Abstract
Metabolic disorders are highly prevalent in kidney transplant candidates and recipients and can adversely affect post-transplant graft outcomes. Management of diabetes, hyperparathyroidism, and obesity presents distinct opportunities to optimize patients both before and after transplant as well as the ability to track objective data over time to assess a patient’s ability to partner effectively with the health care team and adhere to complex treatment regimens. Optimization of these particular disorders can most dramatically decrease the risk of surgical and cardiovascular complications post-transplant. Approximately 60% of nondiabetic patients experience hyperglycemia in the immediate post-transplant phase. Multiple risk factors have been identified related to development of new onset diabetes after transplant, and it is estimated that upward of 7%–30% of patients will develop new onset diabetes within the first year post-transplant. There are a number of medications studied in the kidney transplant population for diabetes management, and recent data and the risks and benefits of each regimen should be optimized. Secondary hyperparathyroidism occurs in most patients with CKD and can persist after kidney transplant in up to 66% of patients, despite an initial decrease in parathyroid hormone levels. Parathyroidectomy and medical management are the options for treatment of secondary hyperparathyroidism, but there is no randomized, controlled trial providing clear recommendations for optimal management, and patient-specific factors should be considered. Obesity is the most common metabolic disorder affecting the transplant population in both the pre- and post-transplant phases of care. Not only does obesity have associations and interactions with comorbid illnesses, such as diabetes, dyslipidemia, and cardiovascular disease, all of which increase morbidity and mortality post-transplant, but it also is intimately inter-related with access to transplantation for patients with kidney failure. We review these metabolic disorders and their management, including data in patients with kidney transplants.
Keywords: chronic kidney failure, diabetes, diabetic nephropathy, end stage kidney disease, hyperparathyroidism, kidney transplantation, metabolism, organ transplant, transplantation, cadaver organ transplantation, obesity, pediatric nephrology, phosphate binders, Parathyroidectomy, Cardiovascular Diseases, diabetes mellitus, Hyperglycemia, Renal Insufficiency, Chronic, Dyslipidemias, Hyperparathyroidism, Secondary, parathyroid hormone, Risk Assessment
Introduction
Metabolic disorders are highly prevalent in kidney transplant candidates and recipients and can adversely affect post-transplant graft outcomes. Thoughtful management of diabetes, hyperparathyroidism, and obesity is critical to optimize the waitlisted population and the patient after transplant. Furthermore, the ability of a patient to comply with complex treatment regimens for aggressive management of their underlying metabolic disorders is an important consideration in assessing the future adherence with immunosuppressive (1,2).
Knowledge of the current literature and tools available to assist in the optimization of these complex patients is needed for success during all phases of transplant care. We provide an overview of the contemporary management of the most complicated metabolic disorders pre-, peri-, and post-transplant with an emphasis on the most pertinent and recent data.
Pretransplant Management of Metabolic Disorders
One of the challenges in managing patients prior to transplant is the degree of cardiovascular disease already present. This is compounded by metabolic disorders that may develop during this time. Data demonstrate that existing cardiovascular disease leads to increased rates of cardiovascular events post-transplant (3–6). In addition, diabetes and obesity both have significant overlap in their presentation and risk for poor outcomes post-transplantation.
Data from the US Renal Data System demonstrate that patients with elevated hemoglobin A1c prior to transplant have an increased risk of post-transplant diabetes mellitus, which in turn, is associated with increased rates of cardiovascular events and decreased patient and graft survival (7,8). Hemoglobin A1c is poorly correlated with fasting blood glucose in patients with kidney failure and those with severe anemia, likely underestimating patients at risk of post-transplant hyperglycemia (9).
The risk of post-transplant diabetes mellitus should be considered when evaluating patients for transplant candidacy, and patients should be counseled on expectations regarding hyperglycemia management post-transplant. Preexisting diabetes itself is a risk factor for increased rejection rates post-transplant (10). Chakkera et al. (11) found seven factors associated with increased risk of new onset diabetes after transplant in their patient population, including age, planned corticosteroid therapy post-transplant, prescription for gout medicine, body mass index (BMI), fasting glucose and triglycerides, and family history of type 2 diabetes, all of which are readily available when listing patients for transplant. Other factors that have been associated with new onset diabetes after transplant include race, hepatitis C, and use of tacrolimus over cyclosporin (12). At our center, a risk assessment has been built into our electronic medical record, and it is used by the pharmacist at the time of assessment for transplant so that risk of developing new onset diabetes after transplant is available to the team at the time of transplant.
