Unrepaired DNA damage in mouse oocytes leads to apoptosis, in part via CHK2 signaling to TRP53 and TRP63. Here, Rinaldi, Bloom, and Schimenti provide evidence that CHK1 can also be involved, especially...
Keywords: checkpoints, meiosis, mouse, oocytes, transducer kinases
Abstract
Eukaryotic organisms have evolved mechanisms to prevent the accumulation of cells bearing genetic aberrations. This is especially crucial for the germline, because fecundity and fitness of progeny would be adversely affected by an excessively high mutational incidence. The process of meiosis poses unique problems for mutation avoidance because of the requirement for SPO11-induced programmed double-strand breaks (DSBs) in recombination-driven pairing and segregation of homologous chromosomes. Mouse meiocytes bearing unrepaired meiotic DSBs or unsynapsed chromosomes are eliminated before completing meiotic prophase I. In previous work, we showed that checkpoint kinase 2 (CHK2; CHEK2), a canonical DNA damage response protein, is crucial for eliminating not only oocytes defective in meiotic DSB repair (e.g., Trip13Gt mutants), but also Spo11−/− oocytes that are defective in homologous chromosome synapsis and accumulate a threshold level of spontaneous DSBs. However, rescue of such oocytes by Chk2 deficiency was incomplete, raising the possibility that a parallel checkpoint pathway(s) exists. Here, we show that mouse oocytes lacking both p53 (TRP53) and the oocyte-exclusive isoform of p63, TAp63, protects nearly all Spo11−/− and Trip13Gt/Gt oocytes from elimination. We present evidence that checkpoint kinase I (CHK1; CHEK1), which is known to signal to TRP53, also becomes activated by persistent DSBs in oocytes, and to an increased degree when CHK2 is absent. The combined data indicate that nearly all oocytes reaching a threshold level of unrepaired DSBs are eliminated by a semiredundant pathway of CHK1/CHK2 signaling to TRP53/TAp63.
OOCYTE development in females begins in utero, when primordial germ cells enter and complete early stages of meiosis, including recombination, before arresting perinatally in a stage called dictyate. In the first few days after birth, the oocytes undergo folliculogenesis, in which they become surrounded by flattened granulosa cells (Peters 1969). The resulting “primordial follicles” constitute the finite oocyte pool present in women and female mice of reproductive age (Findlay et al. 2015).
Meiocytes have developed mechanisms for minimizing the production of gametes with genetic anomalies such as unrepaired double-strand breaks (DSBs) and meiotic chromosome asynapsis. Mouse oocytes bearing mutations that prevent repair of programmed SPO11/TOPOVIBL-induced DSBs, which are essential for recombination-mediated pairing and synapsis of homologous chromosomes (Baudat et al. 2000; Romanienko and Camerini-Otero 2000; Mahadevaiah et al. 2001; Robert et al. 2016), are eliminated by a DNA damage checkpoint (Di Giacomo et al. 2005). The molecular nature of this checkpoint was first revealed as involving signaling of CHK2 to TRP53 and the oocyte-specific TransActivation domain of p63, known as TAp63 (Suh et al. 2006; Livera et al. 2008), by studies exploiting a hypomorphic allele of Trip13 (Bolcun-Filas et al. 2014). This allele (Trip13Gt) causes sterility in both males and females and is useful because it is defective for DSB repair but not synapsis (Li and Schimenti 2007). Deficiency of Chk2 protected against oocyte loss and restored fertility of Trip13Gt/Gt females. Chk2 also plays a role in the DNA damage checkpoint in spermatocyte meiosis (Pacheco et al. 2015).
Defects in chromosome synapsis during meiotic prophase I also triggers death of most oocytes. There are at least two mechanisms underlying this “synapsis checkpoint.” One is meiotic silencing of unsynapsed chromatin (MSUC), a process of extensive heterochromatinization and transcriptional downregulation, which appears to function primarily in situations where only about one to three chromosomes are unsynapsed (Kouznetsova et al. 2009; Cloutier et al. 2015). A second mechanism pertains to oocytes that are highly asynaptic, in which the silencing machinery is presumably overwhelmed (Kouznetsova et al. 2009). Surprisingly, this mechanism is also highly dependent on the DNA damage checkpoint. The mechanistic basis for this is the formation of a threshold level (∼10) of SPO11-independent, spontaneously arising DSBs (Carofiglio et al. 2013; Rinaldi et al. 2017). Approximately 61% of Spo11−/− oocytes, which do not form programmed meiotic DSBs and consequently are defective for homologous chromosome synapsis (but do exhibit some nonhomologous synapsis), reach this threshold, leading to depletion of the entire ovarian reserve (primordial oocytes) by a few weeks after birth (Baudat et al. 2000; Romanienko and Camerini-Otero 2000). Chk2 deletion rescued oocyte numbers to ∼25% of wild type (WT), indicating that most are either eliminated by an alternative pathway or succumb nonspecifically from a catastrophically high number of DSBs (up to ∼100, with an average of ∼50/cell) (Rinaldi et al. 2017). Similarly, Chk2 deficiency rescued Trip13Gt/Gt oocytes to around one-third of WT levels (Bolcun-Filas et al. 2014), raising the possibility that the same CHK2-independent pathway may be active in both cases.
