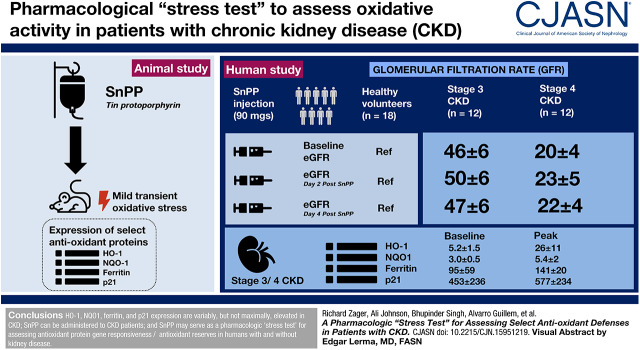
Visual Abstract
Keywords: mice, creatinine, CST3 protein, human, cystatin C, albuminuria, antioxidants, lipocalin-2, tin, ferritins, tin protoporphyrin IX, protoporphyrins, hexosaminidases, healthy volunteers, exercise test, blood urea nitrogen, HAVCR1 protein, human
Abstract
Background and objectives
Oxidative stress is a hallmark and mediator of CKD. Diminished antioxidant defenses are thought to be partly responsible. However, there is currently no way to prospectively assess antioxidant defenses in humans. Tin protoporphyrin (SnPP) induces mild, transient oxidant stress in mice, triggering increased expression of select antioxidant proteins (e.g., heme oxygenase 1 [HO-1], NAD[P]H dehydrogenase [quinone] 1 [NQO1], ferritin, p21). Hence, we tested the hypothesis that SnPP can also variably increase these proteins in humans and can thus serve as a pharmacologic “stress test” for gauging gene responsiveness and antioxidant reserves.
Design, setting, participants, & measurements
A total of 18 healthy volunteers and 24 participants with stage 3 CKD (n=12; eGFR 30–59 ml/min per 1.73 m2) or stage 4 CKD (n=12; eGFR 15–29 ml/min per 1.73 m2) were injected once with SnPP (9, 27, or 90 mg). Plasma and/or urinary antioxidant proteins were measured at baseline and for up to 4 days post-SnPP dosing. Kidney safety was gauged by serial measurements of BUN, creatinine, eGFR, albuminuria, and four urinary AKI biomarkers (kidney injury molecule 1, neutrophil gelatinase-associated lipocalin, cystatin C, and N-acetyl glucosaminidase).
Results
Plasma HO-1, ferritin, p21, and NQO1 were all elevated at baseline in CKD participants. Plasma HO-1 and urine NQO1 levels each inversely correlated with eGFR (r=−0.85 to −0.95). All four proteins manifested statistically significant dose- and time-dependent elevations after SnPP injection. However, marked intersubject differences were observed. p21 responses to high-dose SnPP and HO-1 responses to low-dose SnPP were significantly suppressed in participants with CKD versus healthy volunteers. SnPP was well tolerated by all participants, and no evidence of nephrotoxicity was observed.
Conclusions
SnPP can be safely administered and, after its injection, the resulting changes in plasma HO-1, NQO1, ferritin, and p21 concentrations can provide information as to antioxidant gene responsiveness/reserves in subjects with and without kidney disease.
Clinical Trial registry name and registration number
A Study with RBT-1, in Healthy Volunteers and Subjects with Stage 3–4 Chronic Kidney Disease, NCT0363002 and NCT03893799
Introduction
It is well recognized that CKD is a pro-oxidant state (1–10). This has been documented by elevated clinical markers of oxidative stress (e.g., increases in plasma malondialdehyde, protein carbonyl, F2-isoprostane) and by evidence of oxidative kidney damage in a variety of experimental nephropathies (2,10–13). Both increased reactive oxygen species production and reductions in antioxidant defenses, e.g., most notably within the Nrf2 pathway, are thought to be responsible (2–6).
Our laboratory recently demonstrated that tin protoporphyrin (SnPP), a reversible heme oxygenase 1 (HO-1) inhibitor, induces transient oxidant stress in mice and activates Nrf2-inducible antioxidant genes (14,15). This conclusion was based on a number of experimental observations: (1) SnPP injection into mice transiently increased oxidative stress markers (e.g., malondialdehyde, protein carbonyl); (2) Nrf2 nuclear translocation resulted; (3) Nrf2-driven gene transcription was induced (e.g., increases in HO-1, NAD[P]H dehydrogenase [quinone] 1 [NQO1], SRXN1, GCLC mRNAs); and (4) these SnPP responses were not observed in Nrf2-deficient (−/−) mice (15), indicating Nrf2-dependent mechanisms were involved.
We have previously reported that elevations of plasma HO-1, an Nrf2 activation marker, can serve as a semiquantitative index of oxidative stress in both experimental animals and humans (16). Given that SnPP has been safely administered to children to prevent kernicterus (e.g., 17,18), we queried whether SnPP can be used to activate antioxidant (e.g., Nrf2) responsive genes. If so, then degrees of SnPP-induced plasma and/or urinary antioxidant protein increases (e.g., of HO-1, NQO1, ferritin, p21) might serve as semiquantitative guides for assessing antioxidant reserves in clinical settings, for example, CKD.
Given these considerations, this study addressed the following questions and hypotheses. First, are the above antioxidant proteins elevated or suppressed in CKD, providing possible insights into degrees of Nrf2 activity? Second, can SnPP administration be used as a “stress test” to measure antioxidant gene reserves? In other words, are these genes already maximally expressed in CKD or can further increases be achieved? Third, are specific antioxidant proteins/reserves suppressed in CKD compared with those that exist in healthy volunteers, suggesting new therapies? This study was undertaken to gain insights into these matters.
