Abstract
It has been proposed that Complex Regional Pain Syndrome (CRPS) is a post-traumatic autoimmune disease. Previously we observed that B cells are required for the full expression of CRPS-like changes in a mouse tibia fracture model and that serum IgM antibodies from fracture mice have pronociceptive effects in muMT fracture mice lacking B cells. The current study evaluated the pronociceptive effects of injecting CRPS patient serum or antibodies into muMT fracture mice by measuring hindpaw allodynia and unweighting changes. CRPS serum binding was measured against autoantigens previously identified in the fracture mouse model. Both CRPS patient serum or IgM antibodies had pronociceptive effects in the fracture limb when injected systemically in muMT fracture mice, but normal subject serum and CRPS patient IgG antibodies had no effect. Furthermore, CRPS serum IgM antibodies had pronociceptive effects when injected into the fracture limb hindpaw skin or intrathecally in the muMT fracture mice. Early (1–12 months post injury) CRPS patient (n=20) sera were always pronociceptive after systemic injection and chronic (>12 months post injury) CRPS sera were rarely pronociceptive (2/20 patients), while sera from normal subjects (n=20) and from patients with uncomplicated recoveries from orthopedic surgery and/or fracture (n=15) were never pronociceptive. Increased CRPS serum IgM binding was observed for keratin 16, histone 3.2, gamma actin, and alpha enolase autoantigens. We postulate that CRPS patient IgM antibodies bind to neoantigens in the fracture mouse skin and spinal cord to initiate a regionally restricted pronociceptive complement response potentially contributing to the CRPS disease process.
Keywords: antigen, autoimmunity, pain, fracture, immunoglobulin, complex regional pain syndrome
Summary sentence for the table of contents: CRPS patient IgM-mediated autoimmunity supports nociceptive sensitization in the mouse fracture model.
1. Introduction
Complex regional pain syndrome (CRPS) is an enigmatic syndrome that typically develops after limb injury or surgery and presents with distal limb nociceptive, vascular, and bone changes that exceed the expected clinical course of the inciting injury, frequently resulting in significant motor impairment and disability.[41] Distal limb fracture is the most common cause of CRPS [9,35] and we have developed a tibia fracture rodent model closely resembling CRPS.[5] Distal tibia fractured rats and mice casted for 3–4 weeks develop hindpaw allodynia, unweighting, warmth, edema, increased spontaneous protein extravasation, and regional periarticular bone loss.[17] The fracture CRPS model has been successfully used to study the effects of fracture on neuropeptide signaling,[17,19,46,48] the sympathetic nervous system,[24] mast cell infiltration,[26] keratinocyte [39,46,49] and microglia [28,38] inflammatory mediator (IL-1, IL-6, TNF, CCL2, NGF) production,[10,25,29,32,33,47] and other CRPS-related phenomena.[16]
Accumulating evidence suggests that CRPS may involve both autoinflammatory and autoimmune components.[8,12] Previously we investigated the effects of B cell depletion in the fracture model using anti-CD20 antibodies and in muMT fracture mice lacking both B cells and immunoglobulin, and observed that; 1) wildtype (WT) mice treated with intravenous anti-CD20 antibody had virtually no mature B cells and exhibited attenuated hindpaw allodynia, unweighting, warmth, and edema, 2) muMT mice had attenuated nociceptive and inflammatory changes at 3 weeks post-fracture, 3) IgM-containing immune complexes were deposited in skin and sciatic nerve at 3 weeks after fracture in WT mice but not in muMT fracture mice, and 4) complement system membrane attack complex deposition in skin and sciatic nerve after fracture was partially reversed by anti-CD20 treatment.[27] Furthermore, when serum or IgM antibodies collected from WT fracture mice were injected into muMT fracture mice, they gradually developed increased hindpaw allodynia and unweighting, peaking at 7 days and resolving by 14 days after injection, consistent with the half-life of IgM.[18] Serum from nonfractured WT mice or IgG from fractured WT mice had no pronociceptive effects in the muMT fracture mice. IgM antibody levels gradually increased in the WT mouse fracture limb hind paw skin, sciatic nerve, and corresponding lumbar cord, peaking at 12 to 18 weeks after fracture and then declining to baseline levels at 23 weeks post fracture, consistent with the time course of post fracture nociceptive sensitization.
Based on these experiments we postulated that fracture induced the regional expression of novel antigens in the mouse fracture limb hindpaw skin and corresponding spinal cord, and that CRPS patient autoantibodies might be capable of binding to those antigens and initiating a antibody-antigen-complement response resulting in complement sensitization of nociceptive neurons.[22] The current study tested the hypothesis that the systemic injection of serum antibodies from CRPS patients can induce regionally restricted pain behaviors in muMT B cell deficient fracture mice, and then identified the immunoglobulin isotype responsible for these pronociceptive effects. Additional experiments tested the hypothesis that CRPS autoantibodies can induce pronociceptive effects after injection in hindpaw skin or intrathecal injection in muMT fracture mice. Lastly, CRPS patient serum binding was measured against autoantigens previously identified in the fracture model.
2. Materials and methods
2.1. Subjects and clinical data collection
The study protocols were approved by the respective local institutional review boards in accordance with the Declaration of the World Medical Association. Four cohorts of subjects were evaluated for pronociceptive serum effects in muMT fracture mice; 1) normal control subjects (n=20), 2) early CRPS (n=20), 3) chronic CRPS (n=20), and 4) post limb trauma without CRPS (n=15). The normal control subject sera were collected by a commercial company using our serum processing protocol and all control subjects were tested negative for HIV and HCV antibodies and non-reactive for HBSAG, HIV-1 RNA, HCV RNA, HBV DNA, and STS (BioreclamationIVT, Westbury, NY). All early and chronic CRPS patient sera were collected in Mainz and Giessen Germany (by F.B., F.E., M.H. and H.K) except for 12 chronic CRPS patient sera that were collected in Liverpool United Kingdom (by A.G.). The post limb trauma without CRPS patient sera were collected in Palo Alto California (by P.S.). All patient subjects were enrolled after giving written informed consent. Inclusion criteria for the early CRPS cohort included meeting the Budapest clinical scientific criteria for CRPS [20] at the time of serum collection and a duration of CRPS between 1 and 12 months post injury. Inclusion criteria for the chronic CRPS cohort included meeting the Budapest criteria and a duration of CRPS greater than 12 months post injury. The inclusion criteria for the resolved CRPS cohort were that the patients met the Budapest CRPS criteria when the initial serum samples were collected but when the patients were re-evaluated 2 or more years later for repeat serum collection they no longer met CRPS diagnostic criteria. The inclusion criteria for the orthopedic trauma without CRPS cohort were that patients must be 1–12 months post orthopedic surgery and/or fracture and fail to meet the Budapest clinical diagnostic criteria for CRPS at the time of serum collection. Patient demographics and clinical data were recorded, including age, gender, CRPS duration and etiology, involved limb, pain medications, numerical 11 point (0–10, no pain to the worst pain possible) pain scale, and allodynia. Allodynia was tested by applying 3–4 light strokes with a small brush to the affected skin and asking patients if this evoked a normal or abnormal sensation. If the sensation was described as abnormal the patient was asked to give a qualitative description of the sensation. Descriptions of the brushing as uncomfortable, scratchy, or painful were regarded as allodynia.
2.2. Animals
These experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the animal subjects guidelines of the International Association for the Study of Pain. Three-month-old male muMT mice lacking mature B cells and immunoglobulin, on a C57BL/6J congenic background (#002288, Jackson Laboratory, Bar Harbor, ME), were used in these experiments (n=330). The animals were housed 4 per group under pathogen-free conditions with soft bedding and were given food and water ad libitum, with a 12:12 light:dark cycle. During the experimental period the animals were fed Teklad lab rodent diet 2018 (Harlan Laboratories, Indianapolis, IN), which contains 1.0% calcium, 0.7% phosphorus, and 1.5 IU/g vitamin D3, and were kept under standard conditions with a 12-h light-dark cycle. Data collection was conducted blind to group assignment. All animal and biochemical experiments described in these studies were performed in Palo Alto California (by T-Z.G., T.W., M.T., and W.L.)
2.3. Surgery
The fracture model was performed in 3 month-old male mice as previously described [19]. Under isoflurane anesthesia a hemostat was used to make a closed fracture of the right tibia just distal to the middle of the tibia. The hindlimb was then wrapped in casting tape (Delta-Lite, BSN Medical, Hamburg, Germany) so the hip, knee and ankle were all fixed. After fracture and casting, the mice were given subcutaneously 2 days of buprenorphine (0.1 mg/kg) and enrofloxacin (5 mg/kg) as well as 1.0 ml of normal saline. At 3 weeks after surgery the mice were anesthetized with isoflurane and the cast removed. All mice had union at the fracture site by manual inspection.
2.4. Hindpaw nociceptive testing
Mechanical allodynia was assayed using nylon von Frey filaments according to the “up-down” algorithm as previously described [6]. The mice were placed on wire mesh platforms in clear cylindrical plastic enclosures 10 cm in diameter and 40 cm in height, and after 15 minutes of acclimation von Frey fibers of sequentially increasing stiffness were applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads, and pressed upward to cause a slight bend in the fiber and left in place for 5 sec. Withdrawal of or licking the hindpaw after fiber application was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until 4 fibers had been applied after “negative+positive or positive+negative” response. Hindpaw testing was performed bilaterally. Estimation of the mechanical withdrawal threshold by data fitting algorithm permitted the use of parametric statistics for analysis [31]. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a negative value represents a reduction in the fracture hindpaw withdrawal threshold.