Here, we tested the possibility that the incomplete rescue of oocytes mentioned above is due to the existence of another pathway either distinct or complementary to that involving CHK2, but which also involves TRP53 and TAp63. Our results indicate that this is indeed the case, and that most Spo11−/− and TRIP13-deficient oocytes are ultimately eliminated by the combined activation of TRP53 and TAp63.
Materials and Methods
Mice
Alleles used in this study and their genetic backgrounds were previously described (Bolcun-Filas et al. 2014). Comparisons of compound mutants and controls utilized littermates whenever possible, otherwise animals from related parents or different litters from the same parents were used. Animal work was approved by Cornell’s Institutional Animal Care and Use Committee, under protocol 2004-0038 to J.C.S.
Histology and follicle quantification
Ovaries were fixed in Bouin’s solution, embedded in paraffin, serially sectioned at 6 μm, and stained with hematoxylin and eosin. Follicle identification (Myers et al. 2004) and quantification was as described (Bolcun-Filas et al. 2014). Graphs and statistical analysis were performed with GraphPad Prism8. Comparisons of follicle numbers across genotypes were performed using an ordinary one-way ANOVA test.
Western blot analysis of protein phosphorylation
Ovaries from postnatal (3–5 day old) mice were collected and divided into control and treatment groups. Treated groups were exposed to 3 Gy of ionizing radiation (IR) as described above and proteins were extracted 3 hr post irradiation. Ovaries from all the females in the litter were dissected and individually frozen while genotyping was performed. Proteins from ovaries of selected genotypes were pooled into groups of four and extracted with lysis buffer containing: 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, protease inhibitors (#11836153001; Complete Mini-Roche), and phosphoprotease inhibitors (#04906845001; PhosSTOP-Roche). Proteins were resolved on 4–20% gradient acrylamide gels (#4561093; Bio-Rad, Hercules, CA), transferred to PVDF transfer membranes (#IPVH00010; Millipore, Bedford, MA) and blocked with 5% BSA or 5% nonfat milk according to the manufacturer datasheet for the corresponding antibody. Membranes were probed with rabbit anti-phospho-Chk1 (Ser345) (1:750, 133D3; Cell Signaling Technology), rabbit anti-p53 (rodent-specific 1:750, D2H90; Cell Signaling Technology), mouse anti-p63 (1:500, CM163A; Biocare Medical), and rabbit anti-DDX4/MVH (1:750, 13840; Abcam).
Data availability statement
Mouse strains that were not obtained from others will be made available upon request. Supplemental Material, Figure S1 is a Western blot that is a biological replicate of Figure 2. Table S1 contains primordial and total follicle counts for the genotypes included in Figure 1. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12092187.
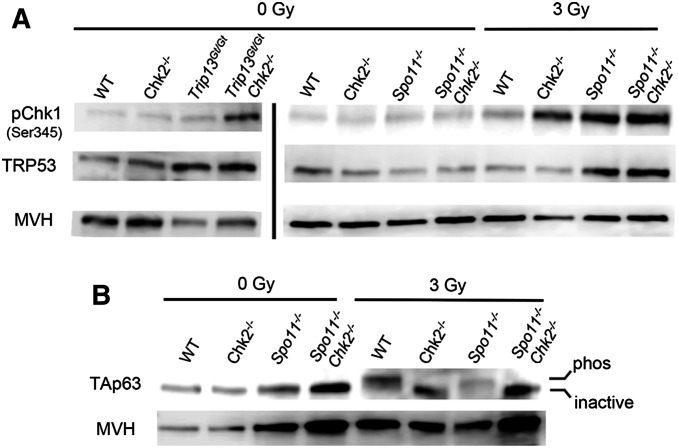
Figure 2.
