Abstract
Objective
To determine risk factors for pediatric hospital-associated venous thromboembolism (HA-VTE) in noncritically ill children to derive a novel HA-VTE risk model for this population.
Study design
Patients with HA-VTE were identified retrospectively via the electronic health record at All Children’s Hospital Johns Hopkins Medicine from April 10, 2013 through January 1, 2006. Seven contemporaneous, noncritically ill control children were randomly selected for each case of HA-VTE. The association between putative risk factors and HA-VTE was estimated with ORs and 95% CIs, which were calculated using the Wald method. A P-value threshold ≤.2 was used in univariate analysis for inclusion into a multivariate (adjusted) model.
Results
Fifty cases of HA-VTE occurred in noncritically ill children. The presence of a central venous catheter (OR 27.67, 95% CI, 8.40–91.22), infection (OR 10.40, 95% CI, 3.46–31.25), and length of stay ≥4 days (OR 5.26, 95% CI, 1.74–15.88) were found to be statistically significant risk factors for HA-VTE. An 8-point risk score was derived in which scores of 8 points, 7 points, and ≤6 points corresponded to venous thromboembolism risks of 12.5%, 1.1%, and 0.1%, respectively.
Conclusion
The presence of a central venous catheter, infection, and length of stay ≥4 days are significant risk factors for HA-VTE in noncritically ill children, forming the basis for a new risk score that could inform venous thromboembolism prophylaxis decision-making. These findings warrant prospective validation.
Venous thromboembolism (VTE), consisting of deep venous thrombosis (DVT) and pulmonary embolism (PE), affects an estimated 300 000–500 000 Americans annually, prompting a Call-to-Action by the US Surgeon General in 2008.1 Although prophylactic anticoagulation for medically ill hospitalized adults has become a standard of care,2 corresponding recommendations and data are lacking in pediatrics, where historical population-based estimates of the incidence of VTE using the National Hospital Discharge Survey were reported at 5 per 10 000 per year,3 approximately 100-fold less frequent than in adults. Yet, more recent findings from the Pediatric Hospital Information Survey database suggest that the incidence of pediatric VTE is dramatically increasing,4 likely the result of improved survival, heightened awareness, and enhanced sensitivity of imaging.
The clinical consequences of VTE are significant, with 25% of children with limb DVT developing signs and/or symptoms of chronic venous insufficiency (the post-thrombotic syndrome),5 16%–20% having objectively confirmed PE,6 and a PE-specific mortality rate ranging between 2% and 9%.7,8 To date, only 2 published randomized controlled clinical trials of anticoagulant primary prevention against thromboembolism have been conducted in children, and these trials have appropriately focused upon the highly selective pediatric settings of femoral arterial catheterization9 and post-Fontan procedure in children with single-ventricle cardiac physiology.10
A risk-stratified approach to the prevention of hospital-associated VTE (HA-VTE) is necessary to optimize the risk/benefit ratio of pharmacologic and mechanical prophylactic interventions. Such a risk-stratified strategy requires the development and validation of risk models and associated risk scores.
In recent years, several single-institutional case-control studies have been conducted.11–17 Two of these studies found a significant increase in the risk of HA-VTE for critically ill children receiving complex care for acute lymphoblastic leukemia14 and extensive cardiac disease.15 In another study, the risk of HA-VTE was found to differ between critically and noncritically ill children.13 This and previous studies have been insufficiently powered for the development of distinct risk models for critically and noncritically ill children. Given the heightened incidence of pediatric HA-VTE in the intensive care unit (ICU) setting, it is likely that current knowledge of HA-VTE risk factors is driven by critical illness factors. The objective of the present work was therefore to develop a HA-VTE risk model and associated risk score specific to noncritically ill children.