Materials and Methods
Subject Recruitment
Both healthy volunteers (n=18) and participants with either stage 3 CKD (CKD3; n=12; or stage CKD 4 (CKD4; n=12) were recruited for this investigation. eGFRs were calculated using the CKD Epidemiology Collaboration formula. All participants resided in Central Florida. The study received institutional review board approval from Advarra (Columbia, MD) and informed consent was obtained from each participant. Institutional review board approval was waived by the Fred Hutchinson Cancer Research Center because only laboratory analyses of deidentified plasma and urine samples were conducted at this site. The study was conducted in adherence with the Declaration of Helsinki. Healthy volunteer inclusion criteria included male and female participants age 18–80 years, a body weight <125 kg, and the absence of any acute or chronic disease or chronic drug administration. Female participants must have had a negative pregnancy test or be postmenopausal, post-tubal ligation, or regularly use effective contraception. CKD inclusion criteria included participants aged 18–80 and body weight <125 kg. All participants must have been able to comply with all study procedures. Study exclusion criteria included pregnancy, any significant illness other than CKD or diabetes, or parenteral iron or any experimental drug administered within the prior 30 days. Specific demographic and baseline clinical/laboratory data are presented in Table 1. This study was undertaken as part of a series of three sequential studies that were enrolled in Clinicaltrials.gov before subject recruitment (NCT0363002; NCT03893799). The parts included the following: (1) pharmacokinetics and responses to a novel iron sucrose formulation; (2) evaluation of the independent effect of SnPP (this study); and (3) a future study to evaluate the combined effects of SnPP with iron sucrose on antioxidant gene expression, with the ultimate goal of using these agents to prevent cardiovascular surgery associated AKI. This report is based solely on the results of SnPP administration. There is no subject crossover between these three arms.
Table 1.
Clinical characteristics of participants in three clinical trial arms evaluating intravenous tin protoporphyrin as a stress test of antioxidant capacity
| Criterion | Healthy Volunteers (N=18) | CKD3 (30–59 ml/min per 1.73 m2) (N=12) | CKD4 (15–29 ml/min per 1.73 m2) (N=12) |
|---|---|---|---|
| Age (yr) | 43 (12) | 71 (8) | 71 (5) |
| Sex (M/F) | 72/28% | 34/66% | 67/33% |
| White race (%) | 50% | 75% | 75% |
| Weight (kg) | 85 (15) | 85 (17) | 94 (20) |
| Diabetes (%) | 0% | 42% | 66% |
| BP systolic/diastolic | 124/78 (18/9) | 139/76 (17/11) | 139/72 (24/9) |
| eGFR (ml/min per 1.73 m2) | 87 (4) | 42 (5) | 21 (4) |
| BUN (mg/dl) | 13 (3) | 30 (10) | 52 (20) |
| Creatinine (mg/dl) | 1.0 (0.2) | 1.5 (0.4) | 2.8 (0.5) |
| U albumin/creatinine (mg/g) | <10 | 223 (480) | 519 (640) |
| Medications (%)a | |||
| Statins | 0% | 50% | 67% |
| Antihypertensives | 0% | 67% | 83% |
| Diuretics | 0% | 35% | 8% |
Demographic and baseline clinical data for the three study cohorts. Mean values and SDs (numbers in parentheses) are presented. CKD3, CKD stage 3 (eGFR of 15–29 ml/min per 1.73 m2); CKD4, CKD stage 4 (eGFR of 30–59 ml/min per 1.73 m2); M, male; F, female; U albumin/creatinine, urinary albumin-creatinine ratio.
For the three classes of medications, the percentages of participants within each group that were taking them are presented.
Clinical Protocol
The 18 healthy volunteers were divided into three treatment groups to receive either 9, 27, or 90 mg SnPP (Cascade Custom Chemistry, Portland, OR). The drug was administered intravenously over 1 hour with 100 ml saline. Participants with CKD received either a 27-mg or 90-mg SnPP injection. Timed plasma and urine samples were collected at baseline (0 hours) and at 4, 12, 24, 48, and 96 hours postinfusion. Participants remained overnight at the study site (Riverside Clinical Research, Edgewater, FL) to screen for potential adverse events. In addition, potential adverse events were recorded throughout the study and for up to 28 days thereafter which included monitoring routine hematology, liver and cardiac enzymes, and electrocardiograms. A four-member data safety monitoring board confirmed no adverse changes in these parameters. BUN, creatinine, urine albumin-creatinine ratios, and plasma ferritin levels were determined by Halifax Laboratory Services (Daytona Beach, FL), which also undertook the above safety studies. Urine AKI biomarkers (neutrophil gelatinase-associated lipocalin [NGAL], kidney injury molecule 1 [KIM1], cystatin C, N-acetyl glucosaminidase) were measured by Nexelis Laboratories (Seattle, WA). Baseline and selected post-SnPP infusion plasma or urine samples were assayed for the following antioxidant proteins using ELISAs: HO-1 (Enzo Life Sciences, Farmingdale, NY), NQO1 (ADI-960-800; G-Biosciences, St. Louis, MO), and p21 levels (ab212063; Abcam, Cambridge, MA). Plasma values are reported as nanograms per milliliter. Urine values were factored by urine creatinine. Based on initial screening of samples, different time-course measurements were selected for analyses of individual proteins (see Results).
Study Outcomes
The primary outcomes were antioxidant protein levels (HO-1, NQO1, ferritin, and p21) at baseline and post-SnPP administration. Secondary outcomes were kidney safety parameters, including eGFR, serum creatinine, BUN, and urinary AKI biomarkers (KIM-1, NGAL, cystatin C, N-acetylglucosaminidase).
Statistical Analyses
All values in the text are given as arithmetic means±1 SD. Urine values were expressed both before and after log10 conversion. No missing data points existed. Baseline values for healthy volunteers versus participants with CKD were compared by unpaired t test. For more than two sets of data, ANOVA with Tukey honest significant difference post hoc test was used. Assessments of changes over time were analyzed by ANOVA for repeated measures. Variance is depicted in figures as 95% confidence intervals. For some comparisons, the participants with CKD3 and CKD4 were combined and treated as a single CKD group to increase sample size for statistical power. Statistical significance was judged by a P value of <0.05.
Results
Baseline Demographics/Clinical Assessments
The CKD populations were significantly older than the healthy volunteers (mean of 71 versus 43 years). As expected, with progressive kidney failure (as gauged by eGFRs), progressive increases in BUN, plasma creatinine, and urine albumin-creatinine values were observed among the groups (Table 1). There was a 54% prevalence of type 2 diabetes in the CKD population. Systolic, but not diastolic, BPs were modestly higher in the CKD versus the healthy volunteer group. All participants completed the SnPP infusion which was well tolerated. The only adverse effect was a mild erythematous rash in sun-exposed areas in 15% of participants, particularly in those receiving the 90-mg SnPP dosage. This sun sensitivity has been previously described by other investigators (17,18).
