Abstract
Background:
Leptin replacement in patients with leptin gene mutations improves hypogonadotropic hypogonadism. The effects of leptin replacement on LH secretion in patients with lipodystrophy are unknown.
Aim:
We examined nocturnal LH secretory dynamics on and off exogenous leptin therapy using a 2 period, non-randomized study that included leptin-naïve and -treated subjects with lipodystrophy.
Methods:
In period 1 (5 days) the leptin-treated group (n=4) continued leptin; leptin was withdrawn for the next 14 days (period 2). Leptin-naïve subjects (n=8) were studied without leptin in period 1, and with leptin replacement in period 2. LH secretory dynamics were assessed [23:00–7:00, q10 min sampling, analyzed by multiparameter deconvolution algorithm] at the end of each period.
Results:
Mean (on vs. off: 5.0±3.1 U/L vs. 3.2±1.3, p=0.04) and integrated LH concentrations (2403 ± 1495 U·L−1·min−1 vs. 1534 ± 642, p=0.04) were higher on leptin therapy. Leptin treatment increased burst mass (9.7± 15.4 U/L vs. 7.0±11.2, p=0.03) and tended to non-significantly increase LH burst frequency (0.77±0.26 hr−1 vs. 0.67±0.24, p=0.08). Consequently, leptin therapy increased pulsatile production rate (64±101 U·L−1·8hr−1 vs. 57±73, p=0.01). On leptin, testosterone (507±286 ng/dL vs. 360±174, p=0.09) and estradiol levels (74±36 pg/mL vs. 29±24, p=0.01) were higher, in males and females, respectively.
Conclusions:
Leptin increases spontaneous nocturnal LH secretion in patients with lipodystrophy. This is consistent with rodent and in vitro studies showing a direct stimulatory effect (hypothalamic, pituitary or both) of leptin on LH secretion. These novel findings may explicate some of the salutary effects of leptin therapy on the hypothalamic-pituitary-gonadal axis in lipodystrophy.
Keywords: Leptin, Lipodystrophy, Luteinizing hormone
Introduction
Since its identification in 1994 [1], the adipokine leptin has been found to play a major role in regulation of body weight [2], glucose and lipid metabolism [3], immune function [4], and reproduction [5–7]. Complete leptin deficiency in patients with leptin gene or leptin receptor mutations causes hypogonadotropic hypogonadism, which is corrected with leptin replacement [8,9]. Leptin replacement for 2 weeks in women with hypothalamic amenorrhea secondary to strenuous exercise or low weight, states of relative leptin deficiency, normalizes the pulsatility of LH secretion [7].
Lipodystrophies, in which there is partial or complete absence of adipose tissue (and hence, adipokines such as leptin), result in variable leptin deficiency, ranging from severe to moderate, and represent another model for understanding the actions of leptin in states of deficiency and replacement. Women with lipodystrophy have impaired reproduction both due to ovarian hyperandrogenism (secondary to hyperinsulinemia), and abnormal gonadotropin secretion secondary to low leptin levels [10]. Leptin replacement in lipodystrophy both reduces hyperandrogenism by lowering insulin levels, and improves LH response to stimulation by GnRH [10], but the effects of leptin replacement on spontaneous LH secretion in this population have not been studied. In this study, we examined the effects of leptin replacement and withdrawal on overnight LH pulsatility in subjects with lipodystrophy.
Subjects and Methods
Subjects
We prospectively studied patients with lipodystrophy under a protocol designed to study the short-term effects of initiation or withdrawal of recombinant human methionyl leptin (metreleptin; Bristol Myers Squibb and Astra Zeneca) replacement (NCT01778556). The study was approved by the NIDDK Institutional Review Board. Patients withdrawn from metreleptin were previously enrolled in another protocol using doses of metreleptin adjusted over time to optimize triglycerides and hemoglobin A1c while minimizing excessive weight loss (NCT00025883). Inclusion criteria for both studies included a clinical diagnosis of lipodystrophy, fasting leptin <12.0 ng/mL (females) or <8.0 (males), and ≥ 1 metabolic complication of lipodystrophy, including: diabetes (defined by 2007 American Diabetes Association criteria), fasting insulin >30 mcU/mL, or fasting triglycerides >200 mg/dL. Exclusion criteria included HIV infection, infectious liver disease, drug or alcohol abuse, and active inflammatory, psychiatric, or kidney disease.
