Abstract
Purpose
Limited data exist to guide the treatment technique for reirradiation of recurrent or second primary squamous carcinoma of the head and neck. We performed a multi-institution retrospective cohort study to investigate the effect of the elective treatment volume, dose, and fractionation on outcomes and toxicity.
Methods and Materials
Patients with recurrent or second primary squamous carcinoma originating in a previously irradiated field (≥40 Gy) who had undergone reirradiation with intensity modulated radiation therapy (IMRT); (≥40 Gy re-IMRT) were included. The effect of elective nodal treatment, dose, and fractionation on overall survival (OS), locoregional control, and acute and late toxicity were assessed. The Kaplan-Meier and Gray’s competing risks methods were used for actuarial endpoints.
Results
From 8 institutions, 505 patients were included in the present updated analysis. The elective neck was not treated in 56.4% of patients. The median dose of re-IMRT was 60 Gy (range 39.6–79.2). Hyperfractionation was used in 20.2%. Systemic therapy was integrated for 77.4% of patients. Elective nodal radiation therapy did not appear to decrease the risk of locoregional failure (LRF) or improve the OS rate. Doses of ≥66 Gy were associated with improvements in both LRF and OS in the definitive re-IMRT setting. However, dose did not obviously affect LRF or OS in the postoperative re-IMRT setting. Hyperfractionation was not associated with improved LRF or OS. The rate of acute grade ≥3 toxicity was 22.1% overall. On multivariable logistic regression, elective neck irradiation was associated with increased acute toxicity in the postoperative setting. The rate of overall late grade ≥3 toxicity was 16.7%, with patients treated postoperatively with hyperfractionation experiencing the highest rates.
Conclusions
Doses of ≥66 Gy might be associated with improved outcomes in high-performance patients undergoing definitive re-IMRT. Postoperatively, doses of 50 to 66 Gy appear adequate after removal of gross disease. Hyperfractionation and elective neck irradiation were not associated with an obvious benefit and might increase toxicity.
Summary
For patients with squamous cell carcinoma of the head and neck undergoing reirradiation with intensity modulated radiation therapy, doses of ≥66 Gy might be associated with improved outcomes. Postoperatively, doses of 50 to 66 Gy appear adequate after removal of gross disease. Hyperfractionation and elective neck irradiation were not associated with an obvious benefit and might increase toxicity.
Introduction
Although the development of recurrence or second primary (RSP) squamous carcinoma in the head and neck after a previous course of radiation therapy (RT) is relatively uncommon, this clinical scenario occurs frequently enough to form the basis of ≥3 prospective clinical trials testing the efficacy of reirradiation (1–3). In general, these trials prescribed radiation in altered schedules (eg, 1.5 Gy twice daily, given every other week) to a dose of 60 Gy and targeted gross disease (or the resection bed) with a 2-cm margin and no elective (uninvolved) volume. Although these approaches are understandable given the first forays into reirradiation with 2-dimensional or 3-dimensional techniques in the prospective setting, these fractionation schemes and doses are not often used in the current era, especially for gross disease.
With the adoption of conformal RT techniques such as intensity-modulated RT (IMRT) and volumetric-modulated arc therapy, the therapeutic ratio of reirradiation might have changed, facilitating higher doses and minimizing the incidence and severity of acute or late toxicities (4). Additionally, evidence from the initial definitive RT setting has cast doubt on the utility of accelerated fractionation with concurrent systemic therapy (5, 6). Furthermore, given the high risk of failure at the treated site, the benefit of elective nodal treatment remains an open question (7). Therefore, we performed a multi-institution study of patients with RSP squamous cell carcinoma in the head and neck who had undergone reirradiation with IMRT techniques (re-IMRT) to investigate these questions of treatment volume, dose, and fractionation.
Materials and Methods
After institutional review board and legal approval, 9 institutions agreed to participate and formed the multi-institution reirradiation (MIRI) consortium. Eight centers contributed to the present analysis: Memorial Sloan-Kettering Cancer Center (New York, New York), Moffitt Cancer Center (Tampa, Florida), the Josephine Ford Cancer Institute at Henry Ford Health System (Detroit, Michigan), the University of Louisville (Louisville, Kentucky), University Hospitals Case Medical Center (Cleveland, Ohio), the Winship Cancer Institute at Emory University (Atlanta, Georgia), University of Alabama at Birmingham, and the Taussig Cancer Institute, Cleveland Clinic (Cleveland, Ohio). In the present updated analysis, an additional 93 patients were identified from 4 of the institutions. At each institution, patients previously irradiated to the head and/or neck to doses of ≥40 Gy, who then subsequently developed RSP squamous cell carcinoma without evidence of distant metastasis and underwent re-IMRT to a prescribed dose of ≥40 Gy with overlapping 40-Gy volumes were retrospectively identified. The demographic, treatment, and outcome data were centrally reviewed and analyzed at the Cleveland Clinic using a repository maintained with REDCap Software, version 5.8.2 (Vanderbilt University, Nashville, Tennessee).
Elective neck irradiation was generally defined as ≥1 nodal stations at risk of regional spread deliberately targeted in the absence of gross or microscopic disease found on clinical examination, imaging studies, or available pathology results at the time of treatment. In the data dictionary provided, institutions were asked to retrospectively code “no elective neck, unilateral or bilateral neck” coverage. Further granularity was not collected. For analysis, the effect of elective neck irradiation was studied among 2 groups: those without evidence of nodal involvement (N0) and those with known nodal disease (N+). The N0 cohort provides a clear perspective on “elective” nodal coverage. The N+ cohort, which included patients with resected or intact nodal disease, provides evidence regarding the role of additional levels of coverage (≥1 uninvolved nodal stations in either the ipsilateral or contralateral neck). For patients in the N+ cohort, if institutions reported either unilateral or bilateral neck coverage, as defined, the patients were included in the “elective neck irradiation group.” If reported as no elective neck coverage, they were included in the “no elective neck irradiation group.” Similarly, for patients with recurrence at the primary and neck, if any nodal levels were treated beyond the gross tumor, the institutions were asked to code that as “elective neck,” and if coded as such, these patients were included in the N+ elective neck cohort.