Baseline eGFRs and Plasma HO-1 Concentrations
Mean baseline eGFRs for the healthy volunteers, the participants with CKD3, and those with CKD4 were 87±16, 42±5, and 21±4 ml/min per 1.73 m2, respectively (Table 2). Baseline plasma HO-1 concentrations were modestly elevated in the participants with CKD, stepwise increases were observed with increasing degrees of kidney failure (healthy volunteers, 3.43±1.4 ng/ml; CKD3, 4.7±2.1 ng/ml; CKD4, 5.65±2.8 ng/ml; P<0.03 for healthy volunteers versus CKD3+4). A strong inverse correlation between mean eGFRs and mean baseline plasma HO-1 concentrations was observed (r=−0.95).
Table 2.
eGFR after administration of 90 mg tin protoporphyrin
| Group | eGFR (ml/min per 1.73 m2) | ||
|---|---|---|---|
| Baseline | Day 2 Post-SnPP | Day 4 Post-SnPP | |
| Healthy volunteers | 84±6 | 87±5 | 88±4 |
| CKD3 | 46±6 | 50±6 | 47±6 |
| CKD4 | 20±4 | 23±5 | 22±4 |
The values are given are means±1 SD. There were no significant eGFR changes from baseline in response to the highest test dose of SnPP (90 mg). CKD3, stage 4 CKD (eGFR of 30–59 ml/min per 1.73 m2); CKD 4, stage 4 CKD (eGFR of 5–29 ml/min per 1.73 m2); SnPP, tin protoporphyrin.
Plasma HO-1 Responses to SnPP Injection
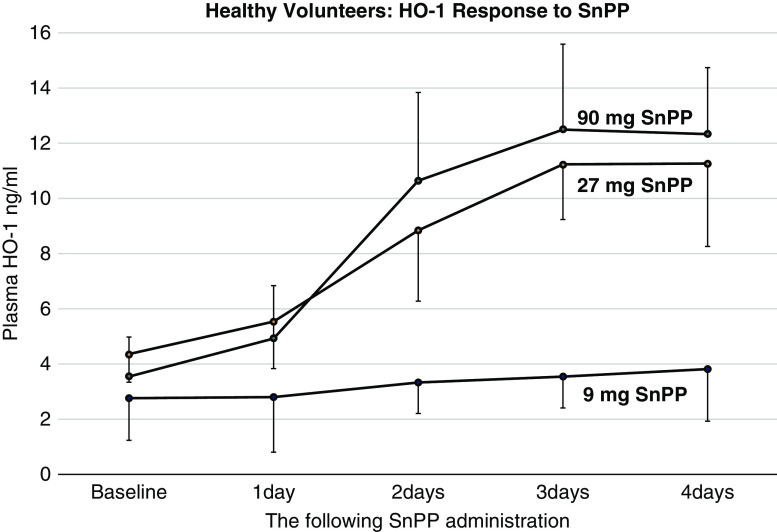
All healthy volunteers demonstrated dramatic, dose-dependent increases in plasma HO-1 levels in response to SnPP injection, rising progressively over a 4-day period of observation (Figure 1). At the highest test dose (90 mg), an approximate 250% HO-1 increase over baseline values was observed by the end of the 4-day observation period.
Figure 1.
Plasma heme oxygenase 1 levels rise in a dose-dependent fashion after SnPP injection in healthy volunteers. Plasma heme oxygenase 1 (HO-1) levels were measured at baseline and from 1 to 4 days after the 9-, 27-, or 90-mg tin protoporphyrin (SnPP) injection. Time- and dose-dependent increases were observed (mean values/95% confidence intervals depicted). Significant increases of HO-1 values over time were observed in each subject group. All P values were <0.001 as determined by ANOVA/repeated measures; within-group analyses.
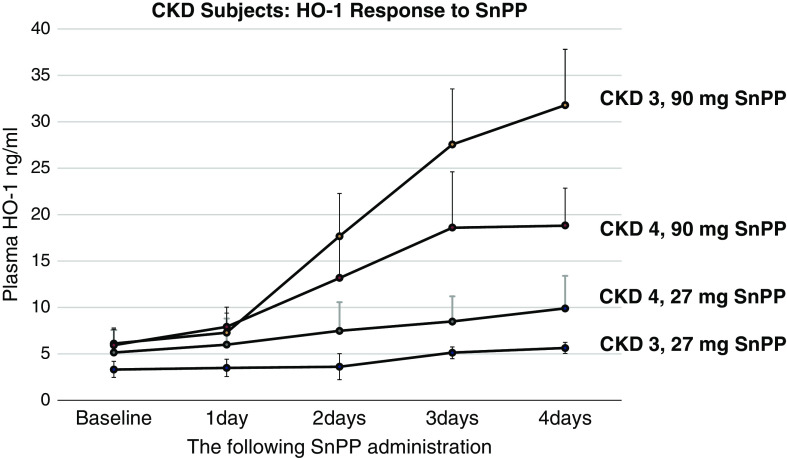
Progressive, dose-dependent plasma HO-1 increases were also observed in participants with CKD3 and CKD4 after SnPP injection (Figure 2). With the 27-mg SnPP dose, the participants with CKD manifested a smaller HO-1 increase versus the healthy volunteers: whereas the healthy volunteers had a 2.7±1.1-fold increase over baseline values at day 4, the combined CKD group (stages 3+4) had only a 1.8±0.4-fold increase (P<0.04). However, with the 90-mg SnPP dose, comparable HO-1 elevations were observed in the healthy volunteer and participants with CKD. Thus, it appears that participants with CKD have a diminished HO-1 response to low-dose SnPP (27 mg), although this blunted response can be completely overcome with higher SnPP dose administration.
Figure 2.
