Abstract
Objective
To determine hospital-associated venous thromboembolism (HA-VTE) risk factors in critically ill neonates.
Methods
We conducted a case-control study in the neonatal intensive care unit (NICU) of All Children’s Hospital Johns Hopkins Medicine (St. Petersburg, FL), from January 1, 2006 – April 10, 2013. We identified HA-VTE cases using electronic health record. Four NICU controls were randomly selected for each HA-VTE case. Associations between putative risk factors and HA-VTE were estimated using odds ratios (ORs) and ninety-five percent confidence intervals (95%CIs) from univariate and multivariate regression analyses.
Results
Twenty-three HA-VTE cases and 92 controls were included. The annual HA-VTE incidence was approximately 1.4 HA-VTE cases per 1,000 NICU admissions. In univariate analyses, mechanical ventilation (OR = 7.27, 95%CI = 2.02–26.17, P = 0.002), central venous catheter (CVC; OR = 52.95, 95%CI = 6.80–412.71, P < 0.001), infection (OR = 7.24, 95%CI = 2.66–19.72, P < 0.001), major surgery (OR = 5.60, 95%CI = 1.82–17.22, P = 0.003) and length of stay ≥15 days (OR = 6.67, 95%CI = 1.85–23.99, P = 0.004) were associated with HA-VTE. Only CVC (OR = 29.04, 95%CI = 3.18–265.26, P = 0.003) remained an independent risk factor in the multivariate analysis. Based on this result, the estimated risk of HA-VTE in NICU patients with a CVC was 0.9%.
Conclusion
This study identifies CVC as an independent risk factor for HA-VTE in critically ill neonates. However, the level of risk associated with CVC is below the conventional threshold for primary anticoagulation thromboprophylaxis. Larger studies are needed to substantiate these findings and identify novel putative risk factors to further distinguish NICU patients at highest HA-VTE risk.
Keywords: thrombosis, venous thromboembolism, risk factor, neonates
Introduction
Venous thromboembolism (VTE), consisting of deep venous thrombosis (DVT) and pulmonary embolism (PE) is a rare disease in children that is usually diagnosed as a secondary complication of primary underlying diseases. Recent evidence indicates that the incidence of pediatric VTE, including neonates, is dramatically increasing [1]. The annual rate of VTE among infants <28 days of age in the Pediatric Health Information System database rose from 44 to 75 cases per 10,000 admissions (an increase of 70%) between 2001 and 2007 [2]. Due to the increasing occurrence of VTE in neonates, it is important to identify risk factors for HA-VTE in this group to facilitate identification of the highest-risk patients that may benefit from thromboprophylaxis, because size constraints limit conventional mechanical prophylaxis options in neonates.
In hospitalized adults, anticoagulant prophylaxis reduces the incidence of VTE by 50–75% [3–6]. Therefore, the 2012 American College of Chest Physicians (ACCP) practice guideline recommends pharmacological VTE prophylaxis in high-risk adult inpatients [7], in whom the bleeding risk is judged to be low. However, due to paucity of high-quality evidence in children, the ACCP makes no specific recommendations regarding HA-VTE prevention in neonates and older children [8].
Previous studies in children have identified mechanical ventilation, systemic infection, malignancy, surgery, congenital heart defects, thrombophilia, central venous catheter (CVC) and hospitalization of five days or more as important risk factors for HA-VTE [4,9–12]. These and other studies have indicated that in the setting of at least three concomitant risk factors, the risk of HA-VTE in children approaches that observed in hospitalized medically-ill adults, in whom prophylactic anticoagulation is indicated [9,13]. However, limited information is available regarding risk factors for VTE in critically ill neonates. In a case series of 25 infants, Demirel and colleagues observed the placement of CVC as the most prevalent underlying risk factor for VTE in neonates [14]. In a previous case-control study in which most of the cases were neonates (25 of 38 cases), Tuckuviene and colleagues identified preterm birth, low Apgar score, and multiple births as risk factors for VTE in infants [15].
The purpose of this study was to identify risk factors for HA-VTE in critically ill neonates by evaluating the association between patient and clinical characteristics and HA-VTE development in the large neonatal intensive care unit (NICU) of a single institution.