All outcomes were assessed from the start date of re-IMRT to the date of the event. Patients who did not complete treatment were only included in the acute toxicity analyses and were omitted from the subset survival analysis as detailed in the next paragraphs. Overall survival (OS) was calculated using the Kaplan-Meier technique, with differences between groups assessed using the log-rank test. Locoregional failure (LRF) was defined as any tumor persistence or progression above the clavicles whether detected by clinical examination, imaging studies, or biopsy. The actuarial cumulative incidence of LRF was calculated accounting for death as a competing risk, with differences assessed using Gray’s test. Multivariable Cox modeling was performed when necessary to assess for confounding. Variable selection was performed based on clinical relevance, with all measured potential confounders included in the multivariable models. Collinearity between covariates was assessed using hierarchal cluster analysis with clusters generated on Spearman’s rank order coefficient (8). When collinearity was present, the variable inflation factor was used for quantification. Interaction terms were investigated and included when significant, and unobserved differences in institutional baseline hazard rates related to patient selection were assessed using a mixed-effects Cox model. Nonlinear effects of continuous covariates were also investigated using penalized smoothing splines, when relevant.
Physician-assessed acute (≤90 days after treatment completion) and late toxicity (>90 days after treatment completion) was classified retrospectively by a review of the medical records by the treating institution and scored using the Common Terminology Criteria for Adverse Events, version 4.0, criteria. Pre-existing feeding tube or tracheostomy dependence was not considered toxicity nor was tracheostomy use after laryngectomy. The acute events specifically evaluated included admission to the hospital for aspiration pneumonia, new tracheostomy use, new feeding tube placement, esophageal stricture dilation, neutropenic fever, and soft tissue necrosis. The potential factors associated with acute toxicity were assessed using binomial logistic regression. The effects of the elective treatment volume, prescription dose, fractionation scheme, and systemic therapy were investigated. Interaction terms were considered in multivariable models to identify potential differential effects in the definitive and postoperative settings.
Late toxicity was defined as developing ≥90 days after re-IMRT completion. Toxicity events developing after locoregional recurrence were considered disease-related and not included. Events specifically investigated included osteoradionecrosis, aspiration pneumonia, esophageal stricture, carotid blowout syndrome, and fistula. Feeding tube and tracheostomy dependence for >1 year in the absence of disease was also considered late toxicity. The cumulative incidence of grade ≥3 late toxicity was calculated using Gray’s method, with disease recurrence and death considered competing risks. All analyses were performed using R software, version 3.2.3 (R project, Vienna, Austria).
Results
Patients
In this updated analysis, a total of 505 patients from the 8 participating institutions met the inclusion criteria. The patient, disease, and treatment characteristics of the study population are listed in Table 1. For surviving patients (n = 176), the median follow-up was 21.5 months (range 0–128.1). The initial approach to the RSP tumor consisted of surgery in 49.2%. Fractionation of re-IMRT was once daily for 79.6%. The target volume included elective treatment to the unilateral neck in 25.0%, the bilateral neck in 17.0%, and no elective neck in 56.4%. Systemic therapy was delivered to 77.4% of the patients and primarily consisted of platinum-based therapy (58.5%) in the concurrent setting (66.7%). Systemic therapy was more commonly used in the definitive setting than in the postoperative setting (84.8% definitive vs 69.8% postoperative; χ2 test, P < .001). The prescribed course of treatment was completed in 96.2% of patients.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Institution | |
| 1 | 166 (32.9) |
| 2 | 117 (23.2) |