Plasma HO-1 levels rise after SnPP injection in participants with stage 3 CKD (15–29 ml/min per 1.73 m2) and stage 4 CKD (30–59 ml/min per 1.73 m2). Plasma HO-1 levels were measured at baseline and from 1 to 4 days after 27- or 90-mg SnPP injection. Time- and dose-dependent HO-1 increases were noted in both CKD stage 3 (CKD3) and CKD stage 4 (CKD4) groups. P<0.001, all in-group comparisons; ANOVA, repeated measures. The mean values/95% confidence intervals are depicted.
Plasma Ferritin Responsiveness to SnPP
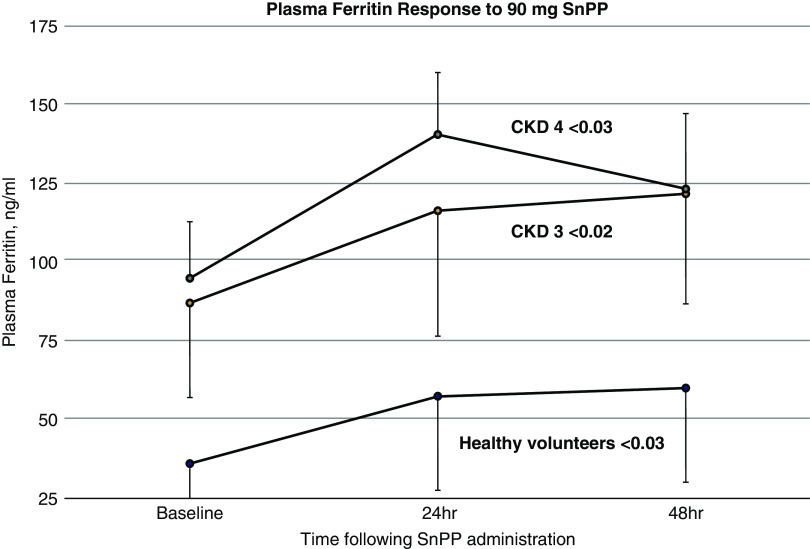
Baseline plasma ferritin levels were approximately three times higher in the participants with CKD (95±59 ng/ml for CKD stages 3+4 combined) versus the healthy volunteers (36±18 ng/ml; P<0.01). In response to the low-dose (27-mg) SnPP injection, only small and inconsistent increases in plasma ferritin levels were observed (data not shown). In contrast, the healthy volunteers and the two CKD groups manifested significant plasma ferritin increases after the 90-mg SnPP injection (Figure 3). The absolute ferritin increases were 33±25 ng/ml for the combined CKD groups, compared with 24±15 ng/ml for healthy volunteers (P=0.22). However, when these increases were expressed as fold increases over baseline values, comparable increases were observed (1.6-fold versus 1.5-fold for healthy volunteers versus the combined CKD groups, respectively).
Figure 3.
Plasma ferritin levels increase in response to SnPP injection. Plasma ferritin levels were measured at baseline and 24 and 48 hours after 90-mg SnPP injection in healthy volunteers and in participants with CKD3/CKD4. The participants with CKD had significantly higher baseline ferritin levels versus the healthy volunteers (P<0.005 between-group comparisons). All three groups manifested significant plasma ferritin increases after SnPP injection. P values by ANOVA, repeated measures; within-group analyses. The increases over baseline values were comparable for the CKD and the healthy volunteer groups. Means/95% confidence intervals are depicted.
NQO1 Assessments
Baseline.
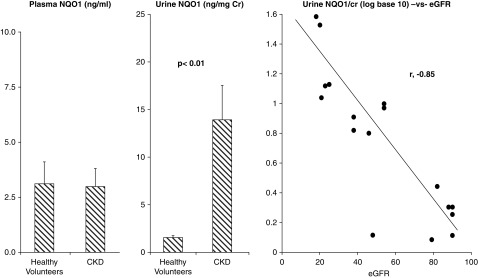
As shown in Figure 4 (left panel) baseline plasma NQO1 values were nearly identical in the healthy volunteers and CKD groups. Despite the comparable plasma values, the participants with CKD manifested approximately ninefold higher baseline urinary NQO1/creatinine levels (Figure 4, middle panel). After log transformation, the urinary NQO1/creatinine ratios and eGFRs showed a strong inverse relationship (r=−0.85).
Figure 4.
Urine NqO1 levels are increased in CKD patients despite comparable plasma concentrations to healthy volunteers. The combined CKD cohorts and the healthy volunteers had virtually the same plasma NAD(P)H dehydrogenase (quinone) 1 (NQO1) concentrations. However, participants with CKD had markedly elevated urinary NQO1/creatinine levels (P<0.01; unpaired t test). When the urine NQO1/creatinine values were converted to log10, the individual values showed a strong inverse correlation with the corresponding baseline eGFRs for each subject (r=−0.85).
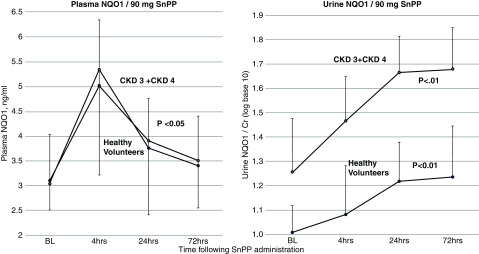
Post-SnPP.
Both the healthy volunteers and the participants with CKD (combined CKD3+4) had an almost identical (approximately 60%) increase in plasma NQO1 within 4 hours of SnPP injection, with values slowly declining thereafter (Figure 5, left panel). Urinary NQO1 levels also rose post-SnPP injection and throughout the period of observation (Figure 5, right; log10 transformation). The absolute urinary increases from baseline to 3 days of observation were 1.85±0.57 to 3.23±1.87 ng/mg for the healthy volunteers, and from 13.9±11.6 to 21.4±14.1 ng/mg for the combined CKD group. The slopes of the log-transformed urinary increases were highly comparable for the CKD and healthy volunteer groups. The SnPP-induced urinary NQO1 increases could not be ascribed to a nonspecific increase in urinary protein excretion given that urine albumin-creatinine ratios tended to slightly fall, rather than rise, over the 3-day observation period (baseline, 391±651 mg/g; 24 hours, 233±244 mg/g; 72 hours, 372±569 mg/g) for participants with CKD.