An incapacitance device (IITC Life Science, Woodland Hills, CA) was used to measure bilateral hindpaw weight bearing. The mice were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates and the entire weight of the mouse was supported on the hindpaws. The duration of each measurement was 6 s and 6 consecutive measurements were taken at 10 s intervals. All 6 readings were averaged to calculate the bilateral hindpaw weight-bearing values. Right hindpaw (fracture side) weight-bearing data were analyzed as a ratio between the right hindpaw weight and the average of right and left hindpaw values ((2R/(R + L)) × 100%), thus a value less than 100% represents a decrease in weight bearing in the fracture limb.
2.5. Hindpaw temperature testing
The temperature of the bilateral hindpaws was measured using a fine wire thermocouple (Omega Engineering, Norwalk, CT) applied to the paw skin, as previously described [29]. The investigator held the wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw: the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a positive value represents increased temperature in the fracture paw.
2.6. Hind paw thickness testing
A laser sensor technique was used to determine the dorsal-ventral thickness of the bilateral hind paws, as we have previously described [29]. For laser measurements each mouse was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. The paw was gently held flat on the table with a small metal rod applied to the top of the ankle joint. Using optical triangulation, a laser with a distance-measuring sensor was used to determine the distance to the table top and to the top of the hindpaw at the midpoint of the third metatarsal and the difference was used to calculate the dorsal-ventral paw thickness. The measurement sensor device used in these experiments (Limab, Goteborg, Sweden) has a measurement range of 200 mm with a 0.01 mm resolution. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a positive value represents increased paw thickness in the fracture paw.
2.7. CRPS serum and immunoglobulin injection experiments in the fracture mouse CRPS model
This experiment examined the pronociceptive (hindpaw mechanical allodynia and unweighting) and inflammatory (hindpaw warmth and edema) effects of early (1–12 month post injury) CRPS patient serum after intraperitoneal injection into 3 weeks post fracture muMT mice (Fig. 1). After clinical evaluation CRPS patient blood was collected in 10ml red top tubes and left undisturbed at room temperature for 60 min to allow clotting, then refrigerated overnight at 4°C and then the blood samples were centrifuged at 2,200g for 20 min at 4°C and the serum supernatants were aliquoted and frozen at − 80°C. The muMT mice underwent baseline behavioral testing (measuring hindpaw von Frey allodynia, unweighting, warmth, and edema) and then, under isoflurane anesthesia, the right distal tibia was fractured and casted. At 3 weeks post-fracture the casts were removed and behavioral testing was repeated the next day and then the mice were injected with the CRPS patient serum or with normal control subject serum (500 ul, i.p.). Further behavioral testing was performed at 1, 5, 7, 9, 14, and 21 days after injection. Each CRPS patient (n=5) or control (n=5) serum was injected into 3 different muMT fracture mice and the test results were averaged for each subject.
Figure 1. CRPS patient serum and IgM had pronociceptive effects in B cell deficient fracture mice.
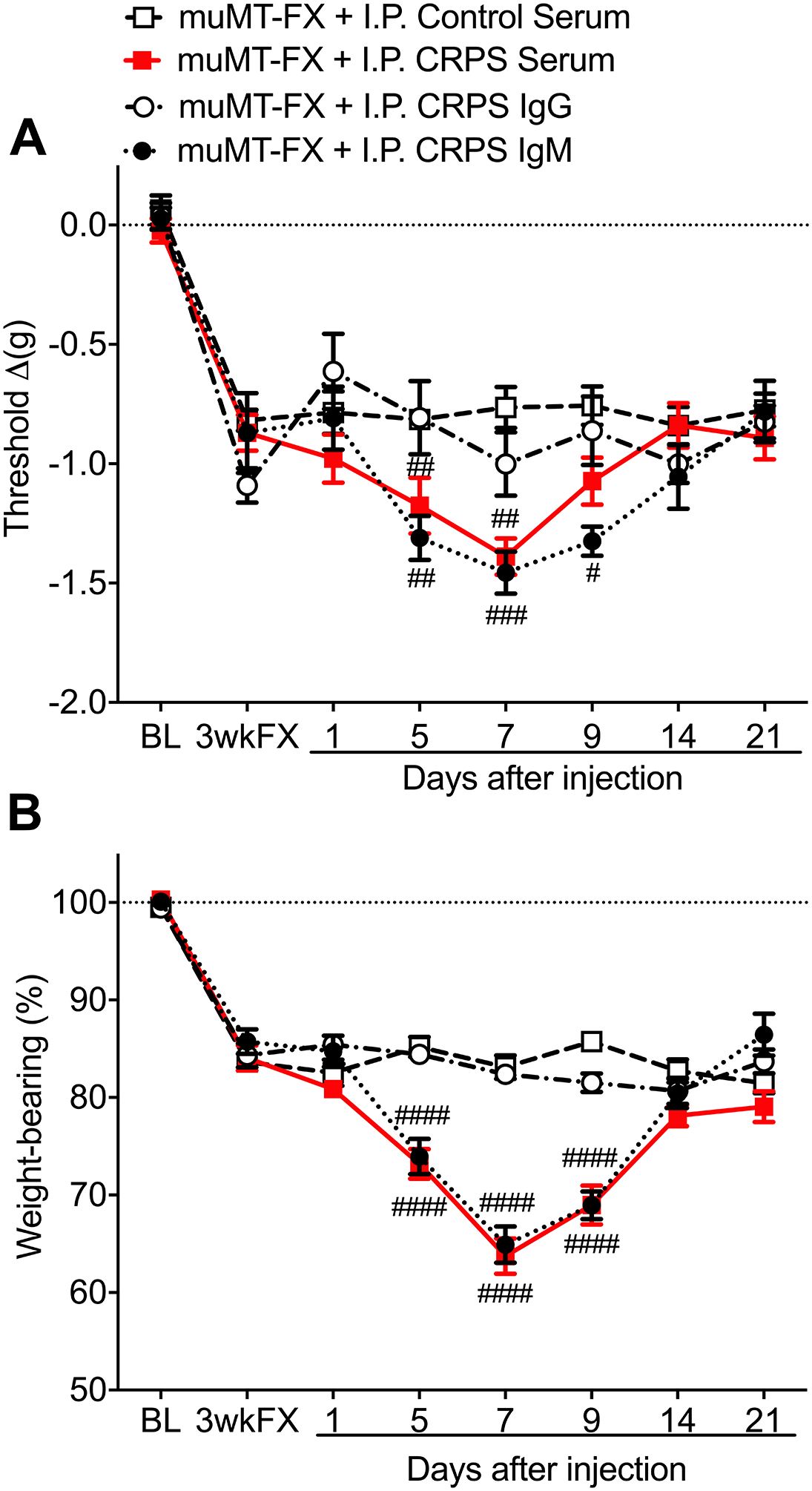
At 3 weeks after tibia fracture and casting (FX) muMT mice lacking B cells and IgM exhibited unilateral hindpaw von Frey allodynia (A) and unweighting (B). After intraperitoneal injection of early (1–12 months post injury) CRPS patient serum (0.5ml, I.P) or IgM (500ug/1ml, I.P.) into 3 weeks post FX muMT mice, the mice gradually developed increased allodynia and unweighting over the ensuing week, and consistent with the 6 day half-life of IgM, these pronociceptive effects resolved by 2 weeks post-injection. The pronociceptive effects of the CRPS serum were restricted to the FX limb and there was no serum effect on post FX hindpaw edema or warmth (data not shown). No pronociceptive effects were observed after intraperitoneal injection of early CRPS patient IgG (5mg/1ml, I.P.) or after injection of control subject serum (0.5ml, I.P.) in 3 weeks post-FX mice. Measurements for (A) represent the difference between the FX side and contralateral paw, thus a negative value represents a decrease in mechanical withdrawal thresholds on the affected side. Measurements for (B) represent weight-bearing on the FX hind limb as a ratio to half of the total bilateral hind limb loading, thus, a percentage lower than 100% represents hindpaw unweighting. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 5 patients per cohort and each patients serum or immunoglobulin was injected into 3 mice for a total n of 15 mice. #P < 0.05, ## P < 0.01, and ### P < 0.001 for each injection cohort vs the control serum treatment group. MuMT: mice lacking B cells, FX: fracture, BL: baseline, 3wkFX: 3 weeks after fracture
Another experiment was designed to identify the immunoglobulin isotype responsible for the pronociceptive effects of early CRPS patient serum in 3 weeks post fracture muMT mice (Fig. 1). IgM was extracted from the CRPS serum using a polypropylene column (BioRad, Hercules, CA) pre-packed with POROS CaptureSelect™ IgM Affinity Matrix (Thermo fisher Scientific, Leiden, Netherlands). The bound IgM was eluted using 100mM glycine pH 3, then the elute pH was adjusted to 7.4 using 1M Tris pH 8.0, then Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) were used to remove glycine from protein and the IgM quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). The dose of IgM used in the current study (500 ug, i.p.) is 20% less than the amount of IgM that would be predicted in 500 ul of adult human serum.[13] IgG was extracted from CRPS serum using a POROS CaptureSelect™ IgG Affinity Matrix (Thermo Fisher Scientific, Leiden, Netherlands) with the pH adjusted to 7.4 using 1M Tris pH 8.0, and then Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) were used to remove glycine from protein, and IgG quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). The dose of IgG injected in the current study (5 mg, i.p.) is consistent with-, and at least equal to the IgG dose injected with 500 ul of adult human serum,[13] thus we were confident that a negative response to 5 mg of CRPS IgG would be physiologic and not attributable to insufficient dosage.
To test the pronociceptive effects of the immunoglobulin isotypes, muMT mice underwent baseline nociceptive behavioral testing (measuring hindpaw von Frey allodynia and unweighting), and then, under isoflurane anesthesia, the right distal tibia was fractured and casted. At 3 weeks post fracture the casts were removed and the next day nociceptive behavioral testing was repeated and the mice were injected with either IgM (500 ug, i.p.) or IgG (5 mg, i.p.) from early CRPS patients (n=5). Further nociceptive behavioral testing was performed at 1, 7, 14, and 21 days after injection. Each CRPS patient’s IgM or IgG was injected into 3 different muMT fracture mice and the tests results were averaged for each subject’s immunoglobulin isotype.
