Laura Dwyer-Lindgren and colleagues have recently published an important mapping study that shows changes in the prevalence of HIV across Africa between 2000 and 2017.1 They used multiple data sets and statistical modeling to estimate both the prevalence of HIV among adults aged 15–49 years old and the number of HIV-infected adults in this age group, at a spatial resolution of five by five kilometers. They are the first to map HIV epidemics across the entire continent of Africa; previous mapping studies have focused on specific countries, e.g., Lesotho2 and Zimbabwe.3
The continental map shows that HIV epidemics in sub-Saharan Africa are not distinct entities contained within a country, but cross national borders (Figure A). Neighboring countries that have a similar prevalence of HIV at their national borders have a strong epidemiologic linkage: e.g., Botswana and Zimbabwe, or Botswana and South Africa. This indicates that their borders are “porous”, and individuals can move relatively freely between the countries; notably, HIV prevalence is high in all three countries. To be successful in eliminating HIV, Governments of epidemiologically-linked countries will need to coordinate their efforts and develop a regional control strategy. However, there is a substantial difference in prevalence between some neighboring countries; e.g., in Namibia the average prevalence is 13%, whereas in Angola it is 2%.1 In such cases, it may not be necessary for the countries to coordinate their HIV control strategies. Taken together, the results shown in Figure A highlight the necessity of determining the strength of connectivity among the country-specific HIV epidemics that traverse Africa.
The continental prevalence map provides a static picture of the current African epidemic, but it also shows the importance of human mobility. The observed large-scale spatial diffusion of HIV across southern and eastern Africa reflects historical movement patterns and migration flows.4 HIV began spreading in the 1920’s from Kinshasa, the capital of the Democratic Republic of Congo, DRC.4 At that time, rail transportation networks were already well established and heavily used: in the DRC, in 1922, ~300,000 passengers traveled by train.5 Human mobility continues to drive the spatial diffusion of HIV in Africa, as shown by phylogenetic6–8 and epidemiologic9–11 studies. However, UNAIDS’ and WHO’s recommended HIV control strategies are based on a static view of the epidemic and do not consider mobility. Novel interventions that are capable of reducing mobility-driven HIV transmission are clearly needed. Interventions that take mobility into account are already widely used for the control of other infectious diseases: e.g., malaria.
The density of infection (DoI) map (Figure B) shows the spatial distribution of HIV-infected individuals throughout Africa; the density varies from one HIV-infected individual to ~38,000 HIV-infected individuals, per 25 square kilometers. The distribution reflects the spatial demographics of African countries, where populations are predominantly rural: for example, in Lesotho only ~20% of the population live in urban centers.2 In rural areas, settlements are small and widely dispersed; consequently, population density - and the density of HIV infection - is low (Figure A). In the urban centers, population density - and the density of HIV infection - is high (Figure A). Prevalence is lower in rural areas than in urban centers; however, as a consequence of the level of urbanization, the majority of HIV-infected individuals live in rural areas. Notably, if the DoI map is used as a health policy tool to design resource allocation strategies, difficult ethical decisions will need to be made as to whether to maximize cost-effectiveness or to reduce urban-rural inequities in access to healthcare.2 Current UNAIDS and PEPFAR strategies are based on maximizing cost-effectiveness by preferentially allocating resources to urban centers.12
The maps constructed by Laura Dwyer-Lindgren and her colleagues clearly show the magnitude of the problem of reducing HIV transmission in Africa. However, the maps mask important gender differences. In all sub-Saharan African countries, HIV prevalence is significantly higher in women than in men, and the highest incidence rate is in adolescent girls and young women. This implies that prevention resources should be preferentially allocated to protect women, rather than men, against infection. As the maps show, in order to achieve elimination, it will be necessary to develop interventions that take into account movement/migration patterns and spatial demographics. Notably, these interventions should reduce current urban-rural inequities in access to healthcare in Africa.