| 3 | 59 (11.7) |
| 4 | 58 (11.5) |
| 5 | 48 (9.5) |
| 6 | 24 (4.8) |
| 7 | 17 (3.4) |
| 8 | 16 (3.2) |
| Race | |
| Black | 33 (6.5) |
| Asian | 3 (0.6) |
| White | 285 (56.4) |
| Hispanic | 11 (2.2) |
| Indian | 1 (0.2) |
| Other | 1 (0.2) |
| Unknown | 171 (33.9) |
| Gender | |
| Male | 369 (73.1) |
| Female | 136 (26.9) |
| Smoking | |
| Current | 69 (13.7) |
| Former (quit >3 mo) | 234 (46.3) |
| Never | 108 (21.4) |
| Unknown | 94 (18.6) |
| Charlson comorbidity | |
| 0 | 294 (58.2) |
| 1 | 102 (20.2) |
| ≥2 | 106 (21) |
| Unknown | 3 (0.6) |
| KPS | |
| 90–100 | 250 (49.5) |
| 70–80 | 202 (40) |
| 50–60 | 12 (2.4) |
| Unknown | 41 (8.1) |
| Type | |
| Recurrent | 381 (75.4) |
| Second primary | 124 (24.6) |
| Site | |
| Base of skull/retropharyngeal | 6 (1.2) |
| Hypopharynx | 20 (4) |
| Larynx | 73 (14.5) |
| Major salivary glands | 8 (1.6) |
| Nasal cavity | 6 (1.2) |
| Nasopharynx | 42 (8.3) |
| Neck | 94 (18.6) |
| Oral cavity | 84 (16.6) |
| Oropharynx | 141 (27.9) |
| Paranasal sinus | 19 (3.8) |
| Skin | 11 (2.2) |
| Other | 1 (0.2) |
| HPV status (RSP oropharynx) | |
| Positive | 38 (22.2) |
| Negative | 27 (15.8) |
| Unknown | 106 (62) |
| rT stage | |
| X/0 | 94 (18.6) |
| Is | 1 (0.2) |
| 1 | 47 (9.3) |
| 2 | 81 (16) |
| 3 | 62 (12.3) |
| 4 | 164 (32.5) |
| T+/NOS | 11 (2.2) |
| Unknown | 45 (8.9) |
| rN stage | |
| 0 | 228 (45.1) |
| 1 | 55 (10.9) |
| 2 | 152 (30.1) |
| 3 | 8 (1.6) |
| N+/NOS | 8 (1.6) |
| Unknown | 54 (10.7) |
| RSP treatment approach | |
| Definitive re-IMRT alone | 39 (7.7) |
| Definitive re-IMRT with systemic therapy | 218 (43.2) |
| Postoperative re-IMRT with gross disease | 74 (14.7) |
| Postoperative re-IMRT without gross disease | 174 (34.5) |
| Dose (definitive)*,† | |
| <60 Gy | 61 (25) |
| 60–65.9 Gy | 95 (38.9) |
| ≥66 Gy | 88 (36.1) |
| Dose (postoperative without gross disease)* | |
| 50–59.4 Gy | 25 (16.1) |
| 60 Gy | 87 (56.1) |
| 60.1–66 Gy | 43 (27.7) |
| Fractionation | |
| BID | 102 (20.2) |
| DAHANCA | 1 (0.2) |
| Once daily | 402 (79.6) |
| Fraction size‡ (Gy) | |
| Hyperfractionation | 1.2 (1.09–1.8) |
| Once daily | 2.0 (1.4–3.7) |
| Elective neck irradiation (all) | |
| Treated | 212 (42.0) |
| Not treated | 285 (56.4) |
| Elective neck irradiation (N0 patients) | |
| Treated | 49 (22.7) |
| Not treated | 167 (77.3) |
Abbreviations: BID = twice daily; DAHANCA = Danish Head and Neck Cancer Group; HPV = human papillomavirus; IMRT = intensity modulated radiation therapy; KPS = Karnofsky performance status; NOS = not otherwise specified; RSP = recurrent or second primary.
Patients completing prescribed course.
Not inclusive of postoperative patients with gross residual disease.
Data presented as median (range).
Elective neck irradiation
We first assessed the effect of the treatment volume on LRF and OS, because this could affect the selection of dose and fractionation. Data on neck irradiation were available for 497 of 505 patients (98.4%).
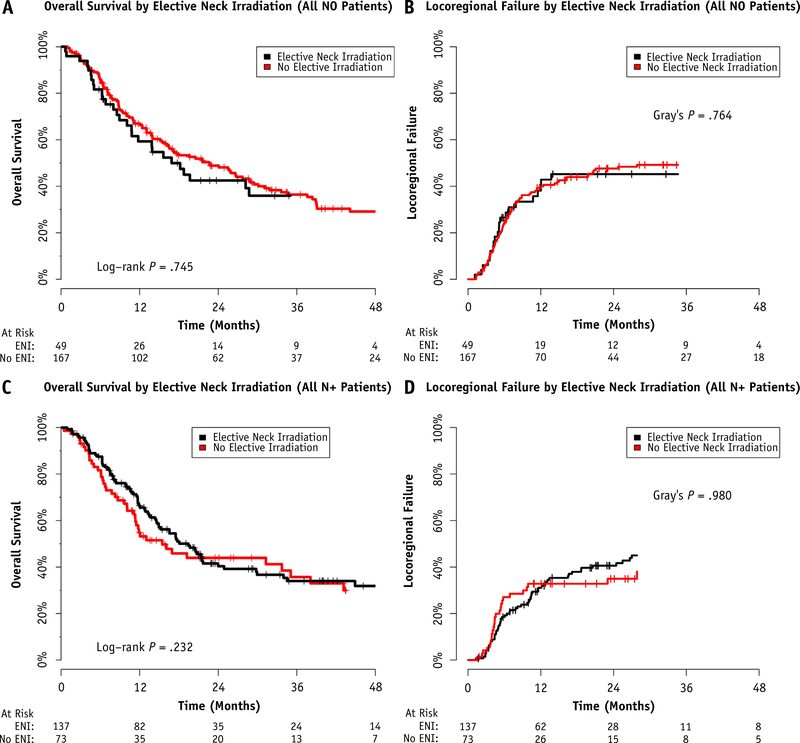
The indications for elective neck treatment were difficult to ascertain in this multi-institutional, retrospective cohort and were at the treating physician’s discretion. Elective neck treatment was more common for postoperative patients (54.9% vs 29.2%), patients with larynx (45.2%), oral cavity (31.0%), or oropharyngeal (33.4%) tumors, and in the N+ setting (62.8%). Information regarding the radiation dose to the elective nodal volume was not collected. Of the node-negative patients (N0), 167 were treated without elective nodal irradiation (ENI), 20 were treated to the unilateral neck, and 29 were treated to the bilateral neck. Of the node-positive patients (N+), 73 did not receive ENI, 89 were treated to the unilateral neck, and 48 were treated to the bilateral neck.
ENI did not appear to reduce the risk of 2-year LRF or improve 2-year OS in either the node-negative (Fig. 1A and 1B) or node-positive (Fig. 1C and 1D) subgroups. The results were similar when stratifying by postoperative or definitive intent re-IMRT and when extending to all patients treated, regardless of rN classification (data not shown).
Fig. 1.
Overall survival and locoregional failure outcomes stratified by the use of elective nodal irradiation (ENI) among (A,B) node-negative and (C,D) node-positive patients.