2.8. CRPS IgM intrathecal and intraplantar injection experiments in fracture mice
In this experiment the pronociceptive effects of injecting early (1–12 month post injury) CRPS patient IgM into the lumbar spinal fluid or hindpaw plantar skin of fracture mice were evaluated (Fig. 2). Under isoflurane anesthesia, muMT mice underwent right distal tibia fracture and casting, and at 3 weeks post fracture the casts were removed and the next day behavioral testing (measuring hindpaw von Frey allodynia, unweighting, warmth, and edema) was performed, then the mice were injected with pooled IgM (5ug/ul intrathecal or 7.8ug/ul, intraplantar) from early CRPS patients (n=8) or normal control subjects (n=8). Further behavioral testing was performed at 0.5, 1, 3, 6, and 24 hours after injection and at 7 days post injection.
Figure 2. CRPS patient IgM had pronociceptive effects in both the spinal cord and skin of B cell deficient fracture mice.

After intrathecal injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, I.T.) into 3 weeks post fracture (FX) muMT mice, the mice rapidly developed increased von Frey allodynia (A) and unweighting (B) within 30 minutes that resolved over the ensuing 6 hours. The pronociceptive effects of the intrathecal IgM injection were restricted to the FX limb and there were no pronociceptive effects when early CRPS IgM was injected intrathecally into nonfracture wildtype mice. No pronociceptive effects were observed after intrathecal injection of control subject IgM. Likewise, after intraplantar injection of early CRPS patient IgM (7.8ug/5ul, I.PL.) into the fracture hindlimb paw of 4 weeks post FX muMT mice, the mice rapidly developed increased von Frey allodynia (C) and unweighting (D) within 1 hour that resolved over the ensuing 6 hours. The pronociceptive effects of the intraplantar IgM injection were restricted to the FX limb and there was no IgM effect on post-FX hindpaw edema or warmth (data not shown). No pronociceptive effects were observed after intraplantar injection of control subject IgM. Furthermore, there were no pronociceptive effects after intraplantar injection of early CRPS IgM in nonfracture wildtype mice. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = IgM was eluted from sera pooled from 8 early CRPS patients or 8 control subjects and injected intrathecally or intraplantarly into 7 mice per cohort. #P < 0.05, ## P < 0.01, and ### P < 0.001 for the CRPS IgM cohort vs the control IgM treatment group, *P < 0.05 and *** P < 0.001 for each post injection time point vs it’s respective baseline (e.g. 3wkFX or 4wkFX). WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, NonFX: nonfractured mice, 3wkFX: 3 weeks after fracture, 4wkFX: 4 weeks after fracture
2.9. Testing sera from control subjects, early CRPS patients, chronic CRPS patients, and limb trauma patients without CRPS for pronociceptive effects in fracture mice.
This experiment examined the pronociceptive effects (hindpaw mechanical allodynia and unweighting) of injecting sera from normal control subjects (n=20), early (1–12 months post injury) CRPS patients (n=20), chronic (> 12 months post injury) CRPS patients, and orthopedic limb trauma patients without CRPS (n=15) into 3 weeks post fracture muMT mice (Fig. 3). After clinical evaluation patient blood was collected and processed for sera. All sera were processed as previously described, except for 12 chronic CRPS patients whose sera were collected in orange top tubes containing a clotting activator and a gel-separator and were processed within 30min by centrifuging at 2000g for 10 min. Under isoflurane anesthesia, muMT mice underwent right distal tibia fracture and casting. At 3 weeks post-fracture the casts were removed and behavioral testing (hindpaw allodynia and unweighting) was performed the next day and then the mice underwent intraperitoneal injection with human sera (500 ul, i.p.). Further behavioral testing was performed at 7 days after injection (time of peak pronociceptive effects, Fig.1). Each patient’s serum was injected into 3 different muMT fracture mice and the test results were averaged for each subject. A significant (25%) reduction in hindpaw von Frey withdrawal thresholds and/or hindpaw weight bearing at day 7 post fracture was considered indicative of nociceptive sensitization.
Figure 3. All early CRPS patient sera were pronociceptive in B cell deficient fracture mice.

Serum was collected from 4 different groups of subjects normal control subjects (Controls, n=20), early (1–12 months post injury) CRPS patients (Early CRPS, n=20), chronic (> 12 months post injury) CRPS patients (Chronic CRPS, n=20), and orthopedic trauma patients without CRPS (1–12 months post injury, Trauma No CRPS, n=15). At 3 weeks after tibia fracture and casting in muMT mice, the cast was removed and the next day the mice underwent hindpaw von Frey allodynia (A) and unweighting (B) testing, then each subject’s serum was intraperitoneally injected into 3 fracture mice (0.5ml per mouse, I.P) and then the mice were retested at 1 week post-injection (time point of peak pronociceptive effect, Fig. 1). The change in von Frey threshold differences and percent weight-bearing between the 3 week post fracture testing and day 7 post injection testing were calculated for each mouse and averaged for the 3 mice injected with each subject’s serum. An increase of greater than 25% (red line) in allodynia or unweighting was considered pronociceptive. Early CRPS patient serum usually (18/20) caused increased hindpaw allodynia (A) and always increased hindlimb unweighting in fracture mice (B). Only 2 of the 20 chronic CRPS sera caused increased allodynia and none of the chronic CRPS sera changed unweighting in fracture mice. Injection of sera from control subjects or orthopedic trauma patients without CRPS had no effects on allodynia and unweighting. A one-way repeated measures analysis of variance was performed followed by a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SD. ***P<0.001 for vs Controls, ###P<0.001 vs Early CRPS
2.10. Dot blot assays for screening CRPS IgM binding reactivity to potential autoantigens
Recombinant human proteins were purchased for dot blotting to measure specific IgM binding reactivity in the sera of normal controls, early CRPS patients, chronic CRPS patients, and resolved CRPS patients. The 9 protein candidates initially screened included; 1) alpha enolase, 2) gamma actin, 3) glutamate ionotropic receptor delta type subunit 2 (GRID2), 4) eukaryotic translation elongation factor 1 alpha 1(EEF1A1), 5) beta tubulin, 6) interleukin 1 receptor associated kinase 1(IRAK1), and 7) synaptotagmin were all obtained from Abcam, Burlingame, CA, 8) keratin 16 (Novus Biologicals, Centennial, CO), and 9) histone 3.2 (New England Biolabs, Ipswich, MA). Full length proteins were utilized whenever commercially available. These potential autoantigens were selected based on results of our previous liquid chromatography-tandem mass spectroscopy studies in fracture mouse skin.[42] Each recombinant protein (2ul) was applied to a nitrocellulose membrane and incubated for 1 hour with human sera (1:1000 dilution in TPBS) followed by 1hour incubation with anti-human IgM-Dylight800 secondary antibody (1:10000 dilution, Thermo Fisher Scientific, Waltham, MA). The signals were detected using Odyssey Near-Infrared Fluorescence Imaging System and quantified using Image Studio Lite (LI-COR Biosciences, Lincoln, NE).
2.11. Statistical analysis
Statistical analysis was performed using a two-way repeated measures ANOVA (Figs. 1,2) or a one-way ANOVA (Fig. 3) with Holm-Sidak multiple comparisons test for post-hoc contrasts. Figure 4 data were not normally distributed so statistical analysis was performed using a Kruskal-Wallis one-way analysis of variance followed by Dunn’s multiple comparisons tests for post hoc contrasts. Data are presented as the mean ± standard error of the mean (Figs. 1,2) or scatter plots (Figs. 3,4), and differences are considered significant at a P value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA).
Figure 4. Early CRPS patient IgM autoantigen binding was enhanced.

Sera was collected from normal control subjects (Controls, n=20), early (1–12 months post injury) CRPS patients (Early CRPS, n=20), chronic (> 12 months post injury) CRPS patients (Chronic CRPS, n=20), and patients that initially had CRPS and pronociceptive serum effects in fracture mice, but were re-evaluated at least a year later and at that point had resolved CRPS and resolved pronociceptive serum effects (Resolved CRPS, n=15). Sera from all four groups were tested with IgM binding dot blot studies using 4 recombinant human proteins (keratin 16, histone 3.2, gamma actin, and alpha enolase). These potential autoantigens had been identified as promising candidates from our previous liquid chromatography-mass spectrometry studies in fracture mouse skin.[42] Early CRPS patient IgM binding was increased for all 4 candidate autoantigens, relative to control subject IgM immunoreativity (A-D). Chronic CRPS IgM binding was increased only for gamma actin proteins, relative to control IgM immunoreactivity (C). Interestingly, the 2 chronic CRPS patients with pronociceptive serum effects in fracture mice (Gi-1, WK-7, Table 3) had the highest IgM immunoreactivity levels to histone 3.2, gamma actin, and alpha enolase of any chronic CRPS patients tested (B-D, red symbols). Resolved CRPS patient IgM binding levels were similar to controls (A-D). A one-way repeated measures analysis of variance was performed followed by a Sidak correction test for post hoc contrasts. Data expressed as mean values ± SD. ***P<0.001, **P<0.01, and *P<0.05 vs Controls, ##P<0.01, and #P<0.05 vs Early CRPS.