Along with uncontrolled diabetes, elevated parathyroid hormone (PTH) may also be a marker of pretransplant nonadherence and could help indicate patients in whom targeted interventions pretransplant might improve post-transplant outcomes (13). There is an opportunity to ensure optimization of patients with elevated PTH prior to transplant because one of the most important risk factors for postkidney transplant hyperparathyroidism is elevated pretransplant PTH levels along with calcium, phosphate, dialysis vintage, and graft function post-transplant (14).
Data suggest that parathyroidectomy prior to transplant increases bone mineral density more effectively than parathyroidectomy after transplant (15). In a retrospective analysis at our center, patients who were transplanted with PTH≥6× normal experienced an increased risk of graft failure, but those who underwent pretransplant parathyroidectomy had a much lower risk of graft failure (odds ratio, 0.547; 95% confidence interval, 0.327 to 0.913) (16). This further adds to the data suggesting that patients who are poorly managed pretransplant have worse outcomes, and it suggests that parathyroidectomy pretransplant can improve post-transplant outcomes.
Obesity is a metabolic disorder that is intimately inter-related with access to transplantation for patients with kidney failure (6). Most transplant centers require that BMI criteria be met prior to listing; this is supported by a survey by the American Society of Transplant surgeons in which 66 of 67 centers used BMI of 35–45 kg/m2 as the upper limit allowable to initiate an evaluation (17). Studies using United Network for Organ Sharing (UNOS) data have demonstrated decreased likelihood of receiving a transplant with increasing BMI >25 kg/m2 as well as increased likelihood of being bypassed when an organ became available with a BMI>25 kg/m2 (18). These UNOS data also demonstrated that 20% of all transplant centers have not listed a single morbidly obese patient with BMI>40 kg/m2 and that only 15% of centers list severely obese patients with BMI>35 kg/m2. This risk-averse listing criteria are driven by not only inferior outcomes demonstrated in the morbidly obese population but also, the increased technical challenges and costs associated with higher levels of care. This barrier to transplant access is compounded by the reluctance of bariatric surgeons to offer surgical weight loss strategies to patients with kidney failure to support them in the effort to meet the BMI goals (19).
Studies confirm that patients listed inactive because of obesity achieved their weight loss goals and were activated only half of the time. This was attributed to a variety of barriers to effective medically supervised weight loss, including comorbidities preventing activity, dietary restrictions associated with kidney disease management, fluid balance, and psychosocial challenges (20,21). New pharmacologic agents support weight loss through thermogenesis, supporting decreased caloric intake or directly interrupting the absorption of calories taken in (22). However, there are limited data on their safety in kidney disease. One promising study reporting 2-year follow-up of a medical weight loss program with orlistat (alli), nutrition education, diet, and exercise for kidney transplant candidates with BMI>28 kg/m2 demonstrated a 5- to 6-kg weight loss benefit observed within the first 6 months that persisted (23). This study was nonrandomized and limited by potential bias, but it does support safety of this weight loss strategy in the kidney transplant candidate population. Bariatric surgery is an alternative option for durable weight loss in this population (19,21,24). The specific experience of bariatric surgery as a bridge to kidney transplantation has been explored with encouraging results, both with sleeve gastrectomy and with Roux-en-Y gastric bypass as surgical options (25–27). However, all weight loss programs are resource intensive, and broad feasibility remains limited for patients with activity restrictions, disability, and psychosocial barriers.
When considering a patient’s transplant candidacy, transplant centers should consider not only the burden of preexisting comorbid disease and the effect on graft survival but also, a patient’s ability to understand and comply with complex treatment regimens. The track record of their successful management of diabetes or hyperparathyroidism as well as demonstrating medically supervised weight loss can all be considered as surrogate markers for health care literacy and adherence.
