Abstract
Background:
Early life exposure to triclosan, an antimicrobial chemical and suspected endocrine disruptor, may adversely affect neurodevelopment. No studies have examined gestational and early childhood exposure to triclosan and children’s academic achievement.
Methods:
Using data from 193 mother-child pairs from the HOME Study, we quantified triclosan in maternal and child urine samples up to nine times between the second trimester of gestation (16-weeks) and age 8 years. At age 8 years, we administered the reading and math components of the Wide Range Achievement Test-4 (WRAT-4) to children. Using multiple informants models, we estimated covariate-adjusted associations of triclosan concentrations during each time period with WRAT-4 scores. We also tested whether associations differed by exposure period and child sex.
Results:
There was evidence that timing of exposure modified the associations between triclosan and reading composite scores (triclosan-exposure period interaction p-value=0.20), but not math scores (interaction p-value=0.72). Each 10-fold increase in triclosan concentrations at delivery was associated with lower reading composite scores (β:−2.6; 95% CI:−5.0, −0.1). Additionally, we observed weaker and less precise inverse association of math scores with triclosan concentrations at delivery (β:−1.9; 95% CI:−4.6, 0.8) and at age 1 year (β:−2.0; 95% CI:−6.0, 2.1). There was not strong evidence that child sex modified the pattern of associations between repeated triclosan measures and WRAT-4 reading composite or math scores (sex-triclosan-exposure period interaction p-values>0.20).
Conclusion:
Urinary triclosan concentrations at delivery and at age 1 year, but not other times during gestation or childhood, were associated with lower reading composite and to a lesser extent math test scores at age 8 years in this cohort of U.S. children.
1. Introduction
Triclosan is a synthetic antimicrobial chemical found in some soaps, toothpaste, and personal care products (Food and Drug Adminnistration, 2016; Rodricks et al., 2010). The addition of triclosan to consumer products has led to widespread exposure in the U.S., including among pregnant women and children (Calafat et al., 2008; Stacy et al., 2017). Although the Food and Drug Adminnistration banned using triclosan in over the counter hand washing soaps in 2016 (Food and Drug Adminnistration, 2016), recent reports indicate that triclosan exposure still occurs in the US population (CDC, 2019).
Triclosan may interfere with thyroid hormone metabolism and homeostasis (Braun et al., 2018; Dann and Hontela, 2011; Johnson et al., 2016; Paul et al., 2012). Given that thyroid hormones play a critical role in neurodevelopment during gestation and early childhood, triclosan-induced alterations in thyroid hormone homeostasis may adversely affect cognition and behavior in children (Álvarez-Pedrerol et al., 2007; Gilbert et al., 2012; Haddow et al., 1999; Heyer and Meredith, 2017; Korevaar et al., 2016; Lazarus et al., 2014). Two previous studies reported that increasing urinary triclosan concentrations during gestation or at delivery were associated with decreased maternal or neonatal thyroid hormone concentrations (Braun et al. 2017b; Wang et al. 2017).
A number of epidemiologic studies have examined the association of gestational or childhood urinary triclosan concentrations with cognitive and behavioral outcomes in children (Braun et al., 2017; Etzel et al., 2018; Jackson-Browne et al., 2019, 2018; Nakiwala et al., 2018; Philippat et al., 2017). We previously reported inverse associations between maternal urinary triclosan concentrations and intelligence quotient (IQ) and adverse behavioral outcomes in children at age 8 years (Jackson-Browne et al., 2019, 2018). However, other studies reported no associations of urinary triclosan concentrations during pregnancy with children’s IQ scores (Etzel et al., 2018; Nakiwala et al., 2018) or other cognitive outcomes such as visual spatial abilities (Braun et al., 2017). Given the inconclusive results related to triclosan and IQ, we extended our prior findings by examining the potential impact of triclosan exposure on academic achievement. Academic achievement is correlated with measures of cognitive abilities (Kaya et al., 2015) and an important predictor of later life career success, health, and wellbeing (Fiscella and Kitzman, 2009; Lê-Scherban et al., 2014).
Using the HOME Study we sought to identify periods of susceptibility to the potential impact of triclosan exposure using repeated measures of gestational and childhood urinary triclosan concentrations and academic achievement test scores in children. Our objective was to determine if the pattern of associations of repeated urinary triclosan concentrations with academic achievement scores were similar to our previously observed associations between repeated urinary triclosan measurements and child IQ scores at age 8 years.
2. Materials and Methods
2.1. Study Participants
We used data from a prospective pregnancy and birth cohort in Cincinnati, Ohio, the Health Outcomes and Measures of the Environment (HOME) study, previously described (Braun et al., 2016). Inclusion and eligibility criteria has been previously reported (Braun et al., 2016; Jackson-Browne et al., 2019, 2018). Briefly, women age 18 or older whom were in their second trimester of pregnancy living in Cincinnati, OH and planned on delivering at a HOME study participating hospital were eligible. Of the 389 eligible women, 193 mother-child pairs had at least one triclosan measurement, academic achievement test scores, and all covariates. The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved this study. The Centers for Disease Control and Prevention (CDC) and Brown University deferred to the CCHMC IRB as the IRB of record. All women provided written informed consent for themselves and their children.