Figure 5.
Plasma and urinary NQO1 increases in response to 90-mg SnPP infusion. The healthy volunteers and participants with CKD showed acute, near-identical increases in plasma NQO1 after 90-mg SnPP injection. Furthermore, the healthy volunteers and the participants with CKD (combined CKD3+4) demonstrated significant and progressive increases in urinary NQO1/creatinine levels over the 72-hour observation period. Although the healthy volunteers had lower baseline (BL) urinary NQO1 levels than the participants with CKD, the slopes of the SnPP-induced urinary NQO1 increases over time were comparable for the healthy volunteer and CKD groups. Values are means/95% confidence intervals; urine values are after log10 conversion; statistics by ANOVA repeated measures, in-group comparisons.
p21 Assessments
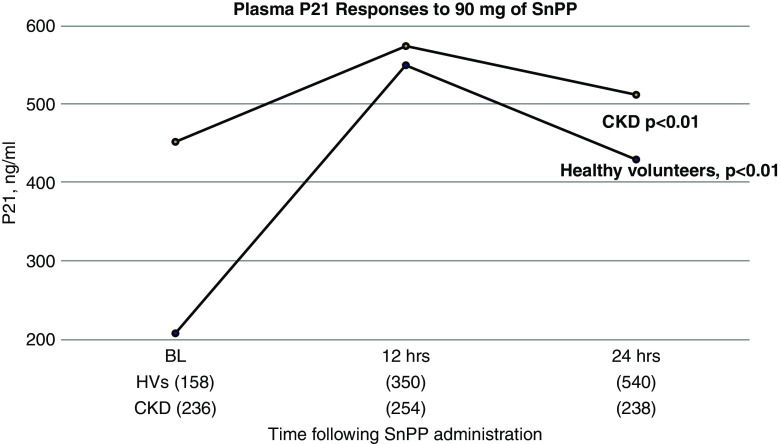
There were wide variations in baseline plasma p21 levels in both the healthy volunteer and CKD cohorts (see Figure 6), with the mean values being approximately twice as high in the combined CKD versus the healthy volunteer group (453±236 ng/ml versus 230±158 ng/ml, respectively; P=0.06). Both groups manifested significant p21 increases in response to 90-mg SnPP dosing (see Figure 6). However, the degree of increase was markedly blunted in the CKD versus the healthy volunteer group (fold increases over baseline: healthy volunteers 2.65±1.1; CKD, 0.51±1.0; P<0.005). With the 27-mg SnPP dose, only minimal p21 changes were observed in either group (data not shown).
Figure 6.
Plasma p21 concentrations increase from baseline after SnPP injection. There were marked variations in p21 values between participants, as reflected by large SDs (SDs shown as numbers in parentheses at the bottom of the graph). However, despite this variation, the healthy volunteer (HV) and participants with CKD demonstrated plasma p21 increases at 12 and 24 hours post-SnPP injection (CKD participants combined, P<0.01; healthy volunteers, P<0.01; ANOVA, repeated measures, in-group comparisons). The fold increase over baseline values was significantly higher in the healthy volunteer versus the CKD group (means of 2.65 versus 0.51, respectively; P<0.001; see text).
Kidney Safety Markers
SnPP infusion, when given at the highest test dose (90 mg), did not significantly alter eGFR in any of the test groups (see Table 2). BUN and creatinine values also did not significantly change post-SnPP injection (data not shown). SnPP safety at the highest (90 mg) test dose was also evidenced by the urinary biomarker assessments (Table 3). Although occasional participants manifested a transient single biomarker elevation at a particular time point post-SnPP infusion, participants did not demonstrate either consistent changes over time, or matching directional changes among the different biomarkers tested (i.e., no combined increases in NGAL, N-acetyl glucosaminidase, cystatin C, and KIM-1 at a single time point).
Table 3.
Urine biomarkers of kidney injury after administration of 90 mg tin protoporphyrin
| Analyte per Participant Subgroup | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | P Value |
|---|---|---|---|---|---|---|
| NAG (mU/mg Cr) | 2.0±1.5 | 2.83±1.167.5 | 3.16±1.16 | 2.51±0.64 | 2.66±0.8 | 0.35 |
| HVs | ||||||
| CKD3 | 4.4±3.52 | 5.66±3.44 | 8.2±3.44 | 6.5±1.7 | 5.9±2.1 | 0.11 |
| CKD4 | 9.0±4.7 | 12.5±11.34 | 10.6±7.5 | 10.5±5.6 | 6.2±3.4 | 0.21 |
| NGAL (ng/mg Cr) | ||||||
| HVs | 14.3±18.2 | 21.1±28.7 | 14.8±13.1 | 14.0±8.7 | 10.2±5.6 | 0.9 |
| CKD3 | 71.2±64.1 | 46.5±35.3 | 35.8±23.2 | 49.8±39.7 | 42.6±51.7 | 0.41 |
| CKD4 | 46.5±58.7 | 58.0±79.1 | 64.8±91.0 | 47.3±60.2 | 48.0±49.5 | 0.49 |
| Cystatin C (ng/mg Cr) | ||||||
| HVs | 30.0±19.5 | 32.1±15.1 | 34.8±7.2 | 39.6±9.4 | 40.4±9.3 | 0.49 |
| CKD3 | 31.0±7.7 | 26.8±12.9 | 32.2±9.8 | 34.0±9.4 | 33.2±33.2 | 0.35 |
| CKD4 | 298±438 | 332±519 | 638±965 | 186±213 | 207±238 | 0.47 |
| KIM-1 (ng/mg Cr) | ||||||
| HVs | 0.97±0.5 | 0.7±0.5 | 0.8±0.7 | 0.7±0.4 | 0.6±0.4 | 0.5 |
| CKD3 | 0.6±0.2 | 0.8±0.2 | 1.5±0.6 | 1.4±0.6 | 0.9±0.2 | 0.08 |
| CKD4 | 0.8±0.5 | 0.8±1.0 | 1.2±1.0 | 1.2±1.0 | 1.0±0.8 | 0.11 |
The urinary biomarker values, as factored by urine creatinine. The eGFRs were assessed at baseline and days 1–4 post–tin protoporphyrin injection. No significant changes over time were observed for any of the analytes (see P values). All values=means±1 SD. The P values are given for changes over time. NAG, N-acetyl glucosaminidase; Cr, creatinine; HVs, healthy volunteers; CKD3, CKD stage 3 (eGFR of 30–59 ml/min per 1.73 m2); CKD4, CKD stage 4 (eGFR of 15–29 ml/min per 1.73 m2); NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1.
