Background and aim
Recent clinical trials have confirmed that Helicobacter pylori infection is positively associated with nonalcoholic fatty liver disease (NAFLD), although some research has shown a negative association. Therefore, to confirm whether H. pylori eradication treatment is feasible for NAFLD patients in our hospital, we aimed to establish the association between H. pylori infection and NAFLD.
Methods
We enrolled 91 patients with NAFLD diagnosed by abdominal B-mode ultrasonography between January and December 2018. H. pylori infection was confirmed by C13 urea breath test, and liver function, glycometabolism, insulin sensitivity, lipid metabolism, as well as inflammatory reaction were assessed through blood biochemical analyses.
Results
A minority of NAFLD patients had liver dysfunction, increased fasting glucose and insulin levels, a score of insulin-resistance (HOMA-Ir), lipid metabolism, slight inflammatory response, fasting hyperglycemia and hypertension. Most patients were complicated with overweight/visceral obesity and dyslipidemia. Moreover, these abnormal indicators were closely associated with the severity of NAFLD and H. pylori infection. Notably, the prevalence of H. pylori infection showed a significant difference between mild, moderate and severe NAFLD, and hepatic steatosis with coexistent NAFLD also revealed a striking difference between H. pylori-positive and H. pylori-negative patients (P < 0.01).
Conclusion
Our results suggest that H. pylori infection may be an independent risk factor in NAFLD progress.
Keywords: glycometabolism, Helicobacter pylori infection, inflammatory reaction, lipid metabolism, liver function, metabolic syndrome, nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common chronic disease worldwide. The disease is characterized by excessive fat deposition in liver cells, and fatty, diffuse and bullous pathological alterations in liver cells, with the absence of alcohol and other liver-damaging factors [1–3]. If NAFLD is not actively prevented or treated, it gradually progresses toward liver fibrosis and cirrhosis [4]. Recently, NAFLD has been reported to affect 20–45% of people worldwide, 60–75% of obese individuals [5], and ~25% of Chinese [6,7]. A meta-analysis published in 2016 reported the prevalence data of NAFLD in different districts, with global prevalence (25%), North America (24%), Europe (24%) and Asia (27%) [8]. A new meta-analysis about massive epidemiological studies from 1999 to 2019 in Asian reported 30% prevalence and 50.9 cases/1000 person/years of incidence of NAFLD [9]. It is worth noting that the pooled nationwide prevalence of NAFLD was 29.2% in China and showing a increased trend from 25.4% in 2008–2010 to 32.3% in 2015–2018, with 14–25% NAFLD sufferers developed advanced fibrosis, which rooted in a updated Chinese NAFLD epidemiology of systematic review and meta-analysis that analyzed 392 studies including more than two million participants from 28 provinces and regions [10]. It is predicted the largest number of liver-associated deaths from NAFLD will appear in China by 2030 [11]. NAFLD is widely recognized as a serious public health issue and has been paid increased attention by researchers because of its high prevalence, unclear etiology and difficult treatment. The risk of NAFLD is also increased in patients with severe chronic diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease, chronic kidney disease and chronic liver disease [5,12–14]. Additionally, NAFLD is regarded as a hepatic component of metabolic syndromes, based on its high association with overweight/visceral obesity, dyslipidemia, insulin resistance/fasting hyperglycemia, T2DM and hypertension [15] Because of the increasing prevalence, and complexity of NAFLD in China, it has been a focus on looking for novel risk factors for prevention and treatment.
Helicobacter pylori was first isolated and cultured from the human gastric mucosal epithelium by researchers Marshall and Warren in 1983. H. pylori is a Gram-negative microaerophilic bacterium affecting >50% of humans worldwide, with a higher prevalence in developing countries [16,17]. A JGHF Marshall and Warren Lecture in 2017 about the epidemiology of H. pylori infection in Malaysia (three major Asian races living together – Malay, Chinese and Indian) reported that the H. pylori prevalence was high in Indians (>50%) and Chinese (40–50%), while a comparatively low prevalence occurred in Malays (10–20%) [18]. Moreover, at the same year, a systematic review and meta-analysis mentioned that the H. pylori infection is highly endemic in East Asian countries, with 54% of prevalence in China, Japan, Taiwan and Korea in 2015 [19]. Therefore, H. pylori is considered to be the major cause of gastric diseases, including chronic gastritis, gastric ulcer disease, gastric lymphoma such as gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and even gastric cancer such as gastric adenocarcinoma [20–22]. Subsequently, H. pylori was also detected in intestinal diseases such as duodenal ulcer [20], and jejunal metaplasia and dysplasia [23], and is reported to be a hazard in several extragastrointestinal diseases including: neurological disorders, such as Parkinson’s disease and Alzheimer’s disease; cardiovascular diseases such as ischemic heart disease; metabolic diseases or metabolic syndrome such as lipid metabolism disorder, obesity and T2DM; and hematological and immune disorders such as idiopathic thrombocytopenic purpura [24–29]. Recently, H. pylori infection has been proved pivotal in liver diseases, including NAFLD, nonalcoholic steatohepatitis, liver fibrosis and cirrhosis, and even liver cancer [30]. Thereby, H. pylori may be a novel risk factor for prevention and treatment of NAFLD.
Although several clinical and experimental large sample or cross-sectional studies from different countries have reported that H. pylori infection has a positive correlation with NAFLD [12,31,32], some high-quality clinical studies showed a negative correlation [33], or even no association [34–36]. The conclusions about the correlation between H. pylori infection and NAFLD are inconsistent, and the pathological mechanisms of H. pylori in NAFLD are unclear. Moreover, a case of the H. pylori association with NAFLD, the first report on the improvement of metabolic profile after H. pylori eradication in a NAFLD patient in 2013, which suggests a possible hypothesis: H. pylori treatment may be a promising strategy for liver steatosis [37]. Thus, to establish clinical experiment evidence of our next study about whether H. pylori eradication might be a simple and effective therapeutic strategy in NAFLD patients, we primarily and preliminarily investigated the relationship between H. pylori infection and NAFLD by analyzing clinical cases of NAFLD in Tianjin Medical University General Hospital, Tianjin, China.