Figure:
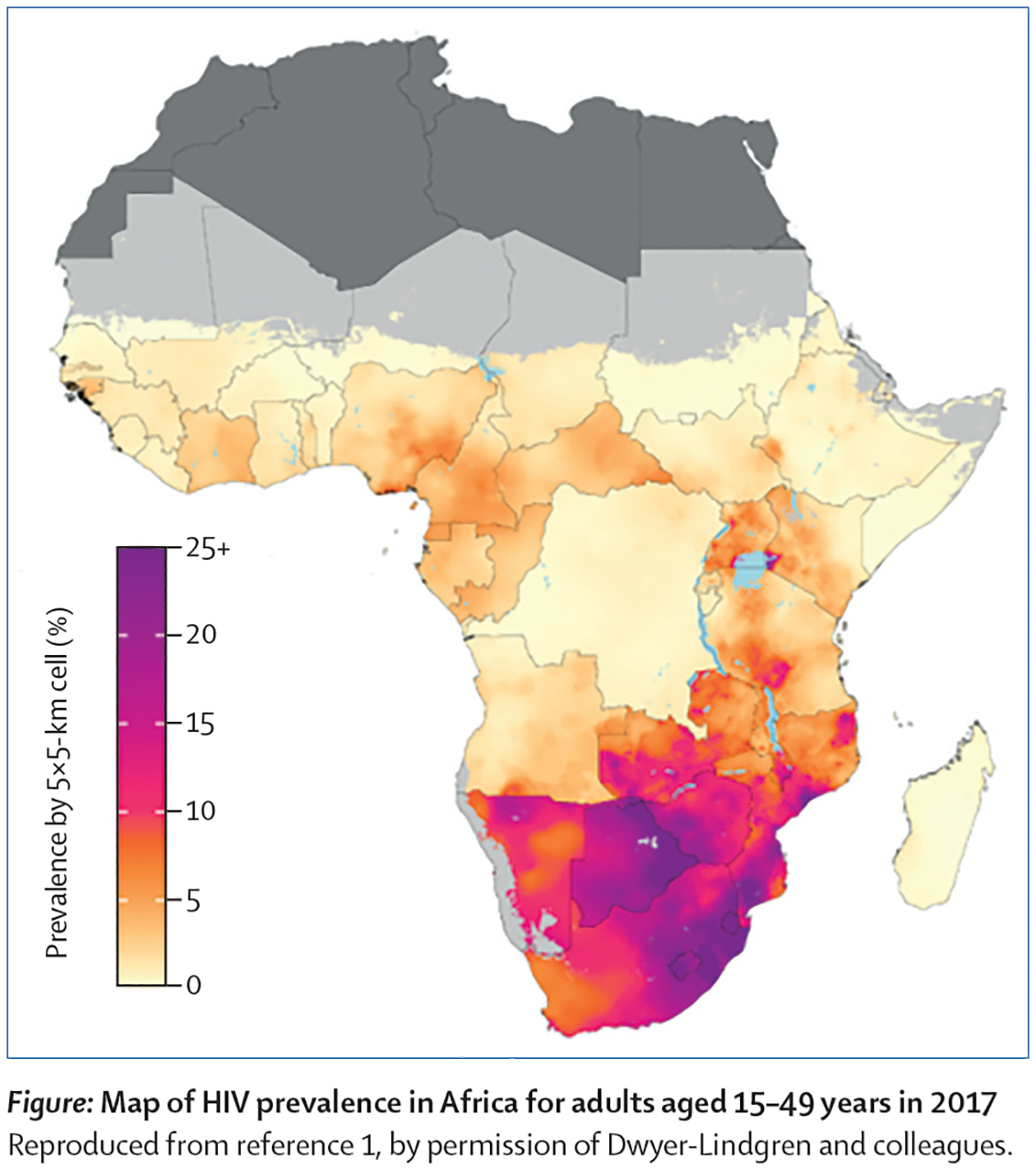
Results from the study by Dwyer-Lindgren et al.1 (A) Map of HIV Prevalence in Africa for adults 15–49 years old in 2017 (B) DoI map of number of HIV-infected adults 15–49 years old in 2017. Requested permission to republish from Nature.
Acknowledgements
SB and JTO acknowledge the financial support of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI116493).
Footnotes
Declaration of interests
SB and JTO declare that they have no conflicts of interest.
References
- 1.Dwyer-Lindgren L, Cork MA, Sligar A, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coburn BJ, Okano JT, Blower S. Using geospatial mapping to design HIV elimination strategies for sub-Saharan Africa. Sci Transl Med 2017; 9(383). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuadros DF, Li J, Mukandavire Z, Musuka GN, Branscum AJ, Sartorius B, Mugurungi O, Tanser F. Towards UNAIDS Fast-Track goals: targeting priority geographic areas for HIV prevention and care in Zimbabwe. AIDS 2019; 33(2): 305–14. [DOI] [PubMed] [Google Scholar]
- 4.Faria NR, Rambaut A, Suchard MA, Baele G, Bedford T, Ward MJ, Tatem AJ, Sousa JD, Arinaminpathy N, Pepin J, Posada D, Peeters M, Pybus OG, Lemey P. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science 2014; 346(6205): 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huybrechts A Transports et Structures de Développement au Congo: Etude du Progrès Economique de 1900–1970. Paris: Mouton; 1970. [Google Scholar]
- 6.Ratmann O, Grabowski MK, Hall M, et al. Inferring HIV-1 transmission networks and sources of epidemic spread in Africa with deep-sequence phylogenetic analysis. Nat Commun 2019; 10(1): 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abeler-Dorner L, Grabowski MK, Rambaut A, Pillay D, Fraser C. PANGEA-HIV 2: Phylogenetics And Networks for Generalised Epidemics in Africa. Curr Opin HIV AIDS 2019; 14(3): 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski MK, Lessler J, Redd AD, et al. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med 2014; 11(3): e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palk L, Blower S. Mobility and circular migration in Lesotho: implications for transmission, treatment, and control of a severe HIV epidemic. J Acquir Immune Defic Syndr 2015; 68(5): 604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobra A, Barnighausen T, Vandormael A, Tanser F. Space-time migration patterns and risk of HIV acquisition in rural South Africa. AIDS 2017; 31(1): 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS 2015; 10(6): 430–8. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS. Location, location: connecting people faster to HIV services. Geneva: UNAIDS, 2013. [Google Scholar]


