Abstract
Background
Technology-based physical activity (PA) interventions have been shown to improve daily step counts and health-related quality of life, but their effect on long-term clinical outcomes like acute exacerbations (AEs) is unknown in persons with COPD.
Methods
U.S. Veterans with stable COPD were randomized (1:1) to either pedometer alone (control) or pedometer plus a website with feedback, goal-setting, disease education, and a community forum (intervention) for 3 months. AEs were assessed every 3 months over a follow-up period of approximately 15 months. Pedometer-assessed daily step counts, health-related quality-of-life (HRQL), and self-efficacy were assessed at baseline, end-of-intervention at 3 months, and during follow-up approximately 6 and 12 months after enrollment. Zero-inflated Poisson models assessed the effect of the intervention on risk for AEs, compared to controls. Generalized linear mixed-effects models for repeated measures examined between-group and within-group changes in daily step count, HRQL, and self-efficacy.
Results
There were no significant differences in age, FEV1% predicted, baseline daily step count, AEs the year prior to enrollment, or duration of follow-up between the intervention (n = 57) and control (n = 52) groups. The intervention group had a significantly reduced risk of AEs (rate ratio = 0.51, [95%CI 0.31–0.85]), compared to the control group. There were no significant between-group differences in change in average daily step count, HRQL, or self-efficacy at 6 and 12 months after enrollment.
Conclusions
A 3-month internet-mediated, pedometer-based PA intervention was associated with reduced risk for AEs of COPD over 12–15 months of follow-up.
ClinicalTrials.gov identifier
Keywords: COPD, Physical activity, Exacerbations, Randomized controlled trial
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide [1,2]. Low levels of physical activity (PA) in COPD patients are present early in the course of the disease [3] and are associated with an increased risk of acute exacerbations (AEs) [4], higher rates of healthcare utilization [5–7], higher levels of systemic inflammation [8], and increased mortality [6,9,10], independent of lung function. Increasing PA, a modifiable risk factor, is recommended by the Global Initiative for Obstructive Lung Disease (GOLD) guidelines as a non-pharmacological treatment of COPD [11].
Technology-based interventions that employ pedometers and accelerometers, the internet, and smartphone applications have emerged to promote PA in COPD [12–16]. Studies have demonstrated the efficacy of these programs to increase PA and 6-min walk test (6MWT) distance and improve health related quality of life (HRQL) [14–20] over the short term (3–4 months). However, the impact of technology-mediated PA interventions on clinical outcomes such as risk for acute exacerbations (AEs) and the long-term (>12 months) durability of improvements in PA are less well established. In one study that used a resource-intense home-based telephone coaching intervention, increased PA was reported over 18 months of follow-up relative to usual care, and lower rates of lung-related healthcare utilization were observed in a subset of subjects with severe airflow obstruction [21]. To date, only limited number of studies have examined the long-term effects of technology-based PA interventions on risk for AEs in COPD [21,22].
We previously reported on the efficacy of a technology-based PA intervention in two studies, (Taking Healthy Steps (THS) [20] and Every Step Counts (ESC), to improve daily step counts and HRQL [23]). The intervention group used a platform that combined a pedometer and website that provided goal setting, feedback, motivational and educational messages, and social support to improve PA, while the control group used a pedometer alone [20,23,24]. In both studies, the intervention increased average daily step count at 3–4 months [20,23]. In THS, the gains in PA were not sustained at 12 months [25], but there was minimal contact with study personnel in the automated study and limited assessment of clinical outcomes. In ESC, in contrast, subjects participated in a minimum of two in-person clinic visits and were followed closely for an additional 12 months after the 3-month intervention period. In this secondary analysis of ESC, we examine the effect of use of a pedometer plus website versus pedometer alone on (1) risk for AEs and (2) long-term changes in daily step count, HRQL, and exercise self-efficacy.
2. Methods
We used data from the ESC study, a single-site randomized controlled trial (RCT) which showed that an intervention combining a pedometer plus a website that provided goal setting, feedback, motivational and educational content, and social support (intervention) is efficacious compared to a pedometer alone (control) [23]. A previous publication, which adhered to the Consolidated Standards of Reporting Trials (CONSORT) checklist for reporting randomized trials, described the research methods in detail and reported the results for the primary outcome of daily step count at 3 months [23]. The protocol was approved by the VA Boston Healthcare System Committee on Human Research. All participants provided written informed consent. The randomized clinical trial was registered on ClinicalTrials.gov (NCT01772082).
