Abstract
Smart polymers as ideal drug nanocarriers have attracted much attention due to the effective drug delivery, internalization and release once triggered by intracellular stimuli, as well as reduced cytotoxicity. We here reported the anionic micelle consisting of copolymer (PEG-b-PAsp) and a PBE (Phenylboronic Ester) group grafted, which can achieve fast response to intracellular ROS and enhanced anti-tumor activity. With this, PEG-b-PAsp-g-PBE/DOX system showed better tumor growth inhibition when studied on HeLa cell lines with high level of intracellular ROS and its subcutaneous tumor models. Additionally, the administration of PEG-b-PAsp-g-PBE/DOX did cause significantly lower systemic toxicity in comparison with free DOX. Hence, PEG-b-PAsp-g-PBE could be a highly efficient and safe nanocarrier to improve the efficacy of chemotherapeutic.
Keywords: Anionic micelles, ROS-Stimuli response, drug delivery system
1. Introduction
Over the past decades, cancer has been the most common cause of death second only to heart diseases. Chemotherapy plays a vital role in inhibiting many malignant tumor growth. However, the clinical application of chemotherapeutics was limited by the extremely serious adverse effect. Therefore, various biodegradable nanocarriers (Ulbrich et al., 2016; Cabral et al., 2018; Liu et al., 2019) have been designed as drug vehicles for cancer therapy, including liposomes (Wijetunge et al., 2018; Dai et al., 2019), micelles (Sun et al., 2018; Zhang et al., 2018; Hua et al., 2019), polymersomes (Liang et al., 2018; Einfalt et al., 2018). Ideal nanocarriers should be able to efficiently protect chemotherapeutics from degradation and clearance during circulation, specifically deliver chemotherapeutics to tumor cells, and precisely control and release drug (Zhang et al., 2019). To achieve this, a variety of stimuli-responsive nanocarriers have been designed and utilized for cancer therapy, which can become cleavable and physical chemical properties in response to tumor microenvironment triggers (Timko et al., 2010; Hu et al., 2017; Lu et al., 2017; Chen et al., 2018).
ROS (Reactive Oxygen Species) and oxidative stress as an excellent triggers is associated with distinct pathological conditions, especially cancer (Li et al., 2018). Compared with normal cells, the level of ROS in cancer cells are evidenced to be 100 times higher. Therefore, the increased level of ROS provides an emerging biomaterial basis in the field of internal biological stimuli (Xu et al., 2016; Xiang et al., 2018). Recently, PBA(phenylboronic acid)-based ROS-responsive vesicles implicated in nanomedicine for cancer targeting have aroused scientific interest because of an important superiority of high drug loading capacity, fast responsiveness, and excellent biocompatibility. A pioneering work by Liu was to construct the PBA contained-polymersome for intracellular delivery nanocarriers and nanoreactors to enhance imaging/drug release features. Upon cellular uptake, PBE group can be selectively degraded by intracellular H2O2 triggers, generating phenol and boronic acid as the oxidation products (Xu et al., 2016).
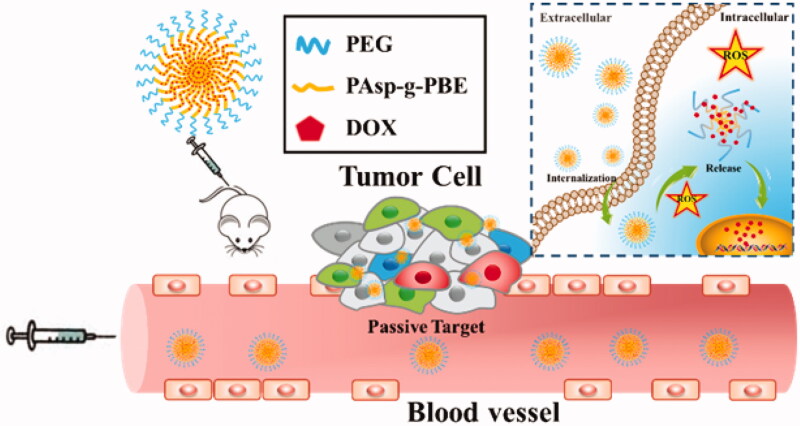
Herein, we reported the fabrication of ROS-responsive micelles exhibiting intracellular triggered and enhanced cancer-targeting features (Scheme 2). During optimization of the chemical design in terms of loading capacity and biocompatibility, we mainly synthesized the block copolymers of polyethylene glycol (PEG)-poly aspartic acid (m-PEG-b-PAsp) with proper PAsp chain lengths. The micelles are self-assembled from block copolymer incorporated with PEG and phenylboronic ester (PBE)-conjugated PAsp (PEG-b-PAsp-g-PBE) and have DOX encapsulated through electrostatic interaction in the interior. The copolymer PEG-b-PAsp-g-PBE loses its PBE side chains and becomes water soluble, leading to release of the preloaded DOX under the high level of ROS in HeLa cells. They hold great promise for controlled drug delivery due to its large loading capacity of DOX and fast responsiveness. Afterwards the drug efficiency of micelles was investigated both in vitro and in vivo. It is anticipated that the micelles can be utilized for cancer-target delivery with using chemically modified PBE as an ROS-degradable and biocompatible polypeptides material.
Scheme 2.
Schematic illustration of PEG-b-PAsp-g-PBE/DOX as an antitumor drug delivery system.
2. Results and discussion
2.1. Synthesis of diblock copolymer PEG-b-PAsp-g-PBE
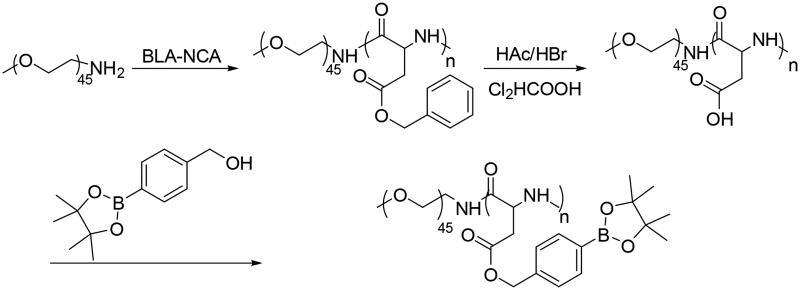
The PEG-b-PAsp-g-PBE copolymer was synthesized through two steps as shown in Scheme 1. The first step was to prepare copolymer PEG-b-PAsp. Copolymers PEG-b-PBLA were synthesized via the amine-initiated ring-opening polymerization (ROP). The amphiphilic PEG-b-PAsp diblock copolymers were obtained after the deprotection of PEG- b-PBLA by HBr/HAc in dichloroacetic acid solution. Finally, according to the length of PAsp chain, PEG-b-PAsp-g-PBE was performed through esterification reaction, conjunction of the phenylboronic acid into the PAsp chain.
Scheme 1.
Synthesis of PEG-b-PAsp-g-PBE Copolymers.