Methods
This study was approved by the Institutional Review Board at All Children’s Hospital Johns Hopkins Medicine (ACH JHM, St. Petersburg, Florida), with waiver of informed consent. Cases of HA-VTE were identified retrospectively via the electronic health record-derived data warehouse (EHR-DW) at ACH JHM from April 10, 2013 through January 1, 2006, and validated by review of the radiologic record. Inclusion criteria consisted of the following: (1) diagnosis of VTE by International Classification of Diseases, 9th Revision (ICD-9) at time of discharge coding, or at coding from an encounter within 30 days of discharge; and (2) verification of VTE diagnosis by review of radiologic reports. Exclusions included the following: (1) admission to an ICU; (2) signs or symptoms of VTE noted in admission history/exam; or (3) radiologic confirmation of VTE within 24 hours of admission to hospital or emergency center, without history of hospital admission in the preceding 30 days. ICD-9 codes for VTE included 325 (phlebitis and thrombophlebitis of intracranial venous sinuses); 437.6 (nonpyogenic thrombosis of intracranial venous sinus); 452 (portal vein thrombosis); 453.2 (inferior vena cava thrombus); 453.4 (VTE of deep vessels of the lower extremity); 453.8 (embolism or thrombosis of other specified veins); 453.9 (embolism and thrombosis of unspecified site); and 415.1 (PE and pulmonary infarction). For each VTE case, seven contemporaneous noncritically ill control patients were randomly selected from the EHR-DW.
For both VTE cases and controls, clinical data on demographics and putative risk factors for VTE were extracted from the EHR-DW, including age; sex; length of stay (LOS); previous history of VTE; previous hospitalization within 30 days; presence of a central venous catheter (CVC); major surgery; congenital heart disease; cardiac catheterization; dehydration; malignancy; infection; chronic inflammatory disease; cystic fibrosis; nephrotic syndrome; and obesity. Chronic inflammatory diseases included diabetes mellitus, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), systemic lupus erythematosus, juvenile rheumatoid arthritis, graft vs host disease, and autoimmune encephalitis. Infection included meningitis, abscess, pneumonia, osteomyelitis, bacteremia, fungemia, and pyelonephritis.
Obesity was defined on the basis of its notation in the admission history due to of a high degree of missing data on patient height by which to calculate an accurate body mass index percentile. Dehydration was identified on the basis of its notation in the admission history, and the presence of CVC was further categorized into long-term CVC (eg, Broviac, Mediport) vs short-term CVC (eg, peripherally inserted central catheter line, temporary subclavian, or femoral line). To capture postdischarge VTE episodes, patients in whom radiologic diagnosis of VTE was made within 24 hours of admission were evaluated for a preceding admission within 30 days; for those in whom this was the case, data on the aforementioned VTE risk factors were extracted from the EHR-DW for the preceding admission.
Statistical Analyses
Dichotomous or categorical variables were compared between cases and controls by the use of χ2 analysis or the Fisher exact test, as appropriate. Continuous variables were compared between groups via the Mann-Whitney U test. A risk model was derived via logistic regression, with 95% CIs calculated for ORs using the Wald method. Although P < .05 were used to define statistical significance for all inferential statistics, a P-value threshold of ≤.2 was used in univariate analysis for inclusion of putative risk factors into the multivariate (adjusted) model. Estimation of HA-VTE risk associated with the risk model and associated risk score was based upon post-test probability of HA-VTE using the model, which was calculated using the pre-test probability (based on calculated incidence) and the likelihood ratio derived from model sensitivity and specificity measures, as previously described,13 and using the formula: pre-test odds × likelihood ratio = post-test odds.
Results
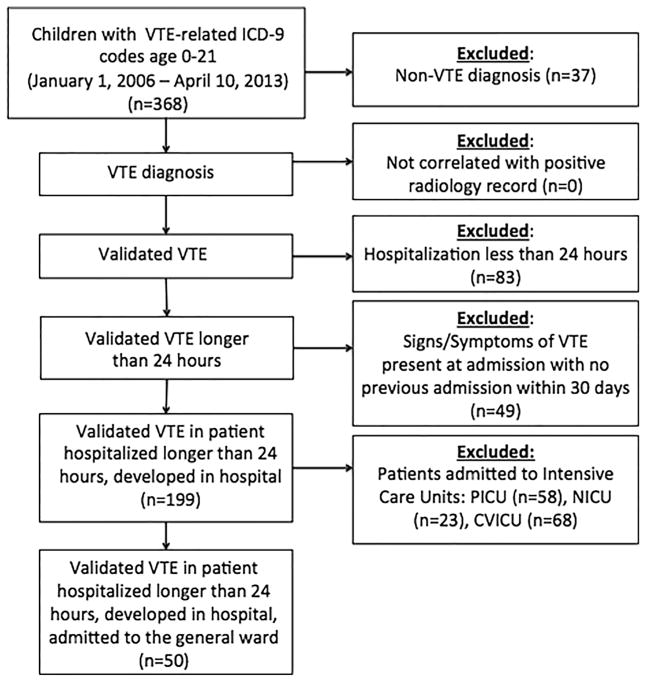
During the 7-year period of observation at ACH JHM, 50 cases of HA-VTE in noncritically ill children were confirmed via the use of the inclusion and validation criteria delineated previously. A flow diagram of included and excluded patients is provided in the Figure. To derive a current estimate on VTE incidence in noncritically ill children at ACH JHM, we determined an average annual number of non-ICU admissions of 6094 in the 2 most recent calendar years of the study (January 2011 to December 2012, inclusive); given an annual average of 10 cases of HA-VTE during this period, the estimated annual occurrence rate of HA-VTE was approximately 1 in 500 hospitalized noncritically ill children. Of the 50 cases, 16 (34%) had infection, as follows: pneumonia, osteomyelitis, or fungemia, n = 4 each; and abscess or meningitis, n = 2 each (Table I).