Briefly, eligible participants were enrolled from the pulmonary clinics at VA Boston Healthcare System between 2012 and 2016, and were 1) > 40 years old, 2) had >10 pack years of smoking, 3) had COPD defined as an ratio of FEV1/FVC <0.70 or emphysema on a clinical chest CT, and 4) had access to the internet. Exclusion criteria included inability to ambulate without assistance and unstable cardiovascular disease.
Subjects completed spirometry, a 6MWT, the St. George’s Respiratory Questionnaire (SGRQ) [26] and Exercise Self-Regulatory Efficacy Scale (Ex-SRES) [27] questionnaire at enrollment (Visit 1). Lower scores were indicative of better HRQL and worse exercise self-efficacy, respectively. The Omron HJ-720 ITC pedometer (Omron Healthcare, Inc., Bannockburn, IL, USA) collected baseline daily step count for 7 days prior to randomization. Subjects in the pedometer plus website group were instructed to wear the pedometer daily during all waking hours and to upload step counts weekly. They received access to a website which provided individualized goal-setting, iterative step-count feedback for self-monitoring, educational and motivational content to enhance self-efficacy, and an online community forum for social support. Subjects received weekly updated step-count goals.
Subjects randomized to the pedometer alone group were given a pedometer (with instructions to wear the pedometer daily while awake) and written materials about exercise but were not assigned step-count goals and did not have access to the website content. Both groups uploaded step-count data to the same study server via the website and completed an in-person study visit at 3 months (Visit 2).
2.1. Long-term follow-up
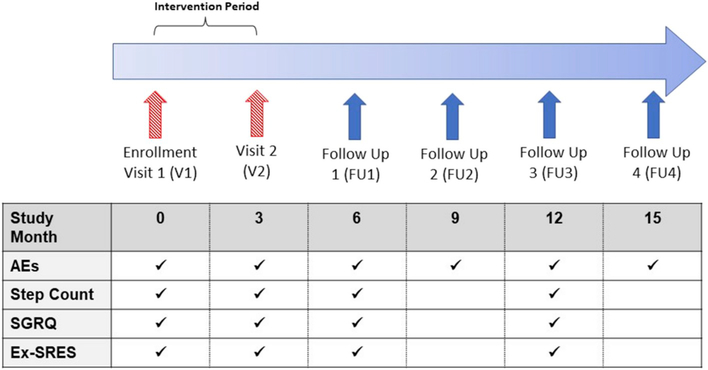
After Visit 2, all participants returned their pedometers and those in the intervention group no longer had access to the content of the website. Subjects in both groups were contacted via telephone by study staff approximately every 3 months (FU1-FU4) for approximately 15 months from the time of enrollment (Fig. 1).
Fig. 1.
Overview of study and follow-up timeline.
Study consisted of in-person visits (red hatched arrow) and telephone interviews (blue solid arrows) over 15 months. Semi-structured questionnaires at all follow-up time points assessed occurrence of self-reported AEs over the preceding 3 months. Daily step count was assessed with the Omron pedometer at V1, V2, FU1, and FU3. AEs = acute exacerbations; SGRQ = St. George’s Respiratory Questionnaire; Ex- SRES = Exercise Self-Regulatory Efficacy Scale. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At Follow-up 1 (FU1) and Follow-up 3 (FU3), subjects were mailed an Omron pedometer and questionnaires which included the SGRQ and Ex-SRES. Subjects were instructed to wear the pedometer for 2 weeks, complete the questionnaires, and return the materials to the study staff in a prepaid envelope. The criteria for a valid “wear day” were >8 h and >100 steps per day; wear times and step counts below those thresholds were considered indicative of failure to wear the pedometer on that particular day. Daily step counts were averaged over a minimum of 5 valid wear days during the 14-day monitoring period at FU1 and FU3. Season at enrollment and at each visit for step count monitoring (for V2, FU1, and FU3) was ascertained based upon whether the majority of days during the monitoring period fell into the calendar month groupings as follows: winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November).