Patients and methods
Patients
We enrolled 91 patients (nonsmokers and nondrinkers, 56 males, 35 females, mean age 52.8 ± 14.7 years, range 18–83 years) who were diagnosed with NAFLD by abdominal B-mode ultrasonography between January and December 2018 at the Tianjin Medical University General Hospital, Tianjin, China. Each patient was immediately required to undergo a face-to-face interview, including symptom description, age, gender, history of becoming overweight, smoking, drinking, structured diet, lifestyle and anamnesis in the preceding 6 months before initiation of the study, as well as a series of detailed medical examinations after hospitalization. NAFLD patients were analyzed for H. pylori infection, liver function, carbohydrate metabolism, lipid metabolism and inflammatory response, as well as the presence of metabolic syndrome. This clinical protocol was approved by our Hospital Ethics Committee and conformed to the 2008 Helsinki Declaration and Good Clinical Practice guidelines. Written informed consent was obtained from all patients before study onset.
The diagnosis or inclusion criteria of NAFLD complied with the Clinical Diagnostic Standards of the Chinese Liver Disease Association in 2011 [38] and the guidelines from the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology in 2012 [39]. The required diagnostic criteria for NAFLD were as follows [40,41]: (1) hepatic steatosis detected by abdominal B-mode ultrasonography; (2) echo near the liver was diffuse enhancement, and stronger than that of the kidney and spleen; (3) the liver far-field echo gradually attenuated; (4) the intrahepatic duct structure was not clear; (5) no significant alcohol consumption or <140 g (male) or <70 g (female) alcohol consumption per week; and (6) no coexisting causes of chronic liver disease, such as hepatitis C, medication, parenteral nutrition, Wilson’s disease or severe malnutrition.
The exclusion criteria were as follows [40]: (1) ≥140 g (male) or ≥70 g (female) alcoholic consumption per week; (2) any other chronic liver disease; (3) history of gastric surgery; (4) history of cancer or current cancer; (5) use of bismuth, antibiotics, proton pump inhibitors or H2 blockers within the prior 4 weeks; (6) severe infection; (7) significant mental or neurological disorder; (8) pregnancy and lactation; (9) missing data; and (10) steatogenic medication such as methotrexate and corticosteroids.
Classification of nonalcoholic fatty liver disease
NAFLD was detected by an abdominal high-resolution B-mode topographic ultrasound system (ACUSON X300, Siemens, Germany) by two well-trained examiners. The fitness status and laboratory test outcomes of the patients were not revealed to the examiners. NAFLD was divided into three types: mild, moderate and severe based on the Chinese Ultrasonic Grading Criteria of NAFLD published in 2003, as follows [42]. Mild fatty liver: the intrahepatic echo is enhanced, and the boundary of the hepatic vessels and diaphragm muscle can be seen; moderate fatty liver: the intrahepatic echo is moderately increased, and the appearance of intrahepatic ducts or diaphragm muscle is slightly attenuated; severe fatty liver: the intrahepatic echo is significantly increased, and the intrahepatic duct, diaphragm muscle, or posterior right lobe is poorly visible or invisible.
Assessment of H. pylori infection
The C13 urea breath test (HCBT-01 tester; Shenzhen Zhonghe Headway Biological Technology Co. Ltd., China) was utilized by experienced examiners for detecting the H. pylori infection. The specific steps and diagnostic criteria were as described previously [41].
Blood biochemical detection
Venous blood samples were collected after a 12-hour fast, and centrifuged at 3000 r/min for 10 minutes. The serum supernatant and plasma were collected for lipid metabolism and liver function tests, sugar metabolism, insulin sensitivity and inflammation index detection, using a Beckman Coulter LXi725 automatic biochemical analyzer and other kits. Several representative indexes for each test were randomly selected. The specific indexes were: liver function: alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), total protein, albumin (ALB) and globulin (GLB); glycometabolism: fasting plasma glucose (GLU) and glycosylated hemoglobin (HbAlc); insulin sensitivity: fasting plasma insulin (INS) and a score of insulin-resistance, as a homeostasis model assessment of insulin resistance (HOMA-Ir); lipid metabolism: total cholesterol (CHOL), triglyceride, high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C); inflammation: fasting plasma white blood cell (WBC), lymphocyte (LYMPH), middle cell (MID) and granulocyte (GRAN) counts. The specific check methods were carried out as described previously [43]. HOMA-Ir was calculated as fasting glucose (mmol/L) × fasting insulin level (mIU/L)/22.5 [44].
Diagnosis of metabolic syndrome
Metabolic syndrome was evaluated on the basis of the Japanese criteria published in 2005 [45]. The metabolic syndromes of overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension were most common and co-occurred with NAFLD, and thus, the metabolic syndromes above were chosen for our study. BMI and resting blood pressure were measured by experienced medical nurses, and the diagnosis of these metabolic syndromes was made as previously described [31,46].
Statistical analysis
Statistical analysis was performed using SPSS version 16.0, and all the outcomes were expressed as numbers, percentages and the means ± SD. For significant differences, the quantitative data between two groups were assessed by independent-samples t tests. Quantitative data between three groups were evaluated by one-way analysis of variance. For equal variance assumed data, the least significant difference t test and Student–Neuman–Keuls q test were utilized. For equal variance unassumed data, Dunnett’s T3 test was used. For comparison of differences of rate, data between two or more groups, the nonparametric Mann–Whitney U test or Pearson’s χ2 test were carried out. P <0.05 was deemed statistically significant.