Dose
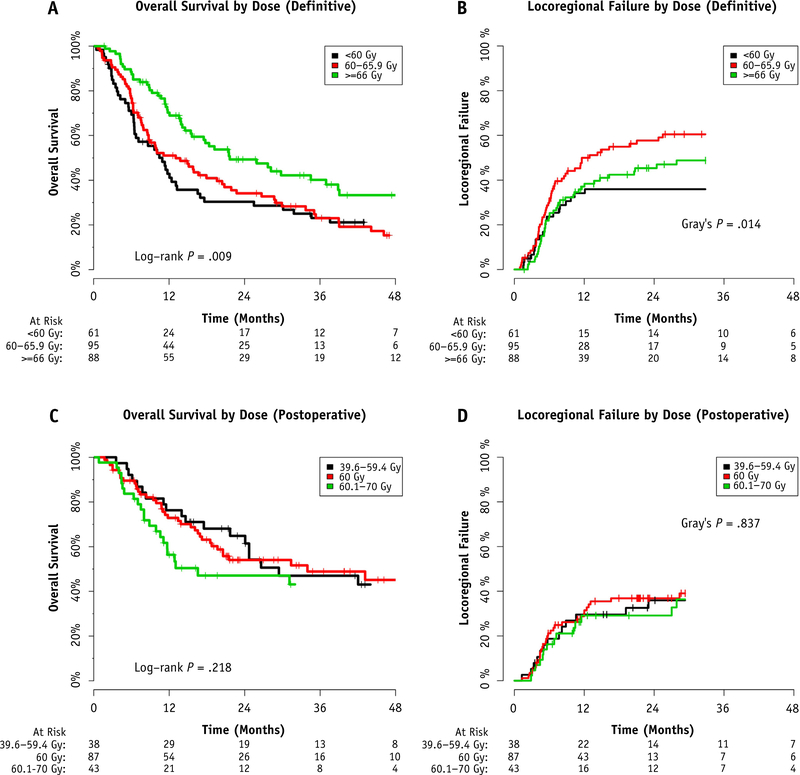
The median received dose of reirradiation to the high-dose planning target volume for the entire cohort was 60 Gy (range 1.8–79.2). For those who completed the prescribed course, the median dose was 60 Gy (range 39.6–79.2). A higher dose was more common for those with gross disease. Thus, 16.1% of those treated postoperatively without gross disease (27 of 168), 52.7% of those treated postoperatively with gross disease (39 of 74), and 36.1% of those treated definitively (88 of 244) received doses of ≥66 Gy.
In the definitive setting, dose escalation to ≥66 Gy was numerically more common in patients with disease of the oropharynx (42.1%), oral cavity (39.3%), larynx/hypopharynx (34.0%), and neck (31.4%). Sinonasal and base of skull/nasopharynx tumors were treated with dose escalation to ≥66 Gy less often (28.6% and 19.0%, respectively). The relationship between location and higher dose did not meet statistical significance (χ2 test, P = .193). All institutions performed dose escalation to ≥66 Gy for ≥15% of definitive patients, and 2 institutions (reporting 25 patients) performed dose escalation to ≥66 Gy for >85% of definitive patients. Dose escalation to ≥66 Gy was more common in the later years of the study (5.9% of definitive patients from 1998 to 2003, 33.7% from 2004 to 2009, and 48.1% from 2010 to 2015; Cochran-Armitage trend test, P < .001). Finally, the time between the courses of RT was longer for definitive patients treated to doses of ≥66 Gy (median time between courses: 4.48 years for ≥66 Gy, 2.42 years for 60–65.9 Gy, and 2.72 years for <60 Gy; Kruskal-Wallis test, P = .013). The use of systemic therapy was more common in those treated to <66 Gy (89.7%) than in those treated to ≥66 Gy (79.5%). The median follow-up period for survivors was not significantly different between those treated to <66 Gy and those treated to ≥66 Gy (26.2 vs 21.4 months, respectively; Wilcox test, P = .771).
For patients who completed the planned course of definitive re-IMRT (n = 244), a dose of ≥66 Gy was associated with improved OS compared with a dose of 60 to 65.9 Gy or <60 Gy (2-year OS 49.3% vs 34.2% vs 30.4%, respectively; global: P = .009; pairwise 60–65.9 Gy vs ≥66 Gy: P = .006; Fig. 2A). A multivariable Cox regression confirmed this association in the presence of potential confounding covariates (Table 2). A statistically significant correlation was noted on hierarchal cluster analysis between the dose of reirradiation and the covariates of time between RT courses, tumor site, and year of re-treatment. However, in each case, the variable inflation factor was ≤1.5, suggesting minimal inflation in covariate effect.
Fig. 2.
Relationship of prescribed dose of definitive reirradiation using intensity modulated radiation therapy (IMRT) with (A) overall survival and (B) locoregional failure. Relationship of prescribed dose of postoperative re-IMRT with (C) overall survival and (D) locoregional failure.
Table 2.
Effect of reirradiation dose on overall survival when controlling for potential confounders among definitive patients who completed treatment (multivariable Cox regression)*
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Dose of reirradiation (continuous, per Gy) | 0.957 | 0.937–0.978 | <.0001 |
| Systemic therapy (yes vs no) | 1.383 | 0.869–2.202 | .170 |
| Time between RT courses (continuous, per y) | 0.982 | 0.955–1.010 | .200 |
| Year of retreatment (continuous, per y) | 1.003 | 0.959–1.048 | .900 |
| Organ dysfunction (yes vs no) | 1.586 | 1.140–2.208 | .006 |
| Tumor site | |||
| Nasopharynx, base of skull, sinonasal, other | Ref | Ref | Ref |
| Oral cavity, oropharynx, larynx, hypopharynx | 2.044 | 1.303–3.205 | .002 |
Abbreviations: CI = confidence interval; HR = hazard ratio; Ref = reference; RT = radiation therapy.