Study design
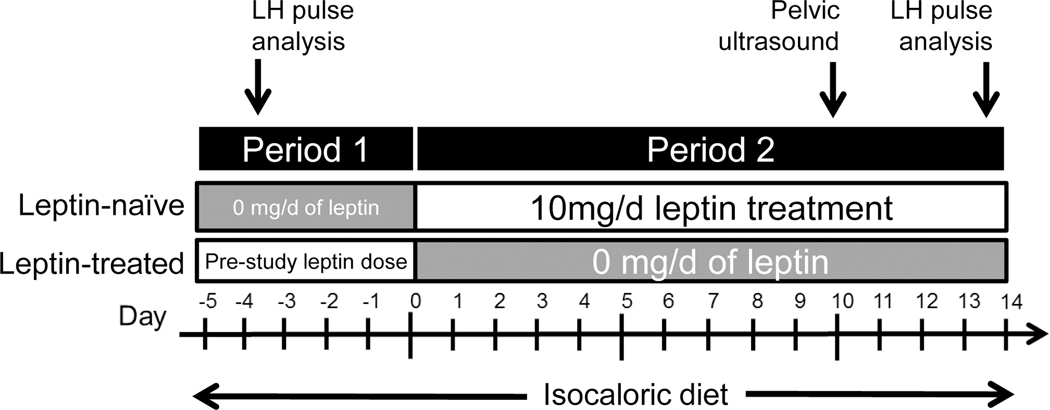
This was a 2 period, non-randomized study in which all subjects were studied twice in a paired design. In period 1 (5 days), metreleptin-treated patients continued metreleptin. Metreleptin was discontinued for the next 14 days (period 2). Leptin-naïve patients were studied without metreleptin in period 1 then were administered 5 mg metreleptin twice daily for the next 14 days (period 2) (Fig. 1). Throughout the study, patients consumed an isocaloric diet of fixed macronutrient composition in order to assess the effects of metreleptin independent of energy intake. With the exceptions of insulin and metreleptin, patients continued all other medications used prior to the study.
Figure 1.
Schematic of the study design. Patients treated with metreleptin prior to the study continued their regimen during period 1 and then completely discontinued metreleptin during period 2. Patient never treated with metreleptin prior to the study were studied without metreleptin in period 1 and then administered 10 mg of metreleptin each day during period 2. Frequent sampling to measure reproductive hormones was done during period 1 and 14 days after the start of period 2. Pelvic ultrasound was done between frequent sampling studies.
At the end of periods 1 and 2, LH was measured in blood (0.5 mL) drawn every 10 minutes from 2300–0700 h. During their period of leptin treatment, patients undergoing leptin-initiation received their last dose of leptin at 2100 h and patients undergoing leptin-withdrawal received their last dose at 0900 h. Early morning (0600–0700 h) FSH in all patients, 17-β estradiol (E2) and progesterone (P4) in females, and total testosterone (T) in males were measured. Trans-abdominal pelvic ultrasounds were obtained during period 2, and read by a single gynecologist (PS). Due to frequent oligo/amenorrhea in women with lipodystrophy, periods 1 and 2 were not synchronized with menstrual cycle phase. Cycle phase at each visit was assigned retrospectively, when possible, based on date of last menstrual period, LH, FSH, E2, P4, and ovarian ultrasonographic appearance. Endogenous leptin was measured after an overnight fast prior to metreleptin treatment. QUICKI was calculated as: 1 / [log (fasting insulin, mcU/mL) + log (fasting glucose, mg/dL)].
Hormone measurements
LH was measured in duplicate by enzyme immunoassay (EIA) (Cayman Chemical Company, Ann Arbor, MI) with minimum detectable concentration of 0.5 IU/L. Intra- and inter-assay coefficients of variation (CVs) were 7.3 and 3.4%, respectively. FSH was measured by EIA (Siemens Immulite 2000XPi, Malvern, PA) with minimum detectable concentration of 0.1 IU/L, intra-assay and inter-assay CVs were 3.1–4.2 % and 4.2–16.6 %. Leptin was measured by RIA using a commercial kit (Linco Research) with minimum detectable concentration of 0.5 ng/ml, intra-assay and inter-assay CVs were 2.2 % and 9.9 %, respectively. All other measurements were done in the NIH Clinical Center laboratory using standard methodology.