3. Results
3.1. Intraperitoneal injections of CRPS serum or IgM had pronociceptive effects in fracture mice
At 3 weeks after right tibia fracture and casting muMT mice lacking B cells and IgM exhibited unilateral hindpaw von Frey allodynia and unweighting the day after cast removal (Fig. 1). Intraperitoneal injection of early (1–12 months post injury) CRPS patient serum (500ul/1ml, i.p.) into 3 weeks post fracture muMT mice caused a gradual increase in hindpaw von Frey allodynia and unweighting over the ensuing week that peaked at day 7 and, consistent with the 6 day half-life of IgM, these pronociceptive effects resolved by 2 weeks post injection. The pronociceptive serum effects were restricted to the fracture limb. Similarly, intraperitoneal injection of early CRPS patient IgM (500ug/1ml, i.p.) into muMT fracture mice caused a gradual increase in allodynia and unweighting that resolved within 2 weeks. Intraperitoneal injection of serum (500ul, i.p.) from normal control subjects and IgG (5mg//1ml, i.p.) from CRPS patients had no pronociceptive effects. Supplemental Figure 1 presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws are presented as side-to-side difference scores in Figure 1A.
The effects of injecting IgM from individual early CRPS patients (n =5) were evaluated in 3 weeks post fracture muMT mice and all 5 CRPS patient IgMs were pronociceptive (Table 1). Furthermore, the individual effects of injecting IgGs from 5 early CRPS patients (n = 5) were evaluated in muMT fracture mice and none of the IgGs were pronociceptive (Table 1). The CRPS duration ranged from 1–8 months in the IgM patient group and from 2–5 months in the IgG group, with no evidence of pronociceptive immunoglobulin class switching from IgM to IgG.
Table 1.
Early (1–12m) CRPS patient IgM/IgG pronociceptive effects in muMT fracture mice
| CRPS Patient ID | CRPS Duration (months) | Sex | Age | CRPS Cause | IgM or IgG | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (%Δ) | Unweighting (%) | (%Δ) | ||||||
| IgM | −0.8 | 13 | −1.3 | 63 | 38 | 200 | |||||
| IgM | −0.6 | 16 | −1.7 | 186 | 35 | 119 | |||||
| Igm | −0.9 | 15 | −1.5 | 55 | 32 | 119 | |||||
| IgM | −0.8 | 14 | −1.5 | 80 | 27 | 90 | |||||
| IgM | −0.8 | 16 | −1.3 | 65 | 43 | 165 | |||||
| IgG | −1.1 | 15 | −0.7 | −38 | 17 | 21 | |||||
| IgG | −1.1 | 17 | −1.2 | 15 | 18 | 7 | |||||
| IgG | −1.0 | 18 | −0.9 | 9 | 17 | −5 | |||||
| IgG | −0.8 | 15 | −0.6 | −19 | 13 | −12 | |||||
| IgG | −1.2 | 18 | −1.1 | −6 | 12 | −35 | |||||
All fracture (FX) mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. The average increase FX mouse hindpaw allodynia at 7 days (7d) after patient IgM intraperitoneal injection was 89 ± 21% and the increase in hindlimb unweighting was 139 ± 19%. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, SX: surgery, CTS: carpal tunnel syndrome, wk: week, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting after serum injection.
At 3 weeks after fracture the muMT mice did not develop warmth and edema in the fracture hindpaw and the injection of CRPS patient serum or IgM had no effect on hindpaw temperature or thickness (data not shown).
3.2. Intrathecal or intraplantar CRPS IgM injections were pronociceptive in fracture mice
Intrathecal injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, i.t.) into the 3 weeks post fracture muMT mice caused an increase in hindpaw von Frey allodynia and unweighting between 0.5 and 6 hours post injection, resolving by 24 hours (Fig. 2). Intrathecal injection of normal control subject IgM had no effect. Supplemental Figure 2 presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that are presented as side-to-side difference scores in Figure 2A. Similarly, intraplantar injection of early (1–12 months post injury) CRPS patient IgM (7.8ug/5ul, i.pl.) into the fracture limb hindpaw of 3 weeks post fracture muMT mice caused an increase in hindpaw von Frey allodynia and unweighting between 1and 6 hours post injection, resolving by 24 hours (Fig. 2). Intraplantar injection of normal control subject IgM had no effect. The pronociceptive effects of intrathecal or intraplantar CRPS IgM were restricted to the fracture limb and when nonfractured control mice were injected intrathecally or intraplantarly with CRPS IgM there were no effects (Fig.2). Supplemental Figure 3 presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that are presented as side-to-side difference scores in Figure 2C of this paper. Intraplantar injection of early CRPS patient IgM in muMT fracture mice had no effect on hindpaw temperature or thickness (data not shown).
3.3. Patient demographics, clinical presentation, and serum pronociceptive effects
Table 2 presents the demographics and clinical presentation of the normal control subjects (n=20) evaluated in this study. Half of the subjects were female and the average age was 49 ± 3 years. None of the 20 normal control subject’s sera had pronociceptive effects at 7 days after intraperitoneal injections into muMT fracture mice (Fig. 3).
Table 2.
Control serum (n=20) had no effect in muMT fracture mice
| Control Subject ID | Sex | Age | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (%Δ) | Unweighting (%) | (%Δ) | |||
| WK | M | 62 | −1.0 | 18 | −0.9 | −13 | 15 | −18 |
| DC | M | 52 | −1.2 | 16 | −0.8 | −33 | 14 | −10 |
| WL | M | 52 | −1.2 | 17 | −0.9 | −29 | 14 | −18 |
| TG | M | 65 | −1.2 | 17 | −0.9 | −17 | 14 | −15 |
| JW | F | 50 | −0.8 | 16 | −0.8 | −6 | 14 | −12 |
| AG | M | 49 | −1.1 | 18 | −0.6 | −42 | 14 | −23 |
| PO3,CO | F | 52 | −0.9 | 17 | −1.0 | −11 | 14 | −21 |
| BRH-43 | M | 49 | −0.9 | 16 | −0.6 | −30 | 17 | 0 |
| BRH-44 | M | 61 | −0.9 | 17 | −0.9 | 0 | 17 | −2 |
| BRH-45 | M | 75 | −1.0 | 16 | −0.7 | −23 | 15 | −8 |
| BRH-46 | M | 52 | −0.7 | 17 | −0.6 | −23 | 16 | −2 |
| BRH-47 | M | 36 | −0.8 | 15 | −0.7 | −14 | 13 | −13 |
| BRH-48 | F | 29 | −0.7 | 15 | −0.7 | −5 | 16 | 4 |
| BRH-49 | F | 18 | −0.9 | 16 | −0.7 | −28 | 15 | −6 |
| BRH-50 | F | 38 | −0.8 | 17 | −0.7 | −16 | 15 | −10 |
| BRH-51 | F | 56 | −0.9 | 17 | −0.9 | 7 | 16 | −2 |
| BRH-52 | F | 58 | −0.9 | 16 | −0.9 | −5 | 14 | −11 |
| BRH-53 | F | 40 | −0.9 | 16 | −0.8 | −14 | 16 | −2 |
| BRH-54 | F | 35 | −1.0 | 20 | −0.8 | −21 | 20 | 0 |
| BRH-55 | F | 46 | −0.8 | 17 | −0.8 | 0 | 16 | −3 |
Average age of the control subjects was 48.8 ± 3.0 years, and 50% were female. All FX mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. The average change in FX mouse hindpaw allodynia at 7 days (7d) after control subject serum intraperitoneal injection was −16 ± 3% and the change in hindlimb unweighting was −9 ± 2%. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting after serum injection.
Table 3 presents the demographics and clinical presentation of the early (1–12 months post injury) CRPS patients (n=20) evaluated in this study. The majority (75%) of the subjects were female, the average age was 49 ± 2 years, and the average duration of CRPS was 4.3 ± 0.6 months post injury. Allodynia was present in 50% of patients, limb edema was present in 90% of patients, and the average numerical pain score on an 11-point scale was 6.9 ± 0.3 at the time of serum collection. Distal limb fracture was the most common etiology of early CRPS, accounting for 50% of patients. When the early CRPS patient serum was intraperitoneally injected into muMT fracture mice, every patient’s serum caused a significant (25%) reduction in hindpaw von Frey withdrawal thresholds and/or hindpaw weight bearing at 7 days after fracture, indicative of nociceptive sensitization (Fig. 3).
Table 3.
