Abstract
Primary sclerosing cholangitis (PSC) is a rare, immune-mediated, chronic cholestatic liver disease associated with a unique phenotype of inflammatory bowel disease that frequently manifests as pancolitis with right-sided predominance. Available data suggest a bidirectional interplay of the gut-liver axis with critical roles for the gastrointestinal microbiome and circulating bile acids (BAs) in the pathophysiology of PSC. BAs shape the gut microbiome, whereas gut microbes have the potential to alter BAs, and there are emerging data that alterations of BAs and the microbiome are not simply a consequence but the cause of PSC. Clustering of PSC in families may suggest that PSC occurs in genetically susceptible individuals. After exposure to an environmental trigger (e.g., microbial byproducts or BAs), an aberrant or exaggerated cholangiocyte-induced immune cascade occurs, ultimately leading to bile duct damage and progressive fibrosis. The pathophysiology can be conceptualized as a triad of (1) gut dysbiosis, (2) altered BA metabolism, and (3) immune-mediated biliary injury. Immune activation seems to be central to the disease process, but immunosuppression does not improve clinical outcomes or alter the natural history of PSC. Currently, orthoptic liver transplantation is the only established life-saving treatment, whereas antimicrobial therapy or fecal transplantation is an emerging therapeutic option for PSC. The beneficial effects of these microbiome-based therapies are likely mediated by a shift of the gut microbiome with favorable effects on BA metabolism. In the future, personalized approaches will allow to better target the interdependence between microbiome, immune function, and BA metabolism and potentially cure patients with PSC.
INTRODUCTION
Definitions and clinical presentation
Primary sclerosing cholangitis (PSC) is an uncommon chronic cholestatic liver disease characterized by inflammation and fibrosis of intra- and extrahepatic bile ducts, leading to the formation of multifocal bile duct strictures and progressive fibrotic transformation of bile ducts (1). Hepatic fibrosis is promoted by these biliary changes, ultimately leading to cirrhosis and liver failure (2). There is a strong association between PSC and inflammatory bowel disease (IBD), particularly ulcerative colitis (UC). Between 2.5% and 7.5% of individuals with IBD will eventually develop PSC. Conversely, between 60% and 70% of patients with PSC have IBD (3). Furthermore, the phenotype of IBD occurring in PSC is unique and differs from the typical phenotype seen in patients with UC or Crohn's disease (CD) (4). Patients with PSC-IBD typically have mild intestinal disease activity and an increased incidence of extensive colitis typically pancolitis, with a right-sided predominance, rectal sparing, and backwash ileitis.
Diagnosis
In the clinical setting, a diagnosis of PSC (1) is made in patients with a cholestatic biochemical profile when cholangiography (e.g., magnetic resonance cholangiography or endoscopic retrograde cholangiography) shows characteristic bile duct changes with multifocal strictures and segmental dilatations, and secondary causes of sclerosing cholangitis have been excluded (5).
Epidemiology
PSC occurs more commonly in men than in women (2:1). The mean age at the time of diagnosis is approximately 40 years (6). Using a random-effects model, a systematic review and meta-analysis concluded that the pooled incidence rate for PSC was 0.77 (0.45–1.09) per 100,000 person-years (7).
Morbidity and mortality
PSC is often progressive, leading to fibrosis, cirrhosis, and end-stage liver disease. In the absence of any pharmacological therapy, orthotopic liver transplantation (OLT) represents the only curative option (8,9). The median life expectancy after a diagnosis of PSC is 13.2–21.3 years without liver transplantation (6). There is a 3-fold mortality rate increase (hazard ratio 2.92, 95% CI 2.16–3.94) and a 2-fold increase in risk of any malignancy (hazard ratio 2.23, 95% CI 0.88–6.11) in patients with PSC compared with the general population; (10). However, cancer-related death far exceeds death caused by end-stage liver disease and other nonmalignant complications of the disease (11).
PATHOPHYSIOLOGY
The pathophysiology of PSC is multifactorial, with genetic and environmental factors implicated.
Genetics
It is now believed that PSC occurs in genetically susceptible individuals (12) after exposure to an environmental trigger (13). This exposure initiates a series of events involving complex interactions between the innate and adaptive immune systems, ultimately leading to gut-derived activated lymphocytes migrate to the liver, cholangiocyte damage, and progressive fibrosis (8). The obvious association between PSC and IBD points toward a possible role of autoimmunity (2). Indeed, characteristic human leukocyte antigen haplotype associations have been recognized in PSC (2), and large-scale genome-wide association studies have identified genotypic associations in cohorts with PSC (14,15). More than 70% of patients with PSC also have IBD, and at least 2 common gene loci have been identified in genome-wide association studies for PSC and UC (14). Overall, the most plausible explanation for the pathogenesis of PSC is immunogenic priming in a genetically predisposed individual, leading to the characteristic phenotype, that is likely further influenced by other host or environmental factor (8).
Host/patient immune response
Several important observations, coupled with the strong association between certain human leukocyte antigen haplotypes and frequency of concurrent extrahepatic autoimmune disorders, support the concept that PSC is an immune-mediated phenomenon. The possible role of liver-gut crosstalk in PSC and UC pathogenesis may be related to the enterohepatic circulation of lymphocytes (16) referred to as the gut lymphocyte homing hypothesis. This theory postulates that a subset activated T cells from the gut appear home in on the liver, then initiate and inflict damage (17).