Increased CHK1 activation and p53 stabilization, but not TAp63 activation, in CHK2-deficient oocytes. (A) CHK1 phosphorylation in oocytes is stimulated by induced or meiotic DSBs. Shown are Western blots probed with indicated antibodies. Each lane contains total protein extracted from four ovaries (postnatal day 3–5) that were either exposed or not to 3 Gy of ionizing radiation (IR). Ovaries were harvested for protein extraction 3 hr post-IR. The blots on the left, separated by a vertical bar from those on the right, were from a different blot and different protein samples and mice. The same two blots (left and right) were stripped and reprobed sequentially with the three antibodies. A biological replicate is shown in Figure S1. Note that the decreased MVH levels in Trip13Gt/Gt ovaries is due to reduction in oocytes. (B) Activation of the TAp63 isoform is dependent on DNA damage and CHK2 signaling, not asynapsis. Shown is a Western blot probed sequentially for TAp63 and the germ cell marker MVH. Each lane contains protein extracted from ovaries as described in A. An upward shift in the band indicates the presence of the active (phosphorylated) vs. inactive TAp63. A biological replicate is shown in Figure S1.
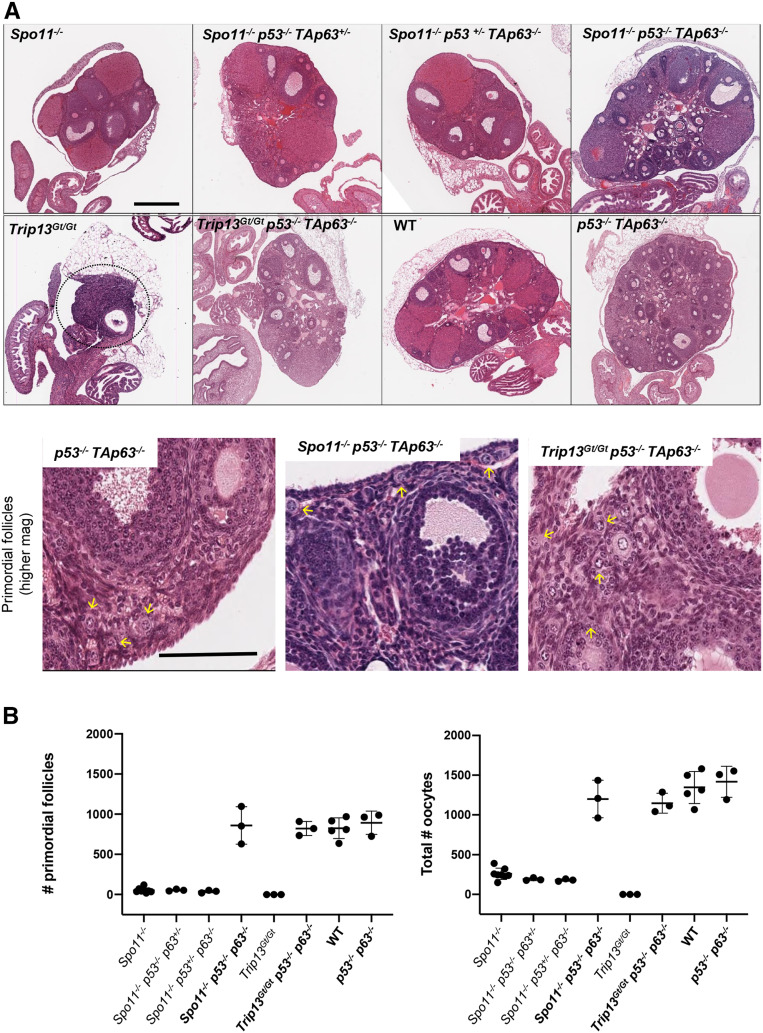
Figure 1.
Rescue of SPO11- and TRIP13-deficient oocytes by compound deletion of p53 and TAp63. (A) Hematoxylin and eosin stained ovaries from 2-month-old mice of the indicated genotypes. The top two rows are images are from histological sections through the approximate center of the ovaries. Bar, 500 µm. The dashed circle indicates the residual Trip13Gt/Gt ovary. The bottom row shows higher magnification images of selected genotypes. Bar, 100 µm. Yellow arrows indicate examples of primordial follicles. (B) Oocyte quantification. To the left is the quantification of primordial follicles [P < 0.0001 for all oocyte rescued (in bold) genotypes compared to nonrescued genotypes; P = 0.92 for oocyte rescued genotypes vs. WT and Trp53−/− TAp63−/− ovaries]. To the right are total oocytes (from all stages of follicles) from individual ovaries (P < 0.0001 for all oocyte rescued genotypes compared to nonrescued genotypes; P = 0.33 for oocyte rescued genotypes vs. WT and Trp53−/− TAp63−/− ovaries). Oocyte quantification data are presented in Table S1. Genotype abbreviations are as follows: TAp63 is abbreviated as p63; WT, wild type.