Analysis of LH secretion
LH secretory profiles were assessed using multiparameter deconvolution as previously described [11]. The following LH secretory parameters were characterized: secretory burst frequency (number of secretory peaks over the sampling period), mean burst amplitude (average of calculated maximal rates of secretion for all secretory episodes), mass/burst (average amount of hormone secreted/burst), pulsatile production rate, and integrated concentrations.
Data analysis
Results are presented as mean ± SD. Differences between reproductive hormone characteristics on and off-leptin were evaluated using 2-tailed, paired t tests for normally distributed data or Wilcoxon matched-pairs tests for nonparametric data. Differences between baseline parameters of leptin-naïve and leptin-treated patients were evaluated using 2-tailed, unpaired t tests or Mann-Whitney tests, as appropriate. To assess if changes on and off leptin were different between leptin-naïve and leptin-treated patients, we examined the period effect for each variable effect using mixed models. A P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Twelve lipodystrophy patients (4 male, 8 female; age 31 ± 13 years; and body weight 70 ± 19 kg) were studied. Four patients (2 male, 2 female) were currently treated with metreleptin (dose 4.0±0.5 mg/day) for 6.6 ± 5.8 years; the remaining eight (2 male, 6 female) were leptin-naïve. Three had congenital generalized lipodystrophy, two had acquired generalized lipodystrophy, and seven had familial partial lipodystrophy. Mean endogenous leptin was 7.8±11.5 ng/mL. Seven of eight leptin-naïve patients were treated with diabetic and hypolipidemic agents (4 with fibrates, and 4 with statins). Three of four leptin-treated patients took diabetic medications in conjunction with metreleptin. Triglycerides were higher in leptin-naïve (2108 ± 3888mg/dL) versus leptin-treated patients (175 ± 121mg/dL, p=.048). A1c tended to be higher in leptin-naïve (8.8 ± 1.8%) versus leptin-treated patients (6.7 ± 1.6%, p=.07). QUICKI was not different between the two groups, but indicated insulin resistance in the overall cohort (0.29±.03). Metreleptin treatment for 2 weeks decreased triglyceride levels while all the other metabolic parameters remained unchanged (suppl. table 2)
All males had testicular volumes >20 mL. All females had achieved menarche >1 year previously, were pre-menopausal, and had histories of oligomenorrhea (<9 periods/year) or amenorrhea prior to metreleptin. No patient took exogenous reproductive hormones within the prior 3 months. Seven of eight women had ultrasound features of polycystic ovarian morphology (>12 follicles 2–9 mm/ovary). Only one subject had menstrual cycle dating and progesterone values confirming the luteal phase at the on-leptin visit. One other subject was possibly in the luteal phase with a progesterone >2 ng/mL at the off-leptin visit. In all other cases, hormonal and ultrasound parameters suggested the follicular phase or hypogonadotropic hypogonadism. Thus, as usually noted in women with polycystic ovarian syndrome, women were unlikely to be in the luteal phase during the on-leptin or off-leptin visits.
Effects of leptin on nocturnal LH pulsatility and sex steroids
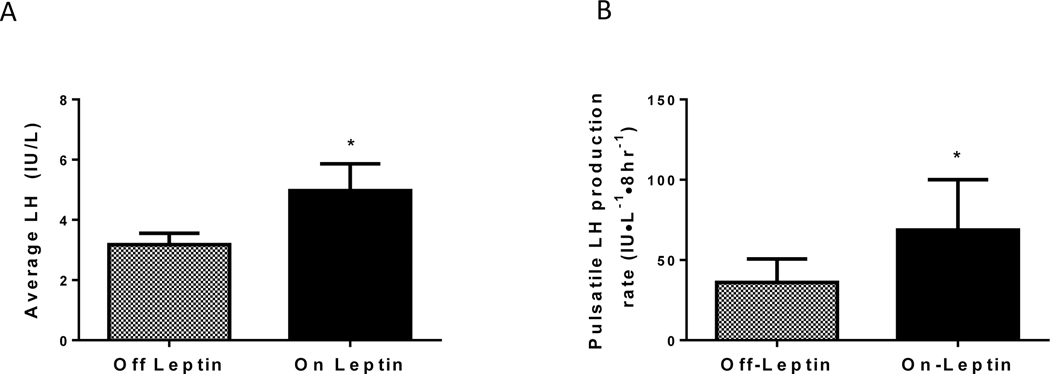
There were no differences in changes between the on and off leptin periods for leptin-naïve versus leptin-treated patients, so data were analyzed in aggregate (period effect >0.05 for all parameters). Average LH over the sampling period was significantly higher on metreleptin (on: 5.0 ± 3.1 IU/L, off: 3.2 ± 1.3, p=0.04). FSH was higher on metreleptin (p=0.02) (Table 1). Leptin treatment had no significant effect on testosterone levels in males (on: 507±286 vs. off: 360±174 ng/dL, p=0.09) or females (43±28 vs. 53 ± 39 ng/dL, p=0.34). In females, E2 was significantly higher on metreleptin (p=0.02) but, as most sampling was not done in the luteal phase, P4 was not different (on: 0.9 ± 1.0 ng/mL, off: 1.1 ± 2.3, p=0.41).