Results
A small number of nonalcoholic fatty liver disease patients showed liver function damage, increased glucose metabolism, lipid metabolism and inflammatory reactions, while most developed metabolic syndromes
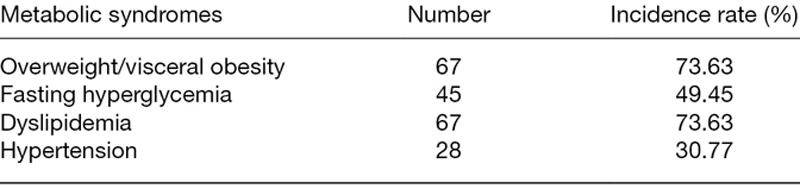
For liver function measurements, 26.37% of NAFLD patients displayed an abnormal increase in ALT in the range of 41–79 U/L, which exceeded the normal reference range of 5–40 U/L. There was an abnormal rise of TBIL in 10.99% of NAFLD patients, and abnormal increase in DBIL in 7.69%. Total protein, ALB and GLB were normal in all NAFLD patients. For carbohydrate metabolism, GLU, HbAlc, INS and HOMA-Ir were all abnormally enhanced in 45.05, 31.87, 36.26 and 45.05% of NAFLD patients, respectively. For lipid metabolism, 42.86% of NAFLD patients showed an abnormal rise in TC compared with the normal reference value, while 5.49% showed an abnormal decrease in TC. A total of 43.96% of NAFLD patients revealed a preternatural increase in triglyceride. Compared with the normal reference ranges, HDL-C showed an abnormal decline in 9.89% of NAFLD patients, while LDL-C showed an exceptional increase in 29.67%. For inflammatory response estimates, WBC count was abnormally increased in 9.89% of NAFLD patients and preternaturally decreased in 2.20%, compared to the normal reference range. A total of 9.89% of NAFLD patients showed an exceptional increase in GRAN count, while 3.30% had an abnormal reduction. MID count was abnormally increased in 8.79% of NAFLD patients, while LYMPH count was within the normal reference ranges (Table 1). As for metabolic syndrome decisions, the incidence rates of overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension were 73.63, 49.45, 73.63 and 30.77%, respectively (Table 2). The above abnormal parameters were analyzed further, as follows.
Table 1.
Estimate of the liver function, carbohydrate metabolism, lipid metabolism and inflammatory response in 91 nonalcoholic fatty liver disease patients
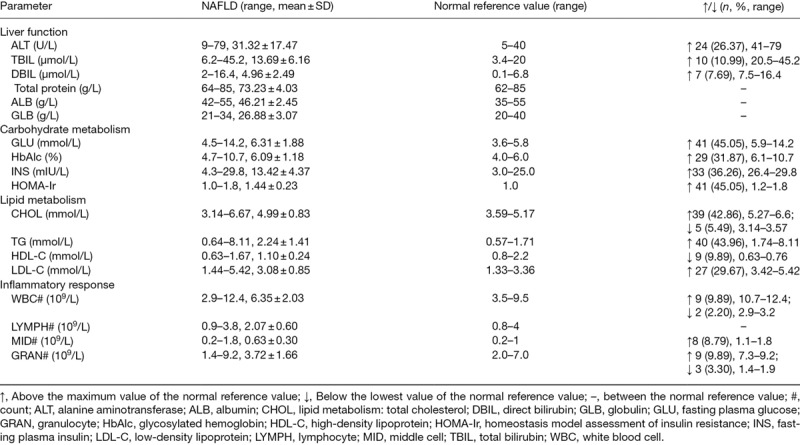
Table 2.
Some nonalcoholic fatty liver patients are complicated with metabolic syndromes like overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension in 91 nonalcoholic fatty liver disease patients
Severity of nonalcoholic fatty liver disease is associated with liver function, carbohydrate metabolism, lipid metabolism and inflammatory reaction, as well as incidence of metabolic syndromes
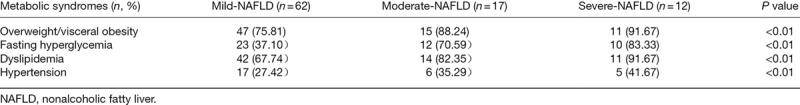
From mild to severe NAFLD, excluding a linear decrease in HDL-C, the indexes of liver function, carbohydrate metabolism, lipid metabolism and inflammatory response, as well as the incidence of disparate metabolic syndromes showed a linear upward trend. For liver function, compared with mild NAFLD, moderate NAFLD showed a higher level of ALT (P = 0.000), and ALT was higher in severe than in mild NAFLD (P = 0.000) and moderate NAFLD (P = 0.027). Severe NAFLD patients displayed added TBIL content compared with mild NAFLD (P = 0.001) and moderate NAFLD (P = 0.013), while DBIL showed no significant difference between each two groups of mild, moderate and severe NAFLD (P > 0.05). For carbohydrate metabolism, comparisons of each two groups of mild, moderate and severe NAFLD revealed no significant differences in GLU and HbAlc (P > 0.05), and a distinct differences in INS and HOMA-Ir (P < 0.05 or 0.01). For lipid metabolism, severe NAFLD patients showed higher LDL-C levels compared with mild NAFLD (P = 0.000) and moderate NAFLD (P = 0.008) patients. TC, triglyceride and HDL-C showed no significant differences between mild, moderate and severe NAFLD patients (P > 0.05). For inflammatory reaction, compared with mild NAFLD patients, both moderate (P = 0.015 and 0.023) and severe NAFLD (P = 0.006 and 0.007) patients showed higher WBC and GRAN counts. MID count showed no change between each two groups of mild, moderate and severe NAFLD patients (P > 0.05) (Table 3). For metabolic syndromes, the severity of NAFLD was worse, and the incidence of overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension was higher (P < 0.01) (Table 4).
Table 3.