Materials and Methods
Patients
We conducted a case–control study between January 1, 2006 and April 10, 2013 using data from the electronic health record-derived Data Warehouse (EHR-DW) at All Children’s Hospital Johns Hopkins Medicine (ACH JHM; St. Petersburg, FL). The study was approved by the Institutional Review Board, with waiver of informed consent.
ACH JHM has a 97-bed, level 3 NICU that serves as a regional referral center for premature neonates and those with complex medical and surgical conditions. HA-VTE cases were identified retrospectively via the EHR-DW using ICD-9 codes and validated by review of radiologic records. ICD-9 codes for VTE included: 325 (phlebitis and thrombophlebitis of intracranial venous sinuses); 437.6 (non-pyogenic thrombosis of intracranial venous sinus); 452 (portal vein thrombosis); 453.2 (inferior vena cava thrombus); 453.4 (venous thromboembolism of deep vessels of the lower extremity); 453.8 (embolism or thrombosis of other specified veins); 453.9 (embolism and thrombosis of unspecified site); and 415.1 (pulmonary embolism and infarction).
Patients with a VTE diagnosis by ICD-9 at time of discharge coding, or at coding from an encounter within 30 days of discharge, were included. Patients with any of the following were excluded: admission to non-NICU units; signs or symptoms of VTE noted in admission history/exam; and radiological confirmation of VTE within 24 hours of admission, without history of hospital admission in the preceding 30 days (Fig. 1). For each HA-VTE case, we randomly selected four controls (with no diagnosis of VTE) admitted during the study period.
Fig. 1.
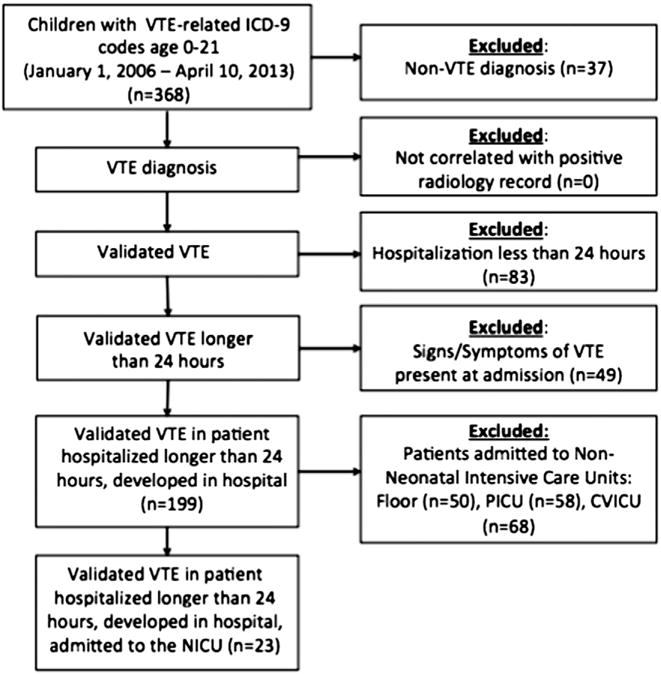
Flow diagram showing the inclusion and exclusion criteria for selection of HA-VTE cases.
Data Collection
For both HA-VTE cases and controls, clinical data on demographics and putative risk factors for VTE were systematically extracted from the EHR-DW, including: gestational age, mechanical ventilation, prematurity, length of stay (LOS) in the hospital, presence of CVC, major surgery, congenital heart disease, cardiac catheterization, dehydration, malignancy, infection, and necrotizing enterocolitis. Infection included meningitis, abscess, pneumonia, osteomyelitis, bacteremia, fungemia, and pyelonephritis. Dehydration was identified based upon its notation in the admission history. The presence of CVC was further categorized into long-term CVC (e.g. Broviac, Mediport) versus short-term CVC (e.g. umbilical vein catheter, PICC line, temporary subclavian or femoral line), and the location of CVC was also collected. For CVC-related DVT cases and controls who had CVCs, data were further extracted on number of lumens in the CVC, catheter days (measured as days from CVC placement to the earlier of HA-VTE diagnosis or CVC removal), and the number of attempts for CVC insertion.