Number of AE’s were assessed 1) at V1 for the year prior to enrollment and 2) prospectively after enrollment via semi-structured questionnaire in-person at Visit 2 and, 3) by telephone interviews and medical record review every 3 months at FU1-FU4 (Fig. 1). An AE was defined as new or increased respiratory symptoms with >2 the following criteria: increased cough, sputum, wheezing, dyspnea, or chest tightness for >3 days requiring antibiotics or new/increased systemic steroid use [8]. AEs were considered distinct events if courses of oral corticosteroids, antibiotics, or COPD-related hospitalizations were separated by > 14 days [8]. When the participant could not be reached by telephone (minimum 3 attempts), AE assessment was conducted using medical record review alone. All AE events were then adjudicated by > 2 study personnel (including a pulmonary physician), with a 3rd adjudicator in the event of discrepancies. Events adjudicated as meeting the criteria for an AE outlined above were included in the analysis. Research staff conducting Visit 2 (in-person visit) and the adjudicators were blinded to randomization group assignment. Research staff conducting follow-up calls and medical record reviews were not formally blinded, but did not have immediate access to randomization group assignment during the periods when AE data were acquired.
2.2. Statistical analysis
Univariate comparisons between groups were made using a Chi- square or Fisher’s exact test for categorical data and a Wilcoxon rank- sum test for continuous data. To assess the effect of the intervention on risk for AEs, zero-inflated Poisson models were constructed with the sum of the number of AEs (that occurred during the period from V1 up through at most 15 months at FU4) as the dependent variable and randomization group as the independent variable. The model adjusted for FEV1% predicted, smoking status, number of AEs in the year prior to enrollment, and season of enrollment. Duration of follow-up was included as the offset, and the zero-model included FEV1% predicted as an independent variable. A p-value <0.05 was considered statistically significant.
To evaluate the effect of the intervention on change in daily step count at 6 and 12 months, generalized linear mixed effects models (PROC MIXED, SAS v9.4, Cary, NC) for repeated measures employing a first order auto-regressive covariance matrix were constructed. Data from the three follow-up time points (V2, FU1, and FU3) were included in the model, with change in daily step count from baseline (V1) as the dependent variable and randomization group as the independent variable with covariate adjustment for FEV1% predicted, season of step count assessment and the number of days between assessments (which were not exactly 3 months apart due to subject availability and responses to follow-up efforts). Similar models were constructed to evaluate the effect of the intervention on SGRQ and Ex-SRES scores at 6 and 12 months, where change in score from baseline was the dependent variable and randomization group the independent variable, with covariate adjustment for FEV1% predicted and the number of days between assessments.
3. Results
3.1. Subject characteristics
One-hundred and fourteen subjects were enrolled and randomized, of which 109 participants who contributed at least 1 week of step-count data following randomization were included in the analyses [23]. There were no between-group differences in age, FEV1% predicted, average daily step count or 6MWT distance at baseline (Table 1), or in the proportion of participants lost to follow-up at FU1 and FU3 (Table 2). In the pedometer alone group, subjects who were lost to follow-up at FU1 and FU3 had higher average daily step counts at baseline (p-values 0.03 and 0.06, respectively) relative to subjects who completed follow-up for step counts. In the pedometer-plus-website group, there were no significant differences between those who were lost to follow-up versus those who completed follow-up. Four subjects (2 pedometer-alone, 2 pedometer-plus-website) missed one or more follow-up calls and had AE assessment performed by medical record review alone; there were no significant differences in age, sex, current smoking status, pack-years FEV1% predicted, or average daily step count at enrollment between subjects who completed all AE telephone assessments and the four subjects who did not. A total of 89 AEs were analyzed, 57 of which were ascertained by both self-report and medical record review, and 32 of which were ascertained by medical record review alone. An additional 72 self-reported AE events were not included in the analyses because of lack of medical record validation, symptoms that were not attributable to COPD AE, or the self-reported event was temporally indistinct from a previous AE event. There were no differences in the distribution of the 72 AE events not included in the analyses by randomization group.
Table 1.
Baseline cohort characteristics by randomization group.
| Pedometer alone | Pedometer + Website | P-value | |
|---|---|---|---|
| N | 52 | 57 | – |
| Age | 68.7 (7.9) | 68.4 (8.7) | 0.91 |
| Sex (male) | 51 (98.1) | 56 (98.3) | 1.00 |
| Race (white) | 47 (90.4) | 53 (93) | 0.73 |
| Current smoker | 18 (34.6) | 22 (38.6) | 0.7 |
| Pack-years | 61.1 (48.6) | 61.9 (33.9) | 0.24 |
| FEV1% predicted | 65.2 (21.9) | 60.2 (21.1) | 0.12 |
| SGRQ | 32 (16.2) | 34.9 (16.9) | 0.60 |
| Ex-SRES | 66.5 (21.2) | 60.9 (22.8) | 0.21 |
| 6MWT distance (meters) | 394.2 (71.8) | 382.5 (89.4) | 0.61 |
| Average daily step count | 3769.5 (2386.3) | 3148.6 (2469.0) | 0.12 |
| Average rate of AE (year prior to enrollment) | 0.27 (0.63) | 0.42 (0.98) | 0.33 |
Data are shown as mean (SD) or n (percent). Significance testing was performed using the Wilcoxon rank sum (continuous) or Chi-square or Fisher’s exact test (categorical) as appropriate.