2.2. Quantification of triclosan urinary concentrations
We assessed triclosan exposure in mothers and children using urinary triclosan concentrations. For urine collection, storage, and analysis, we followed previously described protocols to minimize potential external contamination (Jackson-Browne et al., 2018; Stacy et al., 2017; Ye et al., 2013). Women provided up to two spot urine samples around 16-and 26-weeks of pregnancy and another within 48 hours of delivery. Childhood samples were collected annually from ages 1–5 years, and again at age 8 years. Collection and storage of urine samples were previously described (Jackson-Browne et al., 2018; Stacy et al., 2017). Triclosan concentrations were quantified using online solid phase extraction-high performance liquid chromatography isotope-dilution tandem mass spectrometry methods (Kuklenyik et al., 2005; Zhou et al., 2014). The limit of detection (LOD) was 2.3 ng/ml for all samples except for the 8-year visit which was 1.0 ng/ml. LOD/√2 was assigned for concentrations below the LOD (Hornung and Reed, 1990). Urinary creatinine was quantified and used in regression models to account for urine dilution (Larsen, 1972).
2.3. Child Academic Achievement.
Trained research assistants, who were blinded to women and children’s urinary triclosan concentrations, administered the math and reading subtests of the Wide Range Achievement Test-4 (WRAT-4) to children at age 8 years (Wilkinson and Robertson, 2006).The WRAT-4 is an achievement test that measures foundational academic skills including word reading, sentence comprehension, and math. The reading composite is made up of word reading and sentence comprehension subtests. These scores are normalized to a mean of 100 and standard deviation of 15. Lower scores on the WRAT-4 are indicative of poorer performance on this test. We analyzed all scores as continuous variables.
2.4. Covariates.
We adjusted for variables associated with urinary triclosan concentrations and child academic achievement or shown in previous studies to confound other environmental chemicals similar to triclosan and neurodevelopmental outcomes similar to academic achievement (Figure S1) (Braun et al., 2011; Jackson-Browne et al., 2018; Stacy et al., 2017, 2016; Textor et al., 2016). We included children’s sex, race/ethnicity, maternal education, household income, marital status, maternal IQ, urine creatinine, and serum cotinine in all models. Serum cotinine was measured from samples collected during pregnancy or at birth (Braun et al., 2010). We assessed the quality and quantity of the caregiving environment (Caldwell and Bradley, 1984) at the 1year visit. Child and maternal IQ were assessed using the Wechsler Intelligence Scale for Children-IV (WISC-IV) and Wechsler Abbreviated Scale of Intelligence (WASI), respectively (Wechsler, 2003, 1999). Lower scores on the WISC-IV and WASI are indicative of poorer performance. The WRAT-4 test was administered after the WISC-IV during the 8-year study visit.
2.5. Statistical Analysis.
We described the distribution of urinary triclosan concentrations across exposure periods. We calculated geometric mean urinary triclosan concentrations and mean child reading composite and math scores according to covariates. We used a multiple informants model to examine the associations between repeated urinary triclosan concentrations and academic achievement test scores (Sánchez et al., 2011). This approach uses generalized estimating equations (GEE) to jointly estimate triclosan-academic achievement associations across all nine exposure periods and determine if these associations also differed by exposure window. We examined the associations between repeated urinary log10-transformed creatinine-standardized triclosan concentrations and reading composite and math scores while adjusting for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), caregiving environment scores (continuous), household income (continuous), and maternal marital status (married vs. unmarried), education (less than high school, high school graduate, and college graduate or more), and IQ (continuous).We examined the interaction term between triclosan concentrations and time period to assess heterogeneity in the exposure-outcome association across the nine time periods. Given the low power of the interaction test (Greenland et al., 2016), we set a p-value ≤ 0.20 significance threshold as indication that associations differed by exposure period. Given that we previously observed sex-specific association between triclosan and child behavior (Jackson-Browne et al., 2019), we examined whether child sex modified the associations between repeated urinary triclosan concentration across exposure periods and WRAT-4 scores.
2.5.1. Secondary and Sensitivity Analyses.
We set an p-value threshold of p≤0.20 as evidence that associations were modified by sex. Additionally, we estimated the variation in WRAT-4 scores explained by child IQ using Pearson’s correlation coefficients given the strong linear relations between IQ and academic achievement (Kaya et al., 2015). Furthermore, we characterized the relation between urinary triclosan concentration at delivery and WRAT-4 scores using natural splines and quartiles of urinary triclosan concentrations, controlling for the same covariates used in the multiple informant analyses. We used analysis of variance to determine whether the regression model fit better with triclosan concentrations characterized as a linear term or with natural splines.