Gastrointestinal microbiota
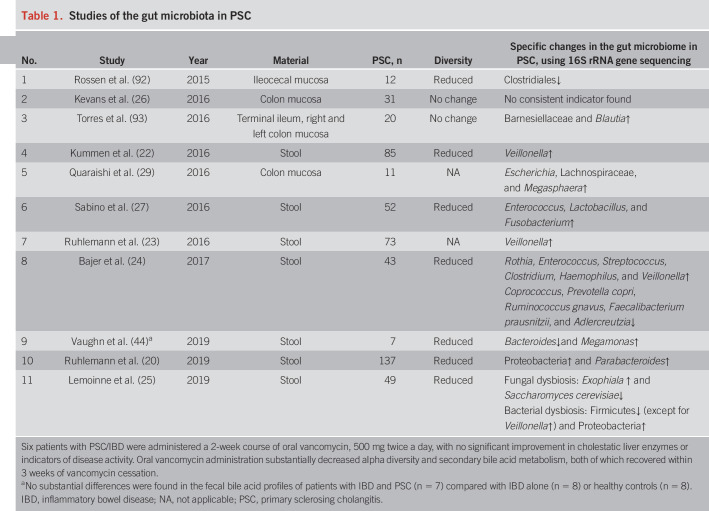
As more than 60% of patients with PSC have coexistent IBD, the pathogenesis of PSC seems to be related to the manifestation of the inflammatory changes of the bowel that seem to mirror UC. It is now widely accepted that the microbiome plays a key role for the manifestation and progression of IBD (18). Similarly, there is now accumulating evidence that alterations of the gastrointestinal microbiome are central to the pathogenesis of PSC (19). Several clinical trials (Table 1) provide evidence that the stool microbiome and the microbiome adherent to the colonic mucosa are distinct in patients with PSC compared with patients with IBD without PSC and non-IBD controls.
Table 1.
Studies of the gut microbiota in PSC
The thus far largest study on the microbiome (20) included 137 patients with PSC and found differences in the microbiota profiles between patients with PSC and UC. Despite the observed dysbiosis, it was not possible to differentiate PSC and UC based on microbial taxonomic characteristics. The taxonomic differences between patients with PSC and UC may have pathophysiological significances (21). Microbes can contribute—either in isolation or collectively via specific metabolic products and/or interference with the mucosal immune system—toward the pathophysiology of PSC (or IBD). Several studies (20,22–27) have shown that patients with PSC have an overrepresentation of Veillonella spp. This taxon (and others enriched in patients with PSC-IBD) can produce amine oxidases. Amine oxidase such as vascular adhesion protein-1 (VAP-1) facilitates adhesion of gut-tropic lymphocytes to the liver endothelium (28) and potentially contributes to the manifestation of PSC-IBD via aberrant lymphocyte tracking between the bowel and liver (29). The alteration in the relative abundance of Veillonella in the gut microbiome is not specific for PSC and is potentially a marker of advanced liver disease (30,31). In addition, Vieria-Silva et al. (32) have very observed that an increase in Enterococcus spp. is positively associated with serum alkaline phosphatase (ALP) levels and bile duct obstruction. They also reported that increases in Veillonella and Fusobacterium spp. are both capable of driving the proinflammatory burden associated with PSC and PSC-IBD but are coexclusive, with members of the latter genus favored in patients with CD or PSC-CD. Lemoinne et al. (25), reported fungal dysbiosis in patients with PSC. They found a decrease in the proportion of the Saccharomycetaceae family, including the species Saccharomyces cerevisiae, known to have anti-inflammatory properties and shown to be decreased in patients with active IBD (33). All these findings suggest that the gut microbiome might be involved in the pathogenesis of PSC (and associated IBD) while patients with PSC and IBD have a microbiome that is distinct from patients with IBD alone. Thus far, no specific microbial signature unique to PSC has been identified based on relative abundances alone, and more studies with comprehensive functional and quantitative characterization of the microbiome are warranted.
Interplay between gastrointestinal microbiome, BA metabolism, and immune function in PSC
The gastrointestinal microbiome and metabolism of BAs.
The human gut microbiota consists of thousands of different bacterial species and other microorganisms with defined functions. This microbial ecosystem maintains homeostasis through a tight balance between the mucosal immune system, cell-to-cell signaling, and subsequent release of antimicrobial peptides to control neighboring bacterial clades (34). Commensal microbial metabolism produces essential vitamins and short-chain fatty acids (SCFAs) and modifies a variety of endogenous and exogenous sources of complex molecules (e.g., BAs and xenobiotics) that can contribute to the gut metabolome (35,36). The SCFAs, particularly butyrate, regulate innate and adaptive immune cell generation, trafficking, and function and have anti-inflammatory effects. Thus, SCFAs play an active role in modulating the mucosal immune system to establish a tolerant phenotype against beneficial commensal microbiota (37). However, the biodiversity of gut microbes implicated in coordinating BA metabolism and their conversion(s) from primary to secondary BAs is narrow and perhaps better defined from a genetic and biochemical context than other aspects of microbial metabolism in the human gut (38).
Hepatocytes synthesize primary BAs, cholic acid (CA), and chenodeoxycholic acid from cholesterol, which are conjugated with taurine or glycine (making them more water soluble) for secretion into bile. Primary BAs are secreted into the bile ducts and are stored in the gallbladder during the interdigestive phase (39). After a meal, cholecystokinin (CCK) released from the pancreas stimulates gallbladder contraction to release BAs into the duodenum. A small proportion of bile secreted into the duodenum reaches the terminal ileum and colon (40), where 95% of BAs are absorbed by active transport and transported via the portal vein to the liver where they are taken up by hepatocytes and re-excreted. This process in known as enterohepatic circulation. Microbes in the ileum and colon transform primary BAs (CA and chenodeoxycholic acid) into hydrophobic secondary BAs (lithocholic acid and deoxycholic acid) by wide array of bacterial transformation including deconjugation catalyzed by the bile salt hydrolase, dehydroxylation by 7-alpha dehydroxylase, oxidation/reduction, and epimerization (41). Some of these secondary BAs are reabsorbed during colonic transit and are also circulated in portal blood to the liver. The enterohepatic circulation of the highly hydrophobic and toxic secondary BAs and increased concentrations in the liver have been linked to inflammation, cholestasis, gallstone formation (42), and carcinogenesis (43). Because of the colonic absorption of BAs, only a small proportion of BAs (0.5 g/d) is excreted in the feces. These are replaced by de novo synthesis of BAs in the liver. This could explain the similarity of fecal BA profiles in patients with IBD and PSC, IBD alone, or healthy controls (44).