Discussion
Given that CKD is associated with both systemic and kidney parenchymal oxidative stress, investigators have sought to quantify this process by measuring oxidant-induced biomarkers (e.g., plasma malondialdehyde, F2 isoprostanes, oxidized glutathione, protein carbonyl levels). However, these tests provide a static assessment of oxidative damage. Furthermore, they provide no information about the current status of antioxidant defenses which are thought to be depleted in participants with CKD (e.g., 2,3,10,13). Hence, the purpose of this study was to test a new hypothesis: the administration of SnPP can serve as a dynamic test of antioxidant defenses or reserves. The underlying physiologic concept is as follows (14,15): (1) SnPP triggers transient oxidative stress; (2) antioxidant pathways, most notably Nrf2, are activated; (3) increased plasma levels of antioxidant proteins (e.g., HO-1, NQO1, ferritin, p21) result; and (4) the degree of their increases may provide dynamic insights into the ability of the body to respond to oxidative stress and the state of antioxidant reserves. If true, such information could conceivably help guide therapy. For example, were a patient with CKD to demonstrate severely decreased antioxidant reserves, administration of potent antioxidants, e.g., tetrahydrocurcumin (19,20), or Nrf2 activators, e.g., bardoxolone methyl (8,21), might be initiated.
A prerequisite for using SnPP as a pharmacologic stress test is safety of administration in patients with CKD. It is noteworthy that HO-1 is widely considered to be a potent antioxidant enzyme. Hence, SnPP-induced HO-1 inhibition could conceivably trigger adverse kidney events (22,23). However, the available data argue strongly against this possibility, given that the highest test SnPP dose (90 mg) had no adverse kidney effects, as assessed by eGFR, albuminuria, and urinary AKI biomarker excretion. As previously noted (14,15), SnPP upregulates the antioxidant Nrf2 cytoprotective pathway, causing marked increases in selected antioxidant proteins (e.g., HO-1, NQO1, SRXN1, GCLC). Thus, these responses would be expected to counter any potential adverse HO-1 inhibitory effects. Furthermore, there is substantial information suggesting that HO-1’s protective actions may be expressed independently of its enzymatic activity. For example, multiple studies have found that SnPP administration can induce a “preconditioning” response (analogous to “ischemic preconditioning”) and thereby confer broad-based tissue protection, rather than an injury-provoking state (24–30). In light of these considerations, it would appear that single-dose SnPP administration can be safely used as a probe of antioxidant reserves. As previously noted, no extrarenal toxicities were observed (as per the data safety monitoring board).
Given the tight mechanistic link between SnPP, Nrf2 activation, and HO-1, we first assessed baseline and plasma HO-1 responses to SnPP injection. The first notable result was that baseline HO-1 levels were elevated in participants with CKD (versus healthy volunteers), with values inversely correlating with eGFRs. These HO-1 increases imply a compensatory response to the CKD pro-oxidant state. To our knowledge, this is the first demonstration of CKD-associated plasma HO-1 increases. Regarding SnPP–HO-1 responsiveness, the healthy volunteers manifested marked and progressive plasma HO-1 increments. The fact that this response was highly SnPP dose dependent (increases of 38%, 157%, and 246% with 9, 27, and 90 mg SnPP, respectively) underscores that the SnPP–HO-1 response can provide quantitative, and not just qualitative, information. A priori, it was predicted that the participants with CKD would have a diminished HO-1 response to SnPP injection compared with healthy volunteers, given the assumption that participants with CKD have reduced antioxidant reserves. Consistent with this hypothesis, at the 27-mg SnPP dose, the participants with CKD manifested only a minimal HO-1 elevation from baseline values, compared with three- to fivefold HO-1 elevations from baseline for the participants with CKD. However, at the 90-mg SnPP dose, comparable CKD and healthy volunteer responses were observed. Thus, diminished SnPP–HO-1 reactivity in CKD is not an absolute phenomenon, given that it can clearly be overcome with an increased SnPP dosage.
A second well described, Nrf2-responsive antioxidant protein is NQO1 (31,32). We therefore measured it in the healthy volunteer and CKD cohorts. Unlike HO-1, baseline plasma NQO1 levels were not elevated in participants with CKD. Hence, we sought urinary elevations as a potential marker of kidney NQO1 expression. Marked (approximately ninefold), but variable, baseline urinary NQO1 elevations were observed in subjects with CKD versus healthy volunteers, with values showing a strong inverse correlation with eGFRs (r=−0.85). This suggests that urinary NQO1 could potentially serve as a marker of kidney dysfunction and associated oxidative stress. In response to the 90-mg SnPP injection, significant urinary and plasma NQO1 increases were observed. However, these SnPP-NQO1 responses were different from those seen with HO-1 in at least two respects: first, compared with HO-1, the plasma NQO1 responses were relatively modest and short lived; and second, unlike the HO-1 results, no discernible differences in SnPP-NQO1 responses were observed between the healthy volunteer and CKD groups. Thus, it would appear that HO-1 is a far more sensitive, and therefore more promising, molecule for SnPP testing.
Ferritin is a third critical antioxidant protein that exerts potent protective effects via a number of mechanisms, such as catalytic iron binding and ferroxidase activities (33,34). Notably, the CKD cohorts had approximately threefold higher baseline ferritin levels than the healthy volunteers, presumably reflecting that CKD is a pro-oxidant and proinflammatory (35) state. Unlike NQO1 and HO-1, ferritin levels are more typically regulated by translation, rather than transcription; hence, Nrf2 would be expected to play a relatively minor role in ferritin expression (34). Nevertheless, SnPP evoked significant, and comparable, plasma ferritin increases in the healthy volunteer and CKD cohorts. This suggests that SnPP may upregulate antioxidant defenses by mechanisms that extend beyond its Nrf2 effects.