Results and Discussion
To address whether a CHK2-independent pathway exists that can eliminate oocytes bearing unrepaired DSBs, we utilized two mutant models, Trip13Gt and a Spo11 null (Spo11-). Virtually all Trip13Gt/Gt oocytes are eliminated due to failure to repair SPO11-dependent DSBs (Li and Schimenti 2007) by the end of pachynema (Rinaldi et al. 2017). Chk2 deficiency rescued around one-third of these oocytes, and these rescued oocytes gave rise to viable offspring (Bolcun-Filas et al. 2014). Although disruption of either of CHK2’s downstream phosphorylation targets, TRP53 and TAp63, enabled little or no rescue of Trip13Gt/Gt oocytes, Trip13Gt/Gt TAp63−/− Trp53+/− mice exhibited oocyte rescue similar to that of Trip13Gt/Gt Chk2−/− mice (Bolcun-Filas et al. 2014). At the time of that report, double mutants (TAp63−/− Trp53−/−; the former allele ablating the TA domain only) were not assayed for the extent to which they could rescue Trip13Gt/Gt oocytes. We hypothesized that the inability to achieve full oocyte rescue in either Trip13Gt/Gt TAp63−/− Trp53+/− or Trip13Gt/Gt Chk2−/− females was due to one of the following: (1) the number of DSBs was so high that elimination of most oocytes occurred in a checkpoint-independent fashion; (2) residual TRP53 activity in the Trip13Gt/Gt TAp63−/− Trp53+/− mice sufficed to trigger apoptosis in many oocytes; and/or (3) a parallel checkpoint pathway is active in Chk2−/− oocytes.
To test these possibilities, we first assessed the ovarian reserve in Trip13Gt/Gt Trp53−/− TAp63−/− mice. Remarkably, the numbers of primordial and later-stage oocytes in the triple mutants were indistinguishable from WT (Figure 1, A and B). This result indicates that essentially all Trip13Gt/Gt oocytes are eliminated by checkpoint signaling to TRP53 and TAp63, thereby eliminating hypothesis 1, but supporting hypothesis 2. This result is also consistent with hypothesis 3, implying that another pathway or kinase is signaling to these two effector proteins.
Next, we tested whether the incomplete rescue of Spo11−/− oocytes by Chk2 deletion is also potentially a consequence of checkpoint signaling to TAp63 and TRP53 via a different transducer. Accordingly, we bred mice that lacked either or both of these proteins in the context of Spo11 deficiency. Oocyte numbers in Spo11−/− mice that were also homozygous for mutations in either Trp53 or TAp63 and heterozygous for a mutation in the other (Trp53−/− TAp63+/− and Trp53+/− TAp63−/−) were indistinguishable from Spo11 nulls; nearly the entire oocyte reserve was depleted after 2 months of age, as is characteristic for Spo11 deficiency (Di Giacomo et al. 2005). However, homozygosity for both Trp53 and TAp63 dramatically restored oocyte numbers to WT levels (Figure 1, A and B). It is unclear why heterozygosity for either Trp53 or TAp63 in the context of nullizygosity for the other gene failed to rescue any Spo11−/− oocytes unlike Trip13Gt/Gt oocytes, but we can speculate that other factors could play a role. These include strain background, enhanced recognition by DNA damage sensors of spontaneous DSBs on asynapsed chromosomes (Spo11−/−) vs. meiotically induced DSBs on synapsed chromosomes (Trip13Gt/Gt), or greater availability of DNA damage signaling factors in Spo11−/− oocytes stimulated by the MSUC response.
These experiments indicate that unrepaired meiotic DSBs, when present at levels above the threshold to trigger their elimination (Rinaldi et al. 2017), ultimately cause DNA damage signaling to both TRP53 and TAp63. Additionally, we conclude that one or both of these proteins can be activated not only by CHK2, but also another kinase. In our previous studies, we suggested that the apical kinase ATM, which when activated by DSBs typically phosphorylates CHK2, is not essential for the meiotic DNA damage checkpoint (Bolcun-Filas et al. 2014). This conclusion was based on the observation that many Atm−/− oocytes, which have extensive DSBs due to ATM’s role in negatively regulating SPO11 (Lange et al. 2011), are eliminated in a CHK2-dependent manner. We proposed (Bolcun-Filas et al. 2014) that the related kinase ATR (ataxia telangiectasia and Rad3 related) might activate CHK2 in oocytes similar to irradiated mitotic cells (Wang et al. 2006), which in turn would phosphorylate TAp63 and TRP53. Since ATR primarily activates CHK1, albeit most notably in the context of damage at DNA replication forks, we speculated that CHK1 can trigger death of DSB-bearing oocytes by activating TRP53 in the absence of CHK2. TRP53 is a known target of CHK1 (Shieh et al. 2000; Ou et al. 2005), and studies have shown that CHK1 can be activated in response to DSBs either in an ATM-dependent (Flaggs et al. 1997; Maréchal and Zou 2013) or ATM-independent (Flaggs et al. 1997; Balmus et al. 2012) manner. Recombinant CHK1 has also been reported to phosphorylate TRP63 in vitro (Kim et al. 2007).