There is emerging evidence that luminal bile acids affect the gastrointestinal microbiome. BAs have antimicrobial activity by damaging the bacterial cell membrane and thus inhibiting bacteria outgrowth (45). Reduced BA concentrations are associated with bacterial overgrowth (46,47). Indeed, a recent systematic review and meta-analysis (48) suggested that advanced chronic liver disease is associated with bacterial overgrowth. Gut bacteria metabolize BAs and thus influence BA composition and BA hydrophobicity (49). These BAs also shape the gut microbiota communities, whereas intestinal microbes alter BAs. Thus, there is a bidirectional interaction between the intestinal microbiota and BAs (50). Thus, there is a link between gastrointestinal microbes and hepatic BA metabolisms. This gut-to-liver axis may play a critical role in the regulation of enterohepatic circulation of BAs, BA pool size, and BA composition. Alterations of the BA homeostasis and excessive intrahepatic accumulation of (potentially toxic) BAs and/or their metabolites are thought to play a pivotal role in mediating the hepatic injury of cholestatic diseases (51).
BA metabolism and mucosal immune function.
The regulatory functions of BAs are mainly the result of activation of intracellular ligand-activated nuclear receptors, such as the farnesoid X receptor (FXR, NR1H4) and cell surface G protein–coupled receptors, specifically the G protein–coupled BA receptor (TGR5 or GPBAR1) (52). Both receptors are highly represented in cells of innate immunity such as intestinal and liver macrophages, dendritic cells, and natural killer T cells. Thus, both FXR and TGR5 are critical regulators of BA metabolism, BA circulation, and intestinal immune function and contribute to the maintenance of a tolerogenic phenotype in enterohepatic tissues (52).
Bile acids also directly shape mucosal immune function via interactions with both dedicated BA receptors (FXR and GPBAR1) and nonspecific BA sensors (pregnane X receptor, vitamin D receptor, and constitutive androstane receptor). In the context of innate immunity (53–55), BAs suppress NF-κB–dependent signaling pathways (56) and inhibit NLRP3-dependent inflammasome activities (55). A recent study by Hang et al. (57) showed that BAs directly regulate the adaptive immune cells via proinflammatory Th17 cells and anti-inflammatory regulatory T cells (Tregs). A successful host immune response is generally the result of both pro- and anti-inflammatory elements that are carefully tuned with the goal of clearing the pathogen while limiting host damage (autoimmunity) (58). Thus, gut microbes can modify BAs, which in turn regulate the gut immunity and inflammation. Furthermore, the impact of mucosal immune function on BA metabolism is currently unknown and will be an area of future investigation. The 3-dimensional interplay between BAs, the microbiota, and the mucosal immune system, proposed by Chen et al. (59), likely plays a crucial role in regulating the mucosal immune homeostasis.
Gut barrier function.
Although alterations of gut barrier function might be related to the mucosa-associated microbiome in the small intestine (60,61), there is also evidence that in patients with PSC, gut barrier dysfunction is associated with a poor outcome (62). Bacterial translocation and/or absorption of bacterial endotoxins such as lipopolysaccharide, lipoteichoic acid, and bacterial DNA/RNA fragments into the portal circulation via a chronically inflamed leaky bowel, combined with Kupffer cell activation, may be crucial in eliciting the proinflammatory, profibrotic hepatobiliary responses that lead to the development of PSC (5,63).
Gut barrier function and host-microbe interactions
The host-microbe interactions may play a pivotal role for the so-called leaky gut and PSC microbiota hypotheses. Indeed, in an animal model of PSC, the epithelial-damaging effect of Klebsiella pneumoniae isolated from patients with PSC was linked to bacterial translocation and increased susceptibility to TH17-mediated injuries of bile ducts (64). These findings are well aligned with the concept that microbes or microbial products may lead to an aberrant or exaggerated immune response of the cholangiocyte (increased induction of cholangiocyte senescence and senescence-associated secretory phenotype), which plays a central role in the pathogenesis of PSC (5). This is further supported by the thought that the epithelial cells lining the bile ducts are not only a target of injury in PSC but also actively involved drivers in the course of disease (65,66).
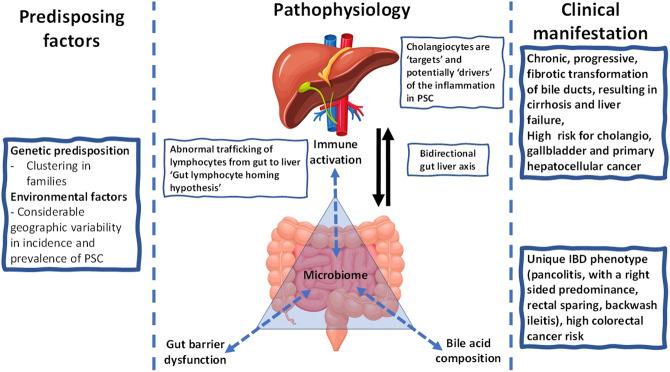
This suggests an interrelationship between gastrointestinal microbes, BAs, and immune-mediated damage to the epithelial lining of the bile ducts, results in an ongoing inflammatory process that defines the pathophysiology of PSC (Figure 1).
Figure 1.
Conceptual framework for primary sclerosing cholangitis (PSC) exploring the interrelationship between bidirectional gut-liver axis.