We performed sensitivity analyses using the word reading and sentence comprehension subscales of the reading composite test to determine if one of these subscales were more strongly associated with triclosan than the other. We also performed sensitivity analyses to assess whether our results differed when adjusting for or standardizing by urinary creatinine concentrations but not both. We previously found triclosan concentrations at delivery to be associated with IQ (Jackson-Browne et al., 2018). Complications during labor and delivery, as well as mode of delivery, may be associated with both increased triclosan exposure and poorer neurodevelopment outcomes. Therefore, we further adjusted regression models for child admission to the neonatal intensive care unit (NICU) and the mode (vaginal vs. caesarean) of delivery. Maternal age at delivery and pre-pregnancy BMI may be associated with both adverse neurodevelopment and academic achievement, as well as pregnancy complications and medical interventions, which in turn could potentially increase triclosan exposure at delivery (Marchi et al., 2015; Pugh et al., 2016). Therefore, we performed further sensitivity analyses adjusting for maternal pre-pregnancy BMI and age at delivery.
3. Results
Baseline characteristics of the study participants included in this analysis were similar to those of the full cohort (Table S1). Among children included in this analysis, 56% were female, 62% were White, non-Hispanic, 77% of the mothers had at least a high school education, and 64% of the mothers were married. Although 60% of study participant had a household income above $40,000 per year, approximately 24% lived in households reported incomes below $20,000 per year (Table 1).
Table 1.
Geometric mean gestational and childhood urinary triclosan concentrations (ng/mL) and mean age 8-year WRAT-4 reading composite and math scores according to sociodemographic, caregiving, and maternal factors among mother-child pairs in the HOME Study.
| Characteristics | Gestational Triclosana | Childhood Triclosanb | Reading Composite | Math | |
|---|---|---|---|---|---|
| N (%) | GM (GSD) | GM (GSD) | Mean (SD) | Mean (SD) | |
| Total | 193 | 17 (3.6) | 11 (2.7) | 109 (16) | 109 (12) |
| Child Sex | |||||
| Female | 108 (56) | 15 (2.8) | 12 (2.8) | 108 (17) | 109(13) |
| Male | 85 (44) | 19 (4.0) | 11 (2.6) | 109 (14) | 109 (11) |
| Child Race | |||||
| White, non-Hispanic | 120 (62) | 17 (3.8) | 12 (2.5) | 114 (13) | 112 (10) |
| Black, non-Hispanic | 63 (33) | 17 (3.2) | 10 (3.1) | 98 (16) | 102 (14) |
| Other | 10 (5) | 18 (3.9) | 9 (2.1) | 113 (14) | 111 (12) |
| Maternal Education | |||||
| < High School Diploma | 45 (23) | 16 (2.7) | 7.1 (2.5) | 98 (16) | 101 (13) |
| High School Diploma | 56 (29) | 17 (3.6) | 13 (2.4) | 104 (14) | 107 (12) |
| College + | 92 (48) | 18 (4.0) | 13 (2.8) | 116 (12) | 114 (9.8) |
| Income ($/year) | |||||
| <20k | 47 (24) | 14 (2.8) | 7.9 (2.5) | 96 (16) | 100 (13) |
| 20–40k | 31 (16) | 18 (3.5) | 9.9 (2.7) | 108 (12) | 109 (11) |
| >40–80k | 62 (32) | 17 (4.1) | 13 (2.5) | 113 (13) | 111 (11) |
| >80k | 53 (28) | 19 (3.8) | 15 (2.9) | 116 (13) | 115 (10) |
| Marital Status | |||||
| Not Married | 70 (36) | 15 (2.7) | 9.1 (2.7) | 99 (15) | 103 (13) |
| Married | 123 (64) | 18 (4.1) | 13 (2.6) | 114 (13) | 113 (11) |
| Caregiving Score | |||||
| <35 | 39 (20) | 15 (3.0) | 9.2 (3.4) | 95 (15) | 100 (13) |
| 35–40 | 53 (27) | 17 (3.4) | 11 (2.4) | 108 (15) | 109 (12) |
| >40 | 101 (52) | 18 (3.9) | 13 (2.6) | 114 (13) | 112 (10) |
| Maternal IQ | |||||
| <96 | 55 (28) | 15 (2.7) | 8.2 (2.6) | 99 (15) | 102 (13) |
| 96–110 | 51 (26) | 17 (3.3) | 12 (2.9) | 107 (15) | 108 (13) |
| >110–117 | 36 (19) | 21 (4.8) | 17 (2.3) | 111 (13) | 111 (9.9) |
| >117 | 51 (26) | 17 (4.0) | 12 (2.6) | 119 (11) | 116 (9.5) |
| Serum Cotinine | |||||
| Unexposed (<0.015 ng/ml) | 86 (45) | 17 (3.4) | 12 (2.7) | 111 (15) | 110 (12) |
| SHS (≥0.15 <3.0 ng/ml) | 90 (47) | 18 (3.8) | 11 (2.8) | 106 (17) | 107 (13) |
| Active (≥3.0 ng/ml) | 17 (8) | 13 (3.1) | 12 (2.5) | 114 (11) | 113 (9.6) |
| NICU Admission | |||||
| No | 182 (94) | 17 (3.5) | 12 (2.7) | 110 (15) | 110 (12) |
| Yes | 11 (6) | 24 (4.8) | 6.8 (1.8) | 96 (20) | 99 (13) |
| Delivery Method | |||||
| Caesarean | 53 (27) | 17 (4.4) | 13 (3.2) | 111 (14) | 111 (11) |
| Vaginal | 140 (73) | 17 (3.3) | 11 (2.5) | 108 (16) | 108 (13) |
Abbreviations: GM: geometric mean, GSD: Geometric Standard Deviation, WRAT-4: Wide Range Achievement Test-4, SHS: secondhand smoke.