Random effects term accounting for baseline institutional differences in hazard demonstrated a variance of 8 × 10−5, suggesting minimal effect; model included 244 patients, 172 events, and 6 degrees of freedom.
A dose of ≥66 Gy was associated with improvements in LRF compared with a dose of 60 to 65.9 Gy (2-year LRF 50.9% vs 67.5%, pairwise Gray’s test, P = .082; Fig. 2B). When analyzing using the Kaplan-Meier technique with the log-rank test, this difference was statistically significant (P = .026), implying that the competing risk of death affects the cumulative incidence of LRF and that for survivors, LRF might be improved when ≥66 Gy was delivered. Patients receiving <60 Gy did not have a significantly different LRF compared with that of those receiving ≥66 Gy; however, the patients in this less-common cohort also demonstrated inferior survival, again suggesting that patient selection and the competing risk of death in those treated <60 Gy accounted for this result.
To further investigate the mechanism behind the association between OS and dose, the patterns of failure and cause of death were analyzed. Investigation of the rate of any distant failure (with or without locoregional failure) stratified by the dose demonstrated statistically similar rates of distant metastases with a 2-year actuarial incidence for the <60 Gy, 60 to 65.9 Gy, and ≥66 Gy groups of 33.3%, 24.3%, and 22.8%, respectively (global log-rank: P = .559; pairwise <60 Gy vs ≥66 Gy: P = .267). The cause of death was attributed to distant progression or a non—head and neck cancer cause for 53.1% of the patients treated to <60 Gy but only 34.1% of those treated to doses ≥60 Gy (χ2 test, P = .03). This again supports the hypothesis that for patients with fewer competing risks of causes other than locoregional progression (eg, those treated to doses ≥60 Gy), doses of ≥66 Gy might improve outcomes.
For the subset of patients cleared of gross disease by surgery (n = 168), the prescription dose was not associated with 2-year LRF and OS (Fig. 2C and 2D). For the 74 patients with gross disease remaining after surgery, doses of ≥66 Gy were not associated with improved OS (2-year OS 33.0% with ≥66 Gy vs 35.0% with <66 Gy; P = .369) or improved LRF (1-year LRF 31.9% with ≥66 Gy vs 32.7% <66 Gy; P = .491).
Fractionation
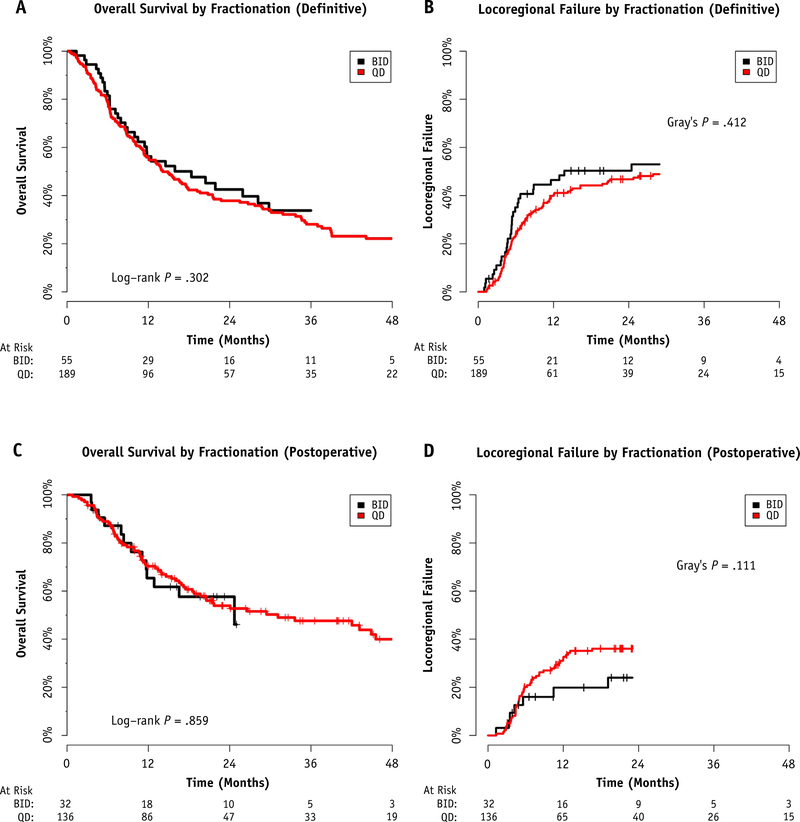
Hyperfractionation (twice daily [BID]) was used at 6 of the participating institutions. Three institutions performed hyperfractionation routinely (>75% of reported patients) and 75% of BID patients were reported by these 3 institutions. Hyperfractionation was more common in the later years of the study: 15.2% received hyperfractionation from 1998 to 2003, 12.1% from 2004 to 2009, and 28.4% from 2010 to 2015. Hyperfractionation was not more commonly used for doses of ≥66 Gy (18.8% of <66 Gy treated BID vs 23.4% of ≥66 Gy treated BID; χ2 test, P = .290).
Selection of fractionation for definitive patients did not appear to improve 2-year LRF (50.3% BID vs 46.8% once daily; P = .412) or 2-year OS (42.5% BID vs 37.9% once daily; P = .302; Fig. 3A and 3B) rates. Similarly, for patients treated postoperatively without gross disease remaining, hyperfractionation did not significantly improve LRF (2-year LRF 24.0% BID vs 37.1% once daily; P = .111) or OS (2-year OS 57.6% BID vs 53.9% once daily; P = .859; Fig. 3C and 3D).
Fig. 3.