Table 1.
LH pulsatility parameters and sex steroid levels during off and on leptin replacement in patients with lipodystrophy.
| Variable | On Leptin | Off Leptin | P-value |
|---|---|---|---|
| Mean LH, IU/L | 4.97 ± 3.10 | 3.17 ± 1.32 | 0.04 |
| Integrated LH, IU•L−1•min−1 | 2403 ± 1495 | 1534 ± 642 | 0.04 |
| LH Burst frequency, hr−1 | 0.77 ± 0.26 | 0.67 ± 0.24 | 0.08 |
| LH Secretory burst mass, IU/L | 9.7 ± 15.4 | 7.0 ± 11.2 | 0.03 |
| LH Pulsatile production rate, IU•L−1•8hr−1 | 63.8 ± 100.7 | 36.0 ± 48.9 | 0.01 |
| LH Total production rate, IU•L−1•8hr−1 | 84.2 ± 99.8 | 57.3 ± 73.1 | 0.06 |
| FSH, IU/L | 5.99 ± 6.18 | 4.40 ± 3.91 | 0.02 |
| Estradiol*, pg/mL | 73.8 ± 36.4 | 29.4 ± 24.4 | 0.02 |
| Total Testosterone†, ng/dL | 507 ± 286 | 360 ± 174 | 0.09 |
Values shown are means ± SD. P values indicate significance for comparisons between “on” and “off” leptin periods using 2-tailed, paired t tests or the nonparametric Wilcoxon rank sum test.
Only females or
males used in analysis.
Metreleptin increased pulsatile LH secretion, due primarily to an increased mass of LH per burst, with a trend to increase burst frequency (Table 1, Fig. 2, suppl figure 1). Metreleptin did not modulate LH basal (time-invariant) secretion rate or orderliness of LH release as measured by approximate entropy (data not shown). Although the small sample size precludes subgroup analysis, post-hoc analysis suggests that there were no sex differences in the effects of leptin on LH pulsatility parameters (Psex*treatment >0.10).
Figure 2.
Mean nocturnal LH concentration (A) and pulsatile LH production rate (B) during the “on” and “off” leptin periods of all patients (n=12, means ± SEM). P value indicates significance for comparison between the two periods.*P<0.05
Discussion
This study demonstrates that, in patients with lipodystrophy, short-term metreleptin treatment or withdrawal modulates LH pulsatility. Metreleptin increases nocturnal integrated LH concentrations by augmenting pulsatile production (secretory burst mass and frequency), increases early morning estradiol concentrations in women, and tends to increase morning testosterone concentrations in men. To our knowledge, this is the first demonstration of enhanced LH production by leptin replacement in patients with lipodystrophy.
Animal studies suggest that leptin acts at multiple levels to modulate the hypothalamic-pituitary-gonadal (HPG) axis [12]. Intraventricular injection of leptin increases plasma LH concentrations in rats due to direct actions on the hypothalamus and pituitary [12]. In the medial basal hypothalamus, leptin increases GnRH. In vitro, leptin acutely stimulates basal and GnRH-induced LH release from the pituitary. Consistent with this, leptin administration to leptin-deficient ob/ob mice reverses hypogonadotropic hypogonadism and anovulation [13].