Mean gestational triclosan concentrations included up to 3 triclosan measurements from maternal urine samples collected during 16-and 26-weeks gestation, and within 48 hours of delivery.
Mean childhood triclosan concentrations included up to 6 triclosan measurements from child urine samples collected annually from ages 1–5 and at 8-years.
Median gestational urinary triclosan concentrations between 16-and 26-weeks decreased from 17 to 12 ng/mL and remained at 12 ng/mL at delivery (Table S2). Geometric mean gestational urinary triclosan concentrations were higher than mean childhood urinary triclosan concentrations (17 vs. 11 ng/mL, Table 1). Gestational urinary triclosan concentrations were higher in males but similar across race/ethnicity and maternal education categories. Gestational urinary triclosan concentrations were lowest among children living in households with an income below $20,000 level (Table 1). Gestational urinary triclosan concentrations increased with increasing caregiving environment scores (Table 1).
Median child urinary triclosan concentrations increased from 3.8 to 17 ng/mL between ages 1 to 4 years and then decreased to 10 ng/mL at age 8 years (Table S2). Childhood urinary triclosan concentrations were similar across sex and race/ethnicity (Table 1). Childhood urinary triclosan concentrations were lower in children with mothers who had less than a high school diploma (7.1 ng/mL) compared to mothers with a high school diploma or greater (13 ng/mL) and lower in children with unmarried (9.1 ng/mL) compared to married (13 ng/mL, Table 1) mothers. Childhood urinary triclosan mothers increased monotonically with increasing household income and caregiving environment scores (Table 1).
Mean reading composite and math scores at age 8-years were similar among males and females, but scores were lower in non-Hispanic Black children compared with other race/ethnicities and scores decreased monotonically with lower maternal education, income, IQ, and caregiving environment scores (Table 1).
3.1. Regression Analyses.
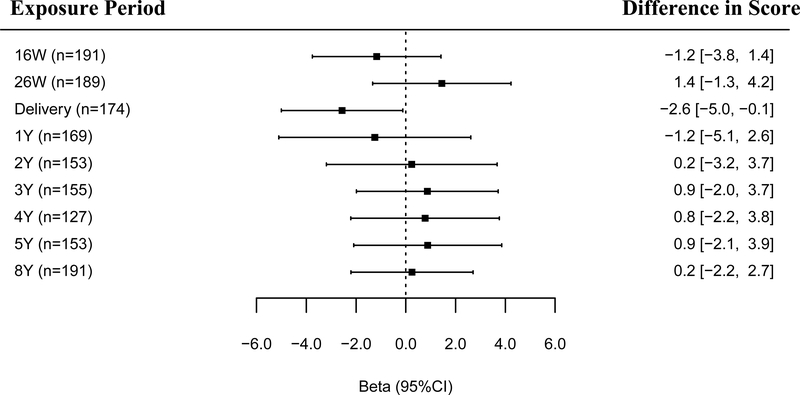
We found evidence that associations of urinary triclosan concentrations and reading composite scores varied by the timing of exposure (triclosan × exposure period interaction p-values = 0.20) but did not with math scores (triclosan × exposure period interaction p-value = 0.72, Figures 1–2). Associations between triclosan concentrations and WRAT-4 scores appeared more heterogeneous for gestational or delivery triclosan measures compared to childhood triclosan measures (Figures 1–2). After adjusting for covariates, associations between triclosan and reading composite scores were strongest at delivery compared to other exposure periods, which were generally null. Specifically, for every 10-fold increase in maternal urinary triclosan concentration at delivery, reading composite score decreased by 2.6 (95% CI: −5.0, –0.1) points (Figure 1).
Figure 1.
Adjusted differences in WRAT-4 reading composite scores at age 8 years per 10-fold increase in gestational and childhood urinary triclosan concentrations.
Betas and 95% CI derived from a multiple informants model.
Abbreviations: W: weeks of gestation, Y: age in years, CI: confidence interval, WRAT-4: Wide Range Achievement Test-4.
Multiple Informant regression model adjusted for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), household income (continuous), marital status (married vs. unmarried), maternal education (less than high school, high school graduate, and college graduate or more), caregiving environment scores (continuous), and maternal IQ (continuous).
Error bars are 95% CI.
Urinary triclosan concentration by exposure window interaction p-value = 0.20.