Peritransplant Management of Metabolic Disorders
Data clearly demonstrate the advantages of transplantation over dialysis in patients with diabetes with decreased mortality and cardiovascular events, and this benefit is noted to strengthen over time (28). Management of metabolic disorders immediately post-transplant is critical to the long-term cardiovascular and graft outcomes of kidney transplantation as well as immediate surgical complications. Observational studies have demonstrated that perioperative hyperglycemia increases the risk for infection, adverse events, reoperative interventions, and mortality even in nonpatients with diabetes (29,30). Obesity also heavily influences surgical approach and expected complications at the time of transplantation, including post-transplant hyperglycemia.
It is estimated that upward of 7%–30% of patients will develop post-transplant diabetes mellitus within the first year post-transplant and will need to start blood glucose management in addition to a new transplant regimen (31). Additionally, hyperglycemia early post-transplant has been shown to increase the risk of rejection and infection post-transplant (32). Along with this, infections, such as hepatitis C and cytomegalovirus, also increase the risk for hyperglycemia post-transplant along with transplant from a deceased donor due to higher levels of proinflammatory markers (33). With all of these risk factors, continuous glucose monitoring (CGM) provides a much clearer picture as to a patient’s true glycemic control post-transplant and can be used in both patients with and without diabetes. In a small population of nondiabetic patients with CGM monitoring for 4 days post-transplant, patients who developed post-transplant diabetes mellitus 3 months after transplant tended to have higher fasting blood glucose on day 1 (34). In pediatric patients with kidney transplants, CGM helped to identify patients with impaired glucose tolerance, which also correlated with hypoglycemic events (35). Kidney transplant recipients have an ideal indication for CGM and perhaps broader availability, and data can aid in the diagnosis of post-transplant diabetes mellitus and long-term management.
Insulin should be used early after transplant to help protect the β-cells and prevent further damage; data suggest that insulin is the best medication for preventing new onset diabetes after transplant (36). Basal-bolus insulin regimens using a combination of short- and long-acting insulins have been validated in postsurgical patients as well as those in a critical care setting (37,38). The traditional oral antihyperglycemic agents metformin and sulfonylureas have limited utility in the early post-transplant population due to adverse effect profiles, but they may be appropriate in select patients. Lactic acidosis can occur in patients treated with metformin experiencing AKI, which is not predictable post-transplant, and it can lead to serious complications. Sulfonylureas are limited by challenges with hypoglycemia, drug interactions, and dosing.
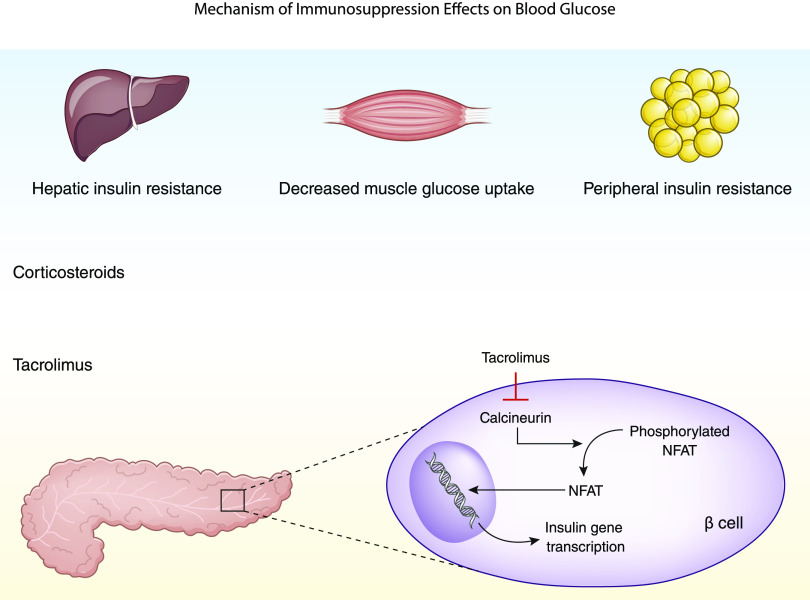
Corticosteroids have also been well documented as a risk factor for developing post-transplant diabetes mellitus. An attempt at modulating this risk by comparing early corticosteroid withdrawal with chronic low-dose corticosteroid administration has failed to demonstrate significant differences in the incidence of post-transplant diabetes mellitus (39). Other induction agents, such as basiliximab and antithymocyte globulin, have not been found to be associated with post-transplant hyperglycemia. Corticosteroids are also used for the treatment of rejection; thus, monitoring after rejection treatment is crucial. It is also important to consider the mechanism of action of corticosteroids in inducing post-transplant dysglycemia with the mechanisms of other immunosuppressive drugs, like tacrolimus, which may raise concern for additive risks of post-transplant diabetes with coadministration of these drugs (Figure 1).