6MWT = 6- minute walk test; SGRQ = St. George’s Respiratory Questionnaire; Ex-SRES = Exercise Self-Regulatory Efficacy Scale.
Table 2.
–Long-term follow-up of AEs and daily step counts by randomization group.
| Pedometer alone | Pedometer + Website | P-value | |
|---|---|---|---|
| Average follow-up time for AEs (days) | 522.0 (79.3) | 537.7 (139.0) | 0.47 |
| Number (%) with valid step counts at: | |||
| Visit 1 | 52 | 57 | – |
| Visit 2 | 49 (94.2) | 57 (100.0) | 0.11 |
| Follow-up 1 (FU1) | 43 (82.7) | 44 (77.2) | 0.63 |
| Follow-up 3 (FU3) | 40 (76.9) | 35 (61.4) | 0.09 |
Data are shown as mean (SD) or n (percent). Significance testing was performed using the Wilcoxon rank sum (continuous) or Fisher’s exact test (categorical) as appropriate.
3.2. Effect of web-based intervention on risk of acute exacerbations
There were no significant differences in follow-up time over 15 months (Table 2) or in rate of AEs in the year prior to enrollment by randomization group (Table 1). The number of AE’s in the year prior to enrollment was moderately correlated with the number of prospective AEs (Spearman’s rho = 0.34, p-value 0.0003) in the entire cohort (combined control and intervention). A plot of the number of AEs experienced by each subject in both randomization groups over approximately 15 months since enrollment demonstrated a large number of individuals with no AEs (Supplementary e-Fig. 1).
In the adjusted zero-inflated Poisson model, the pedometer plus website group had a significantly reduced risk of AEs (rate ratio = 0.51, [95%CI 0.31–0.85]) relative to the pedometer alone group (Table 3). Compared to controls, those in the intervention group had a 49% risk reduction for AEs over the follow-up period. History of AE in the year prior to study entry and season of enrollment were also a significant predictor of future AEs.
Table 3.
Multivariable-adjusted, zero-inflated Poisson model examining the association between randomization group and COPD AEs.
| Rate Ratio [95% CI] | P-value | |
|---|---|---|
| Randomization group (reference = pedometer alone) | 0.51 [0.31, 0.85] | 0.01 |
| FEV1% predicted | 0.99 [0.97, 1.0] | 0.14 |
| Season of enrollment (reference = Winter) | ||
| Spring | 0.73 [0.32, 1.68] | 0.46 |
| Summer | 0.29 [0.12, 0.72] | 7.1 × 10−3 |
| Fall | 1.02 [0.53, 1.95] | 0.95 |
| Current smoking status at enrollment (reference = not currently smoking) | 1.08 [0.66, 1.80] | 0.74 |
| Binary (yes/no) history of AEs in year prior to enrollment (reference = no AEs) | 1.26 [1.09, 1.54] | 2.8 × 10−3 |
3.3. Long-term effect of web-based intervention on daily step counts
There were no significant differences in days of follow-up by randomization group at FU1 and FU3 (Table 2). Fig. 2 shows the change in average daily step count from baseline (least square means from adjusted regression) by randomization group. In the adjusted linear mixed models for repeated measures, no significant between-group differences in change in average daily step count were noted at 6 (FU1) or 12 months (FU3). However, although between-group differences did not reach statistical significance, several within-group observations were notable. In the pedometer along group, a significant decline in average daily step count below baseline was observed at 6 months (FU1). In the pedometer plus website group, a statistically significant decrease in daily step count below baseline was delayed until 12 months (FU3).
Fig. 2.
Change in average daily step count from baseline by randomization group.