TREATMENT
A recent Clinical Guideline of the American College of Gastroenterology (67) concludes that there is “...no approved or proven therapy…” for PSC. On the other hand, there is some evidence that antibiotic therapy is beneficial in patients with PSC. Since an initial case series of a patient with PSC was published in 1958 (68), a number of treatments including immune suppression have been tested, but none have proven to alter the course of disease or cure this disease. Indeed, until now, PSC has a poor prognosis, and OLT is so far the only proven therapy to extend life expectancy.
Treatment targeting the microbiome
Against the background of the American College of Gastroenterology clinical guideline, there is emerging evidence, albeit from relatively small open-label studies that in patients with PSC, targeted modulation of the gastrointestinal microbiome may alter the course of disease to delay or even stop disease progression. The first study on the potential effect of antibiotics in PSC was published in 1959 by Rankin et al. (69). Subsequent case series and studies in adults (70–74) testing various antimicrobial agents in the treatment of PSC demonstrated improvement in ALP and the Mayo PSC Risk Score as accepted markers for the progression of PSC (75,76). In a recent cohort study (77), 17 children were treated with oral vancomycin for UC related to PSC or autoimmune sclerosing cholangitis. In 15 of 17 patients, oral vancomycin therapy achieved mucosal healing (Mayo endoscopic score 0), with 9 achieving histological remission. LFTs improved transiently or normalized in at least 12 patients.
Our recent systematic review and meta-analysis (78) of available studies assessed and compared the efficacy of various antibiotics in adult patients with PSC with or without coexisting IBD with regard to (i) improvement of liver enzymes, (ii) Mayo PSC Risk Score, and (iii) number of adverse drug reactions resulting in discontinuation of antibiotic therapy. In this review, vancomycin seemed to be the most effective antibiotic with regard to clinical improvement and adverse effects. Most recently, a small case series by Dao A et al. (79) found that oral vancomycin was effective for the induction and maintenance of remission of UC in adults with UC-PSC. All had long-standing disease (average duration 15 years), with 5 of 8 patients having undergone OLT for PSC. However, very little is known about the specific mechanisms, and alterations of the gastrointestinal microbiome are associated with response during treatment and relapse after discontinuation of therapy.
Overall, alterations of the gastrointestinal microbiome by antibiotic therapy seem to alter the natural course of PSC and delay the progression of disease. It might even be speculated that favorable permanent changes of the gastrointestinal microbiome could potentially cure PSC.
Evolving disease concepts
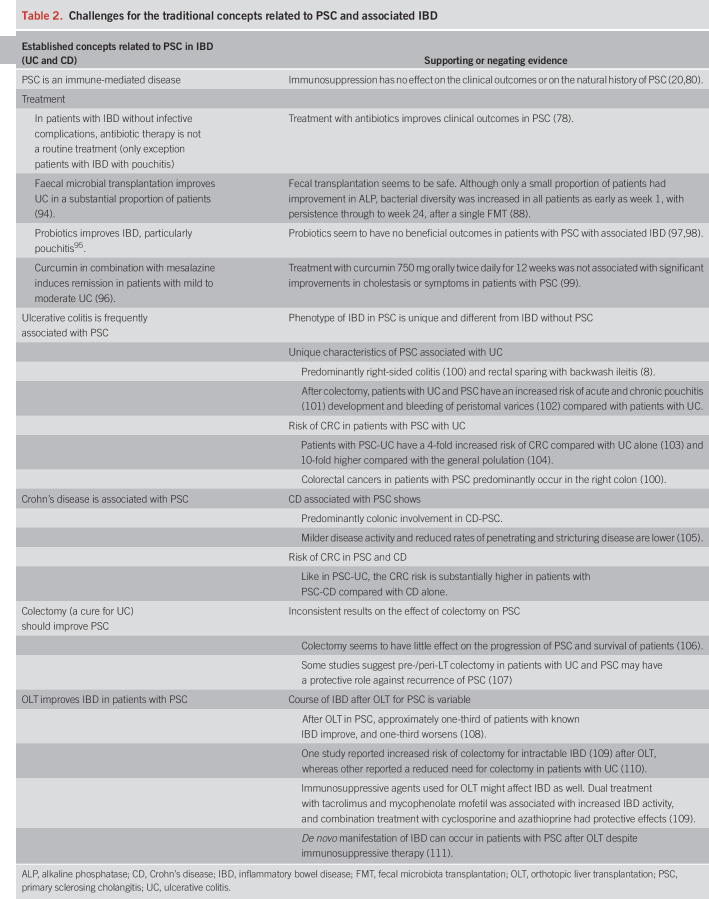
PSC is frequently accompanied by IBD. However, the clinical features of IBD in patients with PSC are unique and distinct from typical patients with IBD (Table 2).
Table 2.
Challenges for the traditional concepts related to PSC and associated IBD
PSC is a progressive chronic inflammatory disease with fibro-obliterative destruction of extra- and intrahepatic bile ducts. These histologic features suggest that PSC is an immune-mediated disorder. However, immune suppression with steroids or other immunosuppressants does not seem to alter the natural history of PSC (80). Thus, other causes for the inflammatory process might be considered. Indeed, a recent systematic review and meta-analysis found that antibiotic therapy and in particular treatment with the nonabsorbable antibiotic vancomycin was associated with clinical improvement. The clinical and biochemical response was better for vancomycin compared with the other antibiotics studied (78). The fact that an antibiotic improved outcomes may further suggest that microbes are involved in the pathophysiology of PSC. Moreover, nonabsorbable antibiotics such as vancomycin were effective, likely suggesting that a gastrointestinal microbe is central to pathogenesis of PSC. Many Gram-positive bacteria are able to deconjugate, dehydroxylate, oxidize, and epimerize primary BAs into secondary BAs (81,82), whereas no deconjugation activity has been detected in Gram-negative intestinal bacteria, with the exception of 2 species of Bacteroides (83). Unlike deconjugation, dehydroxylation is only performed by a minor population of Gram-positive anaerobic Clostridium species (84).