Choice of fractionation in the definitive setting and association with (A) overall survival and (B) locoregional failure. Choice of fractionation in the postoperative setting and association with (C) overall survival and (D) locoregional failure. Abbreviations: BID = twice daily; QD = once daily.
For patients receiving once-daily fractionation, the median fraction size was 2 Gy (range 1.4–3.7). Although patients receiving fractions of 2 to 2.2 Gy daily had a greater rate of toxicity compared with those treated with fraction sizes of 1.6 to 1.9 Gy, they also had a lower rate of failure or death (Fig. E1; available online at www.redjournal.org). However, these differences did not reach statistical significance.
Human papillomavirus and recursive partitioning analysis validation
The present updated analysis allowed for investigation of human papillomavirus (HPV) status and an independent validation of the previously described recursive partitioning analysis (RPA) class, neither of which were described in our previous reports (9, 10). HPV or p16 data were available for 65 of the 171 RSP oropharyngeal cancer patients (38%). Although patients with HPV+ RSP oropharynx cancer demonstrated superior OS at 2 years of 60% compared with 39.5% for those with HPV-negative cancer, this did not reach statistical significance (pairwise, P = .152; Fig. E2; available online at www.redjournal.org). Furthermore, the previously described RPA class remained prognostic for OS in the 93 patients added since the submission of the initial report (2-year OS 68.8%, 50.6%, and 33.0% for RPA class I, II, and III; log-rank test, P = .001; Fig. E3; available online at www.redjournal.org).
Acute toxicity
Complete acute toxicity data were available for 462 patients. The patients experienced a crude grade ≥3 toxicity risk of 22.1% (95% confidence interval [CI] 18.5%−26.1%). The factors investigated for association with acute effects are listed in Table E1 (available online at www.redjournal.org). On multivariable binomial logistic regression (Table E1; available online at www.redjournal.org), a significant interaction was identified between elective neck irradiation (unilateral or bilateral vs none) and the treatment setting (postoperative vs definitive). In the definitive setting, elective neck irradiation was not associated with increased odds of acute effects. However, in the postoperative setting, elective neck irradiation was associated with increased odds of acute grade ≥3 toxicity. Patients classified as having American Joint Committee on Cancer, 7th edition, stage rT4 demonstrated increased odds of acute toxicity (odds ratio 2.320, 95% CI 1.451–3.717; P < .001). No other factors, including dose, fractionation, and systemic therapy, were associated with increased odds of acute toxicity on either univariable or multivariable regression.
Late toxicity
The cumulative incidence of grade ≥3 late toxicity at 2 years was 16.7% (95% CI 13.2%−20.2%), but the competing risk was 64.2% (95% CI 59.7%−68.6%). Patients treated in the postoperative setting experienced greater rates of late toxicity (2-year cumulative incidence of 11.4% for definitive vs 22.3% for postoperative; P = .0056).
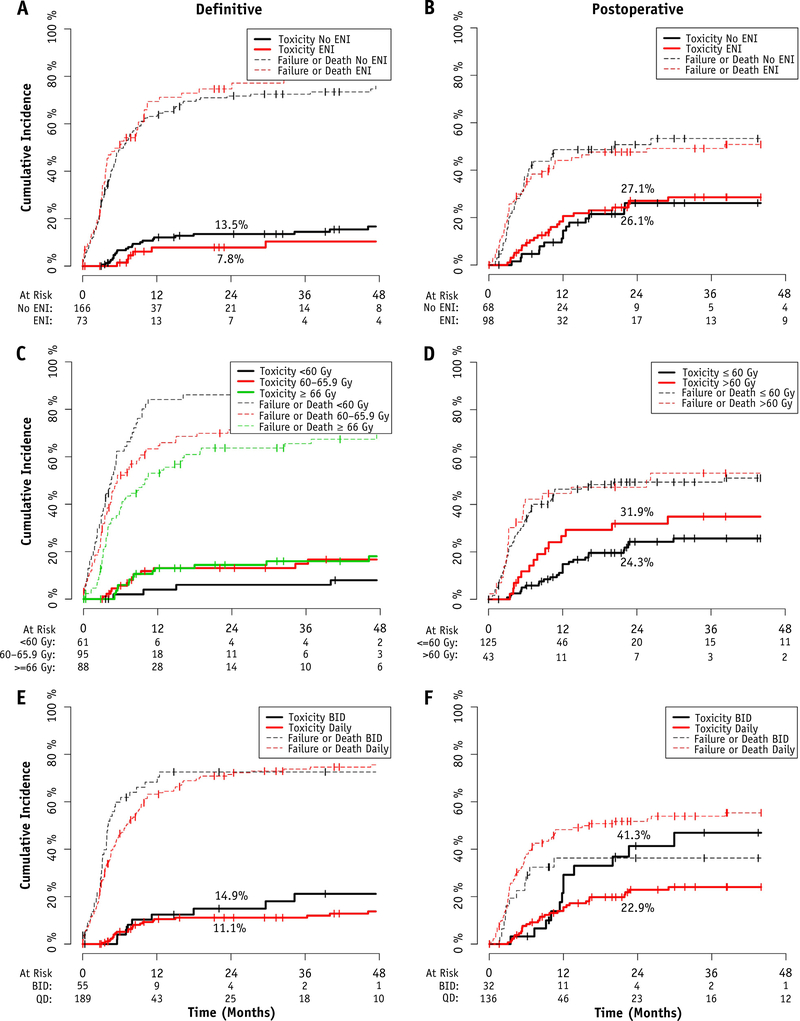
The results of the late toxicity analyses are presented in Fig. 4. In the definitive setting, for those who completed treatment (n = 244), elective neck irradiation was not associated with increased late effects (13.5% for no ENI vs 7.8% with ENI at 2 years; P = .344). Similarly, dose escalation was not associated with increased late effects, with a 2-year cumulative incidence of 5.9% for those treated to <60 Gy, 13.1% for those treated to 60 to 65.9 Gy, and 14.4% for those treated to ≥66 Gy (global: P = .231; pairwise <60 vs ≥66 Gy: P = .126). Hyperfractionation also did not significantly alter the incidence of late effects, with a 2-year rate of 14.9% for those treated twice daily compared with 11.1% for those treated daily (P = .264).