Early (1–12m) CRPS patient serum (n=20) pronociceptive effects in muMT fracture mice
| CRPS Patient ID | CRPS Duration (months) | Sex | Age | CRPS Cause | Allodynia | Edema | NRS | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (%Δ) | Unweighting (%) | (%Δ) | ||||||||
| WK-2 | 1 | F | 53 | CTS SX | No | Yes | 8 | −0.7 | 17 | −1.2 | 82 | 33 | 100 |
| Gi-11 | 1 | M | 50 | Hand Injury | No | Yes | 8.5 | −1.0 | 20 | −1.6 | 59 | 33 | 64 |
| WK-3 | 2 | M | 44 | Hand FX | No | Yes | 7 | −1.2 | 16 | −1.3 | 15 | 31 | 93 |
| WK-10 | 2 | F | 41 | Hand Injury | No | Yes | 7 | −1.1 | 17 | −1.6 | 36 | 41 | 147 |
| WK-11 | 2 | F | 58 | Hand FX | No | Yes | 7 | −1.1 | 19 | −1.6 | 51 | 33 | 75 |
| Gi-13 | 2 | F | 55 | Hand FX | Yes | Yes | 7 | −0.9 | 17 | −1.4 | 53 | 30 | 76 |
| Gi-3 | 2.5 | F | 48 | CTS SX | Yes | Yes | 8 | −0.5 | 17 | −1.3 | 180 | 35 | 104 |
| Gi-9 | 3 | M | 64 | Hand SX | No | Yes | 4 | −1.0 | 20 | −1.6 | 58 | 35 | 77 |
| WK-6 | 4 | M | 30 | Ankle FX | No | Yes | 7 | −1.0 | 14 | −1.3 | 38 | 42 | 196 |
| Gi-2 | 4 | F | 49 | CTS SX | Yes | Yes | 5 | −1.1 | 16 | −1.4 | 33 | 29 | 75 |
| Gi-5 | 4 | F | 24 | Foot SX | Yes | Yes | 6 | −0.8 | 21 | −1.4 | 82 | 31 | 53 |
| Gi-12 | 4 | F | 49 | Hand Injury | Yes | No | 6 | −1.1 | 16 | −1.4 | 22 | 28 | 70 |
| NC-3104 | 4 | F | 60 | Ankle FX | Yes | Yes | 9 | −0.9 | 16 | −1.4 | 56 | 39 | 148 |
| WK-4 | 5 | F | 53 | CTS SX | No | Yes | 8 | −0.6 | 15 | −1.6 | 168 | 33 | 116 |
| NC-3073 | 5 | F | 65 | Wrist FX | Yes | Yes | 4 | −0.7 | 15 | −1.3 | 86 | 31 | 106 |
| WK-5 | 6 | M | 41 | Foot FX | Yes | Yes | 5 | −0.6 | 14 | −1.4 | 127 | 40 | 180 |
| WK-1 | 7 | F | 52 | Hand FX | Yes | Yes | 7 | −0.8 | 16 | −1.5 | 83 | 29 | 84 |
| NC-3218 | 8 | F | 56 | CTS SX | No | Yes | 6 | −0.9 | 17 | −1.5 | 74 | 40 | 134 |
| NC-3124 | 9 | F | 56 | Wrist FX | Yes | No | 9 | −0.7 | 17 | −1.4 | 92 | 39 | 123 |
| Gi-6 | 11 | F | 43 | Hand FX | No | Yes | 9 | −0.9 | 18 | −1.2 | 38 | 33 | 82 |
The mean duration of CRPS was 4.3 ± 0.6 months at the time of serum collection, the average age was 49.6 ± 2.3 years, and the majority of patients were female (75%). The average pain numerical rating score (NRS) was 6.9 ± 0.3. Allodynia was present in 50% of patients, limb edema was present in 90% of patients, and 50% had suffered a fracture. All FX mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. The average increase FX mouse hindpaw allodynia at 7 days (7d) after patient serum intraperitoneal injection was 72 ± 10% and the increase in hindlimb unweighting was 105 ± 9%. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, SX: surgery, CTS: carpal tunnel syndrome, wk: week, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting after serum injection.
Table 4 presents the demographics and clinical presentation of the chronic (>12 months post injury) CRPS patients (n=20) evaluated in this study. The majority (95%) of the subjects were female, the average age was 44 ± 2 years, and the average duration of CRPS was 58.4 ± 8.7 months post injury. Allodynia was present in 55% of patients, limb edema was present in 85% of patients, and the average numerical pain score on an 11-point scale was 6.7 ± 0.4 at the time of serum collection. Distal limb fracture was the most common etiology of chronic CRPS, accounting for 40% of patients. When the chronic CRPS sera was intraperitoneally injected into muMT fracture mice, only 2/20 patients sera (Gi-1, WK-7) caused a significant (25%) reduction in hindpaw von Frey withdrawal thresholds at 7 days after fracture, indicative of nociceptive sensitization (Fig. 3).
Table 4.
Chronic (>12m) CRPS patient serum (n=20) pronociceptive effects in muMT fracture mice
| CRPS Patient ID | CRPS Duration (months) | Sex | Age | CRPS Cause | Allodynia | Edema | NRS | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (% Δ) | Unweighting (%) | (% Δ) | ||||||||
| KN | 14 | F | 29 | Leg Injury | Yes | Yes | 9 | −0.9 | 18 | −0.8 | −19 | 15 | −14 |
| Gi-1 | 15 | F | 53 | Hand SX | Yes | Yes | 4 | −0.8 | 20 | −1.0 | 29 | 17 | −14 |
| WK-9 | 16 | M | 31 | Hand Injury | No | Yes | 6 | −1.0 | 14 | −0.6 | −33 | 13 | −7 |
| WK-8 | 22 | F | 33 | Hand Injury | No | Yes | 6 | −0.9 | 19 | −0.2 | −77 | 13 | −28 |
| WK-7 | 24 | F | 46 | Ankle Injury | Yes | Yes | 7 | −1.0 | 19 | −1.4 | 48 | 14 | −26 |
| Gi-13 | 27 | F | 57 | Wrist FX | Yes | Yes | 4 | −0.9 | 16 | −0.8 | −18 | 15 | −7 |
| WK-1 | 38 | F | 55 | Hand FX | Yes | Yes | 7 | −0.7 | 14 | −0.6 | −12 | 13 | −13 |
| Gi-6 | 41 | F | 45 | Wrist FX | No | Yes | 9 | −0.9 | 14 | −0.8 | −2 | 16 | 14 |
| JR | 41 | F | 55 | Ankle SX | Yes | No | 8 | −1.1 | 18 | −0.8 | −28 | 13 | −27 |
| PO1.SO | 47 | F | 52 | Knee SX | Yes | Yes | 8 | −1.0 | 16 | −0.9 | −12 | 14 | −13 |
| JC | 49 | F | 57 | Toe FX | No | Yes | 9 | −0.9 | 15 | −0.9 | 11 | 14 | −12 |
| SL | 67 | F | 40 | Ankle SX | No | Yes | 9 | −1.0 | 18 | −0.8 | −19 | 14 | −21 |
| NR | 72 | F | 41 | Ankle Injury | No | Yes | 6 | −0.9 | 14 | −0.9 | 9 | 10 | −29 |
| DH | 75 | F | 49 | Foot FX | Yes | Yes | 7 | −0.9 | 17 | −0.7 | −25 | 15 | −11 |
| LMC | 76 | F | 42 | Foot Injury | Yes | Yes | 7 | −1.0 | 16 | −1.2 | 18 | 16 | −3 |
| BW | 83 | F | 30 | Foot Injury | No | No | 7 | −0.9 | 16 | −0.9 | −7 | 14 | −14 |
| Gi-10 | 84 | F | 52 | Ankle FX | Yes | No | 8 | −0.9 | 17 | −0.9 | 6 | 16 | −4 |
| LJ | 89 | F | 28 | Arm Injury | No | Yes | 9 | −1.1 | 20 | −1.2 | 11 | 17 | −15 |
| VP | 125 | F | 30 | Wrist FX | Yes | Yes | 7 | −1.0 | 19 | −0.7 | −28 | 16 | −12 |
| TB | 162 | F | 52 | Ankle FX | No | Yes | 9 | −0.9 | 15 | −0.7 | −20 | 15 | 0 |
The mean duration of CRPS was 58.4 ± 8.7 months at the time of serum collection, the average age was 44.6 ± 2.3 years, and the majority of patients were female (95%). The average pain numerical rating score (NRS) was 7.2 ± 0.4. Allodynia was present in 55% of patients, limb edema was present in 85% of patients, and 40% had suffered a fracture (FX). All FX mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. The average change in FX mouse hindpaw allodynia at 7 days (7d) after patient serum intraperitoneal injection was −8 ± 6% and the change in hindlimb unweighting was −13 ± 3%. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, SX: surgery, CTS: carpal tunnel syndrome, wk: week, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting.
Table 5 presents the demographics and clinical presentation of 8 early and 2 chronic (Gi-1, WK-7) CRPS patients whose serum was initially pronociceptive in the muMT fracture mice. At between 15 and 41 months after the initial evaluation (average interval 28.8 ± 2.7 months) these CRPS patients were clinically re-evaluated and their serum collected for retesting in fracture mice. At follow-up half of the patients previously diagnosed with CRPS no longer met the diagnostic criteria for CRPS and none of those 10 patients’ sera had pronociceptive effects at 7 days after intraperitoneal injections into muMT fracture mice.
Table 5.
Repeat CRPS patient serum (n=10) testing in muMT fracture mice
| CRPS Patient ID | CRPS Persists | Interval BetweenTests (m) | Injury Duration (months) | Sex | Age | CRPS Injury | Allodynia | Edema | NRS | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (%Δ) | Unweighting (%) | (%Δ) | ||||||||||
| WK-1 | Yes | 31 | 38 | F | 55 | Hand FX | Yes | Yes | 7 | −0.7 | 14 | −0.6 | −12 | 13 | −13 |
| WK-2 | No | 32 | 33 | F | 56 | CTS SX | No | No | 0 | −0.9 | 17 | −0.9 | 7 | 14 | −18 |
| WK-7 | Yes | 29 | 53 | F | 48 | Ankle Injury | Yes | Yes | 7 | −0.8 | 15 | −0.7 | −17 | 15 | 0 |
| WK-11 | No | 27 | 29 | F | 60 | Hand FX | No | No | 5 | −0.8 | 15 | −0.7 | −14 | 17 | 12 |
| Gi-1 | Yes | 41 | 56 | F | 56 | Hand SX | Yes | Yes | 4 | −0.9 | 15 | −0.8 | −11 | 15 | 0 |
| Gi-3 | No | 33 | 35 | F | 51 | CTS SX | No | Yes | 3 | −0.9 | 14 | −0.7 | −26 | 15 | 7 |
| Gi-6 | Yes | 31 | 42 | F | 46 | Hand FX | No | Yes | 9 | −0.9 | 14 | −0.8 | −2 | 16 | 14 |
| Gi-9 | No | 18 | 21 | M | 67 | Hand SX | No | No | 0 | −0.9 | 14 | −0.9 | 0 | 15 | 10 |
| Gi-12 | No | 33 | 37 | F | 52 | Hand Injury | No | No | 6 | −0.9 | 14 | −0.6 | −28 | 13 | −7 |
| Gi-13 | Yes | 15 | 17 | F | 57 | Hand FX | Yes | Yes | 4 | −0.9 | 16 | −0.8 | −18 | 15 | −7 |
All 10 patients initially met the IASP CRPS diagnostic criteria at the time of first serum collection and all the initially collected serums were pronociceptive when injected into fracture muMT mice lacking B cells and immunglobulin. When repeat serum was collected at a later time point from these same patients (average interval between collections was 28.8 ± 2.7 months), none of the repeat serums had pronociceptive effects in the fracture mice. At the time the repeat serum was collected 50% of the patients previously diagnosed with CRPS no longer met the IASP CRPS criteria (WK-2, WK-11,Gi-3, Gi-9, Gi-13), the average time elapsed since injury was 35.9 ± 4.4 months, the average age was 54.8 ± 2.1 years, and the average pain numerical rating score (NRS) was 4.2 ± 1.0. Patients with persistent CRPS are labeled in yellow. Allodynia was present in 40% of patients, limb edema was present in 60% of patients, and 40% had suffered a fracture. All FX mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. The average change in FX mouse hindpaw allodynia at 7 days (7d) after control subject serum intraperitoneal injection was −12 ± 4% and the change in hindlimb unweighting was 1 ± 4%. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting after serum injection.