Treatment with vancomycin specifically targets Gram-positive bacteria (85), which includes various Clostridium spp. known to be primarily involved with the dehydroxylation of primary BAs, into the secondary BAs present in the distal small intestine and colon (82,84). This relatively narrow specificity of vancomycin might be a potential explanation for its superior effects on PSC, whereas the other antibiotics (such as rifaximin, metronidazole, and minocycline) trialed in the treatment of PSC are widely considered to be broad spectrum. Thus, it is possible that vancomycin through its effect on gut microbes influences BA metabolism. In support of a selective effect of vancomycin on Gram-positive species, a recent RCT (85) of 20 males with metabolic syndrome showed that treatment with vancomycin—compared with amoxycillin—reduced fecal microbial diversity with a decrease in Gram-positive bacteria (mainly Firmicutes phylum, e.g., species belonging to the Clostridium clusters IV and XIVa) and a compensatory increase in Gram-negative bacteria (mainly Proteobacteria). Treatment with vancomycin not only decreased the fecal secondary BAs and increased the primary BAs but also decreased postprandial plasma concentrations of secondary bile salts and increased that of primary bile salts. Vancomycin induced reduction of the hydrophobic secondary BAs, which are potentially responsible for the right-sided colitis and after absorption for mediating the bile duct injury, could perhaps explain its beneficial role in PSC.
In a mouse model (86), vancomycin was able to reverse mycophenolate mofetil (MMF)-induced GI toxicity (colonic inflammation and weight loss) by potentially restoring the MMF-induced dysbiosis of the microbiota. MMF alters the composition of the gut microbiota, selecting for bacteria expressing the enzyme β-glucuronidase (GUS) and leading to an upregulation of GUS activity, thereby leading to increased concentrations of active mycophenolic acid, which is associated with colonic inflammation. Oral vancomycin depleted classes Bacteroidia and Clostridia, while allowing expansion of Gammaproteobacteria and Bacilli. Although vancomycin has a relatively narrow antibiotic spectrum, it is likely that very specific bacteria are responsible for this anti-inflammatory effect.
Vancomycin, in addition to its effect on the gut microbes, perhaps also has an immunomodulatory effect. A study of 14 children with PSC and UC treated with oral vancomycin (87) showed that in addition to symptom resolution and normalization of liver biochemistry, there was an increase in peripheral CD4+FoxP3+ regulatory T (Treg) cells and transforming growth factor beta (TGF-beta) levels in response to the antibiotic, suggesting an immunomodulatory effect of vancomycin. The immunomodulatory effect of vancomycin was thought to be due to its direct effect on the T-cell inflammatory pathway via the TNF-alpha pathways and downstream Treg induction. This may suggest a immunomodulatory effect of vancomycin via the TNF-alpha inflammatory pathways and/or downstream Treg induction.
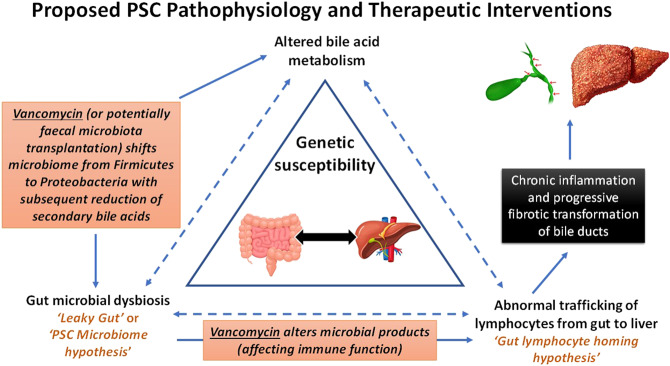
Fecal microbiota transplantation (FMT) refers to the transfer of intestinal microbes from a healthy donor into a recipient with the intent of modifying the recipient's intestinal microbiome. The pilot study (88) of 10 patients with PSC with concomitant IBD in remission who underwent FMT (from a single donor) showed ≥50% reduction in ALP levels in 30% of the treated patients with PSC. This study suggests that FMT treatment is safe and results in an increased stool microbial diversity that persisted after FMT. Engrafting OTUs included genera capable of producing short-chain fatty acids, which are known to be depleted in IBD. Larger controlled studies are needed to define efficacy and define the mechanisms of FMT in PSC. Figure 2 summarizes microbiome-based therapy, targeting key pathophysiologic mechanisms central to PSC. Although FMT increases the overall stool microbial diversity, oral vancomycin therapy is associated with reduced fecal microbial diversity. Thus far, more data point toward improved clinical outcomes in response to antibiotic therapy with vancomycin; both interventions might be effective, and the precise mechanisms of these microbiome-based therapies remain to be explored.
Figure 2.
Pathophysiology of PSC. Against the background of a genetic susceptibility, specific components of microbiome or metabolic products can trigger an exaggerated immune response from cholangiocytes. Alternatively (or in addition), the gut dysbiosis is responsible for altered metabolic function of the gut microbiome with subsequent changes of the bile acid pool or an increased intestinal permeability. Treatment with vancomycin (or potentially fecal microbiota transplantation) shifts the microbiome toward, e.g., Proteobacteria with subsequent effects on the production of secondary bile acids. This affects the bile acid pool and influences favorably cholangiocyte biology. PSC, primary sclerosing cholangitis.