Change in average daily step count from baseline (V1) from adjusted regression model (least square means values). There were no significant between-group differences in change average daily step count from baseline. Blue and red stars denote a significant (p < 0.05) within group change from baseline in the pedometer-only and pedometer plus website groups, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Long-term effects of web-based intervention on HRQL and self-efficacy
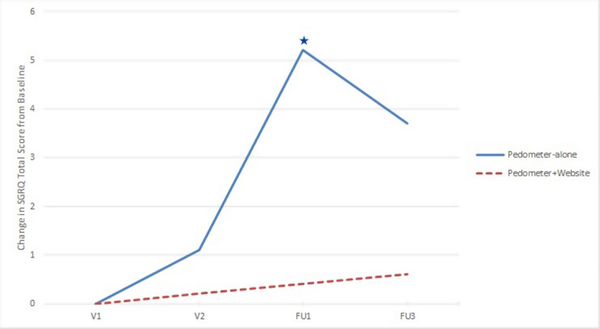
There were no significant differences in baseline SGRQ Total Score (SGRQ-TS) and Ex-SRES scores by randomization group (Table 1). In adjusted mixed models for repeated measures examining change in SGRQ-TS scores over long-term follow-up from baseline, there were no significant between-group differences by randomization group. In the pedometer-alone group, there was a significant within group increase in average SGRQ-TS (indicative of worse quality of life from baseline) at FU1. There was no significant within-group change in SGRQ-TS score in the pedometer plus website group during the entire follow up period, indicating no significant decline in HRQL from baseline (Fig. 3).
Fig. 3.
Change in Saint George’s Respiratory Questionnaire (SGRQ) total score from baseline by randomization group.
Change in St. George’ s Respiratory Questionnaire Total Score (SGRQ-TS) from baseline (V1) from adjusted regression model (least square means values). There were no significant between-group differences in change in SGRQ-TS from baseline. The blue star denotes a significant (p < 0.05) within group change in SGRQ-TS score from baseline in the pedometer-only group at FU1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
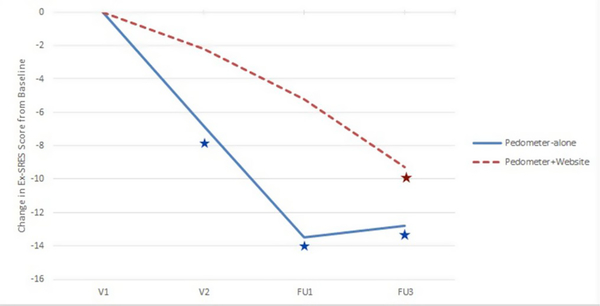
In the adjusted analyses, there were no significant between-group differences in change in Ex-SRES by randomization group. The pedometer-only group exhibited significant within group decreases (indicative of decreasing self-efficacy) in Ex-SRES score from baseline at V2, FU1, and FU3 (Fig. 4). A significant decline in Ex-SRES score from baseline in the pedometer plus website group was not observed until FU3.
Fig. 4.
Change in Exercise Self-Regulatory Efficacy Scale (Ex-SRES) from baseline by randomization group.
Change in Exercise Self-Regulatory Exercise Score (Ex-SRES) from baseline (V1) from adjusted regression model (least square means values). There were no significant between-group differences in change Ex-SRES from baseline. Blue and red stars denote a significant (p < 0.05) within group change from baseline in the pedometer-only and pedometer plus website groups, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In our secondary analysis of the Every Step Counts study [23], a 3-month technology-driven PA intervention that combines a pedometer with a website was associated with a significantly reduced risk of AEs in persons with COPD over 15 months, compared to a control group that used a pedometer alone. Although there were no significant between-group differences in daily PA, HRQL, or exercise self-efficacy during long-term follow-up, within-group changes suggest that the pedometer plus website delayed declines in these outcomes over time. To our knowledge, this is the first study using a RCT design to show that a technology-based PA intervention has a potential causal effect on reducing risk for AEs. To date, based largely on cross-sectional data, it has been assumed that increased PA results in improved clinical outcomes, like reduced risk for AEs [4,5,9,10]. We did not study mechanisms of benefit, but speculate that the benefits in PA and HRQL documented at 3–4 months [20,23] may have led to unmeasured effects-such as improved patient self-management skills through use of the web-based education or changes in markers of systemic inflammation through increased PA at 3 months-that may explain the observed long-term effects on decreasing risk of AEs.
Long-term patterns of change in daily step count suggest that the pedometer and website intervention can slow the natural history of progressive decline in PA among COPD patients [28,29] which may be a possible mechanism by which the protective effects of our intervention on AEs are mediated. Although progressive decline in PA was observed in both groups following the conclusion of the 3-month intervention period (after V2), the initial improvements in PA achieved in the pedometer plus website group effectively set a new, higher baseline PA level and delayed significant declines to below enrollment PA levels until 12 months. In contrast in the pedometer alone group, within-group analyses demonstrated a significant trend towards decline from baseline as early as 6 months.