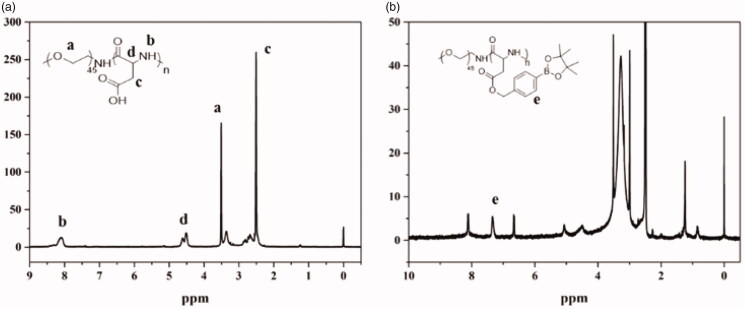
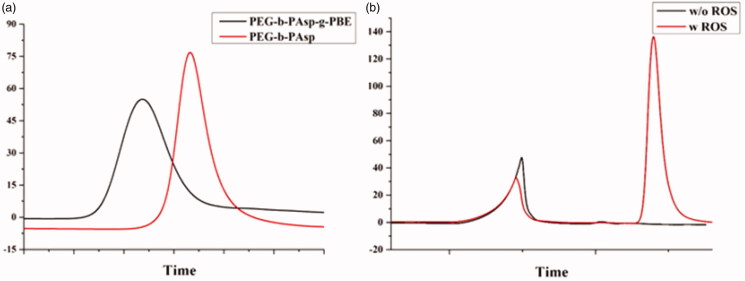
The structure of the copolymers (PEG-b-PAsp and PEG-b-PAsp-g-PBE) was characterized using 1H-NMR spectroscopy. As shown in Figure 1(a), the peaks around 3.51(a) ppm are attributable to the protons (-CH2CH2O) in the PEG chain. The peak around 8.06(b) ppm, 4.5(c) ppm and 2.7(d) ppm are attributable to the protons (-CONH-, -COCHNH-, CH2COOH) in the PAsp chain. The polymerization degrees of PAsp and PEG were determined by calculating the ratio of peak areas of 3.51 ppm to 8.06 ppm, respectively. In Figure 1(b), the peaks around 6.8 ppm, 7.4 ppm and 8.1 ppm (e) attributable to the protons of phenylboronic acid. The degree of PAsp grafted by PBE was calculated by the ratio of peak areas of the protons in the PEG chain and peak of the aromatic protons in the PBE phenyl rings. GPC traces of the block copolymers, PEG45-b-PAsp72 and PEG45-b-PAsp72-g-PBE41 in Figure 2(a) are invariably unimodal with little or no tailing. The compositions of PEG-b-PAsp and PEG-b-PAsp-g-PBE diblock polymers were listed in Table 1. After 4 hours, the GPC result showed one more distinct peak appeared with addition of ROS at pH 7.4, indicating PBA-grafted polymer can be degraded in response to H2O2 triggers (Figure 2(b)).
Figure 1.
(a) 1H NMR spectra of PEG-b-PAsp in DMSO (b) 1H NMR spectra of PEG-b-PAsp-g-PBE in D2O.
Figure 2.
Gel permeation chromatography (GPC) of copolymers (a) PEG-b-PAsp and PEG-b-PAsp-g-PBE (b) PEG-b-PAsp-g-PBE treated with ROS.
Table 1.
Feed Composition and Final Composition of PEG-b-PAsp as well as the CMC Value of the Polymer.
| Diblock Polypeptide | Mna 103 | PDIb | CMC |
|---|---|---|---|
| PEG45-b-PAsp72 | 10.0 | 1.53 | 5.6 mg/L |
| PEG45-b-PAsp72-g-PBE41 | 20.0 | 1.50 | 2.8 mg/L |
Calculated from 1H NMR analysis, the number-average molecular weight (Mn).
Calculated from GPC analysis, the polydispersity index (PDI).
2.2. In vitro cytotoxicity of copolymer
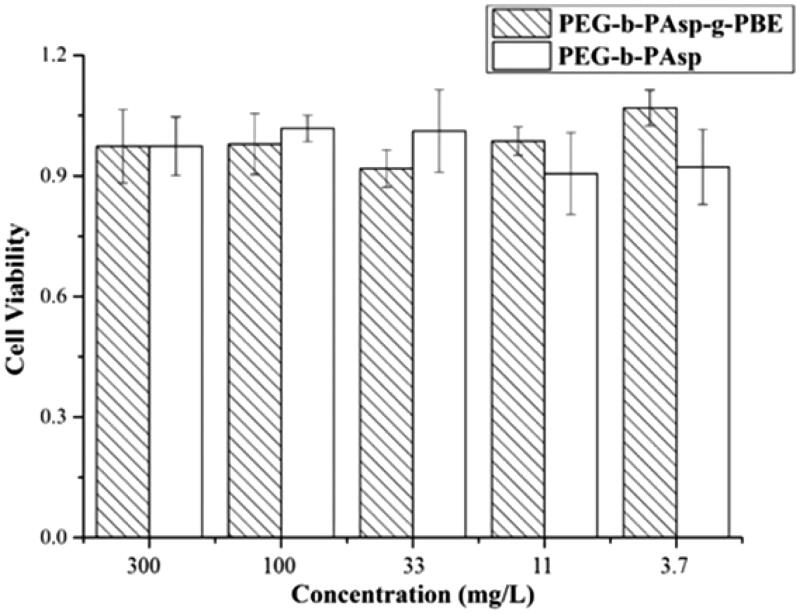
Cytotoxicity of the different copolymer was respectively evaluated by MTT assay and HeLa cells were used in this study. As shown in Figure 3, 3.7–300 mg/l (3.7, 11, 33, 100, 300) of PEG-b-PAsp and PEG-b-PAsp-g-PBE copolymers did not significantly affect the viability of HeLa cell after 48 h of incubation. Consistent with HeLa cell line, the results showed that there was a dramatically high cell viability (more than 95% even at the highest concentration of 300 mg/l) in normal cell line L-O2 (Figures S1 and S2), which displays low cytotoxicity. Inspired by its low cytotoxicity, we finally chose PEG45-b-PAsp72 and PEG45-b-PAsp72-g-PBE41 as the basic diblock copolymer to carry out the further experiments.
Figure 3.
Cell inhibition of PEG-b-PAsp and PEG-b-PAsp-g-PBE on HeLa at different concentrations.
2.3. Preparation of polymeric micelles and drug release in vitro
The PEG-b-PAsp-g-PBE and PEG-b-PAsp-g-PBE/DOX micelles were prepared by dialysis method. The morphology was characterized transmission electron microscope (TEM) and dynamic light scatting (DLS).