If this hypothesis is true, CHK1 would be activated in response to DSBs present in oocytes. To test this, we examined levels of CHK1 phosphorylated at Ser345 (pCHK1; indicative of the active form) and TRP53 (which is stabilized by phosphorylation) in various genotypes of neonatal (3–5 days postpartum) ovaries, and also in ovaries exposed to 3 Gy of IR. This level of IR induces ∼40 DSBs, as measured by RAD51 foci (a proxy for DSBs) on meiotic chromosomes of oocytes (Rinaldi et al. 2017). By way of comparison, DSB repair-defective Trip13Gt/Gt have ∼65 RAD51 foci persisting abnormally on synapsed pachytene cells (Rinaldi et al. 2017). Since ovaries of mutant animals have variable numbers of oocytes, we used the germ-cell-specific marker MVH as a loading reference for the amount of protein corresponding to oocytes in each sample. Ovaries were harvested at 3 hr postirradiation. In unirradiated ovaries, there was no apparent difference between repair-proficient genotypes (WT; Chk2−/−; Spo11−/−; Spo11−/− Chk2−/−) in the levels of pCHK1 or TRP53 (Figure 2A). Both unirradiated Trip13Gt/Gt and irradiated WT ovaries had slightly elevated pCHK1, with the former also having a marked increase in TRP53 (note MVH levels for intersample comparisons). Interestingly, CHK1 phosphorylation was markedly higher in irradiated Chk2−/− and unirradiated Trip13GtGt/Chk2−/− ovaries (Figure 2A and Figure S1). This implies that the ATM and/or ATR kinases have a higher propensity to activate CHK2 than CHK1 in response to DSBs in meiocytes, but that CHK1 becomes a more prominent target in the absence of CHK2, and is able to trigger a TRP53/TAp63 response that results in apoptosis or eventual DSB repair (Bolcun-Filas et al. 2014).
Interestingly, IR also caused a marked increase of pCHK1 in Spo11−/− oocytes compared to WT (Figure 2A and Figure S1). Levels of TRP53 were also higher in IR-treated Spo11−/− ovaries, but the presence or absence of CHK2 had no consequence (Figure 2A). One possible explanation is that repair of IR-induced DSBs by intersister recombination is inhibited in Spo11 mutants, because unsynapsed chromosome axes retain HORMAD1/2 proteins that prevent such repair (Carofiglio et al. 2013; Rinaldi et al. 2017). In contrast, Chk2−/− oocytes would retain intersister repair ability, and thus either delay or minimize signaling to TRP53. A second possible cause of increased pCHK1 in irradiated Spo11−/− oocytes is that asynapsed chromosomes are more susceptible to IR-induced DNA damage than synapsed chromosomes (as in WT and Chk2−/− oocytes). A final possibility is that the presence of ATR on asynapsed chromatin (Turner et al. 2004, 2006) (Perera et al. 2004; Cloutier et al. 2016) facilitates DNA damage signaling to CHK1 under conditions of unrepaired DSBs. This implies that ATR is not only involved in MSUC, but also retains its function as a key component of the DSB repair machinery (Widger et al. 2018).
As discussed earlier, there is evidence for two processes that can trigger death of oocytes progressing through meiosis: MSUC (which functions when only a few chromosomes are asynapsed) and spontaneous DSBs, when there is extensive asynapsis as in Spo11−/− oocytes. While the experiments above revealed that TRP53 is not activated in unirradiated Spo11−/− oocytes, it remained possible that activation of TAp63 could be induced by MSUC or extensive asynapsis. As we previously showed, CHK2 is required for IR-induced phosphorylation of TAp63 (Figure 2B) (Bolcun-Filas et al. 2014), which leads to the conversion of the inactive dimerized to the active tetramer form of TAp63 (Deutsch et al. 2011). However, we found no evidence for activation (phosphorylation) of TAp63 in unirradiated Spo11−/− ovaries (Figure 2B).