Neither the mouse fracture model nor the mechanism of injury for the majority of the CRPS patients in this study involved known nerve damage, but we should acknowledge that the patient population was indeed mixed and that 13% of the CRPS patients in this study were probably CRPS-II patients with injury to the median nerve (WK-2, WK-4, Gi-2, Gi-3, NC-3218).
Table 6 presents the demographics and clinical presentation of orthopedic fracture/and or surgery patients without CRPS (n=15) at the time of evaluation. The mean time since injury was 2.6 ± 0.5 months at the time of clinical evaluation and serum collection. The average age was 59.5 ± 2.5 years and the majority (73%) of the subjects were male. The average numerical pain score on an 11-point scale was 0.6 ± 0.2 at the time of serum collection. None of the patients exhibited allodynia and limb edema was present in 40% of patients. None of the 15 orthopedic trauma no CRPS subject’s sera had pronociceptive effects at 7 days after intraperitoneal injections into muMT fracture mice (Fig. 3).
Table 6.
Orthopedic trauma without CRPS patient serum (n=15) had no effects in muMT fracture mice
| Patient ID | Time Since Injury (months) | Sex | Age | Type of Injury | Allodynia | Edema | NRS | 3 wk post FX mice | FX mice 7d post serum injection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allodynia (Δg) | Unweighting (%) | Allodynia (Δg) | (%Δ) | Unweighting (%) | (%Δ) | ||||||||
| PD-1 | 2 | M | 57 | Foot FX/SX | No | Yes | 2 | −0.9 | 14 | −0.9 | 8 | 15 | 9 |
| PD-2 | 1.5 | F | 58 | Foot SX | No | No | 1 | −0.8 | 14 | −0.7 | −9 | 15 | 10 |
| PD-3 | 1.5 | M | 71 | Foot SX | No | No | 0 | −0.9 | 15 | −0.7 | −17 | 14 | −8 |
| PD-4 | 1 | M | 73 | Foot SX | No | No | 0 | −0.9 | 14 | −0.6 | −36 | 14 | 0 |
| PD-5 | 2 | M | 51 | Ankle FX/SX | No | No | 0 | −0.9 | 17 | −0.7 | −13 | 14 | −15 |
| PD-6 | 2 | M | 47 | Foot FX/SX | Yes | No | 1 | −0.8 | 15 | −0.8 | −1 | 14 | −6 |
| PD-7 | 9 | M | 51 | Ankle SX | Yes | Yes | 2 | −0.8 | 15 | −0.7 | −19 | 13 | −12 |
| PD-8 | 1.5 | M | 72 | Ankle SX | No | No | 0 | −0.8 | 15 | −0.6 | −21 | 15 | −1 |
| PD-9 | 2.5 | M | 75 | Leg FX/SX | No | Yes | 0 | −0.8 | 14 | −0.9 | 8 | 4 | 0 |
| PD-10 | 1.5 | M | 56 | Foot SX | Yes | No | 0 | −0.8 | 14 | −0.7 | −19 | −15 | 5 |
| PD-11 | 2.5 | F | 59 | Foot SX | Yes | No | 0 | −0.9 | 15 | −0.8 | −7 | −14 | 2 |
| PD-12 | 1.5 | M | 46 | Foot FX/SX | Yes | Yes | 2 | −0.9 | 16 | −0.7 | −23 | −14 | −9 |
| PD-13 | 1 | F | 53 | Foot FX/SX | Yes | Yes | 0 | −0.8 | 15 | −0.5 | −38 | −17 | −7 |
| PD-14 | 5 | F | 56 | Foot FX/SX | No | No | 0 | −0.8 | 16 | −0.8 | −6 | −13 | −17 |
| PD-15 | 4 | M | 67 | Ankle FX/SX | No | Yes | 1 | −0.8 | 15 | −0.4 | −54 | −13 | −13 |
The mean time since injury was 2.6 ± 0.5 months at the time of serum collection, the average age was 59.5 ± 2.5 years, and the majority of patients were male (73%). The average pain numerical rating score (NRS) was 0.6 ± 0.2. Allodynia was not present in any of the patients, limb edema was present in 40% of patients, and 53% had suffered a fracture. All FX mice had allodynia and unweighting in the injured hindlimb at 3 weeks post FX. Post injection changes in allodynia or unweighting that reached the 25% increase significance criteria are listed in Bold font. FX: fracture, SX: surgery, (Δg): difference between contralateral hindpaw threshold and fracture hindpaw threshold, thus a negative value represents increased allodynia, (% Δ): % change between 3 wk post FX value and 7d post serum injection value, thus a positive value represents an increase in allodynia or unweighting after serum injection.
3.5. Increased CRPS IgM binding to autoantigens
Preliminary sera IgM binding dot blot studies were performed using the 9 recombinant human proteins identified as the most promising autoantigen candidates from our previous liquid chromatography-mass spectrometry studies in fracture mouse skin.[42] Only 4 of the candidate autoantigens (keratin 16, histone 3.2, gamma actin, and alpha enolase) exhibited increased binding when probed with CRPS IgM. The other 5 potential autoantigens (GRID2, EEF1A1, beta tubulin, IRAK1, and synaptotagmin) with negative preliminary binding results were dropped from further analyses (data not shown). Early CRPS patient IgM immunoreactivity to keratin 16, histone 3.2, gamma actin, and alpha enolase recombinant proteins was increased relative to control subject IgM immunoreativity (Fig. 4). Chronic CRPS IgM binding was increased only for gamma actin proteins, relative to control IgM immunoreactivity (Fig. 4C). Interestingly, the 2 chronic CRPS patients with pronociceptive serum effects in fracture mice (Gi-1, WK-7, Table 3) had the highest IgM immunoreactivity levels to histone 3.2, gamma actin, and alpha enolase of any chronic CRPS patients tested (Fig 4B–C, red symbols). Patients with pronociceptive serum on initial testing in fracture mice who were re-evaluated a year or more later, after resolution of their CRPS symptoms and pronociceptive serum effects (WK-2, WK-11, Gi-3, Gi-9, Gi-12, Table 5), had IgM immunoreactivity levels to keratin 16, histone 3.2, gamma actin, and alpha enolase similar to controls, indicating normalization of antibody titres (Fig.4).
4. Discussion
Witebsky’s criteria for an autoimmune disease include; 1) clinical evidence of an autoimmune or inflammatory disorder, 2) demonstration of autoantigens, and 3) reproduction of clinical features in recipient animals by passive transfer of pathogenic antibodies.[40 ] Clinical evidence suggests that CRPS fulfills the first criteria, with early patients usually exhibiting localized pain, redness, swelling, and warmth.[45] Keratinocytes and mast cells proliferate and express inflammatory cytokines in the CRPS affected limb.[4,15,21,30] Increased numbers of central memory CD4+ and CD8+ T lymphocytes are observed in CRPS patient blood with activation of proinflammatory signaling and, similar to many autoimmune diseases, CRPS displays a female preponderance. [9]
Previously we performed mass spectroscopy on homogenized fracture mouse paw skin run on 2-D gel and probed with fracture mouse sera to identify keratin 16, histone 3.2, gamma actin, and alpha enolase as potential fracture mouse autoantigens.[42] Furthermore, both fracture mouse and CRPS patient sera exhibited enhanced binding to recombinant keratin 16.[42] Now we observe increased early CRPS patient serum binding to these autoantigens, relative to normal control sera (Fig. 4). Chronic CRPS sera also exhibited increased binding to gamma actin, but not keratin 16, histone 3.2, or alpha enolase. Resolved CRPS patient sera antigen binding was similar to controls. We did not compare the pattern of autoantibody reactivity in CRPS versus other autoimmune conditions characterized by painful symptoms. These preliminary results support development of multiplex immunoassays for CRPS autoantigens as a potential biomarker screen for the diagnosis of CRPS.
The current study describes the pronociceptive effects of CRPS serum or IgM injections in fracture mice, supporting Witebsky’s third criteria of autoimmunity that passive transfer of antibodies from patients can induce clinical features of the disease in recipient animals. Remarkably, every early CRPS patient serum (n=20) tested in the fracture mice had pronociceptive effects and none of the sera from normal control subjects (n=20) or orthopedic trauma patients without CRPS (n=15) were pronociceptive (Fig. 3, Tables 2,3,6). The CRPS immunoglobulin isotype responsible for serum pronociceptive effects in fracture mice was IgM (Fig.1), and low doses of intrathecal (5ug) or intraplantar (7.8ug) IgM had pronociceptive effects restricted to the fracture limb (Fig. 2), indicating several sites of CRPS antibody pronociceptive action. We did not assess all components of the serum, and some pronociceptive activity may be carried in non-IgM material. However, early CRPS patient IgM, at a dose equivalent to that found in the volume of serum used for all intraperitoneal injections, fully reproduced the magnitude and time course of the pronociceptive effects of early CRPS patient serum in muMT fracture mice (Fig. 1), which doesn’t support the hypothesis that other serum factors contribute to the pronociceptive effects of CRPS serum.