Liver function, carbohydrate metabolism, lipid metabolism and inflammatory response are relative to severity degree in 91 patients with nonalcoholic fatty liver
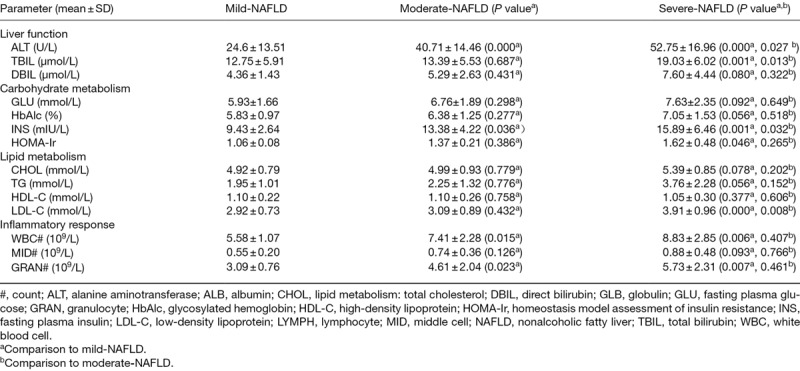
Table 4.
The incidence rate of metabolic syndromes such as overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension is related to the severity degree of nonalcoholic fatty liver
Severity of nonalcoholic fatty liver disease is related to H. pylori infection
The H. pylori infection rate was significantly higher in patients with severe NAFLD (P < 0.01) (Table 5). Furthermore, the striograph of abdominal B-mode ultrasonography revealed a consistent outcome with H. pylori infection rate comparable between mild, moderate and severe NAFLD patients. Under the premises of the same H. pylori infection statuses, the severity degree of NAFLD patients was more fearful, more white background was seen in the image maps, and the intrahepatic ducts were unclear, which suggested that steatosis increased with severity of NAFLD. At the consistent severity degree of NAFLD, patients with positive H. pylori infection showed more severe steatosis with white background and unclear intrahepatic ducts in the B-mode ultrasonography image maps, compared with H. pylori-negative NAFLD patients (Fig. 1).
Table 5.
The severity degree of nonalcoholic fatty liver is associated with H. pylori infection
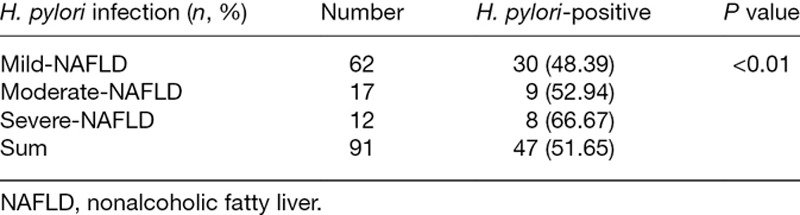
Fig. 1.
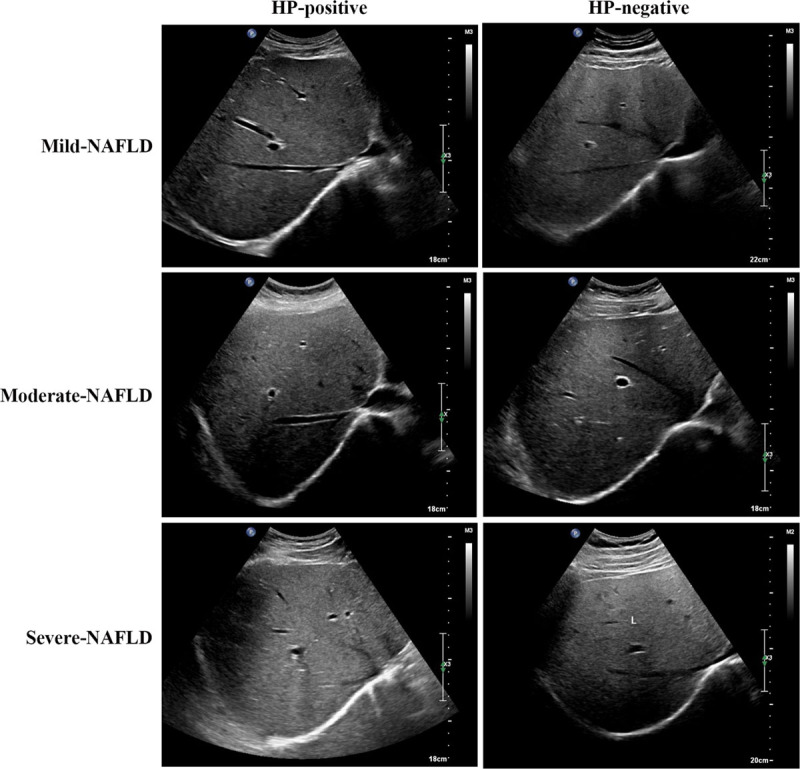
Representative B-mode ultrasonography image maps of different NAFLD patients. The white background represents steatosis tissues, and the black piping shadow represents intrahepatic ducts. The white standard measurement bar on the top right corner is used for evaluating the brightness of the white background.NAFLD, nonalcoholic fatty liver disease.