FUTURE DIRECTIONS
Antimicrobial treatments targeting the gastrointestinal microbiome seem to result in significant improvement in the clinical and cholestatic biomarkers (78) and—based on case reports (87,89–91)—even long-term outcomes in PSC. FMT in PSC seems to be safe and is likely associated with a sustained improvement in the overall microbial diversity and liver enzymes. This points toward the gastrointestinal microbiome as an important disease modifier for PSC. So far, most microbiome research in PSC has focused on the stool microbiome, whereas some studies assessed the colonic mucosa–associated microbiome (Table 1). The bidirectional gut-liver axis makes it equally possible that the mucosa-associated microbiome in the small intestine harbors the microbes that are critical for the pathophysiologic process that causes PSC and the associated IBD. So far, there is cumulating evidence that the stool microbiome and the intestinal microbiome are different in patients with PSC compared with controls (Table 1), and this may explain the altered metabolic functions (e.g., in relation to metabolism of BAs) or immune function that are related to the pathophysiology of PSC.
Although the available data suggest that targeting the gut microbial dysbiosis either by antibiotic therapy (in particular vancomycin) or FMT might be an effective approach in at least a subgroup of patients with PSC, prospective randomized placebo-controlled trials are lacking. These trials also should explore the links between sustained alterations of the gastrointestinal microbiome during treatment and long-term outcomes. These data would be critical to better understand the precise mechanisms by which antibiotic therapy in PSC alters the clinical course and could potentially allow to develop nonantibiotic interventions that ultimately could cure with this currently fatal disease. Understanding the interdependence between microbiome, immune function, BA metabolism, and the changes induced by microbiome-targeting therapies such as antibiotics or FMT will allow us to define new targets for therapy and potentially develop a cure for patients with PSC. The ultimate solution might not necessarily be a treatment that targets the microbiome but a treatment that blocks either the metabolism of a microbial product or the effects of a microbial product(s) that drives the manifestation and progression of PSC.
There is a need for further in‐depth studies using system biology approaches in combination with longitudinal multilevel analyses in patients with PSC (including appropriate controls with and without other cholestatic liver diseases) to elucidate potential targets for interventions. This ultimately will result in a better understanding of the unique clinical pattern of PSC and the factors that drive manifestation and disease progression of patients with PSC with and without concomitant IBD.
CONFLICTS OF INTEREST
Guarantor of the article: Gerald Holtmann, MD, PhD, MBA, FRACP, FRCP, FAHMS.
Financial support: Grant support provided by the Princess Alexandra Hospital Research Foundation.
Specific author contributions: A.S. and G.H.: review idea, concept and design, and drafting of the manuscript. G.A.M. and M.M: drafting of the manuscript and review of the final manuscript.
Potential competing interests: None to report.
REFERENCES
- 1.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet 2013;382:1587–99. [DOI] [PubMed] [Google Scholar]
- 3.de Vries AB, Janse M, Blokzijl H, et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 2015;21:1956–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsaitas C, Semertzidou A, Sinakos E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J Hepatol 2014;6:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabibian JH, Ali AH, Lindor KD. Primary sclerosing cholangitis. Part 1: Epidemiology, etiopathogenesis, clinical features, and treatment. Gastroenterol Hepatol (N Y) 2018;14:293–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58:2045–55. [DOI] [PubMed] [Google Scholar]
- 7.Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: A systematic review and meta-analysis. Hepatology 2011;53:1590–9. [DOI] [PubMed] [Google Scholar]
- 8.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013;145:521–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660–78. [DOI] [PubMed] [Google Scholar]
- 10.Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: A population-based cohort study. J Hepatol 2008;48:939–44. [DOI] [PubMed] [Google Scholar]
- 11.Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol 2019;25:659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley EM, LaRusso NF, Ludwig J, et al. Familial occurrence of primary sclerosing cholangitis and ulcerative colitis. Gastroenterology 1983;85:1160–5. [PubMed] [Google Scholar]
- 13.Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc 2015;90:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinghaus D, Folseraas T, Holm K, et al. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology 2013;58:1074–83. [DOI] [PubMed] [Google Scholar]
- 15.Melum E, Franke A, Schramm C, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet 2011;43:17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 2006;6:244–51. [DOI] [PubMed] [Google Scholar]
- 17.Grant AJ, Lalor PF, Salmi M, et al. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 2002;359:150–7. [DOI] [PubMed] [Google Scholar]
- 18.McIlroy J, Ianiro G, Mukhopadhya I, et al. Review article: The gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol Ther 2018;47:26–42. [DOI] [PubMed] [Google Scholar]
- 19.Karlsen TH. Primary sclerosing cholangitis: 50 years of a gut–liver relationship and still no love? Gut 2016;65:1579–81. [DOI] [PubMed] [Google Scholar]
- 20.Ruhlemann M, Liwinski T, Heinsen FA, et al. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment Pharmacol Ther 2019;50:580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quraishi MN, Shaheen WA. Editorial: Gut microbial profile associated with primary sclerosing cholangitis—what is new and how do we progress from here? Aliment Pharmacol Ther 2019;50:605–6. [DOI] [PubMed] [Google Scholar]
- 22.Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2016;66:611–9. [DOI] [PubMed] [Google Scholar]
- 23.Ruhlemann MC, Heinsen FA, Zenouzi R, et al. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut 2017;66:753–4. [DOI] [PubMed] [Google Scholar]
- 24.Bajer L, Kverka M, Kostovcik M, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol 2017;23:4548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemoinne S, Kemgang A, Ben Belkacem K, et al. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 2019;69:92–102. [DOI] [PubMed] [Google Scholar]