Only 10% of the chronic (>12 months post injury) CRPS patient sera (n=20) tested in the mouse fracture model had pronociceptive effects (Fig. 3, Table 4). Possible explanations for the lack of chronic CRPS serum pronociceptive effects in the fracture mice include; 1) autoimmunity contributes to early CRPS nociceptive sensitization, but by 12 months post injury CRPS autoantibodies resolve and central CNS reorganization or other central nociceptive signaling changes are the primary mechanisms supporting CRPS pain, 2) that fracture mice develop antigens for only some of the autoantibodies expressed in CRPS patients. At 12 months after injury the CRPS antibodies binding to fracture mouse antigens disappear and in the majority of patients symptoms improve or resolve, while a minority of CRPS patients chronically express autoantibodies for CRPS antigens that fracture mice either do not express or that are not conserved across mammalian species. This means that despite chronic CRPS patients having persistent pain their sera would not be pronociceptive in mice, only in the patients themselves, and 3) an IgM to IgG antibody switch occurs after a year in some patients with persistent CRPS and the IgG dose required to elicit nociceptive effects in muMT fracture mice may be higher than the 5 mg of IgG predicted in the 0.5ml chronic CRPS serum used in the current study.
The temporal resolution of pronociceptive autoantibody effects over time may explain the spontaneous attenuation or resolution of symptoms that occurs in most CRPS patients within a year of onset.[1,2,35,36,51] We postulate that CRPS patients usually regain immune tolerance over time and, while still making neoantigens in the injured limb, their adaptive immune system no longer identifies these antigens as “other”. An obvious concern would be that some patients fail to regain immune tolerance, resulting in chronic CRPS.
A previous CRPS antibody transfer study injected IgG collected from chronic (5.3 years average duration) CRPS patients or healthy controls into hindpaw incision mice and observed that CRPS IgG (48mg over 7 days, n=30–37 per cohort) caused increased electronic von Frey allodynia, relative to the control IgG, at day 7 post incision.[43] Each CRPS patient serum (n=6) caused a stronger threshold reduction than their corresponding control; the pooled difference was about 10% absolute threshold reduction, but 2/6 control IgG injections also caused 28–32% threshold reductions. A subsequent study demonstrated that 5/7 IgG samples collected from chronic CRPS patients were pronociceptive when injected (8 mg i.p daily over 3–13 days) in the hindpaw incision model, relative to control IgG. The pooled differences with this higher IgG dose were 15–32% reduction in nociceptive thresholds, suggesting a dose effect compared with the earlier study.[44] A crucial difference between these hindpaw incision studies and the current investigation are the intraperitoneal IgG dosages. The incision studies administered 48–104 mg of chronic CRPS IgG given over 4–13 injections over 7–13 days and our fracture model study utilized a single 5 mg injection of early CRPS IgG (equivalent to the total IgG in 0.5 ml of human serum [13]) injection of early CRPS IgG. Another major difference between these studies are the trauma models utilized. The hind paw incision model induces transient changes in hindpaw inflammation and pain lasting several weeks, while the tibia fracture/cast model induces chronic innate and adaptive immune changes in skin, nerve, and cord with pain behaviors lasting for 5 months.[5,34]
The diagnosis of CRPS is based on clinical examination and history, after ruling out other confounding diagnoses, but has only 70% specificity against other limb pain disorders.[3] Previously proposed serum biomarker assays for CRPS have not been clinically useful, with the best CRPS assay (sensitivity 75%, specificity 100%) requiring a beating cardiomyocyte preparation that has only been used experimentally.[3,23] The CRPS serum passive transfer assay in the muMT fracture mouse had 100% specificity and 100% sensitivity for early (1–12 months post injury) CRPS, compared to normal controls or orthopedic trauma patients without CRPS (Fig. 3), but unfortunately this bioassay isn’t available clinically.
The concept of autoantibodies having pronociceptive effects in chronic pain patients is not CRPS specific. Pronociceptive autoantibody effects in mice have also been observed using IgG obtained from rheumatoid arthritis patients positive for anti-citrullinated protein antibodies (ACPA+).[50] There is a frequent disconnect between pain and inflammation in rheumatoid arthritis patients, with preclinical patients developing joint pain and ACPA+ sera months to years before signs of joint inflammation and diagnosis.[37,44] Injecting rheumatoid arthritis patient ACPA+ IgG (4 mg, i.v.) into normal mice induced hindpaw von Frey allodynia that developed over several days and persisted for at least 28 days, consistent with the 21 days half-life of IgG.[50] Injecting control subject IgG or rheumatoid arthritis patient ACPA− IgG had no effect on von Frey thresholds in mice. Furthermore, depleting B cells and IgM with a single infusion of the B cell antibody rituximab (1000 mg, i.v.) significantly delayed the development of rheumatoid arthritis in ACPA+ subjects in the preclinical stage of arthritis.[11]
In summary, one of the proofs of autoimmune disease is the demonstration of pathogenic autoantibodies. The current study has shown that early CRPS patient sera were always pronociceptive in fracture mice, while sera from normal controls or orthopedic trauma patients without CRPS were never pronociceptive. The IgM immunoglobulin isotype mediated the CRPS serum pronociceptive effects in the fracture limb skin and corresponding spinal cord. Collectively, these data support the hypothesis that fracture with cast immobilization in mice can induce the regionally restricted expression of antigens in the fracture limb and corresponding spinal cord that bind with CRPS patient IgM, thus forming an antigen-IgM complex capable of activating complement pronociceptive processes in the fracture limb skin and corresponding cord.[7,14] We postulate that similar mechanisms mediate nociceptive sensitization and pain in CRPS patients.
Pursuing autoantibody mediated pain as a contributor to CRPS could identify new components of the disease process, including novel mechanisms for activation of adaptive immunity and discovery of new treatment approaches for this disabling condition. Based on the current and prior adaptive immunity studies in the fracture model and in preclinical rheumatoid arthritis patients, perhaps the most promising approach would be performing B cell and IgM depletion in early (<3 months post injury) CRPS patients using anti-CD20 antibodies (Rituximab, 1000 mg i.v. once), which completely reversed allodynia and unweighting in 12 weeks post fracture mice.[18]
Supplementary Material
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 2C of this paper. After intraplantar injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, I.T.) into 3 weeks post fracture (FX) muMT mice, the mice exhibited increased von Frey allodynia in the ipsilateral hindpaw (A), but not the contralateral hindpaw (B) that rapidly developed within 1 hour and resolved over the ensuing 3–6 hours. The pronociceptive effects of the intraplantar IgM injection were restricted to the FX limb and there were no pronociceptive effects when early CRPS IgM was injected intraplantarly into nonfracture wildtype mice. No pronociceptive effects were observed after intraplantar injection of control subject IgM. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = IgM was eluted from sera pooled from 8 early CRPS patients or 8 control subjects and injected intraplantarly into 7 mice per cohort. # P < 0.05, and ## P < 0.01 for the CRPS IgM cohort vs the control IgM treatment group, WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, NonFX: nonfractured mice, 4wkFX: 4 weeks after fracture
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 1A of this paper. At 3 weeks after tibia fracture and casting (FX) muMT mice lacking B cells and IgM exhibited ipsilateral hindpaw von Frey allodynia (A) with no effects in the contralateral hindpaw (B). After intraperitoneal injection of early (1–12 months post injury) CRPS patient serum (0.5ml, I.P) or IgM (500ug/1ml, I.P.) into 3 weeks post FX muMT mice, the mice gradually developed increased allodynia in the ipsilateral hindpaw over the ensuing week, and consistent with the 6 day half-life of IgM, these pronociceptive effects resolved by 2 weeks post-injection. The pronociceptive effects of the CRPS serum were restricted to the FX limb. No pronociceptive effects were observed after intraperitoneal injection of early CRPS patient IgG (5mg/1ml, I.P.) or after injection of control subject serum (0.5ml, I.P.) in 3 weeks post-FX mice. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 5 patients per cohort and each patients serum or immunoglobulin was injected into 3 mice for a total n of 15 mice. ## P < 0.01, and ### P < 0.001 for each injection cohort vs the control serum treatment group. MuMT: mice lacking B cells, FX: fracture, BL: baseline, 3wkFX: 3 weeks after fracture
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 2A of this paper. After intrathecal injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, I.T.) into 3 weeks post fracture (FX) muMT mice, the mice exhibited increased von Frey allodynia in the ipsilateral hindpaw (A), but not the contralateral hindpaw (B) that rapidly developed within 30 minutes and resolved over the ensuing 6 hours. The pronociceptive effects of the intrathecal IgM injection were restricted to the FX limb and there were no pronociceptive effects when early CRPS IgM was injected intrathecally into nonfracture wildtype mice. No pronociceptive effects were observed after intrathecal injection of control subject IgM. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = IgM was eluted from sera pooled from 8 early CRPS patients or 8 control subjects and injected intrathecally into 7 mice per cohort. ## P < 0.01, and ### P < 0.001 for the CRPS IgM cohort vs the control IgM treatment group, WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, NonFX: nonfractured mice, 3wkFX: 3 weeks after fracture
Acknowledgements
This study was supported by the National Institutes of Health grants NS072143 and NS094438, and the Department of Veterans Affairs, Rehabilitation Research and Development Merit grant I01RX001475. Dr. Goebel was supported by grants from the Pain Relief Foundation, Liverpool, and the serum-acquisition from chronic patients was also supported by internal funds of the Walton Centre NHS Foundation Trust, Liverpool. Drs. Birklein and Escolano were supported by the European Commission FP-7 Health-2013-Innovation, Grant no. 602133. The authors do not have financial or other relationships that might lead to conflict of interest.