Liver function, carbohydrate metabolism, inflammatory response and incidence of metabolic syndromes in nonalcoholic fatty liver disease patients are positively correlated with H. pylori infection
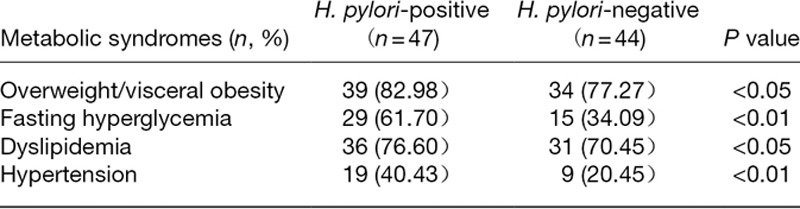
For liver function assessment, in H. pylori-positive NAFLD patients, compared with H. pylori-negative patients, the DBIL level was increased (P = 0.027) and there was no change in ALT and TBIL levels (P > 0.05). For carbohydrate metabolism evaluation, in H. pylori-positive NAFLD patients, compared with H. pylori-negative patients, GLU and HbAlc levels were significantly enhanced (P < 0.01), INS and HOMA-Ir were also increased with P <0.05. For lipid metabolism measurement, in H. pylori-positive NAFLD patients, compared with H. pylori-negative patients, TC, triglyceride, HDL-C and LDL-C levels showed no significant differences (P > 0.05). For inflammatory response detection, in H. pylori-positive NAFLD patients, compared with H. pylori-negative patients, significantly more WBCs (P = 0.029) and GRANs (P = 0.033) were detected. However, compared with the H. pylori-negative NAFLD patients, H. pylori-positive NAFLD patients displayed no changed in MID count (P > 0.05) (Table 6). As for metabolic syndromes, the occurrence of overweight/visceral obesity, dyslipidemia (P < 0.05), fasting hyperglycemia and hypertension (P < 0.01) was higher in H. pylori-positive than in H. pylori-negative NAFLD patients (Table 7).
Table 6.
Liver function, carbohydrate metabolism and inflammatory response are relation with H. pylori infection in 91 patients with nonalcoholic fatty liver
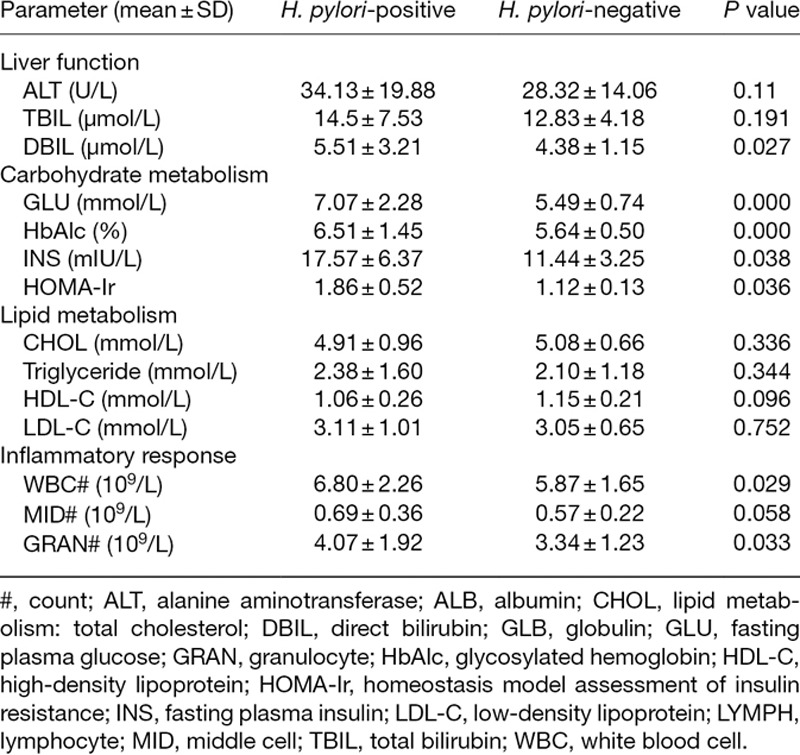
Table 7.
The incidence rate of metabolic syndromes such as overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension is related to the H. pylori infection
H. pylori infection may be involved in the correlation between severity of NAFLD and liver function, carbohydrate metabolism, lipid metabolism and inflammatory response, as well as the incidence of metabolic syndromes.
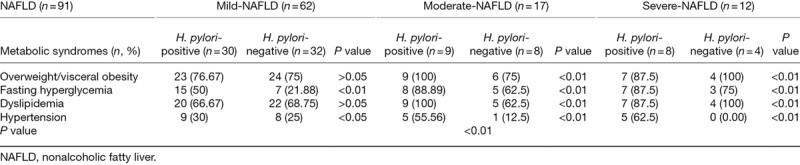
In patients with an equivalent severity of NAFLD, liver function (ALT, TBIL and DBIL), carbohydrate metabolism (GLU, HbAlc, INS and HOMA-Ir), lipid metabolism (TC, TG, HDL-C and LDL-C), inflammatory reaction (WBC, MID and GRAN counts), and metabolic syndromes (overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension) all differed significantly between H. pylori-positive and H. pylori-negative patients (P < 0.05 or P < 0.01). In patients with similar H. pylori infection status, there were marked differences in the above covariates between patients with mild, moderate and severe NAFLD (P < 0.01) (Tables 8 and 9).
Table 8.
H. pylori infection may be involved in the relations between the severity degree of nonalcoholic fatty liver patients and liver function, carbohydrate metabolism, lipid metabolism and inflammatory response
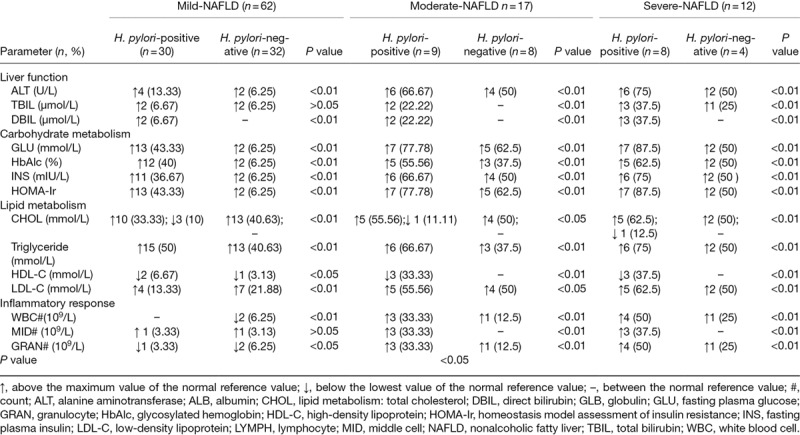
Table 9.