In humans, as in rodents, leptin deficiency (congenital or acquired) is associated with hypogonadism [5,14–16], which improves with metreleptin treatment. In homozygous leptin-deficient adolescents, metreleptin leads to onset of puberty and/or restoration of regular menstrual periods [5,14,17,18], while in adult men, metreleptin leads to higher free and total T, improved muscle strength, increased facial hair, and penile/testicular growth [15]. In two adult women with leptin gene mutations, leptin facilitated ovulation thus raising mid-luteal P4. In two adolescents, metreleptin for 11 weeks [18] or 12 months [17] accentuated LH pulse amplitude and frequency, resulting in significantly elevated mean LH concentrations. Similarly, in an adult with leptin gene mutation, metreleptin therapy for 6 months increased 24-hr average LH, LH pulse amplitude, and T, but did not change LH burst frequency [15].
As anticipated, the effect of leptin replacement on LH pulsatility in patients with lipodystrophy was similar to that previously observed in patients with hypothalamic amenorrhea [7]. In both groups, leptin replacement led to a rise in mean LH with a suggestion of increased LH pulse frequency, but no change in LH pulse amplitude (data not shown for lipodystrophy). Estradiol, likewise rose with leptin replacement in both groups. Grossly improved LH pulse patterns were seen in a similar percentage of female patients with lipodystrophy (7 of 8) and with hypothalamic amenorrhea (6 of 8) [7]. Interestingly, only one of four male patients with lipodystrophy had an improved LH pulse pattern with leptin replacement. Both males and females with congenital leptin deficiency respond to long-term leptin replacement with normal pubertal development, thus the sex differences observed here may be due to chance, or to a different time course of leptin action (requiring greater than 14 day exposure) in males versus females.
Our study demonstrated rapid (14 days) temporal effects of metreleptin on LH secretion, as previously observed in women with hypothalamic amenorrhea [7]. On a more rapid time scale, metreleptin prevented the decrease in LH pulsatility induced by a 72-hour fast in healthy lean men [19]. Thus, in lipodystrophy, replacement of leptin modulates the reproductive axis acutely. Interestingly, these effects on LH appear to be short-lived, as 14-day metreleptin withdrawal promptly attenuated LH secretion even after metreleptin treatment for an average of 7 years. Many studies have shown that there is a nocturnal rise in follicular phase LH pulse amplitude, width, and interpulse interval and LH mean concentrations in eugonadal adult females [20–24]. In healthy women, leptin appears to regulate minute-to-minute oscillations in LH, leading to the suggestion that nocturnal rise in leptin may be a determinant of nocturnal LH profile prior to ovulation [23] [24]. Indeed, similar to our finding, leptin replacement increased nocturnal LH secretion in females with hypothalamic amenorrhea [7] and human congenital leptin deficiency [15].However, understanding the effects of leptin replacement on the circadian LH secretion is important. The design of the current study and blood volume limitations did not allow for a 24-hr, 10 min frequent blood sampling. Thus, the effects of leptin on day-time LH secretion will remain unknown in our study
Patients with lipodystrophy have moderate to severe leptin deficiency. Unlike patients with absolute leptin deficiency from leptin gene mutations, these patients experience normal puberty. However, women manifest hyperandrogenism, amenorrhea, polycystic ovaries, and infertility, and men have slightly low T production [16]. We previously reported that 12 months of metreleptin reduces T, increases E2 levels, and restores regular menstrual cycles in 80% of women [16]. Single morning LH concentrations were not significantly altered by metreleptin, but there was an age-dependent increase in GnRH-stimulated LH release. In men, metreleptin increased T by ~ 67%, with no significant changes in basal or GnRH-stimulated LH [16].
Our current study is open-label, nonrandomized, and lacks a control treatment arm. Ethical and scientific considerations involved in conducting an exploratory study of subjects with a rare disorder with proven therapy precludes are more robust study design. Nevertheless, this study involving a rare disease provides insight into some of the salutary effects of metreleptin on reproductive function. Based on this current study and our studies of long term leptin replacement [16], it appears that leptin replacement restores fertility both by ameliorating insulin resistance and hyperandrogenism, and by augmenting LH pulsatility in patients with lipodystrophy.