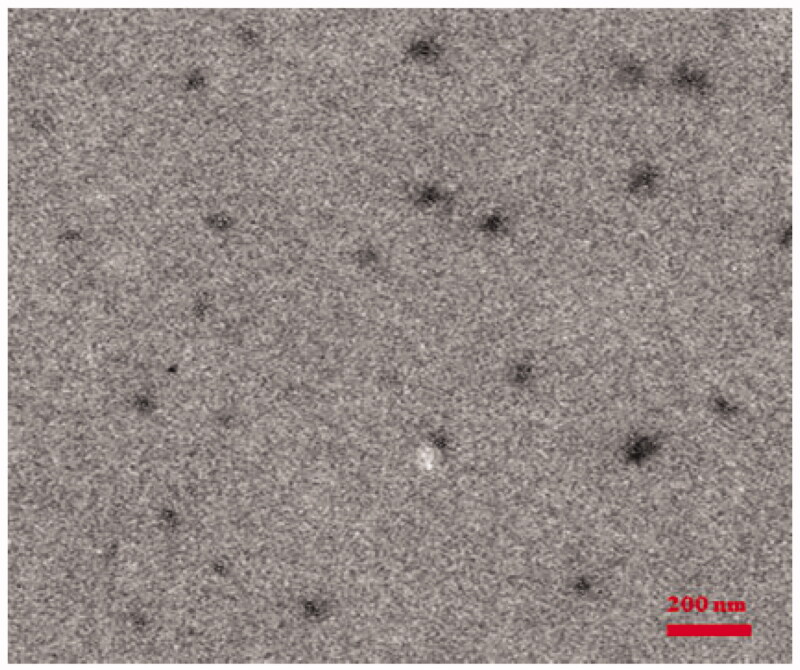
The hydrodynamic diameter of the PEG-b-PAsp micelle was determined as about 70 nm by TEM (Figure S3) which is consistent with its hydrodynamic diameter (Figure S4). Compared with PEG-b-PAsp, its size increased with the modification of PBE group (Figure S5). Moreover, the zeta potential of PEG-b-PAsp-g-PBE micelles changed from −23.9 mV to −15.7 mV (Figure S6), which can further confirm the PBE group has been modified successfully. However, the average diameter of PEG-b-PAsp-g-PBE/DOX was determined as 95 nm by TEM (Figure 4) and DLS (Figure S7), which increase with the effect of the dox encapsulation. Their, surface charges vary from −23.9 mV to −16.1 mV, demonstrating that DOX has been loaded through the electrostatic interaction (Rejinold et al., 2018).
Figure 4.
TEM image of the PEG45-b-PAsp72-g-PBE41/DOX.
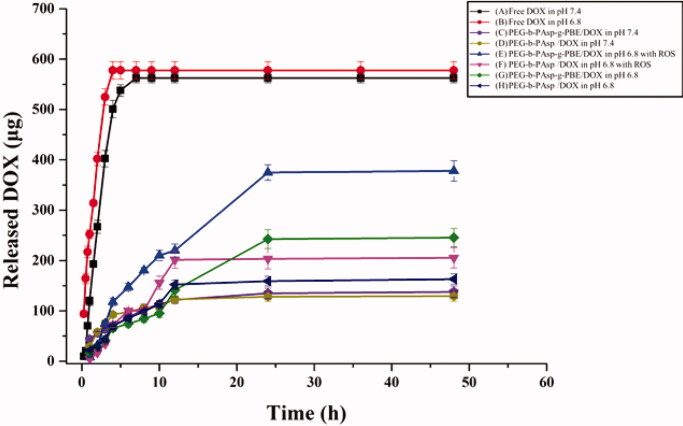
We next assessed the in vitro DOX release profile of the micelles (PEG-b-PAsp/DOX, DLE = 42.0%, DLC = 26.1% and PEG-b-PAsp-g-PBE/DOX, DLE = 43.2%, DLC = 30.0%) in response to ROS by incubating the micelles with PBS, including a control level (pH = 7.4), an acidic pH level (pH = 6.8) and an acidic pH level with ROS (pH = 6.8 with 1 mM H2O2). Free DOX was released quickly in 3 hours at pH 6.8. Even at pH 7.4, it released totally within 6 hours. Since DOX has good water solubility, the free DOX can rapidly exchanges in blood. Compared with the burst release of free DOX, the PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX exhibits a sustained release process and the controlled release behavior. As shown in Figure 5, the release of DOX from PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX was low (22% and 30%) at normal physiological pH 7.4 after 48-h incubation. Acidification of the buffer to pH 6.8 caused a moderately higher release rate (37% and 45%), which could confirm proton can break electrostatic interaction between amino group from DOX and carboxyl group from PAsp, causing drug releasing. Moreover, a significantly higher release rate (70%) of DOX from PEG-b-PAsp-g-PBE/DOX was observed at acidic pH level with H2O2 addition, while DOX release from PEG-b-PAsp/DOX did not obviously increase under the same condition. Therefore, PBA group can be degraded by H2O2 triggers and causing the micelle collapse. Compared with PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX exhibited not only a typical pH-dependent, but also ROS-dependent manner due to the grafted PBE molecules.
Figure 5.
In vitro release profiles of DOX.
2.4. Intracellular ROS generation levels in different cell lines
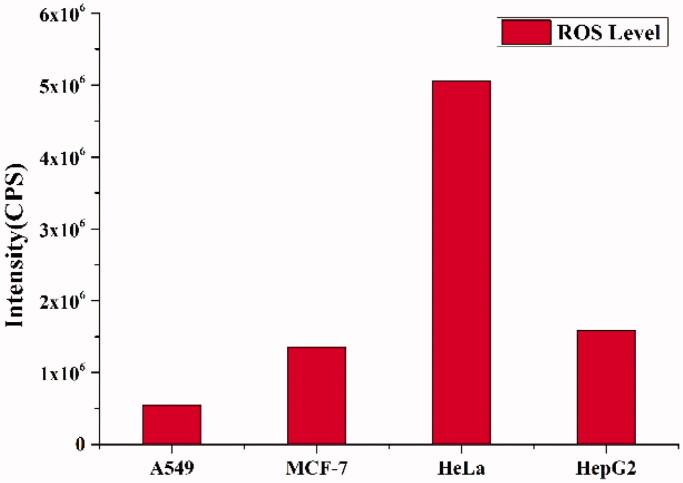
Encouraged by the good ROS-dependent manner of PEG-b-PAsp-g-PBE/DOX, we investigated intracellular ROS levels spontaneously generated in different cell lines (A549, MCF-7, HeLa, and HepG2 cells) by using a ROS Assay Kit (Luan et al., 2019). DCFH-DA, a fluorescence probe for ROS detection, was employed to evaluate the level of intracellular ROS. As depicted, the intracellular ROS levels of HeLa cells were significantly higher in comparison with MCF-7, HepG2 and A549 cells (Figure 6). Encouraged by its level of intracellular ROS, we chose HeLa cell to perform the following experiments.
Figure 6.
ROS levels in A549, MCF-7, HeLa and HepG2 cells quantified using DCFH-DA reagent by fluorometric analysis.
