 240–252. 10.1016/j.cell.2013.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaan U., Yzeiraj E., Meade M., Clark J. F., Rushlow C. A. et al. , 2018. BMP signaling determines body size via transcriptional regulation of collagen genes in Caenorhabditis elegans. Genetics 210: 1355–1367. 10.1534/genetics.118.301631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moribe H., Yochem J., Yamada H., Tabuse Y., Fujimoto T. et al. , 2004. Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J. Cell Sci. 117: 5209–5220. 10.1242/jcs.01403 [DOI] [PubMed] [Google Scholar]
- Murakami M., Koga M., and Ohshima Y., 2001. DAF-7/TGF-beta expression required for the normal larval development in C. elegans is controlled by a presumed guanylyl cyclase DAF-11. Mech. Dev. 109: 27–35. 10.1016/S0925-4773(01)00507-X [DOI] [PubMed] [Google Scholar]
- Nyström J., Shen Z.-Z., Aili M., Flemming A. J., Leroi A. et al. , 2002. Increased or decreased levels of Caenorhabditis elegans lon-3, a gene encoding a collagen, cause reciprocal changes in body length. Genetics 161: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A. P., and Johnstone I. L., 2007. The cuticle (March 19, 2007), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.138.1, http://www.wormbook.org. [Google Scholar]
- Park Y. S., and Kramer J. M., 1994. The C. elegans sqt-1 and rol-6 collagen genes are coordinately expressed during development, but not at all stages that display mutant phenotypes. Dev. Biol. 163: 112–124. 10.1006/dbio.1994.1127 [DOI] [PubMed] [Google Scholar]
- Peixoto C. A., and De Souza W., 1994. Freeze-fracture characterization of the cuticle of adult and dauer forms of Caenorhabditis elegans. Parasitol. Res. 80: 53–57. 10.1007/BF00932624 [DOI] [PubMed] [Google Scholar]
- Popham J. D., and Webster J. M., 1978. An alternative interpretation of the fine structure of the basal zone of the cuticle of the dauerlarva of the nematode Caenorhabditis elegans (Nematoda). Can. J. Zool. 56: 1556–1563. 10.1139/z78-217 [DOI] [PubMed] [Google Scholar]
- Pujol N., Zugasti O., Wong D., Couillault C., Kurz C. L. et al. , 2008. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4: e1000105 10.1371/journal.ppat.1000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual J.-F., Ceron J., Koreth J., Hao T., Nicot A.-S. et al. , 2004. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14: 2162–2168. 10.1101/gr.2505604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org. 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H., Davis E., Abrahams A., Mowla S., Parker M. I. et al. , 2007. A role for Tbx2 in the regulation of the alpha2(1) collagen gene in human fibroblasts. J. Cell. Biochem. 102: 618–625. 10.1002/jcb.21315 [DOI] [PubMed] [Google Scholar]
- Teuscher A. C., Jongsma E., Davis M. N., Statzer C., Gebauer J. M. et al. , 2019. The in-silico characterization of the Caenorhabditis elegans matrisome and proposal of a novel collagen classification. Matrix Biology Plus 1: 100001 10.1016/j.mbplus.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Tanaka S., and Ramirez F., 2005. GATA-4 binds to an upstream element of the human alpha2(I) collagen gene (COL1A2) and inhibits transcription in fibroblasts. Matrix Biol. 24: 333–340. 10.1016/j.matbio.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Watson E., MacNeil L. T., Ritter A. D., Yilmaz L. S., Rosebrock A. P. et al. , 2014. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell 156: 1336–1337 (erratum: Cell 156: 759–770). 10.1016/j.cell.2014.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Pears C., and Woollard A., 2017. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 7: 9839 10.1038/s41598-017-10454-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Madaan U., Park A., Aftab N., and Savage-Dunn C., 2015. Multiple cis elements and GATA factors regulate a cuticle collagen gene in Caenorhabditis elegans. Genesis 53: 278–284. 10.1002/dvg.22847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors confirm that all data necessary for confirming the conclusions presented are within the article, figures, and tables. Figure S1 contains a summary of chromatin immunoprecipitation sequencing (ChIP-seq) data for ELT-3 binding to collagen gene intergenic regions. Table S1 contains a summary of previously published collagen gene expression in response to bacterial and fungal exposures. Table S2 contains a list of TFs that are hypodermally enriched and were knocked down by RNAi. Table S3 contains NanoString probes used and RNAi target sequences. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11946360