In summary, we have shown that mouse oocytes with unrepaired DSBs or extensive asynapsis are culled by a DNA damage response funneling through TRP53 and p63. Some, but not all of the damage signaling to these proteins is transduced by CHK2, and we provide evidence that CHK1 can also perform this function (see model in Figure 3). The relative contributions of these transducer kinases in meiotic DNA damage responses is unclear. Even though the essential nature of CHK1 in embryonic and premeiotic germ cell development (Abe et al. 2018) complicates analyses, CHK1 conditional mutagenesis and depletion experiments indicate that this kinase plays a role in modulating cell cycle progression in spermatocytes during meiotic prophase I (Abe et al. 2018), and in oocytes at the G2/M checkpoint (Chen et al. 2012). A key remaining question is whether CHK1 and CHK2 are the sole direct responders for TRP53 and TRP63, or if another transducer kinase(s), such as casein kinases 1 or 2, function in parallel (Figure 3).
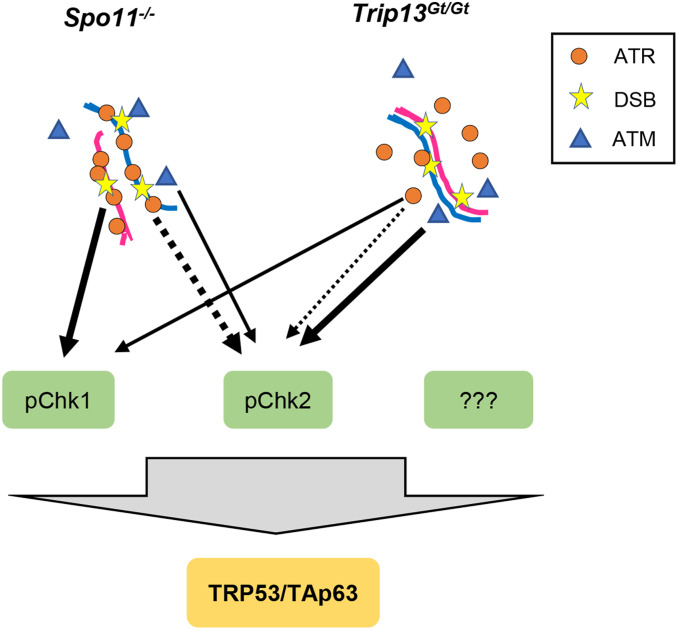
Figure 3.
Model of checkpoint signaling in mouse oocytes. We propose that all DSB damage signaling in oocytes requires activation of TRP53 and TAp63 for complete oocyte elimination. The dashed lines represent noncanonical phosphorylation of CHK2 by ATR, and the thickness of all lines represents the relative amounts of activation in the two indicated mutant situations. We propose that in highly asynaptic Spo11 mutant oocytes, the “preloading” of ATR as part of the MSUC response leads it to play a larger role in signaling to CHK1 and CHK2 than under situations in which DSBs occur on synapsed chromosomes.
Acknowledgments
A. Mills originally provided us with TAp63 mutant mice. This work was supported by National Institutes of Health grant GM45415 to J.C.S. and an institutional training grant (T32HD057854) that supported J.C.B.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.12092187.