H. pylori infection may be involved in the relations between the severity degree of NAFLD patients and the occurrence rate of metabolic syndromes
Discussion
We demonstrated that liver dysfunction, increased glucose and lipid metabolism, slight inflammatory response, fasting hyperglycemia and hypertension occurred in a minority of NAFLD patients, while the majority of patients were complicated with overweight/visceral obesity and dyslipidemia. The abnormalities in the all above indexes can reflect the severity of NAFLD. H. pylori infection is positively correlated with the severity or steatosis status of NAFLD patients, and with increased liver dysfunction, abnormal carbohydrate metabolism and inflammatory reaction, as well as incidence of metabolic syndromes such as overweight/visceral obesity, fasting hyperglycemia, dyslipidemia and hypertension. However, NAFLD has no effect on lipid metabolism. In order to rule out the influence of NAFLD severity or H. pylori infection on the above abnormalities, while independently considering the role of H. pylori infection or NAFLD severity, we performed a further analysis. We discovered that H. pylori infection had an adverse effect on liver function, glycometabolism, insulin sensitivity, lipid metabolism, inflammatory reaction and occurrence of metabolic syndromes in patients with similar severity of NAFLD. In patients with similar H. pylori infection status, NAFLD severity was also positively associated with these parameters. These findings suggest that H. pylori infection is an independent risk factor for NAFLD progress through increasing liver dysfunction, abnormal glycometabolism, insulin sensitivity, lipid metabolism, inflammatory reaction and occurrence of metabolic syndromes.
Previous studies on the relationship between H. pylori infection and hepatic diseases have been inconsistent. A systematic review in 2018 showed that H. pylori infection has a positive correlation with NAFLD, but a negative association has also been reported in large and small cross-sectional studies, retrospective studies, randomized controlled trials, retrospective studies, and meta-analyses. In addition, except for a negative relationship between H. pylori infection and chronic B viral hepatitis identified in a large cross-sectional study in 2011, positive correlations between H. pylori infection and other hepatic diseases such as chronic viral hepatitis, cirrhosis, and hepatocellular carcinoma have been reported in cross-sectional studies and meta-analyses [47]. However, the focuses that H. pylori infection contributing to NAFLD is disparate.
A cross-sectional study of 4081 individuals found that 52.36% were H. pylori positive and 47.82% H. pylori-positive individuals were diagnosed with NAFLD. Moreover, in H. pylori-positive NAFLD patients, women and patients with dyslipidemia showed a significant increase in the fully adjusted odds ratios and 95% confidence intervals [12]. A prospective cohort multicenter pilot study with 2 years of follow-up in 369 adults without NAFLD at baseline indicated that H. pylori infection was positively associated with NAFLD development [increased NAFLD-liver fat score (NAFLD-LFS) and hepatic steatosis index (HSI)]. This was shown by increased markers of insulin resistance, inflammatory mediators such as interleukin-6 and C-reactive protein (CRP), and lipid metabolism such as the leptin/adiponectin ratio, and decreased lipid metabolism marker, HDL. Furthermore, these NAFLD risk factors can be recovered after eradication of H. pylori infection [31]. Additionally, a cohort study carried out in 17 028 adults without NAFLD at baseline showed that H. pylori infection was significantly related to NAFLD development, independent of metabolic and inflammatory risk factors [32]. However, the lipid metabolism markers of TC, triglyceride, HDL-C and LDL-C showed no significant difference between H. pylori-positive and H. pylori-negative NAFLD patients in our study. Notably, a significant difference occurred after removal of the confounding factor of NAFLD status. In a large study of 20 389 Chinese subjects, compared with the control group, the NAFLD group showed a higher prevalence of H. pylori infection, and combination of H. pylori infection and WBC count was positively correlated with NAFLD by multivariate logistic regression [40]. However, the association between H. pylori infection and WBC count was not demonstrated in that study. Moreover, NAFLD patients have a higher incidence of H. pylori infection at genetic, small molecular and clinical levels, as analyzed by the Pathway Studio Res Net Mammalian database and the clinical data of 2263 elderly Southern Chinese subjects [41]. A small clinical study indicated that H. pylori infection (H. pylori IgG seropositivity or C13 urea breath test positivity) may be a contributing factor in the pathogenesis of NAFLD, but not in the progression from NAFLD to nonalcoholic steatohepatitis [48]. Based on these previous results, our findings supported that WBC count was positively correlated with NAFLD [40], and H. pylori infection was highly associated with occurrence of hypertension [32]. Importantly, we also concluded that H. pylori infection was positively related to NAFLD development, but the evidence in our study was distinct. We are the first to demonstrate that H. pylori infection is positively involved in development of NAFLD via increasing steatosis and severity of NAFLD. Furthermore, we revealed that H. pylori infection may be an autocephalous risk factor for forecasting NAFLD progress, including liver dysfunction, exceptionally increased glycometabolism and lipid metabolism, inflammatory reaction and occurrence of metabolic syndromes, as well as decresing insulin sensitivity. Weightily, NAFLD is recognized as hepatic manifestation of metabolic syndromes with the characteristics similar to those of metabolic disorders including obesity, inflammation and insulin resistance [49]. A score of insulin-resistance (HOMA-Ir) was increased in NAFLD patients compared with the control people, and insulin-resistance (HOMA-Ir) was used as a diagnostic and predictive factor of NAFLD [50–52]. Additionally, infection of H. pylori was related to a higher insulin resistance degree [53]. The eradication of H. pylori has been proved beneficial to insulin resistance, atherogenic serum lipids abnormalities and mild inflammation [54]. These previous reports forcefully supported our finds that H. pylori infection is positively associated with NAFLD.