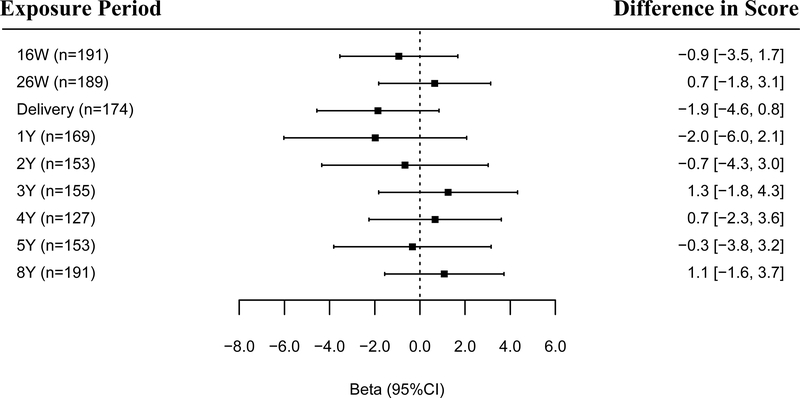
Figure 2.
Adjusted differences in child WRAT-4 math scores at 8 years of age per 10-fold increase in gestational and childhood urinary triclosan concentrations.
Betas and 95% CI derived from a multiple informants model.
Abbreviations: W: weeks of gestation, Y: age in years, CI: confidence interval, WRAT-4: Wide Range Achievement Test-4.
Multiple Informant regression model adjusted for log10-transformed serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (White-non-Hispanic, Black-non, Hispanic, and other), household income (continuous), marital status (married vs. unmarried), maternal education (less than high school, high school graduate, and college graduate or more), caregiving environment scores (continuous), and maternal IQ (continuous).
Error bars are 95% CI.
Urinary triclosan concentration by exposure window interaction p-value = 0.72
Generally, we observed weaker associations of maternal urinary triclosan concentrations during gestational or childhood periods with math scores, except for associations at delivery and age 1 years (Figure 2). Specifically, each 10-fold increase in triclosan concentrations at delivery age 1 years was associated with a 1.9-point (95% CI:–4.6, 0.8) and 2.0-point (95% CI: –6.0, 2.1) decrease in math scores at age 8 years, respectively.
3.2. Secondary and Sensitivity Analyses.
Child sex did not modify the associations between repeated urinary triclosan concentrations and WRAT-4 scores (triclosan × exposure period × sex p-values > 0.20). However, in sex-stratified analyses we observed that urinary triclosan concentrations were generally inversely associated with WRAT-4 scores in males, whereas associations were mostly positive in females (Table S3). Child full scale IQ scores were strongly correlated with reading composite (r = 0.75) and math (r = 0.76) scores (Figure S2). In addition, verbal IQ subscale scores were strongly correlated with reading composite (r = 0.71) and math (r = 0.60) scores (Figure S2). After adjusting for covariates, increasing urinary triclosan concentrations at delivery were associated with increasing mean reading composite scores and we did not observe evidence that the association was non-linear (non-linearity p-value= 0.55, Figure S3). Reading composite scores were lower among children born to mothers with urinary triclosan concentrations at delivery in the second (β: −5.3; 95% CI: −12.0, 1.0), third (β: −3.7; 95% CI: −10, 2.8), and fourth quartiles (β: −3.7; 95% CI: −10, 2.7) compared with children born to women with urinary triclosan concentrations in the first quartile (Figure S4).
The association of triclosan at delivery with reading composite scores did not change after further adjustment for maternal age at delivery and pre-pregnancy BMI (Table S4). Additional adjustment for NICU admission and mode of delivery also did not substantially change the association between maternal urinary triclosan concentrations at delivery and WRAT-4 scores. There were no appreciable differences between creatinine-adjusted and creatinine-standardized model results (Table S5). The associations of triclosan concentrations with the word reading and sentence comprehension subtests were similar to reading composite results (Figures S5–S6).
4. Discussion
In this prospective cohort study, urinary triclosan concentrations at delivery and age 1 year, but not other periods, were inversely associated with both reading composite and math scores on the WRAT-4. These results are consistent with our prior findings from this cohort showing that triclosan concentrations at delivery were inversely associated with child IQ and suggest that triclosan exposure near delivery could be a period of heightened neurodevelopmental susceptibility (Jackson-Browne et al., 2018).
While the underlying biological mechanisms by which triclosan could affect neurodevelopment or academic achievement are not well understood, experimental studies in animals report reductions in thyroid hormone concentrations during pregnancy, infancy, and adulthood associated with triclosan exposure (Johnson et al., 2016; Louis et al., 2017). Lower triiodothyronine (T3) and thyroxine (T4) hormone concentrations during pregnancy and infancy have consistently been associated with poorer neurodevelopmental outcomes (Klein et al., 2001; Lazarus, 1999; Murphy et al., 2015). Additionally, children with congenital hypothyroidism also have poorer neurodevelopmental outcomes (Klein et al., 2001; Lazarus, 1999; Murphy et al., 2015). Gestational triclosan concentrations were also inversely associated with maternal serum free T3 (Aker et al., 2018; Skarha et al., 2019; Wang et al., 2017), maternal free T4 (Berger et al., 2018; Wang et al., 2017), and cord serum total T4 (Braun et al., 2018) in human studies. Thus, we speculate that observed associations may relate to the ability of triclosan to affect thyroid hormone homeostasis during late gestation and early infancy.