Figure.
Flow diagram of included and excluded patients in the study. CVICU, cardiovascular intensive care unit; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Table I.
Demographic and clinical characteristics among cases with HA-VTE and controls
| Cases | Controls | |||
|---|---|---|---|---|
|
|
|
|||
| n | % | n | % | |
| Median age, y (range) | 50 | 11.5 (0.1–20.0) | 350 | 7.7 (6.1–21.4) |
| Birth to <1 | 7 | 14 | 64 | 18.29 |
| 1–5 | 3 | 6 | 98 | 28 |
| 6–10 | 12 | 24 | 70 | 20 |
| 11–15 | 13 | 26 | 49 | 14 |
| 16–21 | 15 | 30 | 69 | 19.71 |
| CVC (any) | 42 | 84 | 50 | 14.29 |
| Short-term CVC | 21 | 42 | 14 | 4 |
| Long-term CVC | 22 | 44 | 37 | 10.57 |
| Infection | 26 | 52 | 42 | 12 |
| Major surgery | 3 | 6 | 85 | 24.29 |
| Malignancy | 18 | 36 | 35 | 10 |
| Obesity | 3 | 6 | 5 | 1.43 |
| Dehydration | 6 | 12 | 29 | 8.29 |
| Chronic inflammatory disease (non-lupus) | 5 | 10 | 5 | 1.43 |
| Hospital days, median (range) | 50 | 14 (2–166) | 350 | 3 (0–35) |
| Hospital days ≥4 | 44 | 88 | 113 | 32.29 |
| Previous hospitalization within 30 d | 17 | 34 | 41 | 11.71 |
| Cardiac catheterization | 2 | 4 | 1 | 0.29 |
| Premature birth | 3 | 6 | 41 | 11.71 |
| History of VTE | 5 | 12.5 | 0 | 0 |
| Congenital heart disease | 1 | 2 | 14 | 4 |
| Nephrotic syndrome | 0 | 0 | 4 | 1.14 |
| Diabetes mellitus | 1 | 2 | 6 | 1.71 |
| Cystic fibrosis | 3 | 6 | 6 | 1.71 |
| Systemic lupus erythematosus | 0 | 0 | 2 | 0.57 |
Cases of HA-VTE were matched with randomly selected, contemporaneous control patients. Univariate logistic regression analyses (Table II) revealed that, among the putative risk factors evaluated, the presence of a CVC (OR 31.49, 95% CI, 13.97–71.02), LOS ≥4 days (OR 15.38, 95% CI, 6.37–37.14), and infection (OR 7.95, 95% CI, 4.18–15.09) were all statistically significantly associated with development of HA-VTE. After adjustment in multiple logistic regression (Table I), each of these remained as statistically significant, independent risk factors for HA-VTE (CVC [OR 27.67, 95% CI, 8.40–91.22], infection [OR 10.40, 95% CI, 3.46–31.25]), and LOS ≥4 days (OR 5.26, 95% CI, 1.74–15.88). Using a risk score with relative weighting (based on the aforementioned ORs) of 5 points for CVC, 2 points for infection, and 1 point for LOS ≥4 days, the estimated risks for HA-VTE among noncritically ill children with scores of 8 points, 7 points, and ≤6 points were 12.5%, 1.1%, and 0.1%, respectively.
Table II.