2.5. In vitro cellular internalization and cytotoxicity of PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX micelles
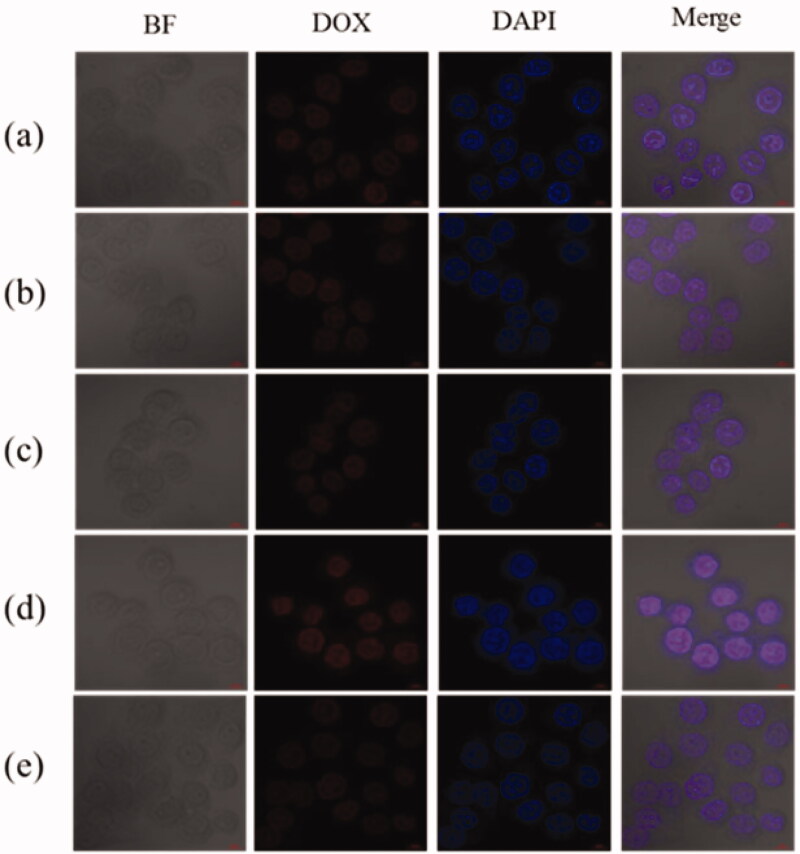
The effect of PBE functionalization on cellular uptake was evaluated by HeLa cells by confocal laser microscopy. For DOX itself is fluorescent, it was used directly to investigate cellular uptake without additional markers in the micelles. Figure 7 showed the cell uptake and intracellular distribution of PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX micelles in HeLa cells after incubation for 4 h. HeLa cells treated with PEG-b-PAsp-g-PBE/DOX (10 μg/mL) presented significantly higher fluorescent signal (Figure 7(d)), indicating a more cellular uptake than that of free DOX (Figure 7(a)) and PEG-b-PAsp/DOX (Figure 7(b)) in the nuclei. Further to prove the role of PBA molecule in ROS response, we pretreat the cells with NAC, acting as a scavenger of ROS, which led to a remarkable decrease of the cellular internalization of PEG-b-PAsp-g-PBE/DOX (Figure 7(e)). However, in PEG-b-PAsp/DOX group (Figure 7(c)), fluorescent signal of DOX did not decrease in pretreatment cell. PEG-b-PAsp-g-PBE effectively facilitated the uptake of DOX through the fast response to the high level of intracellular ROS on HeLa cell.
Figure 7.
Cellular uptake and intracellular localization of DOX in HeLa cells. (a) Free DOX (b) PEG-b-PAsp/DOX (c) PEG-b-PAsp/DOX with NAC pretreatment (d) PEG-b-PAsp-g-PBE/DOX (e) PEG-b-PAsp-g-PBE/DOX with NAC pretreatment.
Besides, DOX released from the PEG-b-PAsp-g-PBE/DOX were quickly transported to the cytoplasm and diffused to the nuclei in response to high concentration of ROS and inherent intracellular acid. Then, the diffused DOX specifically affiliated with DNA and lead to tumor cells damage and apoptosis. As shown in bright field in Figure 7, most HeLa cells treated with the free DOX still survived, while most of cells died with the PEG-b-PAsp-g-PBE/DOX treatment. It indicated that the PEG-b-PAsp-g-PBE/DOX showed the more efficient cell inhibition dependent on the concentration and presented notable cytotoxicity toward HeLa cells, as evidenced with MTT assays.
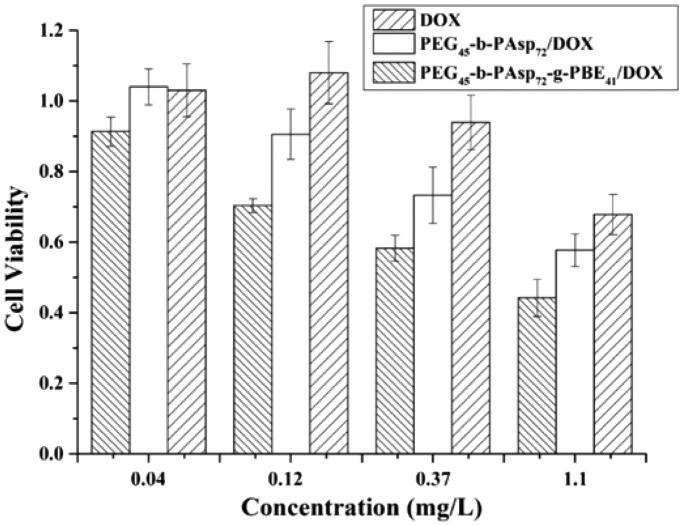
The effect of PBE modification on the cytotoxicity of drug-loaded micelles was studied with the cancer cells HeLa. After 48-h incubation, dose-dependent loss of cell viability was reduced in free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX group (Figure 8). This confirms that the cytotoxicity was due to delivery of the DOX but not the micelles itself. Free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX all exhibited dose-dependent cytotoxicity on HeLa cells. The cytotoxicity of PEG-b-PAsp-g-PBE/DOX was superior to free DOX and PEG-b-PAsp/DOX in cell lines HeLa. Even at the low concentration (0.12 mg/L), PEG-b-PAsp-g-PBE/DOX displays the obvious inhibition, while free DOX and PEG-b-PAsp/DOX does not. The micelles with ROS trigger exhibited an efficient inhibition of HeLa cell growth than that without ROS trigger and free DOX, which is consistent with CLSM result.
Figure 8.
Cell viability rate of HeLa cell lines after co-incubation with DOX, PEG45-b-PAsp72/DOX and PEG45-b-PAsp72-g-PBE41/DOX for 48 h, respectively.
2.6. In vivo tumor inhibition of PEG-b-PAsp-g-PBE/DOX
Encouraged by in vitro effective antitumor activity and uptake on HeLa cells, we proceeded to test the antitumor efficacy of PEG-b-PAsp-g-PBE/DOX in HeLa cell inoculated xenograft mice. Mice were used for the in vivo tumor inhibition experiments, as the tumor volume reached about 120–150 mm3.