It is well recognized that, in response to oxidant injury, tissue levels of the cyclin kinase inhibitor p21 rise, and that p21 can exert potent cytoprotective effects (36–39). Although p21-mediated protection has classically been ascribed to the benefits of transient cell cycle inhibition (36–38), it has recently been demonstrated that p21 can also act as a potent antioxidant (39,40). The latter action is thought to arise, at least in part, via p21-mediated Nrf2 activation due to p21 displacement of the Nrf2 inhibitor Keap1 from the Nrf2 molecule (40). Hence, p21 can be viewed as another antioxidant defense. We previously demonstrated that plasma p21 and kidney cortical p21 levels closely correlate in mouse models of aging and AKI (41). This led us to question whether clinical CKD is associated with plasma p21 elevations and, if so, can this pathway be further stimulated by SnPP? The answer to both questions appears to be yes. As shown in Figure 6, mean baseline plasma p21 values were approximately twice as high in the subjects with CKD versus the healthy volunteers, and both groups manifested p21 increases in response to SnPP injection. However, the degree of p21 responsiveness was markedly reduced in the CKD cohorts. These findings raise a number of interesting questions. First, given this marked differential p21 response, might p21 serve as a useful dynamic marker of oxidant stress or antioxidant reserves in patients with CKD? Second, the antitumor suppressor p53, and not Nrf2, is the prime transcription factor that controls p21 expression (42,43). Because p53 is activated by oxidant stress (42–44), might SnPP activate p53 and thereby increase p21, and ultimately Nrf2 activity? If so, then SnPP might activate Nrf2 by at least two mechanisms: first, direct SnPP-induced oxidant stress effects on Nrf2; and second, indirect Nrf2 activation via an increase in the p53/p21 axis.
In conclusion, this study provides additional evidence that CKD is a pro-oxidant state, as evidenced by increased baseline plasma or urinary levels of HO-1, NQO1, ferritin, and p21. However, none of these antioxidant proteins is maximally expressed in CKD, as evidenced by the fact that SnPP injection abruptly increases the plasma concentrations of each, as indicated by peak values shown in Table 4. However, degrees of SnPP responsiveness vary substantially among test participants. This indicates that substantial differences in susceptibility to oxidant stress exist among individuals which can be probed with SnPP. It is also notable that differences in SnPP responses appear to exist between healthy volunteers and participants with CKD. This is supported by findings of relative suppression of HO-1 and p21 responsiveness to low-dose and high-dose SnPP administration, respectively.
Table 4.
Baseline and peak plasma values of tested proteins demonstrating degrees of responsiveness to the highest tested dose of tin protoporphyrin (90 mg)
| Group | HO-1 | Ferritin | NQO1 | p21 | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Peak | Baseline | Peak | Baseline | Peak | Baseline | Peak | |
| Healthy volunteers | 3.4±1.4 | 11.2±3.8 | 36±18 | 60±25 | 3.1±0.6 | 5.0±1.8 | 208±158 | 551±350 |
| CKD (eGFR 15–59 ml/min per 1.73 m2) | 5.2±1.5 | 26±11 | 95±59 | 141±20 | 3.0±0.5 | 5.4±2.0 | 453±236 | 577±254 |
Baseline and maximal (peak) plasma antioxidant protein concentrations (ng/ml), as generated with the highest test dose of tin protoporphyrin (90 mg). The data indicate the ability of tin protoporphyrin to upregulate HO-1, ferritin, NQO1, and p21 gene/protein expression. The values presented are means and 95% confidence intervals. Please see the figures for change of values over time and statistics by ANOVA for repeated measures. All peak values are significantly higher than baseline values, although the CKD group had a significantly blunted p21 response compared with the healthy volunteer group (see text). The timing of the peak values were 4 d, 12 h, 12 h, and 4 h for HO-1, ferritin, p21, and NQO1, respectively. HO-1, heme oxygenase 1; NQO1, NAD(P)H dehydrogenase (quinone) 1; CKD, CKD stage 3 and CKD stage 4 groups combined.
Clearly, this study has a number of important limitations. First, it was exploratory in nature and relatively small numbers of participants per group were tested. Second, although plasma or urinary test protein elevations were observed at baseline and post-SnPP injection, their tissue origins remain unknown. Although kidney and liver are the primary sites of tissue SnPP uptake (45), direct evidence to support their dominance in mediating SnPP responses are lacking at this time. Third, we only tested participants with a single SnPP dose; therefore, it is unknown whether consistent “within-subject” responses would be expressed over time.
It remains to be determined whether measurements of these four antioxidant proteins, and their responses to SnPP, will ultimately have clinical value. However, the following questions in this regard seem relevant. First, might low baseline levels of these four antioxidant proteins suggest potentially faster CKD progression versus subjects with high levels? Second, does a diminished response to SnPP also have prognostic value given its ability to test untapped antioxidant reserves? Third, might low ferritin, NQO1, HO-1, and p21 levels, either at baseline or post-SnPP, imply the need for aggressive antioxidant treatment (e.g., with Nrf2 activators such as tetrahydrocurcumin or bardoxolone)? Fourth, might measurement of the Nrf2-activated proteins, HO-1 and NQO1, serve as a biomarker for Nrf2-activator drug efficacy, and possibly help guide therapy? Each of these questions seem worthy of further investigation.
Disclosures
Dr. Guillem is the chief executive officer of Renibus Therapeutics. Dr. Keyser is the president and chief operating officer of Renibus Therapeutics. Dr. Guillem, Dr. Keyser and Dr. Singh are Renibus board members and stockholders. Dr. Zager is a paid consultant to Renibus Therapeutics. Dr. Johnson has nothing to disclose.
Funding
This work was supported by a sponsored research agreement between the Fred Hutchinson Cancer Research Center and Renibus Therapeutics, Southlake, Texas.
Data Sharing Statement
Individual deidentified participant data (including data dictionaries) will be shared. Data that form the basis of this study will be shared. Related documents and data will be posted on clinicaltrials.gov. Data will be available after article publication. The access criteria include scientists and clinicians involved in the study of oxidant stress and kidney disease and can be provided by email, fax, or access at clinicaltrials.gov.