Unadjusted OR and aOR for putative risk factors for development of HA-VTE in noncritically ill children from univariate and multiple logistic regression
| Putative risk factors | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| OR | 95% LCL | 95% UCL | P value | OR | 95% LCL | 95% UCL | P value | |
| Age, y | ||||||||
| 1–5 | 0.28 | 0.07 | 1.12 | .07 | 0.38 | 0.05 | 2.75 | .34 |
| 6–10 | 1.57 | 0.58 | 4.23 | .37 | 2.54 | 0.48 | 13.42 | .27 |
| 11–15 | 2.43 | 0.90 | 6.54 | .08 | 2.21 | 0.40 | 12.32 | .37 |
| 16–21 | 1.99 | 0.76 | 5.19 | .16 | 2.32 | 0.45 | 11.87 | .31 |
| CVC | 31.49 | 13.97 | 71.02 | <.001 | 27.67 | 8.40 | 91.22 | <.0001 |
| Infection | 7.95 | 4.18 | 15.09 | <.001 | 10.40 | 3.46 | 31.25 | <.0001 |
| Major surgery | 0.20 | 0.06 | 0.66 | .008 | 0.34 | 0.07 | 1.62 | .17 |
| Malignancy | 5.06 | 2.58 | 9.94 | <.001 | 0.90 | 0.31 | 2.62 | .85 |
| Obesity | 4.40 | 1.02 | 19.03 | .047 | 1.95 | 0.21 | 18.20 | .56 |
| Dehydration | 1.51 | 0.59 | 3.84 | .39 | ||||
| Chronic inflammatory disease (non-lupus) | 7.67 | 2.14 | 27.52 | .002 | 1.00 | 0.16 | 6.32 | 1.00 |
| Hospital days (<4 vs ≥4) | 15.38 | 6.37 | 37.14 | <.001 | 5.26 | 1.74 | 15.88 | .003 |
| Previous hospitalization within 30 d | 3.88 | 1.99 | 7.59 | <.001 | 1.41 | 0.52 | 3.85 | .50 |
| Cardiac catheterization | 14.54 | 1.29 | 163.41 | .03 | 9.17 | 0.34 | 249.81 | .19 |
| History of prematurity | 0.48 | 0.14 | 1.62 | .24 | ||||
| Congenital heart disease | 0.49 | 0.06 | 3.81 | .5 | ||||
| Diabetes mellitus | 1.17 | 0.14 | 9.93 | .89 | ||||
| Cystic fibrosis | 3.66 | 0.89 | 15.13 | .07 | 0.92 | 0.12 | 6.93 | .93 |
LCL, lower confidence limit; UCL, upper confidence limit.
Independent risk factors, as determined by the results of multivariate analysis, are shown in bold type.
Discussion
This study provides novel data on HA-VTE occurrence rate and risk factors in noncritically ill hospitalized children. The occurrence rate for HA-VTE was determined to be approximately 1 in 500 hospitalized noncritically ill children. Our finding that nearly one-half of the cases of HA-VTE occurred during the most recent 2 years is consistent with existing knowledge of a rising incidence of HA-VTE.4 An adjusted risk model was derived via multiple logistic regression in which the presence of a CVC, infection, and LOS ≥4 each served as statistically significant, independent risk factors for HA-VTE in this setting; a risk score also was developed that assigns 5 points, 2 points, and 1 point for these risk factors, respectively, on the basis of their associated ORs from the adjusted model. Of the patients with the risk factor of CVC, 94% of these CVCs were located at the same location of the clot (CVC associated clot). The estimated risk for HA-VTE among noncritically ill children with scores of 8 points, 7 points, and ≤6 points was 12.5%, 1.1%, and 0.1%, respectively. Given the risk thresholds of ≥2% and 1% to <2% modeled in medically ill adults for pharmacologic and mechanical prophylaxis, respectively,18 our previous HA-VTE risk estimates for noncritically ill hospitalized children are of the magnitude for which we propose the following risk-based prophylactic paradigm, for future validation in prospective multicenter studies and subsequent evaluation in clinical trials: a score of 8 points warrants chemoprophylaxis (low-dose anticoagulation) in the absence of bleeding contraindications; a score of 7 points warrants mechanical prophylaxis (pneumatic compression device in children of appropriate size; early ambulation as appropriate); and a score of ≤6 points warrants observation without specific prophylactic intervention.
It should be noted that use of mechanical prophylaxis for moderate-risk patients will only be applicable in the prevention of lower-extremity DVT and will likely not prevent CVC-related DVT in settings where the use of a pneumatic compression device is avoided on a leg with a CVC in place. It also should be noted that chemoprophylaxis for the prevention of HA-VTE in pediatric patients with CVC alone and no additional significant risk factors was not previously found to be effective in the prevention of HA-VTE16; however, our proposed model, if validated in larger prospective studies, would justify the need for new trials of chemoprophylaxis in hospitalized children who have CVCs in combination of other significant risk factors—specifically those with a risk score of 8.