Although many PA interventions for COPD patients have been reported to improve PA over the short-term (3–4 months) [14,19,20,23], a limited number of studies have reported differing results on the durability of these improvements and their effects on other clinical endpoints. In two different pedometer-based interventions, short-term improvements in PA and HRQL achieved during the 3-month intervention period were not sustained at 12–15 months [16,25,30]. In contrast, a 20-week telephone-based coaching program improved PA which was sustained at 18 months [21]. A separate 12-month multi-modality walking intervention did not result in significant differences in the rate of severe AEs (on study) compared to usual care groups [22]. In contrast, in a 20-week telephone-based coaching study, increased PA as well as a reduction in lung-related healthcare utilization was reported in a subset of subjects with severely reduced FEV1 [21]; notably, both PA and healthcare utilization endpoints in this study were self-reported. Heterogeneity in intervention components, outcomes assessment, and baseline patient characteristics likely contributes significantly to the varying long-term results reported.
While the long-term changes in HRQL and exercise self-efficacy also did not exhibit significant between-group differences, some notable within-group trends were observed. There was a trend towards worsening HRQL from baseline by FU1 and FU3 in the pedometer-alone group which was not observed in the pedometer plus website group.
When we examined whether the change in the SGRQ-TS was associated with the number of AEs during follow up, we did not observe a significant association (data not shown). Self-efficacy, defined as the belief in one’s ability to achieve an outcome [31], predicts success with traditional exercise training [32,33] and has been shown to be a determinant of change in PA levels in COPD [34]. Analogous to the long-term PA results, Ex-SRES scores in the pedometer alone group exhibited a significant within-group decline from baseline as early as V2 whereas the pedometer plus website group did not exhibit a significant decline until later at FU3. Thus, the website intervention which contained content to promote self-efficacy may have delayed the natural decline in self-efficacy over time observed in the pedometer alone group.
The strengths of our study include a well-characterized cohort, randomized controlled trial design with objectively-assessed PA data and well-defined, prospective AE events data which were collected using a rigorous and standardized protocol. We acknowledge a high percentage of self-reported AE events which were not validated and attribute this to the relatively low reliability of patient recall with regards to AE events as well as the stringent criteria used to define AE events in our study. Importantly, the distribution of non-validated AE events was not significantly different by randomization group and would therefore, be less likely to introduce bias. We believe the impact of “non-respondents” (subjects who could not be reached by telephone for all follow-up AE assessments) on our reported results was minimal due to the relatively modest number of non-respondents and the balance between randomization groups. Due to integral role of medical record review in our AE event validation/adjudication process and the multiple-payor, multiple provider healthcare system in the United States, we acknowledge that our record review process may not have captured AE events which occurred outside the Veterans Affairs (VA) healthcare system. However, we contend that the impact of this on our results is mitigated by 1) the fact that the VA healthcare system represents the largest integrated healthcare system within the United States, and 2) the frequent import into and availability of outside hospital records through the VA electronic medical record.
Additional potential limitations of our study include the male predominance, lack of racial/ethnic diversity, and single-center nature of our study, all of which may limit the generalizability of our findings. Future studies in larger and more diverse populations with COPD are warranted. Lastly, although the study was powered for the primary outcome of daily step counts at 3 months, the modest sample sizes within each arm of the study as a result of attrition during long-term follow-up may have limited our ability to detect significant between- group differences in daily step counts, HRQL and exercise self-efficacy at 6 and 12 months. Despite these limitations, we contend that our novel finding of a beneficial long-term effect of a 3-month technology-mediated PA intervention to reduce future risk for AEs represents a potentially significant clinical benefit of PA promotion in COPD patients.
Supplementary Material
Acknowledgments
We thank the Veterans for their participation in this research study.
Funding sources
United States Department of Veterans Affairs, Rehabilitation Research and Development Service [Career Development Award 2, F6847W (Moy); CDA2 IK2RX002165 (Wan); Merit O1150-R (Moy)].
Role of the funding Source(s)
The funding body had no role in the design, collection, analysis or interpretation of the data, in writing the manuscript, or in the decision to submit the manuscript for publication.