Conversely, in a randomized open-label clinical trial in 100 patients, H. pylori eradication did not affect liver fat content, liver function tests including serum ALT, AST and ALP, lipid profiles including triglyceride, CHOL, HDL and LDL, and HOMA-IR index in dyspeptic NAFLD patients [55]. H. pylori eradication has also been shown to have no long-term effect on hepatic steatosis, but it did improve NAFLD fibrosis score and HSENSI (homocysteine, serum glutamic oxaloacetic transaminase, erythrocyte sedimentation rate, nonalcoholic steatohepatitis index) in an MRI-based pilot open-label study of 13 adults with biopsy-proven nonalcoholic steatohepatitis [56]. A cross-sectional study of 21 456 subjects who underwent a healthy checkup program in China showed that H. pylori infection increased BMI, blood pressure and lipid metabolism (higher levels of triglyceride, and lower levels of HDL-C), as well as the prevalence of NAFLD in women but not in men. However, H. pylori infection is not associated with NAFLD in the total population after adjusting for the above confounding factors [34]. A large-scale cross-sectional study performed in 13 737 adults in Japan in 2010 revealed that BMI, serum ALT and platelet count were positively correlated with FLD and NAFLD, but not H. pylori infection. In addition, in male patients, metabolic syndrome was significantly associated with FLD and NAFLD [35]. A retrospective study of 3663 healthy people who underwent health screening found that H. pylori infection was closely associated with older age, male gender, hypertension, higher BMI and dyslipidemic profile, whereas NAFLD (HSI and NAFLD-LFS) was not. Moreover, CRP concentration and smoking were identified as significant risk factors for NAFLD [36]. Despite the opposing conclusions reported in these studies, the study participants and analytical approaches also differed from our study.
Although some novel and valuable discoveries were found in our study, there were still some limitations. Due to the limited clinical data, it was difficult to analyze the effects of sex, age or other sensitive factors on H. pylori infection or NAFLD; thus, the outcomes did not rule out these factors having an effect. Additionally, the standards for H. pylori detection and classification of NAFLD are one dimensional. For instance, H. pylori IgG seropositivity is also a highly sensitive and scientific diagnostic method for H. pylori infection [48], and NAFLD-LFS and HSI are frequently used and recognized diagnostic criteria for NAFLD progress or severity [31,36]. Increasing the number of NAFLD patients and further study of therapeutic effects of a follow-up of the patients after H. pylori eradication are needed to confirm the relationship between H. pylori infection and NAFLD.
In summary, H. pylori infection is positively correlated with NAFLD. Considering the limited clinical data in our study, further investigations are necessary to confirm the connection between H. pylori infection and NAFLD, which may be undoubtedly afford novel insights for effective prevention and treatment of NAFLD.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004; 279:32345–32353 [DOI] [PubMed] [Google Scholar]
- 2.Bhala N, Younes R, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Curr Pharm Des. 2013; 19:5169–5176 [DOI] [PubMed] [Google Scholar]
- 3.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007; 11:1–16, vii [DOI] [PubMed] [Google Scholar]
- 4.Fan XF, Deng YQ, Wu GL. Study on the distribution and characteristics of Chinese medicine syndrome in patients with nonalcoholic fatty liver disease Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011; 31:1332–1336 [PubMed] [Google Scholar]
- 5.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015; 313:2263–2273 [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010; 28:155–161 [DOI] [PubMed] [Google Scholar]
- 7.Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012; 61:409–415 [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019; 4:389–398 [DOI] [PubMed] [Google Scholar]
- 10.Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of nonalcoholic fatty liver disease in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019; 70:1119–1133 [DOI] [PubMed] [Google Scholar]
- 11.Lee HW, Wong VW. Changing NAFLD epidemiology in China. Hepatology. 2019; 70:1095–1098 [DOI] [PubMed] [Google Scholar]
- 12.Jiang T, Chen X, Xia C, Liu H, Yan H, Wang G, Wu Z. Association between Helicobacter pylori infection and non-alcoholic fatty liver disease in north Chinese: a cross-sectional study. Sci Rep. 2019; 9:4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikolasevic I, Milic S, Turk Wensveen T, Grgic I, Jakopcic I, Stimac D, et al. Nonalcoholic fatty liver disease - amultisystem disease? World J Gastroenterol. 2016; 22:9488–9505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang MH, Sung J, Gwak GY. The associations between apolipoprotein B, A1, and the B/A1 ratio and nonalcoholic fatty liver disease in both normal-weight and overweight Korean population. J Clin Lipidol. 2016; 10:289–298 [DOI] [PubMed] [Google Scholar]
- 15.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1219 [DOI] [PubMed] [Google Scholar]
- 16.Cheng DD, He C, Ai HH, Huang Y, Lu NH. The possible role of Helicobacter pylori infection in non-alcoholic fatty liver disease. Front Microbiol. 2017; 8:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol. 2009; 15:2839–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh KL. Lessons learnt from the epidemiology of Helicobacter pylori infection in Malaysia: JGHF Marshall and Warren lecture 2017. J Gastroenterol Hepatol. 2018; 33:1177–1184 [DOI] [PubMed] [Google Scholar]
- 19.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017; 153:420–429 [DOI] [PubMed] [Google Scholar]
- 20.Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. Pylori and host immune defenses. Clin Microbiol Rev. 2006; 19:597–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013; 62:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362:1597–1604 [DOI] [PubMed] [Google Scholar]
- 23.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012; 13:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Korwin JD, Ianiro G, Gibiino G, Gasbarrini A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter. 2017; 22Suppl 1 [DOI] [PubMed] [Google Scholar]