This work is important, given the need for appropriately risk-stratified models in children at risk for VTE, to optimize risk/benefit considerations of thromboprophylaxis. Previous work in VTE risk modeling in hospitalized children has mainly consisted of hospital-wide analyses that are limited by heterogeneity of clinically important and distinguishable patient subpopulations, such as noncritically ill children, children with congenital cardiac disease in the cardiovascular ICU, children in the pediatric (noncardiovascular) ICU, and neonates in the neonatal ICU. Previous studies have largely neglected, or have not been sufficiently powered to achieve, risk models specifically applicable to each of these subpopulations. An additional strength of the present study is its use of diagnostic validation of ICD-9 discharge code-based VTE case definition via the radiologic record, seldom used in previous studies that have evaluated pediatric HA-VTE risk factors.13,19
The HA-VTE risk factors determined in this study for noncritically ill hospitalized children are concordant with recent work by Branchford et al13 in an independent hospitalized pediatric population, with respect to identification of infection and LOS as significant independent risk factors for HA-VTE in children. However, the risk model determined here (as well as in the aforementioned pediatric study) differs from that which is established for medically ill hospitalized adults when the International Medical Prevention Registry on Venous Thromboembolism score is used18 in that increasing age and a diagnosis of cancer were found to be important HA-VTE risk factors in adults but not in these children. LOS was a significant risk factor in both pediatric studies but not in the International Medical Prevention Registry on Venous Thromboembolism. Given the retrospective nature of both pediatric studies, duration and degree of immobilization (all important risk factors in medically ill hospitalized adults18) were not accurately assessable. Likewise, the use of a notation of obesity in the admission history in lieu of a body mass index definition (given the high frequency of missing data on height at time of weight measurement, as noted in the Methods) represents a limitation of the present study with regard to evaluating obesity as a potential risk factor. In addition, the inclusion criteria of our study necessitate that patients admitted to the non-ICU floor and later transferred to the ICU during their stay were retained in this study. This characteristic (and potential bias) applies to both cases and controls alike, based upon the uniform application of our inclusion criteria; furthermore, on the basis of administrative data from the ACH JHM EHR-DW, the proportion of patients admitted to non-ICU beds who are subsequently transferred to an ICU represents only 6% of the institutional population and is therefore unlikely to impact our findings.
It was not possible to review records of other hospitals regionally and beyond for possible readmission of patient controls included in this study to assure that they were not misclassified (ie, missed cases of postdischarge VTE within 30 days that would then meet case definition for HA-VTE). However, the likelihood of this potential limitation impacting the validity of our findings is modest because our institutional data show that the vast majority of readmissions occurring within 30 days of an initial admission to our free-standing children’s hospital occur at our institution, rather than other hospitals. The size of our study is such that it is possible that other bona fide risk factors may exist that we were not able to detect. A further limitation of the current work is the fact that few VTE cases include postsurgical patients, such that it was not possible to distinguish a medically ill vs surgical patient VTE risk model in these noncritically ill hospitalized children.
Notwithstanding these limitations, this single-institutional case-control study investigating HA-VTE risk factors is novel in deriving a risk model and associated risk score for HA-VTE in noncritically ill children, who comprise the majority of pediatric inpatients at most medical centers. This risk score warrants prospective evaluation in larger cooperative studies that also seek to evaluate the need for distinct risk scores for upper- vs lower-extremity DVT. If prospectively validated, our risk score and risk-based interventional model would provide a rational, pragmatic approach to the institution of electronic health record-based screening and risk-stratified preventive measures for HA-VTE in noncritically ill hospitalized children. Future work must also seek to develop subpopulation-specific pediatric HA-VTE risk models and associated risk scores for critically ill children hospitalized in cardiovascular, pediatric (noncardiovascular), and neonatal ICUs.
Acknowledgments
C.A. is supported by a 2013 AOA Carolyn L. Kuckein Student Research Fellowship.