Studies examining the relations between intelligence and academic achievement suggest that intelligence test scores explain much, but not all of the variation, in academic achievement scores in children (Gustafsson and Undheim, 1996; Jencks, 1979; Jensen and Rushton, 1998; Lubinski et al., 2001). This may explain differences in the magnitude of associations of triclosan concentrations at delivery between WRAT reading composite (β: −2.6; 95% CI: −5.0, −0.1) compared to IQ scores (β: −4.5; 95% CI: −7.0, −2.0) at age 8-years (Jackson-Browne et al., 2018).
While we are unaware of any studies examining the association between triclosan exposure and academic achievement, two other studies examined associations between early life triclosan exposure and IQ in children (Etzel et al., 2018; Nakiwala et al., 2018). We previously reported that increasing urinary triclosan concentrations measured near delivery were inversely associated with IQ at age 8 years in the HOME Study, but in the Canadian MIREC and French EDEN cohorts, maternal triclosan concentrations during pregnancy were not associated with child IQ at ages 3–4 and 5 years, respectively (Etzel et al., 2018; Nakiwala et al., 2018). However, these studies did not assess triclosan exposure near the time of delivery. Additionally, the MIREC and EDEN studies both only had a single gestational triclosan measurement compared to multiple gestational measurements in the HOME Study, and the EDEN cohort only included mother-son pairs (Braun et al., 2016; Nakiwala et al., 2018). These differences in the study design, specifically the timing of exposure assessment, may explain the divergent results observed among these studies.
We can only speculate as to why neurodevelopmental processes near the time of delivery may be more sensitive to triclosan than exposure at other times. One possible explanation is that triclosan disrupts the neonatal microbiome at the time of delivery, which is a crucial period for microbiota development (DiGiulio et al., 2015; Ribado et al., 2017). Future work could explore the role triclosan plays in differences in microbiota diversity in the mother and newborn at delivery.
Our study has several strengths. First, the prospective study design enabled us to examine associations between child academic achievement test scores and triclosan exposure during nine separate gestational and childhood developmental periods. Second, we capitalized on these repeated exposure measures to formally examine differences in associations of academic achievement with triclosan concentrations measured at different exposure time periods. The use of a well-validated measure of academic abilities was another strength of our study (Wilkinson and Robertson, 2006). An additional strength was the adjustment for sociodemographic, maternal, and child factors that may confound the association between triclosan concentrations and WRAT-4 scores. Moreover, adjustment for these factors resulted in negative confounding of the association of triclosan at delivery and WRAT-4 scores, and the standard errors for the adjusted associations were more precise than the unadjusted. Finally, mean WRAT-4 scores in this cohort were similar to other children living in the US (Wilkinson and Robertson, 2006) and median urinary triclosan concentrations among pregnant women and children in this cohort were also similar to concentrations observed in the U.S. general population (Calafat et al., 2008).
There are some limitations of this study. First, we relied on a single urine sample during each exposure period to assess exposure to triclosan, however exposure assessment at more frequent intervals was not economical. Exposure to non-persistent chemicals, like triclosan, varies within individuals because exposure is episodic and triclosan has a relatively short biological half-life (~21 hours) (Calafat et al., 2008; Jackson-Browne et al., 2018; Sandborgh-Englund et al., 2006; Stacy et al., 2017). We previously reported that maternal urinary triclosan concentrations in this cohort had a intraclass correlation coefficient (ICC) of 0.5 compared to a lower ICC (< 0.4) during childhood (Stacy et al., 2017). Assuming non-differential exposure measurement error, we expect this misclassification would likely bias associations toward the null (Perrier et al., 2016). Second, although we did not observe differences between the characteristics of this study sample and the original cohort, attrition due to loss to follow-up in our cohort resulted in limited sample size for assessing sex-specific effects, and the modest sample size may have limited our ability to identify periods of susceptibility. We acknowledge that our modest sample size may have also been insufficient to meet positivity assumptions of our statistical models and this might be reflected by the differences in the magnitude and precision between our unadjusted and adjusted estimates.
Third, it is still possible that there were unmeasured factors associated with higher triclosan concentrations at delivery and lower academic test scores at age 8 years. However, we only observed inverse associations between triclosan at delivery and WRAT-4 scores after adjusting for covariates, suggesting that there was negative confounding of this association (Mehio-Sibai et al., 2005). One alternative explanation for our findings is that complications during labor and delivery may increase the risk for medical interventions which in turn are associated with both increased triclosan exposure and adverse neurodevelopmental risks; however, adjusting for mode of delivery and NICU admission did not materially change the association between triclosan concentrations at delivery and academic achievement scores. Finally, a decrease of 2 points in academic achievement scores may not translate into a meaningful change in an individual child’s classroom performance. However, on a population level, a 2-point reduction in academic achievement scores can result in a larger proportion of children with poorer scholastic performance at the population level (Bellinger, 2004; Braun et al., 2016a).
5. Conclusions
In this cohort of children, we observed inverse associations of urinary triclosan concentrations measured at delivery and age 1 year with reading and math achievement test scores at age 8 years, but not at other time periods. These results are consistent with our prior findings from this same cohort of associations between triclosan exposure at delivery with reduced cognitive abilities at age 8 years. Taken together, this study and previous investigations in this cohort suggest that triclosan exposures near delivery may influence intelligence and subsequently, academic performance in children.