We divided tumor-bearing mice into five different groups and later intravenously injected into mice with PEG-b-PAsp-g-PBE/DOX from tail vein. Two group treated with PBS and PEG-b-PAsp-g-PBE were recruited as blank group and another two groups treated with DOX and PEG-b-PAsp/DOX positive controls for 18 days. In order to investigate the tumor growth after administration, we periodically measured the tumor sizes after tail injection.
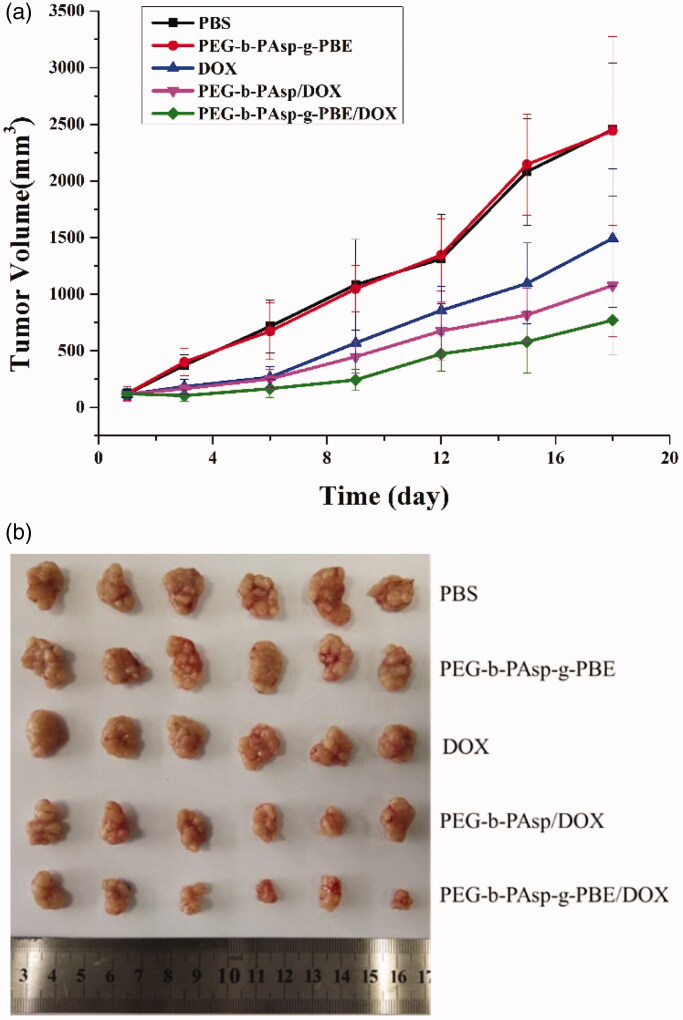
After injection with blank samples (PBS saline and PEG-b-PAsp-g-PBE), no inhibitory effect on tumor growth was observed, and the average tumor volumes of the two control groups at the end of the experiments were 2452.09 and 2442.70 mm3, respectively. However, all DOX groups (DOX, PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX) showed different levels of tumor growth inhibition effect. The tumor volume and tumor growth inhibition of the group PEG-b-PAsp-g-PBE/DOX injected with was 770 mm3 and 68.9% on the 18th day, while positive group treated with DOX and PEG-b-PAsp/DOX were 1492.9 mm3, 39% and 1078.4 mm3, 58.05%, respectively. The holistic tumor photograph after excision was visible in Figure 9. Compared with the saline group, the group treated with PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX and DOX showed smaller tumor burden. Remarkable inhibition in tumor growth was observed with PEG-b-PAsp-g-PBE/DOX during the in vivo study in comparison to free DOX and PEG-b-PAsp/DOX. At the same concentration (2 mg/kg) of DOX, experiments in mice demonstrated that PEG-b-PAsp-g-PBE/DOX did have more efficient antitumor activity in vivo than free DOX which is used as a first-line chemotherapy drug against HeLa tumor. This result can demonstrate PBE groups take advantage of passive targeting to deliver DOX to malignant tumor more efficiently.
Figure 9.
(a) Real-time observation of tumor sizes in vivo after treatment with samples. Error bars represent means ± SD (*p <.05, **p <.01); (b) Photo of tumor tissues from mice treated with PBS, PEG-b-PAsp-g-PBE, DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX at the end of the study.
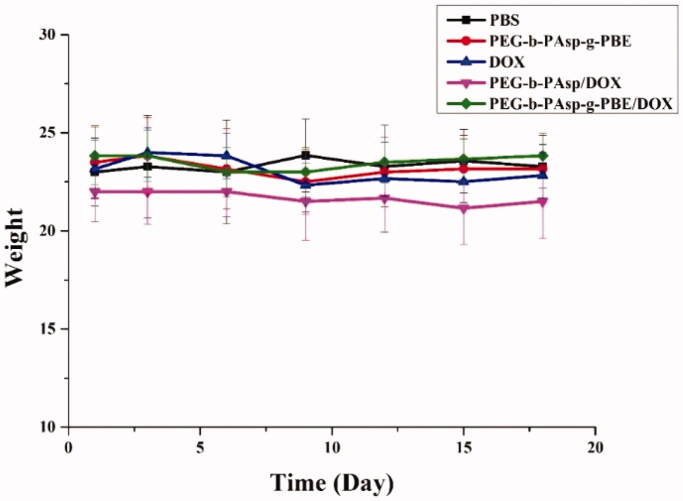
Toxicity is a major concern for in vivo application of chemotherapy. In order to identify the safety of drug delivery, we also measured the changes of mouse weight during the treatment process. We discovered that the weight of all five groups of mice did not change obviously at the end of treatment. (Figure 10) Meanwhile after treatment with PBS, PEG-b-PAsp-g-PBE, free DOX, PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX for 18 days, we sacrificed mice, collected all the tumor tissues and measured them respectively (Liu et al., 2016; Wang et al., 2019).
Figure 10.
Real-time weight analysis of mice after each treatment. Error bars represent means ± SD.
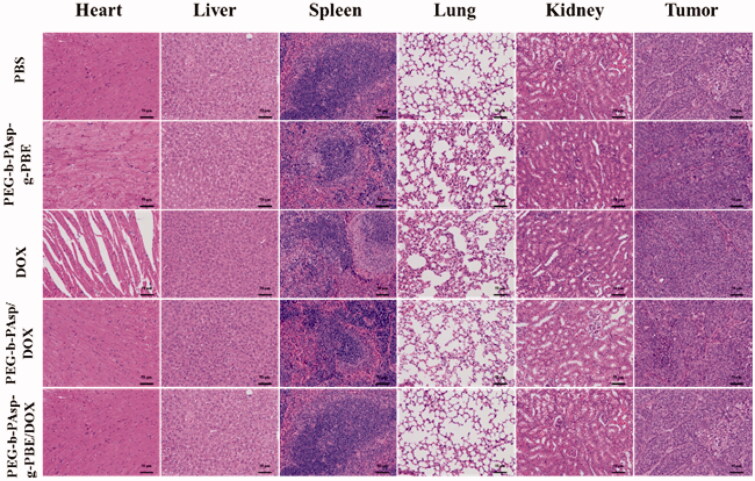
The histological analysis of fixed tissues was showed in Figure 11. DOX·HCl, a classical chemotherapeutic agent, is well known for its severe side effects dominated by heart toxicity. Compared with tissues of blank group, especially in histological observation of heart tissues, puff cardiomyocyte nucleus with vacuole and undense cytoplasm, was observed in free DOX group. While in PEG-b-PAsp-g-PBE/DOX group, no obvious abnormality was found. Obviously, through the reasonable design and the chemical assembly, the damages on heart due to the low selectivity of DOX·HCl were efficaciously relieved by this intelligent drug delivery system.