Fig. 4.
Cumulative incidence of grade ≥3 late toxicity and competing risks of failure or death, in the (A) definitive cohort, (B) postoperative cohort, (C) definitive cohort stratified by dose, (D) postoperative cohort stratified by dose, (E) definitive cohort stratified by fractionation, and (F) postoperative cohort stratified by fractionation. No differences were statistically significant, with the exception of late toxicity in the postoperative patients treated with hyperfractionation (Gray’s test, P = .031). Greater competing risks were observed in definitive patients treated to <60 Gy (global Gray’s, P = .002). Abbreviations: BID = twice daily; ENI = elective nodal irradiation; QD = once daily.
In the postoperative setting, for those with no gross disease remaining who completed treatment (n = 168), elective neck irradiation was not significantly associated statistically with increased late effects (26.1% no ENI vs 27.1% ENI at 2 years; P = .444). Dose escalation was not significantly associated with increased late effects, with a 2-year cumulative incidence of 24.3% for those treated to ≤60 Gy compared with 31.9% for those treated to >60 Gy (P = .198). A significant increase was noted in late effects, with a 2-year rate of 41.3% for those treated postoperatively with hyperfractionation compared with 22.9% for those treated daily (P = .031). Fractionation did not significantly alter the late effects in the absence of systemic therapy (n = 108; 2-year cumulative incidence of 16.7% BID vs 15.7% once daily; P = .836).
Discussion
In the accompanying analyses from this multi-institution collaboration, we have devised a prognostic grouping to aid with patient counseling and treatment selection of conventionally delivered RT or stereotactic RT (9, 10). We focused on the details that could affect the volume and prescription of re-IMRT.
In the reirradiation scenario, in which disease is presumed to be inherently radioresistant and normal organs at risk might have already received tolerance doses, the benefit of elective nodal irradiation appears less clear than in the definitive setting. For example, Popovtzer et al (7) demonstrated that 96% of LRFs after reirradiation were within the high-dose volume. In contrast, Margalit et al (11) noted some component of marginal or out of field locoregional recurrence in 23% of patients, although this was primarily seen in the postoperative setting. However, they were unable to demonstrate an improvement in the risk of recurrence with prophylactic neck treatment (11). Similarly, in both patients with a N0 neck and all patients, we were unable to demonstrate a LRF or OS benefit with ENI. Although ENI did not appear to increase the risk of late effects, it was significantly associated with increased acute complications, such as might be expected from the larger volume of treatment.
Prospective reirradiation trials have used altered fractionation schedules, with hyperfractionation at 1.5 Gy BID (2, 3) or conventionally fractionated (1) in a week on/week off manner. Given the potential time needed at the linear accelerator for re-IMRT, many groups have moved to once-daily treatments without a break, owing to the lack of benefit for accelerated fractionation with concurrent chemotherapy (5, 6). In both the definitive and the postoperative re-IMRT setting, it appeared that conventionally fractionated treatment had LRF and OS outcomes equivalent to those with accelerated RT (primarily hyperfractionation). In addition, caution should be exercised when selecting hyperfractionation, because it was associated with greater grade ≥3 late toxicity in the postoperative setting.
The same prospective trials cited previously prescribed a total dose of 60 Gy, whether as definitive or postoperative treatment. In general, 60 Gy would not be considered sufficient for gross disease, and single-institution reports have reflected that bias, prescribing doses of ≥66 Gy (12–14). In developing a nomogram, Riaz et al (15) found that doses in excess of 50 Gy were associated with improvements in both locoregional control and OS. However, the doses for postoperative or definitive intent were not analyzed separately. Takiar et al (12) also examined the role of the re-IMRT dose in a single-institution study, demonstrating an improvement in 5-year locoregional control, progression-free survival, and OS with a dose of 70 Gy, compared with ≤66 Gy, for patients not receiving surgery. In contrast, the dose was not significant for patients after salvage surgery. Our results appear to agree, in general, with these findings, with improved LRF and OS in definitive re-IMRT patients receiving ≥66 Gy compared with patients receiving 60 to 65.9 Gy. Although patients receiving <60 Gy did not appear to have worse LRF, their OS was still significantly lower than that of patients receiving ≥66 Gy, implying that the competing risk of death might be a driver of this nonsignificant difference. In addition, lower doses might have been chosen for patients with suboptimal function and/or late sequelae of previous RT and, as such, might have been at a greater risk of other causes of death. Postoperative re-IMRT patients appeared to have little difference in either LRF or OS using doses of 50 to 66 Gy.
Evidence has shown that patients with recurrent or metastatic HPV-related head and neck cancer have better outcomes than do those with HPV-negative cancer (16, 17). Our results support this view; however, despite a 20.5% absolute difference in favor of HPV-positive patients, we were unable to demonstrate statistical significance for this comparison, perhaps owing to the small numbers available for this subset analysis.
The significant acute and late toxicity associated with re-IMRT is well known. It is hoped that with re-IMRT or other advanced RT approaches the therapeutic index will widen. However, the risk of acute grade ≥3 complications was 22.1% exclusively using re-IMRT in our study, although this compares favorably with the 28% to 63% risk seen in prospective trials with older RT techniques. This acute toxicity rate was also similar to that from more modern single-institution studies of ~30% (14, 18). The late grade ≥3 toxicity rate was 16.8% at 2 years, also comparable to those from recent reports of single-institution series using re-IMRT (12, 14, 19, 20), including proton reirradiation (18, 21). Also, the late toxicity rates will continue to increase for ≤5 years after re-IMRT, plateauing at 48% to 66% (12, 14), warranting caution in the assessment of late toxicity at earlier points.