Abbreviations
- 6MWT
6-Minute Walk Test
- AE
Acute Exacerbation
- COPD
Chronic Obstructive Pulmonary Disease
- ESC
Every Step Counts
- Ex-SRES
Exercise Self-Reported Efficacy Scale
- FEVi
Forced Expiratory Volume in the first second
- FVC
Forced Vital Capacity
- HRQL
Health-Related Quality of Life
- mMRC
modified Medical Research Council
- PA
Physical Activity
- THS
Taking Healthy Steps
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Moy reports receiving an honorarium for consulting from AstraZeneca, outside the submitted work. The remaining authors have no relevant conflicts to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2020.105878.
Use of an internet-mediated pedometer-based physical activity intervention is associated with a reduction in acute exacerbations of COPD.
References
- [1].Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E, Smoking prevalence and cigarette consumption in 187 countries, 1980–2012, J. Am. Med. Assoc. 311 (2) (2014) 183–192. [DOI] [PubMed] [Google Scholar]
- [2].Xu J, Murphy SL, Kochanek MA, Brigham Bastian BS, Arias E, Deaths: final data for 2016, Natl. Vital Stat. Rep 67 (5) (2018). [PubMed] [Google Scholar]
- [3].Van Remoortel H, Hornikx M, Demeyer H, Langer D, Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T, Daily physical activity in subjects with newly diagnosed COPD, Thorax 68 (10) (2013) 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E, Daily step count predicts acute exacerbations in a US cohort with COPD, PLoS One 8 (4) (2013), e60400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen HQ, Chu L, Amy Liu IL, Lee JS, Suh D, Korotzer B, Yuen G, Desai S, Coleman KJ, Xiang AH, Gould MK, Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease, Ann. Am. Thorac. Soc 11 (5) (2014) 695–705. [DOI] [PubMed] [Google Scholar]
- [6].Durheim MT, Smith PJ, Babyak MA, Mabe SK, Martinu T, Welty-Wolf KE, Emery CF, Palmer SM, Blumenthal JA, Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group, Ann. Am. Thorac. Soc 12 (3) (2015) 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zanoria SJ, ZuWallack R, Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease, Chronic Respir. Dis 10 (4) (2013) 207–213. [DOI] [PubMed] [Google Scholar]
- [8].Moy ML, Teylan M, Weston NA, Gagnon DR, Danilack VA, Garshick E, Daily step count is associated with plasma C-reactive protein and IL-6 in a US cohort with COPD, Chest 145 (3) (2014) 542–550. [DOI] [PubMed] [Google Scholar]
- [9].Moy ML, Gould MK, Liu IA, Lee JS, Nguyen HQ, Physical activity assessed in routine care predicts mortality after a COPD hospitalisation, ERJ Open Res. 2 (1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, Magnussen H, Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study, Chest 140 (2) (2011) 331–342. [DOI] [PubMed] [Google Scholar]
- [11].Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2019 Report, 2019.
- [12].Barberan-Garcia A, Vogiatzis I, Solberg HS, Vilaro J, Rodriguez DA, Garasen HM, Troosters T, Garcia-Aymerich J, Roca J, Consortium N, Effects and barriers to deployment of telehealth wellness programs for chronic patients across 3 European countries, Respir. Med 108 (4) (2014) 628–637. [DOI] [PubMed] [Google Scholar]
- [13].Hornikx M, Demeyer H, Camillo CA, Janssens W, Troosters T, The effects of a physical activity counseling program after an exacerbation in patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot study, BMC Pulm. Med. 15 (2015) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mendoza L, Horta P, Espinoza J, Aguilera M, Balmaceda N, Castro A, Ruiz M, Diaz O, Hopkinson NS, Pedometers to enhance physical activity in COPD: a randomised controlled trial, Eur. Respir. J. 45 (2) (2015) 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, van der Weijden T, de Witte, It’s LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial, J. Med. Internet Res. 17 (7) (2015) e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vorrink SN, Kort HS, Troosters T, Zanen P, Lammers JJ, Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation, Eur. Respir. J. 48 (4) (2016) 1019–1029. [DOI] [PubMed] [Google Scholar]