Figure 11.
Histological analysis of tissues with H&E after treatments with PBS, PEG-b-PAsp-g-PBE, DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX.
3. Conclusion
In summary, we have constructed the biocompatible drug delivery system with a polypeptide material PEG-b-PAsp-g-PBE, containing PBE chemically modification for effective chemotherapeutic drugs delivery. In comparison with PEG-b-PAsp/DOX, the higher inhibition of fabricated ROS-responsive PEG-b-PAsp-g-PBE/DOX is mainly owing to valid degradation, efficient disassembly and successful intracellular release of DOX once triggered by intracellular ROS. PBE groups can enhanced their tumor targeting ability passively by fast response to tumor microenvironment of ROS rich. Benefiting from the PBE, the entire system showed an enhanced chemotherapeutic efficacy both in vitro and in vivo. Overall, PEG-b-PAsp-g-PBE exhibited a good tumor inhibition effect and low side effects, which will open a new avenue toward the fabrication of smart drug delivery system by taking advantage of biologically relevant intracellular triggering stimuli.
4. Experiment section
4.1. Chemicals and apparatus
All reagents and solvents were commercially available and used without additional treatment. Doxorubicin Hydrochloride (DOX•HCl, denoted as DOX, 99.8%) were purchased from Melone Pharmaceutical Corporation. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin-EDTA, penicillin–streptomycin, dimethyl sulfoxide (DMSO), and 4,6-diamidino-2-phenylindole (DAPI) were obtained from Gibco. 96 well plates, 6-well plates, and 10 mL graduated sterile centrifuge tubes were purchased from KeyGen BioTECH. Other reagents and chemicals were at least analytical reagent grade.
4.2. Characterization
1H NMR spectra of the polymers were recorded on a Bruker 400 MHz nuclear magnetic resonance instrument using DMSO as the solvents. Gel permeation chromatography (GPC) was used to analyze the molecular weights and molecular weight distributions (Mw/Mn) of the polymers. GPC of PEG-b-PAsp was measured at room temperature with a Waters 1525 chromatograph equipped with a Waters 2414 refractive index detector. H2O was used as eluents with a flow rate of 1.0 mL/min and narrowly distributed polyethylene glycol was used as standard. The size and surface charge of the nanocarrier was investigated on Malvern Zetasizer Nano ZS 90 zeta potential analyzer. Ultraviolet-visible (UV-vis) spectra were collected using a LAMBDA-35 spectrometer. Transmission electron microscopy (TEM) was performed on a JEOL-2100 with accelerating voltage of 200 kV. TEM samples were prepared by drop-casting dispersion onto copper grids covered by carbon film. Confocal images were acquired using a Zeiss confocal laser scanning unit mounted on an LSM 710 fixed-stage upright microscope (CLSM).
4.3. Synthesis of diblock copolymers
4.3.1. M-poly (ethylene glycol)-b- poly (aspartate)
The diblock copolymer (PEG-b-polyaspartate) was firstly synthesized via the amine-initiated ring-opening polymerization (ROP) of N-carboxy-α-amino acid anhydrides of β-Benzyl-L-aspartate (BLA-NCA). Briefly, BLA-NCA was dissolved completely in DMF/DCM followed by addition of initiator which had been dissolved in DCM. Then, the reaction mixture was stirred for 5 days at 35 °C under a dry nitrogen atmosphere and the crude products were precipitated in 10-fold excess of cold diethyl ether and isolated by centrifugation. After washed twice with diethyl ether, the products were dried in vacuum. Block copolymers of m-PEG-b-PAsp were obtained by deprotection of m-PEG-b-PBLA in HCCl2COOH/HBr/HAc solution. After stirred for 3 h under ice bath, the solution was precipitated in large amount of cold diethyl ether and isolated by centrifugation. The solid was dissolved in DMSO. The solution was dialyzed against water in a dialysis bag with a proper molecular cutoff and then solid m-PEG-b-PAsp was obtained by lyophilization (Yang et al., 2015; Yavvari et al., 2019).
4.3.2. M-poly (ethylene glycol)-b-poly (aspartate)-g-phenylboronic acid pinacol ester
Partial modification of PEG-b-PAsp with phenylboronic acid pinacol ester (PBE) was prepared according to the esterification reaction. First, PEG-b-PAsp was dissolved in anhydrous DMF at room temperature. According to the length of PAsp chain, PBE with proper molar ratio was added followed by DMAP addition, and the mixture solution was stirred at 40 °C for 12 h followed by being dialyzed against deionized water for 2 days in a dialysis bag with 3500DA cutoff. Finally, PEG-b-PAsp-g-PBE were obtained by lyophilizing the corresponding polymer solution (Hu et al., 2017). 1H NMR spectra of the polymers were recorded on a Bruker 400 MHz nuclear magnetic resonance instrument using DMSO as the solvents. Gel permeation chromatography (GPC) was used to analyze the molecular weights and molecular weight distributions (Mw/Mn) of the polymers. GPC of PEG-b-PAsp was measured by a Waters 1525 chromatograph equipped with a Waters 2414 refractive index detector. The critical micelle concentration (CMC) of PEG-b-PAsp copolymers was determined according to the literature using pyrene as a hydrophobic fluorescent probe (Raimbault et al., 2018).
4.4. Cell lines and culture conditions
HeLa (Cervical cancer cells), L-O2 (Human normal liver cells) A549 (Human lung adenocarcinoma cells) MCF-7 (Human breast cancer cell)and HepG2 (liver hepatocellular carcinoma) cell lines were provided by KeyGEN Biotech and maintained in Dulbecco’s Modified Eagles Medium (DMEM) containing 10% fetal bovine serum (HyClone Laboratories, Inc. Logan, UT, USA) with 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin. The cells were cultured in a humidified incubator at 37 °C, 5% CO2. Female Balb/c mice weighing 18–20 g furnished by Experimental Animal Center, Jiangsu Academy of Traditional Chinese Medicine. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of China Pharmaceutical University and approved by the Animal Ethics Committee of China Pharmaceutical University, Jiangsu, China.