- 26.Kevans D, Tyler AD, Holm K, et al. Characterization of intestinal microbiota in ulcerative colitis patients with and without primary sclerosing cholangitis. J Crohns Colitis 2016;10:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016;65:1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trivedi PJ, Tickle J, Vesterhus MN, et al. Vascular adhesion protein-1 is elevated in primary sclerosing cholangitis, is predictive of clinical outcome and facilitates recruitment of gut-tropic lymphocytes to liver in a substrate-dependent manner. Gut, 2018;67:1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quraishi MN, Sergeant M, Kay G, et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut 2017;66:386–8. [DOI] [PubMed] [Google Scholar]
- 30.Wei X, Yan X, Zou D, et al. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol 2013;13:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira-Silva S, Sabino J, Valles-Colomer M, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol 2019;4:1826–31. [DOI] [PubMed] [Google Scholar]
- 33.Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD Gut 2017;66:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez KB, Leone V, Chang EB. Microbial metabolites in health and disease: Navigating the unknown in search of function. J Biol Chem 2017;292:8553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature 2011;474:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonçalves P, Araújo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis 2018;24:558–72. [DOI] [PubMed] [Google Scholar]
- 38.Hylemon PB, Harris SC, Ridlon JM. Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Lett 2018;592:2070–82. [DOI] [PubMed] [Google Scholar]
- 39.Chiang JY. Bile acids: Regulation of synthesis. J Lipid Res 2009;50:1955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtmann G, Kelly DG, Sternby B, et al. Survival of human pancreatic enzymes during small bowel transit: Effect of nutrients, bile acids, and enzymes. Am J Physiol 1997;273:G553–8. [DOI] [PubMed] [Google Scholar]
- 41.Ridlon JM, Harris SC, Bhowmik S, et al. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016;7:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low-Beer TS, Nutter S. Colonic bacterial activity, biliary cholesterol saturation, and pathogenesis of gallstones. Lancet 1978;2:1063–5. [DOI] [PubMed] [Google Scholar]
- 43.Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: An underrecognized cause of colon cancer. World J Surg Oncol 2014;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaughn BP, Kaiser T, Staley C, et al. A pilot study of fecal bile acid and microbiota profiles in inflammatory bowel disease and primary sclerosing cholangitis. Clin Exp Gastroenterol 2019;12:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurdi P, Kawanishi K, Mizutani K, et al. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol 2006;188:1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clements WD, Parks R, Erwin P, et al. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Gut 1996;39:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah A, Shanahan E, Macdonald GA, et al. Systematic review and meta-analysis: Prevalence of small intestinal bacterial overgrowth in chronic liver disease. Semin Liver Dis 2017;37:388–400. [DOI] [PubMed] [Google Scholar]
- 49.Wahlström A, Sayin SI, Marschall H-U, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 50.Chiang JY. Bile acid metabolism and signaling. Compr Physiol 2013;3:1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolbright BL, Dorko K, Antoine DJ, et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol 2015;283:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorucci S, Biagioli M, Zampella A, et al. Bile acids activated receptors regulate innate immunity. Front Immunol 2018;9:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 2014;11:55–67. [DOI] [PubMed] [Google Scholar]
- 54.Ma C, Han M, Heinrich B, et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 2016;45:802–16. [DOI] [PubMed] [Google Scholar]
- 56.Vavassori P, Mencarelli A, Renga B, et al. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol 2009;183:6251–61. [DOI] [PubMed] [Google Scholar]
- 57.Hang S, Paik D, Yao L, et al. Bile acid metabolites control TH17 and Treg cell differentiation. 2019:576;143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cicchese JM, Evans S, Hult C, et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev 2018;285:147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen ML, Takeda K, Sundrud MS. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol 2019;12:851–61. [DOI] [PubMed] [Google Scholar]
- 60.Jung TH, Park JH, Jeon WM, et al. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract 2015;9:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rios-Covian D, Ruas-Madiedo P, Margolles A, et al. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tornai T, Palyu E, Vitalis Z, et al. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol 2017;23:5412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Hara SP, Tabibian JH, Splinter PL, et al. The dynamic biliary epithelia: Molecules, pathways, and disease. J Hepatol 2013;58:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamoto N, Sasaki N, Aoki R, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 2019;4:492–503. [DOI] [PubMed] [Google Scholar]
- 65.Tabibian JH, O'Hara SP, Splinter PL, et al. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014;59:2263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabibian JH, Trussoni CE, O'Hara SP, et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest 2014;94:1126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindor KD, Kowdley KV, Harrison ME. ACG clinical guideline: Primary sclerosing cholangitis. Am J Gastroenterol 2015;110:646–59; quiz 660. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz SI, Dale WA. Primary sclerosing cholangitis; review and report of six cases. AMA Arch Surg 1958;77:439–51. [PubMed] [Google Scholar]
- 69.Rankin JG, Boden RW, Goulston SJ, et al. The liver in ulcerative colitis; treatment of pericholangitis with tetracycline. Lancet 1959;2:1110–2. [DOI] [PubMed] [Google Scholar]
- 70.Silveira MG, Torok NJ, Gossard AA, et al. Minocycline in the treatment of patients with primary sclerosing cholangitis: Results of a pilot study. Am J Gastroenterol 2009;104:83–8. [DOI] [PubMed] [Google Scholar]
- 71.Farkkila M, Karvonen AL, Nurmi H, et al. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: A randomized placebo-controlled trial. Hepatology 2004;40:1379–86. [DOI] [PubMed] [Google Scholar]
- 72.Tabibian JH, Weeding E, Jorgensen RA, et al. Randomised clinical trial: Vancomycin or metronidazole in patients with primary sclerosing cholangitis—A pilot study. Aliment Pharmacol Ther 2013;37:604–12. [DOI] [PubMed] [Google Scholar]
- 73.Rahimpour S, Nasiri-Toosi M, Khalili H, et al. A triple blinded, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of oral vancomycin in primary sclerosing cholangitis: A pilot study. J Gastrointestin Liver Dis 2016;25:457–64. [DOI] [PubMed] [Google Scholar]