The limitations of the present study included its retrospective nature, with the inherent biases of selection and recall that cannot be overcome simply by a centralized data review. This is particularly applicable to the N+ cohort when investigating elective neck irradiation, because the exact extent and nature of “elective” nodal coverage could not be centrally confirmed as the contour and isodose line data were not collected. However, given that the findings of our larger retrospective and multi-institution series support the conclusions seen with smaller single-institution series, we are cautiously optimistic that our results could be useful in shaping practice going forward and could inform future prospective trials.
Conclusion
The routine use of elective neck irradiation or hyperfractionation during re-IMRT does not appear beneficial. For patients undergoing definitive re-IMRT, doses of ≥66 Gy appear to be relatively safe and might improve outcomes, especially for high-performing patients or those with a prolonged natural history such as HPV-associated RSP oropharynx cancer. For patients receiving postoperative re-IMRT in the absence of gross disease, doses of 50 to 66 Gy appear adequate.
Supplementary Material
Acknowledgments
Conflict of interest: J. Bonner has a consulting role with Merck Serma, Eli Lilly, Bristol-Meyers Squibb, and Cell-Sci, all outside the submitted work. J. Caudell has a consulting role with EMD Serano outside the submitted work. N. Lee is on the advisory board for Merck, Pfizer, and Vertex, all outside the submitted work. M. Machtay has received honoraria and has consulting and advisory roles with Stemnion and Abbvie, all outside the submitted work. N. Riaz has received honoraria from Medimmunue outside the submitted work. F. Siddiqui has received honoraria and has a speaker and travel relationship with Varian Medical Systems, American College of Radiology, Med Dos advisory board, and Wayne State University, all outside the submitted work.
Footnotes
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol 2008;26:5518–5523. [DOI] [PubMed] [Google Scholar]
- 2.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008;30:281–288. [DOI] [PubMed] [Google Scholar]
- 3.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group protocol 9911. J Clin Oncol 2007;25:4800–4805. [DOI] [PubMed] [Google Scholar]
- 4.Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer—Disease control and morbidity outcomes. Int J Radiat Oncol 2009;73:399–409. [DOI] [PubMed] [Google Scholar]
- 5.Ang K, Pajak T, Wheeler R, et al. A phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas (RTOG 0129): Report of efficacy and toxicity. Int J Radiat Oncol 2010;77:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99–02): An open-label phase 3 randomised trial. Lancet Oncol 2012;13:145–153. [DOI] [PubMed] [Google Scholar]
- 7.Popovtzer A, Gluck I, Chepeha DB, et al. The pattern of failure after reirradiation of recurrent squamous cell head and neck cancer: Implications for defining the targets. Int J Radiat Oncol 2009;74:1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrell F Regression Modeling Strategies. 2nd ed. New York: Springer Publications; 2011. [Google Scholar]
- 9.Ward MC, Riaz N, Caudell JJ, et al. Refining patient selection for re-irradiation of head and neck squamous carcinoma in the IMRT era: a multi-institution cohort study by the MIRI collaborative. Int J Radiat Oncol Biol Phys 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargo JA, Ward MC, Caudell JJ, et al. A multi-institution comparison of SBRT and IMRT for definitive re-irradiation of recurrent or second primary head and neck cancer. Int J Radiat Oncol Biol Phys 2017. in press, accepted April 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margalit DN, Rawal B, Catalano PJ, et al. Patterns of failure after reirradiation with intensity-modulated radiation therapy and the competing risk of out-of-field recurrences. Oral Oncol 2016;61:19–26. [DOI] [PubMed] [Google Scholar]
- 12.Takiar V, Garden AS, Ma D, et al. Reirradiation of head and neck cancers with intensity modulated radiation therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys 2016;95:1117–1131. [DOI] [PubMed] [Google Scholar]
- 13.Curtis KK, Ross HJ, Garrett AL, et al. Outcomes of patients with locoregionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol 2016;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez F, Berwouts D, Madani I, et al. High-dose reirradiation with intensity-modulated radiotherapy for recurrent head-and-neck cancer: Disease control, survival and toxicity. Radiother Oncol 2014; 111:388–392. [DOI] [PubMed] [Google Scholar]
- 15.Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol 2014;111: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeken JF, Newkirk K, Harter KW, et al. Effect of multimodality treatment on overall survival for patients with metastatic or recurrent HPV-positive head and neck squamous cell carcinoma. Head Neck 2015;37:630–635. [DOI] [PubMed] [Google Scholar]
- 17.Vermorken JB, Psyrri A, Mesía R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: Retrospective analysis of the phase III EXTREME trial. Ann Oncol 2014;25:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan J, Sio TT, Nguyen TP, et al. Reirradiation of head and neck cancers with proton therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys 2016;96:30–41. [DOI] [PubMed] [Google Scholar]
- 19.Velez MA, Veruttipong D, Wang P-C, et al. Re-irradiation for recurrent and second primary cancers of the head and neck. Oral Oncol 2017;67:46–51. [DOI] [PubMed] [Google Scholar]
- 20.Karam I, Huang SH, McNiven A, et al. Outcomes after reirradiation for recurrent nasopharyngeal carcinoma: North American experience. Head Neck 2016;38(Suppl 1):E1102–E1109. [DOI] [PubMed] [Google Scholar]
- 21.Romesser PB, Cahlon O, Scher ED, et al. Proton beam reirradiation for recurrent head and neck cancer: Multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys 2016;95: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.