Supplementary Material
Highlights.
We measured urinary triclosan concentrations in women up to twice during pregnancy, at delivery, and up to 6 times in children from ages 1–8-years.
We measured children’s academic achievement at age 8 years using the Wide Range Achievement Test (WRAT-4).
We used a multiple informant regression to estimate covariate-adjusted triclosan-reading composite and triclosan-math WRAT-4 test score associations at each visit and tested if triclosan-WRAT-4 associations at each visit differed from one another.
Timing of exposure modified the associations between triclosan and reading composite but not math test scores.
Increasing maternal and childhood triclosan concentrations at delivery and at age 1 year were inversely associated with reading and math scores at age 8 years.
Acknowledgements:
This work was supported by NIEHS grants F32 ES029812, R01 ES024381, R01 ES020349, P01 ES011261, and R01 ES014575. We acknowledge the technical assistance of Z. Zhou, P. Dwivedi, J. Tao, and the late X. Ye (Centers for Disease Control and Prevention CDC) in measuring the urinary triclosan concentrations.
Footnotes
Disclaimer:
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD, 2018. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int 113, 341–349. 10.1016/J.ENVINT.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Pedrerol M, Ribas-Fitó N, Torrent M, Julvez J, Ferrer C, Sunyer J, 2007. TSH concentration within the normal range is associated with cognitive function and ADHD symptoms in healthy preschoolers. Clin. Endocrinol. (Oxf). 66, 890–898. 10.1111/j.1365-2265.2007.02871.x [DOI] [PubMed] [Google Scholar]
- Berger K, Gunier RB, Chevrier J, Calafat AM, Ye X, Eskenazi B, Harley KG, 2018. Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ. Res 165, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, Yolton K, Lanphear BP, 2017. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 58, 75–83. 10.1016/j.neuro.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Hoofnagle A, Papandonatos GD, Jackson-Browne M, Hauser R, Romano ME, Karagas MR, Yolton K, Thomas Zoeller R, Lanphear BP, 2018. Associations of early life urinary triclosan concentrations with maternal, neonatal, and child thyroid hormone levels. Horm. Behav 101, 77–84. 10.1016/j.yhbeh.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, Xia Y, Bearer C, Barr DB, Lanphear BP, 2010. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: The correlation between serum and meconium and their association with infant birth weight. Environ. Heal. A Glob. Access Sci. Source 9, 53 10.1186/1476-069X-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP, 2011. Variability and Predictors of Urinary Bisphenol A Concentrations during Pregnancy. Environ. Health Perspect 119, 131–137. 10.1289/ehp.1002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2016. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int. J. Epidemiol 1–10. 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ. Health Perspect 116, 303–307. 10.1289/ehp.10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH, 1984. Home Observation for Measurement of the Environment, Little Rock, AR: University of Arkansas at Little Rock. [Google Scholar]
- CDC, 2019. Fourth National Report on Human Exposure to Environmental Chemicals: Executive Summary. [Google Scholar]
- Dann AB, Hontela A, 2011. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol 31, 285–311. 10.1002/jat.1660 [DOI] [PubMed] [Google Scholar]
- Etzel T, Muckle G, Arbuckle TE, Fraser WD, Ouellet E, Séguin JR, Lanphear B, Braun JM, 2018. Prenatal urinary triclosan concentrations and child neurobehavior. Environ. Int 114, 152–159. 10.1016/j.envint.2018.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K, Kitzman H, 2009. Disparities in Academic Achievement and Health: The Intersection of Child Education and Health Policy. Pediatrics 123, 1073 LP – 1080. 10.1542/peds.2008-0533 [DOI] [PubMed] [Google Scholar]
- Food H, 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final rule. Fed. Regist 81, 61106–30. [PubMed] [Google Scholar]
- Gilbert ME, Rovet J, Chen Z, Koibuchi N, 2012. Developmental thyroid hormone disruption: Prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 10.1016/j.neuro.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG, 2016. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol 31, 337–350. 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J-E, Undheim JO, 1996. Individual differences in cognitive functions., in: Handbook of Educational Psychology. Prentice Hall International, London, England, pp. 186–242. [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ, 1999. Maternal Thyroid Deficiency during Pregnancy and Subsequent Neuropsychological Development of the Child. N. Engl. J. Med 341, 549–555. 10.1056/NEJM199908193410801 [DOI] [PubMed] [Google Scholar]
- Heyer DB, Meredith RM, 2017. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58, 23–41. 10.1016/j.neuro.2016.10.017 [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 5, 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, Braun JM, 2018. Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environ. Health Perspect 126, 057001. 10.1289/EHP2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Yolton K, Lanphear BP, Braun JM, 2019. Early-life triclosan exposure and parent-reported behavior problems in 8-year-old children. Environ. Int 128, 446–456. 10.1016/j.envint.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks CO, 1979. Who Gets Ahead? The Determinants of Economic Success in America. [Google Scholar]