- 25.Aoshiba K, Tsuji T, Yamaguchi K, Itoh M, Nakamura H. The danger signal plus DNA damage two-hit hypothesis for chronic inflammation in COPD. Eur Respir J. 2013; 42:1689–1695 [DOI] [PubMed] [Google Scholar]
- 26.Wu MS, Lee WJ, Wang HH, Huang SP, Lin JT. A case-control study of association of Helicobacter pylori infection with morbid obesity in Taiwan. Arch Intern Med. 2005; 165:1552–1555 [DOI] [PubMed] [Google Scholar]
- 27.Wong F, Rayner-Hartley E, Byrne MF. Extraintestinal manifestations of Helicobacter pylori: a concise review. World J Gastroenterol. 2014; 20:11950–11961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dogan Z, Sarikaya M, Ergul B, Filik L. The effect of Helicobacter pylori eradication on insulin resistance and hba1c level in people with normal glucose levels: a prospective study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015; 159:242–245 [DOI] [PubMed] [Google Scholar]
- 29.Nasif WA, Mukhtar MH, Nour Eldein MM, Ashgar SS. Oxidative DNA damage and oxidized low density lipoprotein in type II diabetes mellitus among patients with Helicobacter pylori infection. Diabetol Metab Syndr. 2016; 8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waluga M, Kukla M, Żorniak M, Bacik A, Kotulski R. From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol. 2015; 7:2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Razik A, Mousa N, Shabana W, Refaey M, Elhelaly R, Elzehery R, et al. Helicobacter pylori and non-alcoholic fatty liver disease: anew enigma? Helicobacter. 2018; 23:e12537. [DOI] [PubMed] [Google Scholar]
- 32.Kim TJ, Sinn DH, Min YW, Son HJ, Kim JJ, Chang Y, et al. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J Gastroenterol. 2017; 52:1201–1210 [DOI] [PubMed] [Google Scholar]
- 33.Kang SJ, Kim HJ, Kim D, Ahmed A. Association between caga negative Helicobacter pylori status and nonalcoholic fatty liver disease among adults in the United States. Plos One. 2018; 13:e0202325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease: across-sectional study in China. Front Microbiol. 2018; 9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okushin K, Takahashi Y, Yamamichi N, Shimamoto T, Enooku K, Fujinaga H, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. 2015; 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeg MK, Yoon SK, Ko SH, Noh YS, Lee IS, Choi MG. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2016; 22:2592–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abenavoli L, Milic N, Masarone M, Persico M. Association between non-alcoholic fatty liver disease, insulin resistance and Helicobacter pylori. Med Hypotheses. 2013; 81:913–915 [DOI] [PubMed] [Google Scholar]
- 38.Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, Chan LY; Chinese Association for the Study of Liver Disease. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18:163-166). J Dig Dis. 2011; 12:38–44 [DOI] [PubMed] [Google Scholar]
- 39.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012; 142:1592–1609 [DOI] [PubMed] [Google Scholar]
- 40.Yu YY, Cai JT, Song ZY, Tong YL, Wang JH. The associations among Helicobacter pylori infection, white blood cell count and nonalcoholic fatty liver disease in a large Chinese population. Medicine (Baltimore). 2018; 97:e13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CX, Mao YS, Foster P, Zhu ZW, Du J, Guo CY. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl Physiol Nutr Metab. 2017; 42:295–301 [DOI] [PubMed] [Google Scholar]
- 42.Li ZA. Clinical Ultrasound Imaging. 2003, Beijing: People's Health Publishing House; 924–925 [Google Scholar]
- 43.Wang Y, Ge S, Yan Y, Wang A, Zhao Z, Yu X, et al. China suboptimal health cohort study: rationale, design and baseline characteristics. J Transl Med. 2016; 14:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petito-da-Silva TI, Souza-Mello V, Barbosa-da-Silva S. Empaglifozin mitigates NAFLD in high-fat-fed mice by alleviating insulin resistance, lipogenesis and ER stress. Mol Cell Endocrinol. 2019; 498:110539. [DOI] [PubMed] [Google Scholar]
- 45.Japanese A I. Definition and the diagnostic standard for metabolic syndrome–Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nihon Naika Gakkai Zasshi. 2005; 94:794–809 [PubMed] [Google Scholar]
- 46.Wang Y, Jiang T, Wang X, Zhao J, Kang J, Chen M, et al. Association between insomnia and metabolic syndrome in a Chinese han population: across-sectional study. Sci Rep. 2017; 7:10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okushin K, Tsutsumi T, Ikeuchi K, Kado A, Enooku K, Fujinaga H, et al. Helicobacter pylori infection and liver diseases: epidemiology and insights into pathogenesis. World J Gastroenterol. 2018; 24:3617–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E, Zafeiriadou E, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. 2013; 62:121–126 [DOI] [PubMed] [Google Scholar]
- 49.Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016; 78:181–205 [DOI] [PubMed] [Google Scholar]
- 50.Isokuortti E, Zhou Y, Peltonen M, Bugianesi E, Clement K, Bonnefont-Rousselot D, et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: a population-based and inter-laboratory study. Diabetologia. 2017; 60:1873–1882 [DOI] [PubMed] [Google Scholar]
- 51.Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul). 2019; 34:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harsha Varma S, Tirupati S, Pradeep TVS, Sarathi V, Kumar D. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019; 13:1065–1069 [DOI] [PubMed] [Google Scholar]
- 53.Vafaeimanesh J, Bagherzadeh M, Heidari A, Motii F, Parham M. Diabetic patients infected with Helicobacter pylori have a higher insulin resistance degree. Caspian J Intern Med. 2014; 5:137–142 [PMC free article] [PubMed] [Google Scholar]
- 54.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J. 2010; 103:190–196 [DOI] [PubMed] [Google Scholar]
- 55.Jamali R, Mofid A, Vahedi H, Farzaneh R, Dowlatshahi S. The effect of Helicobacter pylori eradication on liver fat content in subjects with non-alcoholic fatty liver disease: a randomized open-label clinical trial. Hepat Mon. 2013; 13:e14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polyzos SA, Nikolopoulos P, Stogianni A, Romiopoulos I, Katsinelos P, Kountouras J. Effect of Helicobacter pylori eradication on hepatic steatosis, NAFLD fibrosis score and HSENSI in patients with nonalcoholic steatohepatitis: a MR imaging-based pilot open-label study. Arq Gastroenterol. 2014; 51:261–268 [DOI] [PubMed] [Google Scholar]