Communicating editor: F. Cole
Literature Cited
- Abe H., Alavattam K. G., Kato Y., Castrillon D. H., Pang Q. et al. , 2018. CHEK1 coordinates DNA damage signaling and meiotic progression in the male germline of mice. Hum. Mol. Genet. 27: 1136–1149. 10.1093/hmg/ddy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmus G., Zhu M., Mukherjee S., Lyndaker A. M., Hume K. R. et al. , 2012. Disease severity in a mouse model of ataxia telangiectasia is modulated by the DNA damage checkpoint gene Hus1. Hum. Mol. Genet. 21: 3408–3420. 10.1093/hmg/dds173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F., Manova K., Yuen J. P., Jasin M., and Keeney S., 2000. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking. Mol. Cell 6: 989–998. 10.1016/S1097-2765(00)00098-8 [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E., Rinaldi V. D., White M. E., and Schimenti J. C., 2014. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 343: 533–536. 10.1126/science.1247671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carofiglio F., Inagaki A., de Vries S., Wassenaar E., Schoenmakers S. et al. , 2013. SPO11-independent DNA repair foci and their role in meiotic silencing. PLoS Genet. 9: e1003538 10.1371/journal.pgen.1003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chao S.-B., Wang Z.-B., Qi S.-T., Zhu X.-L. et al. , 2012. Checkpoint kinase 1 is essential for meiotic cell cycle regulation in mouse oocytes. Cell Cycle 11: 1948–1955. 10.4161/cc.20279 [DOI] [PubMed] [Google Scholar]
- Cloutier J. M., Mahadevaiah S. K., ElInati E., Nussenzweig A., Tóth A. et al. , 2015. Histone H2AFX links meiotic chromosome asynapsis to prophase I oocyte loss in mammals. PLoS Genet. 11: e1005462 (erratum: PLoS Genet. 11: e1005753). 10.1371/journal.pgen.1005462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J. M., Mahadevaiah S. K., ElInati E., Tóth A., and Turner J., 2016. Mammalian meiotic silencing exhibits sexually dimorphic features. Chromosoma 125: 215–226. 10.1007/s00412-015-0568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G. B., Zielonka E. M., Coutandin D., Weber T. A., Schäfer B. et al. , 2011. DNA damage in oocytes induces a switch of the quality control factor TAp63α from dimer to tetramer. Cell 144: 566–576. 10.1016/j.cell.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo M., Barchi M., Baudat F., Edelmann W., Keeney S. et al. , 2005. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc. Natl. Acad. Sci. USA 102: 737–742. 10.1073/pnas.0406212102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay J. K., Hutt K. J., Hickey M., and Anderson R. A., 2015. How is the number of primordial follicles in the ovarian reserve established? Biol. Reprod. 93: 111 10.1095/biolreprod.115.133652 [DOI] [PubMed] [Google Scholar]
- Flaggs G., Plug A. W., Dunks K. M., Mundt K. E., Ford J. C. et al. , 1997. Atm-dependent interactions of a mammalian chk1 homolog with meiotic chromosomes. Curr. Biol. 7: 977–986. 10.1016/S0960-9822(06)00417-9 [DOI] [PubMed] [Google Scholar]
- Kim M.-A., Kim H.-J., Brown A. L., Lee M.-Y., Bae Y.-S. et al. , 2007. Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Exp. Mol. Med. 39: 205–212. 10.1038/emm.2007.23 [DOI] [PubMed] [Google Scholar]
- Kouznetsova A., Wang H., Bellani M., Camerini-Otero R. D., Jessberger R. et al. , 2009. BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J. Cell Sci. 122: 2446–2452. 10.1242/jcs.049353 [DOI] [PubMed] [Google Scholar]
- Lange J., Pan J., Cole F., Thelen M. P., Jasin M. et al. , 2011. ATM controls meiotic double-strand-break formation. Nature 479: 237–240. 10.1038/nature10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. C., and Schimenti J. C., 2007. Mouse pachytene checkpoint 2 (Trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 3: e130 10.1371/journal.pgen.0030130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G., Petre-Lazar B., Guerquin M.-J., Trautmann E., Coffigny H. et al. , 2008. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 135: 3–12. 10.1530/REP-07-0054 [DOI] [PubMed] [Google Scholar]
- Mahadevaiah S. K., Turner J. M., Baudat F., Rogakou E. P., de Boer P. et al. , 2001. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27: 271–276. 10.1038/85830 [DOI] [PubMed] [Google Scholar]
- Maréchal A., and Zou L., 2013. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 5: a012716. 10.1101/cshperspect.a012716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M., Britt K. L., Wreford N. G. M., Ebling F. J. P., and Kerr J. B., 2004. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127: 569–580. 10.1530/rep.1.00095 [DOI] [PubMed] [Google Scholar]