4.5. In vitro cytotoxicity of copolymers
The cytotoxic effect of block copolymers PEG-b-PAsp and PEG-b-PAsp-g-PBE was evaluated using MTT. In brief, L-O2 and HeLa cells were initially seeded into a 96-well cell culture plate at 4 × 103 per well and then incubated for 24 h at 37 °C under 5% CO2. Then, DMEM solutions with 10% FBS of different block copolymers at different concentrations were added under the same condition for 72-h incubation. Four hours before the experiment was stopped, the cells were washed three times with 0.2 mL PBS and culture medium was replaced with MTT solution of 0.2 mL. At the end of the experiment, the medium solution was replaced by 0.15 mL DMSO solution. The optical density of the solution was measured by enzyme linked immunosorbent assay (ELISA) at a wavelength of 490 nm. The absorbance value of untreated cells was set at 100%. Each experiment was repeated three times in sextuplicate. The cell viability was calculated according to following formula (Hao et al., 2018):
4.6. Preparation of polymeric micelles
The PEG-b-PAsp, PEG-b-PAsp-g-PBE and PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX polymeric micelles were prepared by dialysis method (Li et al., 2015).
PEG-b-PAsp (50 mg) and PEG-b-PAsp-g-PBE (50 mg) were dissolved in 4 ml DMF. Then deionized water (10 ml) was added into DMF solution slowly. The above solution was stirred at room temperature for 6 h. The mixture was dialyzed against deionized water for 3 days by using a dialysis bag (MWCO: 3500 Da). The solution in the dialysis bag was lyophilized to obtain micelles.
DOX (20 mg) and PEG-b-PAsp-g-PBE (50 mg) were dissolved in DMSO (4 ml). Then deionized water (15 ml) was added into DMSO. The above solution was stirred at room temperature for 6 h. The mixture was dialyzed against deionized water to remove DMSO and free DOX·HCl. The solution in the dialysis bag was lyophilized to obtain PEG-b-PAsp-g-PBE/DOX micelles. PEG-b-PAsp/DOX was prepared by the same method.
Drug-loading content (DLC) and drug-loading efficiency (DLE) were calculated according to the following formula:
Li et al., 2018.
The UV-visible absorption spectra of free DOX, PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX were scanned at 492 nm against the corresponding solvent blank in a 200 μL quartz cuvette and the calibration curve drug loading was measured in triplicate.
4.7. Dynamic light scattering (DLS) measurement and morphology
The size and zeta potential of polymeric micelles were measured by dynamic light scattering (DLS) using a Malvern ZS90 instrument equipped with a 532 nm laser at a scattering angle of 90° (Yadav et al., 2018). The measurements were performed at 25 °C after diluting the samples to an appropriate concentration with ultrapure water (pH= 7.0).
4.8. In vitro release profile study
To study the in vitro release behavior of ROS-triggered DOX load Polymeric micelles, 1.5 mg of PEG-b-PAsp/DOX and PEG-b-PAsp-g-PBE/DOX aqueous solution was infused into a dialysis bag with 3500 DA cutoff and dialyzed against phosphate-buffered saline (PBS, 1 mM H2O2) at 37 °C on an orbital shaker in the dark. At each interval time point over a period of 48 h (1 h, 2 h, 4 h, 6 h, 10 h, 12 h, 24 h, 48 h), the concentration of DOX in the dialysate was measured by ultraviolet spectroscopy. The total volume of dialysis medium was maintained at 80 mL through the test.
4.9. Measurement of intracellular ROS level
Intracellular ROS generation in different cell lines was quantitatively detected using a ROS Assay Kit. Briefly, tumor cells (1 × 106 cells/mL) A549, HeLa, HepG2, MCF-7 were seeded on a coverslip in a 6-well plate and incubated overnight in a 5% CO2 incubator at 37 °C for attachment, respectively. DCFH-DA was added for ROS detection. The fluorescence of DCF-DA (generated after the oxidation of DCFH-DA by ROS) was measured by fluorescence analysis (Huang et al., 2018; Zhang et al., 2019).
4.10. Confocal laser scanning microscopy (CLSM) imaging analysis
To investigate the general intracellular distribution of free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX, CLSM was utilized to trace the endocytosed free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX according to previously reported procedures. 5 × 104 HeLa cells were seeded on a coverslip in 35 mm dishes and incubated overnight in a 5% CO2 incubator at 37 °C for attachment, respectively. Cells were first washed with phosphate-buffered saline (PBS, pH = 7.4) and treated with free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX. After culturing for 4 h, the cells were washed with PBS three times and mixed with 500 μL 4% paraformaldehyde for 20 min. Subsequently, to further observe cell nuclei, cells were stained with DAPI (2 μg/mL) for 15 min. Cell nuclei and intracellular fluorescent free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX were observed by CLSM (Zhao et al., 2018).
4.11. In vitro antitumor activity
The cell cytotoxicity of free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX on HeLa cells was determined quantitatively by using MTT assay. All the cells were seeded in a 96 well plate at a density of 1 × 104 cells/well and incubated with a series of free DOX, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX containing the same concentration ranging from 3 to 0.04 mg/L of DOX for 48 h under the same conditions.
4.12. Tumor model
Three- to four-week-old Balb/c female nude mice (20–22 g, Nanjing Mu Tu Medical Science and Technology Co.) were inoculated subcutaneously with 2 × 106 HeLa cells (suspended in 100 μl sterile PBS)/mouse in the right side of the flank. The tumor sizes were measured every 2 days by a digital caliper. The tumor volume was figured out according to the following equation: volume = tumor width2×tumor length/2. In vivo experiments were conducted when the tumor average volume reached 120–150 mm3 (Liu et al., 2016; Li et al., 2017; Wang et al., 2019).
4.13. Antitumor efficacy study
To evaluate the curative effects of PEG-b-PAsp-g-PBE/DOX in vivo, nude mice bearing HeLa tumor were administrated twice a week with PBS, free DOX·HCl, PEG-b-PAsp-g-PBE, PEG-b-PAsp/DOX, PEG-b-PAsp-g-PBE/DOX (containing same concentration of DOX) nanoparticles, respectively. Eighteen days later, treated mice were sacrificed, and the tumor tissues were removed from the bodies for measurement. Tumors and major organs (heart, liver, spleen, lung and kidney) were dissected from mice and hematoxylin–eosin (H&E) staining.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the support of Qing Lan Project in Jiangsu Province, “Double First-Class” University project (CPU2018GY25) and Postgraduate Research and Practice Innovation Program of Jiangsu Province.
Disclosure statement
The authors declare no competing financial interest.