- 74.Tabibian JH, Gossard A, El-Youssef M, et al. Prospective clinical trial of Rifaximin therapy for patients with primary sclerosing cholangitis. Am J Ther 2017;24:e56–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 2000;75:688–94. [DOI] [PubMed] [Google Scholar]
- 76.Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther 2014;40:1292–301. [DOI] [PubMed] [Google Scholar]
- 77.Tan LZ, Reilly CR, Steward-Harrison LC, et al. Oral vancomycin induces clinical and mucosal remission of colitis in children with primary sclerosing cholangitis–ulcerative colitis. Gut 2019;68:1533–5. [DOI] [PubMed] [Google Scholar]
- 78.Shah A, Crawford D, Burger D, et al. Effects of antibiotic therapy in primary sclerosing cholangitis with and without inflammatory bowel disease: A systematic review and meta-analysis. Semin Liver Dis 2019;39:432–41. [DOI] [PubMed] [Google Scholar]
- 79.Dao A, Abidian M, Lestrange A, et al. Oral vancomycin induces and maintains remission of ulcerative colitis in the subset of patients with associated primary sclerosing cholangitis. Inflamm Bowel Dis 2019;25:e90–e91. [DOI] [PubMed] [Google Scholar]
- 80.Sarkar S, Bowlus CL. Primary sclerosing cholangitis: Multiple phenotypes, multiple approaches. Clin Liver Dis 2016;20:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Batta A, Salen G, Arora R, et al. Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids. J Biol Chem 1990;265:10925–8. [PubMed] [Google Scholar]
- 82.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 2006;72:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones BV, Begley M, Hill C, et al. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci 2008;105:13580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitahara M, Sakata S, Sakamoto M, et al. Comparison among fecal secondary bile acid levels, fecal microbiota and Clostridium scindens cell numbers in Japanese. Microbiol Immunol 2004;48:367–75. [DOI] [PubMed] [Google Scholar]
- 85.Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol 2014;60:824–31. [DOI] [PubMed] [Google Scholar]
- 86.Taylor MR, Flannigan KL, Rahim H, et al. Vancomycin relieves mycophenolate mofetil–induced gastrointestinal toxicity by eliminating gut bacterial β-glucuronidase activity. Sci Adv 2019;5:eaax2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abarbanel DN, Seki SM, Davies Y, et al. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol 2013;33:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: A pilot clinical trial. Am J Gastroenterol 2019;114:1071–9. [DOI] [PubMed] [Google Scholar]
- 89.Davies YK, Tsay CJ, Caccamo DV, et al. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transpl 2013;2013:314292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cox KL, Cox KM. Oral vancomycin: Treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 1998;27:580–3. [DOI] [PubMed] [Google Scholar]
- 91.Buness C, Lindor KD, Miloh T. Oral vancomycin therapy in a child with primary sclerosing cholangitis and severe ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr 2016;19(3):210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rossen NG, Fuentes S, Boonstra K, et al. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis 2015;9:342–8. [DOI] [PubMed] [Google Scholar]
- 93.Torres J, Bao X, Goel A, et al. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment Pharmacol Ther 2016;43:790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang H, Fu L, Wang J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: A systematic review and meta-analysis. Biomed Research International 2018;2018:8941340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abraham BP, Quigley EMM. Probiotics in inflammatory bowel disease. Gastroenterol Clin North Am 2017;46:769–82. [DOI] [PubMed] [Google Scholar]
- 96.Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2015;13:1444–9.e1. [DOI] [PubMed] [Google Scholar]
- 97.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol 2008;20:688–92. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu M, Iwasaki H, Mase S, et al. Successful treatment of primary sclerosing cholangitis with a steroid and a probiotic. Case Rep Gastroenterol 2012;6:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eaton JE, Nelson KM, Gossard AA, et al. Efficacy and safety of curcumin in primary sclerosing cholangitis: An open label pilot study. Scand J Gastroenterol 2019;54:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jorgensen KK, Grzyb K, Lundin KE, et al. Inflammatory bowel disease in patients with primary sclerosing cholangitis: Clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis 2012;18:536–45. [DOI] [PubMed] [Google Scholar]
- 101.Wasmuth HH, Trano G, Endreseth BH, et al. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch-anal anastomosis. J Gastrointest Surg 2010;14:1099–104. [DOI] [PubMed] [Google Scholar]
- 102.Wiesner RH, LaRusso NF, Dozois RR, et al. Peristomal varices after proctocolectomy in patients with primary sclerosing cholangitis. Gastroenterology 1986;90:316–22. [DOI] [PubMed] [Google Scholar]
- 103.Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol 2016;28:383–90. [DOI] [PubMed] [Google Scholar]
- 104.Khaderi SA, Sussman NL. Screening for malignancy in primary sclerosing cholangitis (PSC). Curr Gastroenterol Rep 2015;17:17. [DOI] [PubMed] [Google Scholar]
- 105.Navaneethan U, G K Venkatesh P, Lashner BA, et al. Severity of primary sclerosing cholangitis and its impact on the clinical outcome of Crohn's disease. J Crohns Colitis 2012;6:674–80. [DOI] [PubMed] [Google Scholar]
- 106.Ong J, Bath MF, Swift C, et al. Does colectomy affect the progression of primary sclerosing cholangitis? A systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench 2018;11:277–83. [PMC free article] [PubMed] [Google Scholar]
- 107.Buchholz BM, Lykoudis PM, Ravikumar R, et al. Role of colectomy in preventing recurrent primary sclerosing cholangitis in liver transplant recipients. World J Gastroenterol 2018;24:3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh S, Loftus EV, Jr, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol 2013;108:1417–25. [DOI] [PubMed] [Google Scholar]
- 109.Dvorchik I, Subotin M, Demetris AJ, et al. Effect of liver transplantation on inflammatory bowel disease in patients with primary sclerosing cholangitis. Hepatology 2002;35:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Navaneethan U, Venkatesh PG, Mukewar S, et al. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2012;10:540–6. [DOI] [PubMed] [Google Scholar]
- 111.Mouchli MA, Singh S, Boardman L, et al. Natural history of established and de novo inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Inflamm Bowel Dis 2018;24:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]