Glossary
- ACH JHM
All Children’s Hospital Johns Hopkins Medicine
- CVC
Central venous catheter
- DVT
Deep venous thrombosis
- EHR-DW
Electronic health record-derived data warehouse
- HA-VTE
Hospital-associated venous thromboembolism
- ICD-9
International Classification of Diseases, 9th Revision
- ICU
Intensive care unit
- LOS
Length of stay
- PE
Pulmonary embolism
- VTE
Venous thromboembolism
Footnotes
The authors declare no conflicts of interest.
References
- 1. [Accessed June 17, 2014];United States Surgeon General’s Call-to-Action for Deep Vein Thrombosis. 2008 [cited 2012]. Available at: http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-ondvt-2008.pdf.
- 2.Kahn S, Lim W, Dunn AS, Cushman M, Dentali F, Akl E, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein PD, Kayali E, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145:563–5. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg NA, Donadini MP, Kahn SR, Crowther M, Nowak-Göttl U, Manco-Johnson MJ. Post thrombotic syndrome in children: a systematic review of frequency of occurrence, validity of outcome measures, and prognostic factors. Haematologica. 2010;95:1952–9. doi: 10.3324/haematol.2010.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monagle P, Adams M, Mahoney M, Ali K, Barnard D, Bernstein M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;476:763–6. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hancock HS, Wang M, Gist KM, Gibson E, Miyamoto SD, Mourani PM, et al. Cardiac findings and long-term thromboembolic outcomes following pulmonary embolism in children: a combined retrospective-prospective inception cohort study. Cardiol Young. 2013;23:344–52. doi: 10.1017/S1047951112001126. [DOI] [PubMed] [Google Scholar]
- 8.Biss TT, Brandão LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142:808–18. doi: 10.1111/j.1365-2141.2008.07243.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanslik A, Kitzmüller E, Thom K, Haumer M, Mlekusch W, Salzer-Muhar U, et al. Incidence of thrombotic and bleeding complications during cardiac catheterization in children: comparison of high-dose vs. low-dose heparin protocols. J Thromb Haemost. 2011;9:2353–60. doi: 10.1111/j.1538-7836.2011.04539.x. [DOI] [PubMed] [Google Scholar]
- 10.Monagle P, Cochrane A, Roberts R, Manlhiot C, Weintraub R, Szechtman B, et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58:645–51. doi: 10.1016/j.jacc.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 11.Sharathkumar AA, Mahajerin A, Heidt L, Doerfer K, Heiny M, Vik T, et al. Risk-prediction tool for identifying hospitalized children with a predisposition for development of venous thromboembolism: Peds-Clot clinical Decision Rule. J Thromb Haemost. 2012;10:1326–34. doi: 10.1111/j.1538-7836.2012.04779.x. [DOI] [PubMed] [Google Scholar]
- 12.Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: A patient-safety and quality improvement initiative. Pediatrics. 2011;127:e1326–32. doi: 10.1542/peds.2010-3282. [DOI] [PubMed] [Google Scholar]
- 13.Branchford BR, Mourani P, Bajaj L, Manco-Johnson M, Wang M, Goldenberg NA. Risk factors for in-hospital venous thromboembolism in children: a case-control study employing diagnostic validation. Haematologica. 2012;97:509–15. doi: 10.3324/haematol.2011.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santono N, Colombini A, Silvestri D, Grassi M, Giordano P, Parasole R, et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J Pediatr Hematol Oncol. 2013;35:348–55. doi: 10.1097/MPH.0b013e31828dc614. [DOI] [PubMed] [Google Scholar]
- 15.Hanson SJ, Punzalan RC, Christensen MA, Ghanayem NS, Kuhn EM, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children with cardiac disease. Pediatr Cardiol. 2012;33:103–8. doi: 10.1007/s00246-011-0098-2. [DOI] [PubMed] [Google Scholar]
- 16.Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–7. doi: 10.1097/TA.0b013e31824964d1. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto CM, Sohi S, Desai K, Bharaj R, Khanna A, McFarland S, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164:332–8. doi: 10.1016/j.jpeds.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Spyropoulos AC, Anderson FA, Jr, Fitzgerald G, Decousus H, Pini M, Chong BH, et al. IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706–14. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 19.Branchford BR, Gibson E, Manco-Johnson MJ, Goldenberg NA. Sensitivity of discharge diagnosis ICD-9 codes for pediatric venous thromboembolism is greater than specificity, but still suboptimal for surveillance and clinical research. Thromb Res. 2012;129:662–3. doi: 10.1016/j.thromres.2011.10.032. [DOI] [PubMed] [Google Scholar]