References
- Cabral H, Miyata K, Osada K, et al. (2018). Block copolymer micelles in nanomedicine applications. Chem Rev 118:6844–92. [DOI] [PubMed] [Google Scholar]
- Chen H, Gu Z, An H, et al. (2018). Precise nanomedicine for intelligent therapy of cancer. Sci China Chem 61:1503–52. [Google Scholar]
- Dai Z, Yu M, Yi X, et al. (2019). Chain-length- and saturation-tuned mechanics of fluid nanovesicles direct tumor delivery. ACS Nano 13:7676–89. [DOI] [PubMed] [Google Scholar]
- Einfalt T, Witzigmann D, Edlinger C, et al. (2018). Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat Commun 9:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Li Q, Wang H, et al. (2018). CAGW modified polymeric micelles with different hydrophobic cores for efficient gene delivery and capillary-like tube formation. ACS Biomater Sci Eng 4:2870–8. [DOI] [PubMed] [Google Scholar]
- Hu X, Yu J, Qian C, et al. (2017). H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano 11:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang Y, Xie Z, et al. (2017). Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 18:649–73. [DOI] [PubMed] [Google Scholar]
- Hua Q, Qiang Z, Chu M, et al. (2019). Polymeric drug delivery system with actively targeted cell penetration and nuclear targeting for cancer therapy. ACS Appl Bio Mater 2:1724–31. [DOI] [PubMed] [Google Scholar]
- Huang CH, Huang CY, Huang MH. (2018). Unsaturated squalene content in emulsion vaccine adjuvants plays a crucial role in ROS-mediated antigen uptake and cellular immunity. Mol Pharmaceutics 15:420–9. [DOI] [PubMed] [Google Scholar]
- Li HM, Dong ZP, Wang QY, et al. (2017). De novo computational design for development of a peptide ligand oriented to VEGFR-3 with high affinity and long circulation. Mol Pharmaceutics 14:2236–44. [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Zhang Y, et al. (2015). Choline derivate-modified doxorubicin loaded micelle for glioma therapy. ACS Appl Mater Interfaces 7:21589–601. [DOI] [PubMed] [Google Scholar]
- Li Y, Bai H, Wang H, et al. (2018). Reactive oxygen species (ROS)-responsive nanomedicine for RNAi-based cancer therapy. Nanoscale 10:203–14. [DOI] [PubMed] [Google Scholar]
- Li Y, Lv S, Song Z, et al. (2018). Photodynamic therapy-mediated remote control of chemotherapy toward synergistic anticancer treatment. Nanoscale 10:14554–62. [DOI] [PubMed] [Google Scholar]
- Liang L, Fu J, Qiu L. (2018). Design of pH-sensitive nanovesicles via cholesterol analogue incorporation for improving in vivo delivery of chemotherapeutics. ACS Appl Mater Interfaces 10:5213–26. [DOI] [PubMed] [Google Scholar]
- Liu LX, Li BX, Wang QY, et al. (2016). An integrative folate-based metal complex nanotube as a potent antitumor nanomedicine as well as an efficient tumor-targeted drug carrier. Bioconjugate Chem 27:2863–73. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu F, Ji Y, et al. (2019). Recent advances in anti-cancer protein/peptide delivery. Bioconjugate Chem 30:305–24. [DOI] [PubMed] [Google Scholar]
- Lu Y, Aimetti AA, Langer R, et al. (2017). Bioresponsive materials. Nat Rev Mater 2:1–17. [Google Scholar]
- Luan T, Cheng L, Cheng J, et al. (2019). Tailored design of an ROS-responsive drug release platform for enhanced tumor therapy via “Sequential Induced Activation Processes”. ACS Appl Mater Interfaces 11:25654–63. [DOI] [PubMed] [Google Scholar]
- Raimbault J, Casier R, Little H, et al. (2018). Hydrophobic and elastic forces experienced by a series of pyrene end-labeled poly(ethylene oxide)s interacting with sodium dodecyl sulfate micelles. Macromolecules 51:5933–43. [Google Scholar]
- Rejinold NS, Yoo J, Jon S, et al. (2018). Curcumin as a novel nanocarrier system for doxorubicin delivery to MDR cancer cells: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 10:28458–70. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang G, Zhang H, et al. (2018). The blood clearance kinetics and pathway of polymeric micelles in cancer drug delivery. ACS Nano 12:6179–92. [DOI] [PubMed] [Google Scholar]
- Timko BP, Dvir T, Kohane DS. (2010). Remotely triggerable drug delivery systems. Adv Mater 22:4925–43. [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Hola K, Subr V, et al. (2016). Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev 116:5338–431. [DOI] [PubMed] [Google Scholar]
- Wang QY, Li HM, Dong ZP, et al. (2019). Peptide-mediated cationic micelles drug-delivery system applied on a VEGFR3-overexpressed tumor. J Mater Chem B 7:1076–86. [DOI] [PubMed] [Google Scholar]
- Wijetunge SS, Wen J, Yeh CK, et al. (2018). Lectin-conjugated liposomes as biocompatible, bioadhesive drug carriers for the management of oral ulcerative lesions. ACS Appl Bio Mater 1:1487–95. [DOI] [PubMed] [Google Scholar]
- Xiang J, Liu X, Zhou Z, et al. (2018). Reactive oxygen species (ROS)-responsive charge-switchable nanocarriers for gene therapy of metastatic cancer. ACS Appl Mater Interfaces 10:43352–62. [DOI] [PubMed] [Google Scholar]
- Xu Q, He C, Xiao C, et al. (2016). Reactive oxygen species (ROS) responsive polymers for biomedical applications. Macromol Biosci 16:635–46. [DOI] [PubMed] [Google Scholar]
- Yadav N, Kumar N, Prasad P, et al. (2018). Stable dispersions of covalently tethered polymer improved graphene oxide nanoconjugates as an effective vector for siRNA delivery. ACS Appl Mater Interfaces 10:14577–93. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang C, Li C, et al. (2015). Glucose-responsive polymer vesicles templated by alpha-CD/PEG inclusion complex. Biomacromolecules 16:1372–81. [DOI] [PubMed] [Google Scholar]
- Yavvari PS, Awasthi AK, Sharma A, et al. (2019). Emerging biomedical applications of polyaspartic acid-derived biodegradable polyelectrolytes and polyelectrolyte complexes. J Mater Chem B 7:2102–22. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kang Y, Liu G, et al. (2019). Poly(cystine–PCL) based pH/redox dual-responsive nanocarriers for enhanced tumor therapy. Biomater Sci 7:1962–72. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li D, Huang J, et al. (2019). Screening of pH-responsive long-circulating polysaccharide–drug conjugate nanocarriers for antitumor applications. J Mater Chem B 7:251–64. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang R, Huang J, et al. (2019). Albumin enhances PTX delivery ability of dextran NPs and therapeutic efficacy of PTX for colorectal cancer. J Mater Chem B 7:3537–45. [Google Scholar]
- Zhang Y, Guo Z, Cao Z, et al. (2018). Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials 183:243–57. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou J, Ma S, et al. (2019). Reactive oxygen species (ROS)-degradable polymeric nanoplatform for hypoxia-targeted gene delivery: unpacking DNA and reducing toxicity. Biomacromolecules 20:1899–913. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ge X, Zhao H, et al. (2018). Double-sensitive drug release system based on mno2 assembled upconversion nanoconstruct for double-model guided chemotherapy. ACS Appl Nano Mater 1:1648–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.