For HA-VTE cases, patients were considered positive for a risk factor only if it was documented prior to the VTE diagnosis. Therefore, patients in whom radiologic diagnosis of VTE was made within 24 hours of admission were evaluated for a preceding admission within 30 days. For patients with a recent hospital admission data on the aforementioned VTE risk factors were extracted from the EHR-DW for the preceding admission.
Statistical Analyses
Dichotomous or categorical variables were compared between cases and controls using chi-squared analysis or Fisher’s exact test, as appropriate. Continuous variables were compared between groups using Mann-Whitney U testing. The association between putative risk factors and HA-VTE was estimated using odds ratios (ORs) and ninety-five percent confidence intervals (95%CIs) from univariate and multivariate logistic regression models. Any and all putative risk factors with a P-value < 0.1 in univariate analyses were included a multivariate model. All analyses were performed using SAS version 9.3. HA-VTE risk in patients possessing the risk factor(s) retained in the final multivariate model was estimated based upon post-test probability. Post-test probability was calculated as previously described [9], using the risk factor-related likelihood ratio and the pre-test probability estimate derived from the observed HA-VTE occurrence rate.
Results
Twenty-three cases of HA-VTE and 92 controls were included in the study. To estimate the annual occurrence of VTE in the NICU at ACH-JHM, we determined the number of NICU admissions (N = 2196) during the two most recent calendar years of the study (January 2011 – December 2012, inclusive). With 6 cases of HA-VTE during this period, the estimated occurrence was approximately 1.4 in 1,000 NICU admissions (0.14%).
All 23 HA-VTE cases were comprised of DVT without PE. A total of 22 cases had a CVC and for 21 (95.5%) of them the DVT was in the same location as the CVC. DVT sites were as follows: 11 (47.8%) in the thoracoabdominal veins, 8 (34.8%) in lower extremities, 3 in (13.0%) the internal jugular veins and 1 (4.4%) in the cerebral sinovenous system. Cases and controls had similar median gestational ages at birth (Table 1). However, compared to controls, HA-VTE cases had a greater LOS and more frequently were premature, on mechanical ventilation or had a CVC, infection, or congenital heart disease (Table 1).
Table 1.
Characteristics and univariate analysis of risk factors for HA-VTE in critically ill neonates.
| Characteristic | Case group (n = 23)
|
Control group (n = 92)
|
||||
|---|---|---|---|---|---|---|
| N | % | N | % | OR (95%CI)* | P-value | |
| Median (range) gestational age at birth (weeks) | 35 (23–41) | 35.5 (23–41) | 0.95 (0.87–1.04) | 0.3 | ||
| Mechanical ventilation | 20 | 87 | 44 | 47.8 | 7.27 (2.02–26.17) | 0.002 |
| Central venous catheter (CVC) | 22 | 95.7 | 27 | 29.4 | 52.95 (6.8–412.71) | <0.001 |
| Short term CVC | 17 | 73.9 | 26 | 28.3 | 7.19 (2.55–20.26) | <0.001 |
| Long term CVC | 8 | 34.8 | 4 | 4.4 | 11.73 (3.14–43.89) | <0.001 |
| Infection | 13 | 56.5 | 14 | 15.2 | 7.24 (2.66–19.72) | <0.001 |
| Major surgery | 8 | 34.8 | 8 | 8.7 | 5.6 (1.82–17.22) | 0.003 |
| Median (range) length of stay (LOS, days) | 87 (8–307) | 14.7 (0.66–187.2) | 1.02 (1.01–1.04) | <0.001 | ||
| LOS ≥ 15 days | 20 | 87 | 46 | 50 | 6.67 (1.85–23.99) | 0.004 |
| Cardiac catheterization | 1 | 4.4 | 1 | 1.1 | 4.14 (0.25–68.75) | 0.32 |
| Prematurity | 15 | 65.2 | 54 | 58.7 | 1.32 (0.51–3.42) | 0.57 |
| Congenital heart disease | 5 | 21.7 | 9 | 9.8 | 2.56 (0.77–8.56) | 0.13 |
odds ratio (OR) and the corresponding 95% con dence interval (95%CI).