Supplementary Material
Acknowledgments
We would like to acknowledge the patients with lipodystrophy, clinical fellows and the nursing staff at the clinical center involved in the care of these patients, the clinical core laboratory of NIDDK, and Bristol Myers Squibb and Astra Zeneca for the metreleptin used in this study. This research was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Eunice Kennedy Shriver Institute of Child Health and Human Development (NICHD).
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM: Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi IS, O’Rahilly S: 20 years of leptin: Human disorders of leptin action. The Journal of endocrinology 2014;223:T63–70. [DOI] [PubMed] [Google Scholar]
- 3.Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, Paruthi J, Mantzoros CS: Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocrine reviews 2013;34:377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procaccini C, Pucino V, Mantzoros CS, Matarese G: Leptin in autoimmune diseases. Metabolism: clinical and experimental 2015;64:92–104. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S: Effects of recombinant leptin therapy in a child with congenital leptin deficiency. The New England journal of medicine 1999;341:879–884. [DOI] [PubMed] [Google Scholar]
- 6.Chehab FF, Lim ME, Lu R: Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature genetics 1996;12:318–320. [DOI] [PubMed] [Google Scholar]
- 7.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS: Recombinant human leptin in women with hypothalamic amenorrhea. The New England journal of medicine 2004;351:987–997. [DOI] [PubMed] [Google Scholar]
- 8.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S: Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997;387:903–908. [DOI] [PubMed] [Google Scholar]
- 9.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B: A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401. [DOI] [PubMed] [Google Scholar]
- 10.Oral EA, Ruiz E, Andewelt A, Sebring N, Wagner AJ, Depaoli AM, Gorden P: Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. The Journal of clinical endocrinology and metabolism 2002;87:3110–3117. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuis JD, Johnson ML: Deconvolution analysis of hormone data. Methods in enzymology 1992;210:539–575. [DOI] [PubMed] [Google Scholar]
- 12.Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM: Role of leptin in hypothalamic-pituitary function. Proceedings of the National Academy of Sciences of the United States of America 1997;94:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA: Leptin is a metabolic signal to the reproductive system. Endocrinology 1996;137:3144–3147. [DOI] [PubMed] [Google Scholar]
- 14.Ozata M, Ozdemir IC, Licinio J: Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. The Journal of clinical endocrinology and metabolism 1999;84:3686–3695. [DOI] [PubMed] [Google Scholar]
- 15.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML: Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America 2004;101:4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P: The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism: clinical and experimental 2005;54:255–263. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S: Beneficial effects of leptin on obesity, t cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. The Journal of clinical investigation 2002;110:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Schnurbein J, Moss A, Nagel SA, Muehleder H, Debatin KM, Farooqi IS, Wabitsch M: Leptin substitution results in the induction of menstrual cycles in an adolescent with leptin deficiency and hypogonadotropic hypogonadism. Hormone research in paediatrics 2012;77:127–133. [DOI] [PubMed] [Google Scholar]
- 19.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS: The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. The Journal of clinical investigation 2003;111:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapen S, Boyar R, Hellman L, Weitzman ED: The relationship of luteinizing hormone secretion to sleep in women during the early follicular phase: Effects of sleep reversal and a prolonged three-hour sleep-wake schedule. The Journal of clinical endocrinology and metabolism 1976;42:1031–1040. [DOI] [PubMed] [Google Scholar]
- 21.Soules MR, Steiner RA, Cohen NL, Bremner WJ, Clifton DK: Nocturnal slowing of pulsatile luteinizing hormone secretion in women during the follicular phase of the menstrual cycle. The Journal of clinical endocrinology and metabolism 1985;61:43–49. [DOI] [PubMed] [Google Scholar]
- 22.Filicori M, Santoro N, Merriam GR, Crowley WF Jr.: Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. The Journal of clinical endocrinology and metabolism 1986;62:1136–1144. [DOI] [PubMed] [Google Scholar]
- 23.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS, McCann SM, Gold PW: Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proceedings of the National Academy of Sciences of the United States of America 1998;95:2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenichel RM, Dominguez JE, Mayer L, Walsh BT, Boozer C, Warren MP: Leptin levels and luteinizing hormone pulsatility in normal cycling women and their relationship to daily changes in metabolic rate. Fertility and sterility 2008;90:1161–1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.