Acknowledgments
The authors thank Renibus Therapeutics for providing clinical information, clinical samples, and financial support for the completion of this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chen DQ, Cao G, Chen H, Liu D, Su W, Yu XY, Vaziri ND, Liu XH, Bai X, Zhang L, Zhao YY: Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/β-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol 12: 505–521, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND: Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant 28: 2038–2045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau WL, Liu SM, Pahlevan S, Yuan J, Khazaeli M, Ni Z, Chan JY, Vaziri ND: Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig Dis Sci 60: 1215–1222, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino D, La Russa D, Marrone A: Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized Patients. Antioxidants (Basel) 8: E594, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo G, Reggiani F, Astori E, Altomare A, Finazzi S, Garavaglia ML, Angelini C, Milzani A, Badalamenti S, Dalle-Donne I: Advanced oxidation protein products in nondiabetic end stage renal disease patients on maintenance haemodialysis. Free Radic Res 53: 1114–1124, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Ito M, Tanaka T, Nangaku M: Nuclear factor erythroid 2-related factor 2 as a treatment target of kidney diseases. Curr Opin Nephrol Hypertens 29: 128–135, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Vaziri ND: Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 13: 93–99, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Vaziri ND: Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol 24: 469–473, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz S, Pergola PE, Zager RA, Vaziri ND: Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS: Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25: 119–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sureshbabu A, Ryter SW, Choi ME: Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol 4: 208–214, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zager RA, Johnson ACM, Becker K: Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and “end-stage” kidney disease. Am J Physiol Renal Physiol 301: F1334–F1345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson ACM, Delrow JJ, Zager RA: Tin protoporphyrin activates the oxidant-dependent NRF2-cytoprotective pathway and mitigates acute kidney injury. Transl Res 186: 1–18, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Johnson ACM, Zager RA: Mechanisms and consequences of oxidant-induced renal preconditioning: An Nrf2-dependent, P21-independent, anti-senescence pathway. Nephrol Dial Transplant 33: 1927–1941, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Zager RA, Johnson AC, Becker K: Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubaltelli FF, Griffith PF: Management of neonatal hyperbilirubinaemia and prevention of kernicterus. Drugs 43: 864–872, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Cooke RW: New approach to prevention of kernicterus. Lancet 353: 1814–1815, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lau WL, Khazaeli M, Savoj J, Manekia K, Bangash M, Thakurta RG, Dang A, Vaziri ND, Singh B: Dietary tetrahydrocurcumin reduces renal fibrosis and cardiac hypertrophy in 5/6 nephrectomized rats. Pharmacol Res Perspect 6: e00385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song KI, Park JY, Lee S, Lee D, Jang HJ, Kim SN, Ko H, Kim HY, Lee JW, Hwang GS, Kang KS, Yamabe N: Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: In vitro and in vivo studies. Planta Med 81: 286–291, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG; BEAM Study Investigators: Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME: Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt BA, Shanley TP, Croatt A, Alam J, Johnson KJ, Nath KA: Glomerular inflammation induces resistance to tubular injury in the rat. A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest 98: 2139–2145, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atef Y, El-Fayoumi HM, Abdel-Mottaleb Y, Mahmoud MF: Quercetin and tin protoporphyrin attenuate hepatic ischemia reperfusion injury: Role of HO-1. Naunyn Schmiedebergs Arch Pharmacol 390: 871–881, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Sutherland BA, Shaw OM, Clarkson AN, Winburn IC, Errington AC, Dixon CL, Lees G, Sammut IA, Appleton I: Tin protoporphyrin provides protection following cerebral hypoxia-ischemia: Involvement of alternative pathways. J Neurosci Res 89: 1284–1294, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Peng PH, Chao HM, Juan SH, Chen CF, Liu JH, Ko ML: Pharmacological preconditioning by low dose cobalt protoporphyrin induces heme oxygenase-1 overexpression and alleviates retinal ischemia-reperfusion injury in rats. Curr Eye Res 36: 238–246, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA: Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol 169: 21–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A: Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int 63: 1393–1403, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Zager RA, Johnson AC, Frostad KB: Combined iron sucrose and protoporphyrin treatment protects against ischemic and toxin-mediated acute renal failure. Kidney Int 90: 67–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zager RA: Marked protection against acute renal and hepatic injury after nitrited myoglobin + tin protoporphyrin administration. Transl Res 166: 485–501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinkova-Kostova AT, Talalay P: NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venugopal R, Jaiswal AK: Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93: 14960–14965, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A: Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson AC, Gooley T, Guillem A, Keyser J, Rasmussen H, Singh B, Zager RA: Parenterial iron sucrose-induced renal preconditioning: Differential ferritin heavy and light chain expression in plasma, urine, and internal organs. Am J Physiol Renal Physiol 317: F1563–F1571, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Kell DB, Pretorius E: Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 6: 748–773, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Megyesi J, Andrade L, Vieira JM Jr, Safirstein RL, Price PM: Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Renal Physiol 283: F810–F816, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Price PM, Safirstein RL, Megyesi J: The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu F, Megyesi J, Safirstein RL, Price PM: Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol 2005: F514–F520, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Vitiello PF, Wu YC, Staversky RJ, O’Reilly MA: p21(Cip1) protects against oxidative stress by suppressing ER-dependent activation of mitochondrial death pathways. Free Radic Biol Med 46: 33–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villeneuve NF, Sun Z, Chen W, Zhang DD: Nrf2 and p21 regulate the fine balance between life and death by controlling ROS levels. Cell Cycle 8: 3255–3266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson AC, Zager RA: Plasma and urinary p21: Potential biomarkers of AKI and renal aging. Am J Physiol Renal Physiol 315: F1329–F1335, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brate A, Giannakakou P: The importance of p53 location: Nuclear or cytoplasmic zip code? Drug Resist Updat 6: 313–322, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, St Clair DK: p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res 65: 3745–3750, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Xu Y: p53, oxidative stress, and aging. Antioxid Redox Signal 15: 1669–1678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson KE, Simionatto CS, Drummond GS, Kappas A: Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther 228: 327–333, 1984 [PubMed] [Google Scholar]