In the univariate logistic regression analyses, mechanical ventilation (OR = 7.27, 95%CI = 2.02–26.17, P = 0.002), CVC (OR = 52.95, 95%CI = 6.80–412.71, P < 0.001), infection (OR = 7.24, 95%CI = 2.66–19.72, P = 0.001), major surgery (OR = 5.60, 95%CI = 1.82–17.22, P = 0.003) and LOS ≥ 15 days (6.67, 95%CI = 1.85–23.99, P = 0.004) showed statistically significant associations with HA-VTE (Table 1). After adjustment for all factors meeting pre-specified criteria for inclusion in a multivariate logistic regression model (see Methods), only CVC (OR = 29.04, 95%CI = 3.18–265.26, P = 0.003) remained a statistically significant, independent risk factor for HA-VTE (Table 2). Both short term (OR = 6.25, 95%CI = 1.43–27.35, P = 0.015) and long term (OR = 11.29, 95%CI = 1.75–72.93, P = 0.011) CVC were statistically significant in multivariate analysis (data not shown). Using these data, the estimated risk (see Methods) of HA-VTE in NICU patients with a CVC was 0.9%.
Table 2.
Multivariate analysis of risk factors for HA-VTE in critically ill neonates.
| Characteristic | OR* | 95% LCL† | 95% UCL‡ | P-value |
|---|---|---|---|---|
| Mechanical ventilation | 1.10 | 0.20 | 6.11 | 0.91 |
| Central venous catheter | 29.04 | 3.18 | 265.26 | 0.003 |
| Infection | 2.63 | 0.0.79 | 8.71 | 0.11 |
| Major surgery | 1.42 | 0.38 | 5.39 | 0.61 |
| LOS ≥ 15 days | 1.71 | 0.33 | 8.78 | 0.52 |
OR = odds ratio.
LCL = lower confidence limit.
UCL = upper con dence limit.
We observed no statistical significant difference in the distribution of CVC locations between CVC-related DVT cases and those controls who had CVCs (See Table 3.). However, multi-lumen (two or more lumens) CVC were present in 46.7% of CVC-related DVT cases, as compared to 0% of controls who had CVCs (P < 0.001). Median (and observed range) number of attempts for CVC insertion and catheter days were 1 (1–3) and 17 (3–93) respectively among CVC-related DVT cases and 1 (1–4) and 10.5 (1–65) among controls who had a CVC.
Table 3.
Additional characteristics of participants with CVC.
| Cases
|
Controls
|
||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | P-value |
| Line location | 0.11 | ||||
| Upper extremity | 10 | 45.5 | 17 | 63 | |
| Lower extremity | 10 | 45.5 | 5 | 18.5 | |
| Umbilical | 2 | 9.1 | 5 | 18.5 | |
| Lumen number | <0.001 | ||||
| Single | 8 | 53.3 | 20 | 100 | |
| Multiple | 7 | 46.7 | 0 | 0 | |
| Number of attempts for line insertion | |||||
| Median (range) | 11 | 1 (1–3) | 17 | 1 (1–4) | 0.31 |
| Catheter days, median (range) | 16 | 17 (3–93) | 24 | 10.5 (1–65) | 0.31 |
Discussion
In this single institutional case-control study, we observed a HA-VTE rate of approximately 1.4 in 1,000 hospitalized children per year in critically ill neonates and identified CVC as an independent risk factor for VTE with a risk of 0.9% in this sub-population of children. Our observed rate of HA-VTE in neonates confirms that the incidence of VTE is increasing in neonates and children. Recent reports from Canada (24 per 10,000 NICU admissions[16] and the U.S. (58 cases per 10,000 admissions) between 2001 and 2007 [2] indicate a 10-fold increase in pediatric VTE compared to the first prospective registry report of an annual incidence of 0.07 per 10,000 children or 5.3 per 10,000 hospital admissions [10], with the report from the US showing that the neonatal subpopulation experienced the highest increase (100%; from 25 to 50 cases per 10,000 admissions) in VTE incidence among the hospitalized population overall [2].