Footnotes
The authors do not have financial or other relationships that might lead to conflict of interest.
Reference
- [1].Bean DJ, Johnson MH, Heiss-Dunlop W, Kydd RR. Extent of recovery in the first 12 months of complex regional pain syndrome type-1: A prospective study. European journal of pain 2016;20(6):884–94. [DOI] [PubMed] [Google Scholar]
- [2].Bickerstaff DR, Kanis JA. Algodystrophy: an under-recongnized complication of minor trauma. Br J Rheumatol 1994;33:240–48. [DOI] [PubMed] [Google Scholar]
- [3].Birklein F, Ajit SK, Goebel A, Perez R, Sommer C. Complex regional pain syndrome - phenotypic characteristics and potential biomarkers. Nature reviews Neurology 2018;14(5):272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM, Dawson LF, Clark JD, Kingery WS. Activation of cutaneous immune responses in complex regional pain syndrome. The journal of pain : official journal of the American Pain Society 2014;15(5):485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Birklein F, Ibrahim A, Schlereth T, Kingery WS. The Rodent Tibia Fracture Model: A Critical Review and Comparison With the Complex Regional Pain Syndrome Literature. The journal of pain : official journal of the American Pain Society 2018;19(10):1102 e1–02 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [7].Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology 2006;104(6):1274–82. [DOI] [PubMed] [Google Scholar]
- [8].David Clark J, Tawfik VL, Tajerian M, Kingery WS. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Molecular pain 2018;14:1744806918799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain 2007;129(1–2):12–20. [DOI] [PubMed] [Google Scholar]
- [10].Gallagher JJ, Tajerian M, Guo T, Shi X, Li W, Zheng M, Peltz G, Kingery W, Clark JD. Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord. Molecular pain 2013;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gerlag DM, Safy M, Maijer KI, Tang MW, Tas SW, Starmans-Kool MJF, van Tubergen A, Janssen M, de Hair M, Hansson M, de Vries N, Zwinderman AH, Tak PP. Effects of B-cell directed therapy on the preclinical stage of rheumatoid arthritis: the PRAIRI study. Annals of the rheumatic diseases 2019;78(2):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goebel A Autoantibody pain. Autoimmunity reviews 2016;15(6):552–7. [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clinical and experimental immunology 2008;151(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci 2007;27(32):8699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord 2006;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guo T, Wei T, Li W, Li X, Clark JD, Kingery W. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture. The journal of pain : official journal of the American Pain Society 2014;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain 2004;108(1–2):95–107. [DOI] [PubMed] [Google Scholar]
- [18].Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain 2017;158(12):2410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Molecular pain 2012;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain medicine 2007;8(4):326–31. [DOI] [PubMed] [Google Scholar]
- [21].Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators of inflammation 2002;11(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jang JH, Clark JD, Li X, Yorek MS, Usachev YM, Brennan TJ. Nociceptive sensitization by complement C5a and C3a in mouse. Pain 2010;148(2):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kohr D, Singh P, Tschernatsch M, Kaps M, Pouokam E, Diener M, Kummer W, Birklein F, Vincent A, Goebel A, Wallukat G, Blaes F. Autoimmunity against the beta2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 2011;152(12):2690–700. [DOI] [PubMed] [Google Scholar]
- [24].Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain 2013;154(8):1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain 2009;147(1–3):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li WW, Guo TZ, Liang DY, Sun Y, Kingery WS, Clark JD. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012;116(4):882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li WW, Guo TZ, Shi X, Czirr E, Stan T, Sahbaie P, Wyss-Coray T, Kingery WS, Clark JD. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain 2014;155(11):2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li WW, Guo TZ, Shi X, Sun Y, Wei T, Clark DJ, Kingery WS. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience 2015;310:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain 2009;144(3):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators of inflammation 2005;2005(6):366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesthesia and analgesia 1998;87:941–48. [DOI] [PubMed] [Google Scholar]
- [32].Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain 2008;137(3):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain 2008;138(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain 2009;145(3):341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103(1–2):199–207. [DOI] [PubMed] [Google Scholar]
- [36].Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br 1993;75(3):450–2. [DOI] [PubMed] [Google Scholar]
- [37].Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Toes RE, Trouw LA, van Schaardenburg D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013;65(4):911–5. [DOI] [PubMed] [Google Scholar]
- [38].Shi X, Guo TZ, Wei T, Li WW, Clark DJ, Kingery WS. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain 2015;156(10):1852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept 2013;186:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sommer C Anti-autonomic nervous system antibodies in CRPS. Pain 2011;152(12):2675–6. [DOI] [PubMed] [Google Scholar]
- [41].Subarrao J, Stillwell GK. Reflex sympathetic dystrophy syndrome of the upper extremity: analysis of total outcome of management of 125 cases. Arch Phys Med Rehab 1981;62:549–54. [PubMed] [Google Scholar]
- [42].Tajerian M, Hung V, Khan H, Lahey LJ, Sun Y, Birklein F, Kramer HH, Robinson WH, Kingery WS, Clark JD. Identification of KRT16 as a target of an autoantibody response in complex regional pain syndrome. Experimental neurology 2017;287(Pt 1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tekus V, Hajna Z, Borbely E, Markovics A, Bagoly T, Szolcsanyi J, Thompson V, Kemeny A, Helyes Z, Goebel A. A CRPS-IgG-transfer-trauma model reproducing inflammatory and positive sensory signs associated with complex regional pain syndrome. Pain 2014;155(2):299–308. [DOI] [PubMed] [Google Scholar]
- [44].van Steenbergen HW, van Nies JA, Huizinga TW, Bloem JL, Reijnierse M, van der Helm-van Mil AH. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Annals of the rheumatic diseases 2015;74(6):1225–32. [DOI] [PubMed] [Google Scholar]
- [45].Veldman PHJM Reynen HM, Arntz IE Goris RJA. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 1993;342:1012–16. [DOI] [PubMed] [Google Scholar]
- [46].Wei T, Guo TZ, Li WW, Hou S, Kingery W, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. Journal of neuroinflammation 2012;9(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. Journal of neuroinflammation 2016;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain 2009;144(3):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain 2009;13(3):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wigerblad G, Bas DB, Fernades-Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, Kato J, Sandor K, Su J, Jimenez-Andrade JM, Finn A, Bersellini Farinotti A, Amara K, Lundberg K, Holmdahl R, Jakobsson PJ, Malmstrom V, Catrina AI, Klareskog L, Svensson CI. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Annals of the rheumatic diseases 2016;75(4):730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zyluk A The natural history of post-traumatic reflex sympathetic dystrophy. Journal of hand surgery 1998;23(1):20–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 2C of this paper. After intraplantar injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, I.T.) into 3 weeks post fracture (FX) muMT mice, the mice exhibited increased von Frey allodynia in the ipsilateral hindpaw (A), but not the contralateral hindpaw (B) that rapidly developed within 1 hour and resolved over the ensuing 3–6 hours. The pronociceptive effects of the intraplantar IgM injection were restricted to the FX limb and there were no pronociceptive effects when early CRPS IgM was injected intraplantarly into nonfracture wildtype mice. No pronociceptive effects were observed after intraplantar injection of control subject IgM. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = IgM was eluted from sera pooled from 8 early CRPS patients or 8 control subjects and injected intraplantarly into 7 mice per cohort. # P < 0.05, and ## P < 0.01 for the CRPS IgM cohort vs the control IgM treatment group, WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, NonFX: nonfractured mice, 4wkFX: 4 weeks after fracture
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 1A of this paper. At 3 weeks after tibia fracture and casting (FX) muMT mice lacking B cells and IgM exhibited ipsilateral hindpaw von Frey allodynia (A) with no effects in the contralateral hindpaw (B). After intraperitoneal injection of early (1–12 months post injury) CRPS patient serum (0.5ml, I.P) or IgM (500ug/1ml, I.P.) into 3 weeks post FX muMT mice, the mice gradually developed increased allodynia in the ipsilateral hindpaw over the ensuing week, and consistent with the 6 day half-life of IgM, these pronociceptive effects resolved by 2 weeks post-injection. The pronociceptive effects of the CRPS serum were restricted to the FX limb. No pronociceptive effects were observed after intraperitoneal injection of early CRPS patient IgG (5mg/1ml, I.P.) or after injection of control subject serum (0.5ml, I.P.) in 3 weeks post-FX mice. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 5 patients per cohort and each patients serum or immunoglobulin was injected into 3 mice for a total n of 15 mice. ## P < 0.01, and ### P < 0.001 for each injection cohort vs the control serum treatment group. MuMT: mice lacking B cells, FX: fracture, BL: baseline, 3wkFX: 3 weeks after fracture
This figure presents the time course of the raw von Frey fiber withdrawal threshold data in the ipsilateral and contralateral hindpaws that were presented as side-to-side difference scores in Figure 2A of this paper. After intrathecal injection of early (1–12 months post injury) CRPS patient IgM (5ug/5ul, I.T.) into 3 weeks post fracture (FX) muMT mice, the mice exhibited increased von Frey allodynia in the ipsilateral hindpaw (A), but not the contralateral hindpaw (B) that rapidly developed within 30 minutes and resolved over the ensuing 6 hours. The pronociceptive effects of the intrathecal IgM injection were restricted to the FX limb and there were no pronociceptive effects when early CRPS IgM was injected intrathecally into nonfracture wildtype mice. No pronociceptive effects were observed after intrathecal injection of control subject IgM. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = IgM was eluted from sera pooled from 8 early CRPS patients or 8 control subjects and injected intrathecally into 7 mice per cohort. ## P < 0.01, and ### P < 0.001 for the CRPS IgM cohort vs the control IgM treatment group, WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, NonFX: nonfractured mice, 3wkFX: 3 weeks after fracture