- Ou Y.-H., Chung P.-H., Sun T.-P., and Shieh S.-Y., 2005. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA-damage-induced C-terminal acetylation. Mol. Biol. Cell 16: 1684–1695. 10.1091/mbc.e04-08-0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco S., Marcet-Ortega M., Lange J., Jasin M., Keeney S. et al. , 2015. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 11: e1005017 10.1371/journal.pgen.1005017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera D., Perez-Hidalgo L., Moens P. B., Reini K., Lakin N. et al. , 2004. TopBP1 and ATR colocalization at meiotic chromosomes: role of TopBP1/Cut5 in the meiotic recombination checkpoint. Mol. Biol. Cell 15: 1568–1579. 10.1091/mbc.e03-06-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H., 1969. The development of the mouse ovary from birth to maturity. Acta Endocrinol. (Copenh.) 62: 98–116. 10.1530/acta.0.0620098 [DOI] [PubMed] [Google Scholar]
- Rinaldi V. D., Bolcun-Filas E., Kogo H., Kurahashi H., and Schimenti J. C., 2017. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol. Cell 67: 1026–1036.e2. 10.1016/j.molcel.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T., Nore A., Brun C., Maffre C., Crimi B. et al. , 2016. The TopoVIB-Like protein family is required for meiotic DNA double-strand break formation. Science 351: 943–949. 10.1126/science.aad5309 [DOI] [PubMed] [Google Scholar]
- Romanienko P. J., and Camerini-Otero R. D., 2000. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6: 975–987. 10.1016/S1097-2765(00)00097-6 [DOI] [PubMed] [Google Scholar]
- Shieh S. Y., Ahn J., Tamai K., Taya Y., and Prives C., 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14: 289–300. [PMC free article] [PubMed] [Google Scholar]
- Suh E.-K., Yang A., Kettenbach A., Bamberger C., Michaelis A. H. et al. , 2006. p63 protects the female germ line during meiotic arrest. Nature 444: 624–628. 10.1038/nature05337 [DOI] [PubMed] [Google Scholar]
- Turner J. M. A., Aprelikova O., Xu X., Wang R., Kim S. et al. , 2004. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14: 2135–2142. 10.1016/j.cub.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Turner J. M. A., Mahadevaiah S. K., Ellis P. J. I., Mitchell M. J., and Burgoyne P. S., 2006. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev. Cell 10: 521–529. 10.1016/j.devcel.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Wang X. Q., Redpath J. L., Fan S. T., and Stanbridge E. J., 2006. ATR dependent activation of Chk2. J. Cell. Physiol. 208: 613–619. 10.1002/jcp.20700 [DOI] [PubMed] [Google Scholar]
- Widger A., Mahadevaiah S. K., Lange J., ElInati E., Zohren J. et al. , 2018. ATR is a multifunctional regulator of male mouse meiosis. Nat. Commun. 9: 2621 10.1038/s41467-018-04850-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mouse strains that were not obtained from others will be made available upon request. Supplemental Material, Figure S1 is a Western blot that is a biological replicate of Figure 2. Table S1 contains primordial and total follicle counts for the genotypes included in Figure 1. Supplemental material available at figshare: https://doi.org/10.25386/genetics.12092187.
Figure 2.
Increased CHK1 activation and p53 stabilization, but not TAp63 activation, in CHK2-deficient oocytes. (A) CHK1 phosphorylation in oocytes is stimulated by induced or meiotic DSBs. Shown are Western blots probed with indicated antibodies. Each lane contains total protein extracted from four ovaries (postnatal day 3–5) that were either exposed or not to 3 Gy of ionizing radiation (IR). Ovaries were harvested for protein extraction 3 hr post-IR. The blots on the left, separated by a vertical bar from those on the right, were from a different blot and different protein samples and mice. The same two blots (left and right) were stripped and reprobed sequentially with the three antibodies. A biological replicate is shown in Figure S1. Note that the decreased MVH levels in Trip13Gt/Gt ovaries is due to reduction in oocytes. (B) Activation of the TAp63 isoform is dependent on DNA damage and CHK2 signaling, not asynapsis. Shown is a Western blot probed sequentially for TAp63 and the germ cell marker MVH. Each lane contains protein extracted from ovaries as described in A. An upward shift in the band indicates the presence of the active (phosphorylated) vs. inactive TAp63. A biological replicate is shown in Figure S1.
Figure 1.
Rescue of SPO11- and TRIP13-deficient oocytes by compound deletion of p53 and TAp63. (A) Hematoxylin and eosin stained ovaries from 2-month-old mice of the indicated genotypes. The top two rows are images are from histological sections through the approximate center of the ovaries. Bar, 500 µm. The dashed circle indicates the residual Trip13Gt/Gt ovary. The bottom row shows higher magnification images of selected genotypes. Bar, 100 µm. Yellow arrows indicate examples of primordial follicles. (B) Oocyte quantification. To the left is the quantification of primordial follicles [P < 0.0001 for all oocyte rescued (in bold) genotypes compared to nonrescued genotypes; P = 0.92 for oocyte rescued genotypes vs. WT and Trp53−/− TAp63−/− ovaries]. To the right are total oocytes (from all stages of follicles) from individual ovaries (P < 0.0001 for all oocyte rescued genotypes compared to nonrescued genotypes; P = 0.33 for oocyte rescued genotypes vs. WT and Trp53−/− TAp63−/− ovaries). Oocyte quantification data are presented in Table S1. Genotype abbreviations are as follows: TAp63 is abbreviated as p63; WT, wild type.