The dramatic increase in the incidence of pediatric and neonatal VTE has been attributed to a number of factors including an increased awareness and recognition of VTE, recent improvements in the care of children with underlying medical conditions associated with VTE that keep these children alive long enough to develop VTE and invasive medical interventions that may disrupt the vascular and/or hemostatic systems and result in the development of VTE [17]. In addition, a disadvantageous catheter-to-vessel-diameter ratio puts neonates at a higher HA-VTE risk compared to older children and adults. However, other yet unidentified factors may also be contributing to this increase. Future larger studies are therefore warranted to identify rare risk factors that may be missed in smaller studies due to insufficient power.
Our finding that CVCs are an independent risk factor for VTE adds to the existing evidence of the role of CVCs in the development of VTE in children. CVCs are associated with N50% of thrombi in all children and are the most common risk factor for pediatric VTE [13,16,18,19]. In a previous study, Male and group observed a high association between CVC and VTE locations, with nearly all cases having VTE and CVC located on the same side (right versus left) [20]. In addition to the frequent association between side of CVC and VTE, 83% of the VTEs in the study were also noted to be located in the same venous segment that the CVC was placed [20]. Previous reports in neonates indicate that 89–94% of VTE are associated with CVCs [16,21], which is similar to the observed 91% in our present study. In addition, we observed that both short-term and long-term CVCs were significantly associated with HA-VTE, with long-term CVCs showing a stronger association than short-term CVCs. Our finding that multiple lumen catheters are associated with HA-VTE substantiates a recent finding by Gray and colleagues [22] and the finding may be attributed to the larger size of multi-lumen CVCs compared to single-lumen CVCs.
Although CVCs are an iatrogenic risk factor for VTE, they are required for the management of critically ill neonates. Since chemoprophylaxis for HA-VTE prevention in otherwise-unselected pediatric patients with CVCs (i.e., no further risk-stratification) is not effective in the prevention of HA-VTE [23], larger studies are warranted to identify additional risk factors that may be used together with CVC to identify patient subgroups that may benefit from chemoprophylaxis. Though other factors were significant in our univariate models, only CVC remained as an independent risk factor in our multivariate model, indicating that the effect of the other factors may be dependent on CVCs.
This study has several strengths, including: validation of case definition via review of the radiologic record following identification by ICD-9 discharge code; detection of cases identified on re-presentation after initial hospital discharge (see Methods); and specificity of the sub-population in order to discern risk factors applicable to critically ill neonates. At the same time, there are a few limitations of this study that need to be considered in the interpretation of its findings. First, we were only able to confirm 23 cases over the seven-year study period, and thus had insufficient power to detect rare risk factors. Hence, we may have been unable to detect an association with some putative risk factors due to the small sample size. The small sample size also limited our ability to explore potential interactions among the risk factors.
In addition, due to our retrospective design, we were unable to examine risk factors such as family history of VTE or familial thrombophilia, which are not routinely documented in the medical record for NICU admissions. Future prospective studies of HA-VTE in critically ill neonates should systematically assess these putative risk factors. Furthermore, our results are generally not applicable to neonates who have undergone surgery for congenital cardiac disease, since such patients, once diagnosed, are managed in a separate cardiovascular intensive care unit (CVICU) at ACH JHM. Therefore, additional studies are warranted to determine risk factors for CVICU-associated VTE, with age as a pre-specified analytic variable.
In conclusion, this study substantiates the role of CVCs in the development of HA-VTE in the NICU setting. However, the level of risk associated with CVC in hospitalized neonates is below the conventional threshold for primary anticoagulation thromboprophylaxis in hospitalized adults. Due to the small number of cases in this study, future work including a larger number of cases is warranted to identify additional risk factors that may help to develop sub-population-specific pediatric HA-VTE risk models and associated risk scores for critically ill children hospitalized in the NICU setting.
Acknowledgments
CMA was supported by a 2013 AOA Carolyn L. Kuckein Student Research Fellowship.
Footnotes
Conflict of Interest
None to declare.