- [17].Voncken-Brewster V, Tange H, de Vries H, Nagykaldi Z, Winkens B, van der Weijden T, A randomized controlled trial evaluating the effectiveness of a web- based, computer-tailored self-management intervention for people with or at risk for COPD, Int. J. Chronic Obstr. Pulm. Dis. 10 (2015) 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO, Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD, Int. J. Chronic Obstr. Pulm. Dis. 4 (2009) 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, Loeckx M, Buttery SC, Rubio N, Van der Molen T, Hopkinson NS, Vogiatzis I, Puhan MA, Garcia-Aymerich J, Polkey MI, Troosters T, Mr Papp Psg, the Pc. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial, Thorax 72 (5) (2017) 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, Nguyen HQ, Cohen MD, Goodrich DE, Giardino ND, Richardson CR, An internet-mediated pedometer-based program improves health-related quality- of-life domains and daily step counts in COPD: a randomized controlled trial, Chest 148 (1) (2015) 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coultas DB, Jackson BE, Russo R, Peoples J, Singh KP, Sloan J, Uhm M, Ashmore J, Blair SN, Bae S, Home-based physical activity coaching, physical activity, and health care utilization in chronic obstructive pulmonary disease. Chronic obstructive pulmonary disease self-management activation research trial secondary outcomes, Ann. Am. Thorac. Soc 15 (4) (2018) 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Balcells E, Benet M, Borrell E, Celorrio N, Delgado A, Jane C, Marin A, Martin-Cantera C, Monteagudo M, Montella N, Munoz L, Ortega P, Rodriguez DA, Rodriguez- Roisin R, Simonet P, Toran-Monserrat P, Torrent-Pallicer J, Vall-Casas P, Vilaro J, Garcia-Aymerich J, Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial, Eur. Respir. J. 52 (4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wan ES, Kantorowski A, Homsy D, Teylan M, Kadri R, Richardson CR, Gagnon DR, Garshick E, Moy ML, Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use, Respir. Med. 130 (2017) 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martinez CH, Moy ML, Nguyen HQ, Cohen M, Kadri R, Roman P, Holleman RG, Kim HM, Goodrich DE, Giardino ND, Richardson CR, Taking Healthy Steps: rationale, design and baseline characteristics of a randomized trial of a pedometer-based Internet-mediated walking program in veterans with chronic obstructive pulmonary disease, BMC Pulm. Med. 14 (2014) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, Nguyen HQ, Cohen MD, Goodrich DE, Giardino ND, Richardson CR, Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial, J. Med. Internet Res. 18 (8) (2016) e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jones PW, Quirk FH, Baveystock CM, Littlejohns P, A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire, Am. Rev. Respir. Dis. 145 (6) (1992) 1321–1327. [DOI] [PubMed] [Google Scholar]
- [27].Davis AH, Figueredo AJ, Fahy BF, Rawiworrakul T, Reliability and validity of the Exercise Self-Regulatory Efficacy Scale for individuals with chronic obstructive pulmonary disease, Heart Lung 36 (3) (2007) 205–216. [DOI] [PubMed] [Google Scholar]
- [28].Moy ML, Danilack VA, Weston NA, Garshick E, Daily step counts in a US cohort with COPD, Respir. Med. 106 (7) (2012) 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sievi NA, Brack T, Brutsche MH, Frey M, Irani S, Leuppi JD, Thurnheer R, Kohler M, Clarenbach CF, Physical activity declines in COPD while exercise capacity remains stable: a longitudinal study over 5 years, Respir. Med. 141 (2018) 1–6. [DOI] [PubMed] [Google Scholar]
- [30].Altenburg WA, ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, B Wempe J, Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial, Respir. Med. 109 (1) (2015) 112–121. [DOI] [PubMed] [Google Scholar]
- [31].Bandura A, Self-Efficacy: the Exercise of Control, W.H. Freeman and Company, New York, 1997. [Google Scholar]
- [32].Blackstock FC, Webster KE, McDonald CF, Hill CJ, Self-efficacy predicts success in an exercise training-only model of pulmonary rehabilitation for people with COPD, J. Cardiopulm. Rehabil. Prev. 38 (5) (2018) 333–341. [DOI] [PubMed] [Google Scholar]
- [33].Liacos A, McDonald CF, Mahal A, Hill CJ, Lee AL, Burge AT, Moore R, Nicolson B, O’Halloran P, Cox NS, Lahham A, Gillies R, Holland AE, The Pulmonary Rehabilitation Adapted Index of Self-Efficacy (PRAISE) tool predicts reduction in sedentary time following pulmonary rehabilitation in people with chronic obstructive pulmonary disease (COPD), Physiotherapy 105 (1) (2019) 90–97. [DOI] [PubMed] [Google Scholar]
- [34].Robinson SA, Shimada SL, Quigley KS, Moy ML, A web-based physical activity intervention benefits persons with low self-efficacy in COPD: results from a randomized controlled trial, J. Behav. Med. 42 (6) (2019) 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.