- Jensen AR, Rushton JP, 1998. The g Factor: The Science of Mental Ability. [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, Campbell M, Donald JM, Sen S, Bero L, Zeise L, Woodruff TJ, 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ. Int 92–93, 716–728. 10.1016/j.envint.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya F, Juntune J, Stough L, 2015. Intelligence and Its Relationship to Achievement. Elem. Educ. Online 14, 1060–1078. 10.17051/io.2015.25436 [DOI] [Google Scholar]
- Klein RZ, Sargent JD, Larsen PR, Waisbren SE, Haddow JE, Mitchell ML, 2001. Relation of severity of maternal hypothyroidism to cognitive development of offspring. J. Med. Screen 8, 18–20. 10.1136/jms.8.1.18 [DOI] [PubMed] [Google Scholar]
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, Steegers EAP, Visser TJ, White T, Tiemeier H, Peeters RP, 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43. 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Ye X, Calafat AM, Kuklenyik Z, Needham LL, Calafat AM, 2005. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Anal. Chem 77, 5407–5413. 10.1021/ac050390d [DOI] [PubMed] [Google Scholar]
- Larsen K, 1972. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 41, 209–217. [DOI] [PubMed] [Google Scholar]
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B, 2014. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur. Thyroid J 3, 76–94. 10.1159/000362597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JH, 1999. Thyroid hormone and intellectual development: a clinician’s view. Thyroid 9, 659–660. [DOI] [PubMed] [Google Scholar]
- Lê-Scherban F, Diez Roux AV, Li Y, Morgenstern H, 2014. Does academic achievement during childhood and adolescence benefit later health? Ann. Epidemiol 24, 344–355. 10.1016/j.annepidem.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski D, Webb RM, Morelock MJ, Benbow CP, 2001. Top 1 in 10,000: A 10-Year Follow-Up of the Profoundly Gifted. J. Appl. Psychol 86, 718–729. 10.1037/0021-9010.86.4.718 [DOI] [PubMed] [Google Scholar]
- Marchi J, Berg M, Dencker A, Olander EK, Begley C, 2015. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- Murphy NC, Diviney MM, Donnelly JC, Cooley SM, Kirkham CH, Foran AM, Breathnach FM, Malone FD, Geary MP, 2015. The effect of maternal subclinical hypothyroidism on IQ in 7-to 8-year-old children: A case-control review. Aust. New Zeal. J. Obstet. Gynaecol 55, 459–463. 10.1111/ajo.12338 [DOI] [PubMed] [Google Scholar]
- Nakiwala D, Peyre H, Heude B, Bernard JY, Béranger R, Slama R, Philippat C, EDEN mother-child study group, T.E. mother-child study, 2018. In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environ. Health 17, 17 10.1186/s12940-018-0359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM, 2012. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: A dynamic and kinetic evaluation of a putative mode-of-action. Toxicology 300, 31–45. 10.1016/j.tox.2012.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C, 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27, 378–388. 10.1097/eDe.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, EDEN Mother–Child Study Group, the E.M.S., 2017. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ. Health Perspect 125, 097014. 10.1289/EHP1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, Bodnar LM, 2016. Child academic achievement in association with pre-pregnancy obesity and gestational weight gain. J. Epidemiol. Community Health 10.1136/jech2015-206800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM, 2010. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol 40, 422–84. 10.3109/10408441003667514 [DOI] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM, 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ. Health Perspect 119, 409–415. 10.1289/ehp.1102453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J, 2006. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health 69, 1861–1873. 10.1080/15287390600631706 [DOI] [PubMed] [Google Scholar]
- Skarha J, Mínguez-Alarcón L, Williams PL, Korevaar TIM, de Poortere RA, Broeren MAC, Ford JB, Eliot M, Hauser R, Braun JM, 2019. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ. Int 122, 256–262. 10.1016/J.ENVINT.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Calafat AM, Chen A, Lanphear BP, Hauser R, Papandonatos GD, Sathyanarayana S, Ye X, Yolton K, Braun JM, 2016. Patterns, Variability, and Predictors of Urinary Bisphenol A Concentrations during Childhood. Environ. Sci. Technol 50, 5981–5990. 10.1021/acs.est.6b00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Etzel T, Papandonatos G, Calafat AM, Chen A, Hauser R, Lanphear BP, Sathyanarayana S, Ye X, Yolton K, Braun JM, 2017. Patterns, Variability, and Predictors of Urinary Triclosan Concentrations during Pregnancy and Childhood. Environ. Sci. Technol 51, 6404–6413. 10.1021/acs.est.7b00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH, 2016. Robust causal inference using directed acyclic graphs: The R package “dagitty.” Int. J. Epidemiol 45, 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Wang XX, Ouyang F, Feng L, Wang XX, Liu Z, Zhang J, 2017. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels : A Prospective Study. Environ. Health Perspect 125, 067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2003. WISC-IV: Wechsler Intelligence Scale for Children, Integrated: Technical and Interpretive Manual. Pearson, Inc, San Antonio, TX. [Google Scholar]
- Wechsler W, 1999. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. Wide range achievement test 4 (WRAT4). Lutz, FL Psychol. Assess. Resour [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM, 2013. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect 121, 283–286. 10.1289/ehp.1206093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X, 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 944, 152–156. 10.1016/j.jchromb.2013.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.