References
- 1.Raffini L, Thornburg C. Testing children for inherited thrombophilia: more questions than answers. Br J Haematol. 2009;147(3):277–88. doi: 10.1111/j.1365-2141.2009.07820.x. [DOI] [PubMed] [Google Scholar]
- 2.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–8. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 3.Andrew M, Marzinotto V, Brooker LA, Adams M, Ginsberg J, Freedom R, et al. Oral anticoagulation therapy in pediatric patients: a prospective study. Thromb Haemost. 1994;71(3):265–9. [PubMed] [Google Scholar]
- 4.Boulet SL, Grosse SD, Thornburg CD, Yusuf H, Tsai J, Hooper WC. Trends in venous thromboembolism-related hospitalizations, 1994–2009. Pediatrics. 2012;130(4):e812–20. doi: 10.1542/peds.2012-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setty BA, O’Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer. 2012;59(2):258–64. doi: 10.1002/pbc.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuckuviene R, Christensen AL, Helgestad J, Johnsen SP, Kristensen SR. Pediatric venous and arterial noncerebral thromboembolism in Denmark: a nationwide population-based study. J Pediatr. 2011;159(4):663–9. doi: 10.1016/j.jpeds.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ, American College of Chest Physicians Antithrombotic T et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e737S–801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branchford BR, Mourani P, Bajaj L, Manco-Johnson M, Wang M, Goldenberg NA. Risk factors for in-hospital venous thromboembolism in children: a case-control study employing diagnostic validation. Haematologica. 2012;97(4):509–15. doi: 10.3324/haematol.2011.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–7. [PubMed] [Google Scholar]
- 11.Chalmers EA. Epidemiology of venous thromboembolism in neonates and children. Thromb Res. 2006;118(1):3–12. doi: 10.1016/j.thromres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Hanson SJ, Punzalan RC, Christensen MA, Ghanayem NS, Kuhn EM, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children with cardiac disease. Pediatr Cardiol. 2012;33(1):103–8. doi: 10.1007/s00246-011-0098-2. [DOI] [PubMed] [Google Scholar]
- 13.Takemoto CM, Sohi S, Desai K, Bharaj R, Khanna A, McFarland S, et al. Hospital-Associated Venous Thromboembolism in Children: Incidence and Clinical Characteristics. J Pediatr. 2013;164(2):332–8. doi: 10.1016/j.jpeds.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Demirel N, Aydin M, Zenciroglu A, Bas AY, Yarali N, Okumus N, et al. Neonatal thromboembolism: risk factors, clinical features and outcome. Ann Trop Paediatr. 2009;29(4):271–9. doi: 10.1179/027249309X12547917868961. [DOI] [PubMed] [Google Scholar]
- 15.Tuckuviene R, Christensen AL, Helgested J, Hundborg HH, Kristensen SR, Johnsen SP. Infant, obstetrical and maternal characteristics associated with thromboembolism in infancy: a nationwide population-based case-control study. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F417–22. doi: 10.1136/archdischild-2011-300665. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics. 1995;96(5 Pt 1):939–43. [PubMed] [Google Scholar]
- 17.Kerlin BA. Current and future management of pediatric venous thromboembolism. Am J Hematol. 2012;87(Suppl. 1):S68–74. doi: 10.1002/ajh.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM. Venous thrombosis in children. J Thromb Haemost. 2003;1(7):1443–55. doi: 10.1046/j.1538-7836.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 19.Journeycake JM, Buchanan GR. Thrombotic complications of central venous catheters in children. Curr Opin Hematol. 2003;10(5):369–74. doi: 10.1097/00062752-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L, et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood. 2003;101(11):4273–8. doi: 10.1182/blood-2002-09-2731. [DOI] [PubMed] [Google Scholar]
- 21.van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001;139(5):676–81. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 22.Gray BW, Gonzalez R, Warrier KS, Stephens LA, Drongowski RA, Pipe SW, et al. Characterization of central venous catheter-associated deep venous thrombosis in infants. J Pediatr Surg. 2012;47(6):1159–66. doi: 10.1016/j.jpedsurg.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72(5):1292–7. doi: 10.1097/TA.0b013e31824964d1. [DOI] [PubMed] [Google Scholar]