Figure 1.
Mechanism of immunosuppression effects on blood glucose. NFAT, nuclear factor of activated T-cells, a family of transcription factors shown to be integral to immune response.
There are many factors that affect a patient’s blood glucose control in the immediate post-transplant phase, including steroid dose, kidney function, and baseline glycemic control. Blood glucose levels can change rapidly on the basis of these different factors, and close follow-up is needed because patients with kidney transplants are discharged within days after transplant. The transplant pharmacist is uniquely positioned to help care for patients who require intense monitoring with frequent dose adjustments of their antihyperglycemic regimen. Studies show that pharmacists’ effect on diabetes management in kidney transplant results in significant reductions in hemoglobin A1c in patients diagnosed with post-transplant diabetes mellitus (40,41). At our center, we have adopted a model optimizing the use of the transplant pharmacist as a transition to the transplant endocrinologist when the patient is stable, about 3 months post-transplant. This has led to a reduction in readmissions and emergency department visits for hyperglycemia management within 90 days post-transplant without increased adverse effects or hypoglycemia. Management of post-transplant hyperglycemia is crucial to the short- and long-term outcomes of both diabetic and nondiabetic patients with transplants.
A recent systematic review and meta-analysis of the literature demonstrated a higher risk of acute rejection, patient death, graft loss, and delayed graft function in patients classified as obese with a BMI>30 kg/m2 (42). Obesity and rapid weight gain post-transplant can also increase the risk for hyperglycemia. A subgroup analysis of children 6–12 years old in the North American Pediatric Renal Transplant Cooperative Study demonstrated an association between obesity and increased risk of death (43). The most definitive risk factor associated with a poor outcome in the obese kidney transplant population is the occurrence of wound infection, which is noted in up to 40% of patients (44). A modified surgical approach has been proposed in select morbidly obese patients to avoid these wound complications by using a robotic approach (45–47). Corticosteroid use has not been associated with weight gain post-transplant (48). Regardless of potentially inferior outcomes compared with nonobese patients undergoing transplantation, the evidence is clear that obese patients have far better outcomes with transplantation compared with dialysis (49).
Management of metabolic disorders during the pretransplant period is critical to the outcome of the transplant and post-transplant management.
Post-Transplant Management of Metabolic Disorders
Diabetes, hyperparathyroidism, and obesity are not metabolic disorders in silos unto themselves. It is important to highlight their long-term risks associated hypertension, proteinuria, and ultimately, cardiovascular disease in addition to their implications on the graft and patient survival. Diabetes itself accelerates atherosclerosis and increases arterial stiffness (50,51). Elevated PTH levels are also thought to be associated with structural changes in resistance vessels and changes in vasodilatory responses, and obesity leads to activation of the sympathetic nervous system and altered renin-angiotensin-aldosterone system responses (52,53). These situations lead to increased BP and other micro- and macrovascular complications, including proteinuria. Although outside the scope of this review, BP control post-transplant is critical to management of metabolic disorders in kidney transplant recipients.
New onset diabetes after transplant is associated with an increased risk of death with a functioning graft and an increased risk of acute rejection, thus making it critical to manage diabetes effectively (54). In addition to diet and exercise, there are over ten classes of medications currently Food and Drug Administration approved for management of patients with type 2 diabetes, but few of those have been studied in the post-transplant population. The most relevant data post-transplant is summarized in Table 1. It is important to note that the Kidney Disease Improving Global Outcomes guidelines recommend that all patients with transplants and diabetes receive cholesterol-lowering therapy with a statin as well to optimize cardiovascular risk.
Table 1.
Summary of studies of post-transplant diabetes medications
| Agent | Study Year | Study Authors | Type of Study | Population Type | No. of Participants | Outcomes | PMID |
|---|---|---|---|---|---|---|---|
| Glipizide | 1998 | Sagedal et al. (55) | Prospective study | Kidney transplant recipients | 11 | Glipizide does not change cyclosporin metabolism | 9850449 |
| Rosiglitazone | 2004 | Baldwin et al. (56) | Prospective cohort study/observational study | Solid organ recipients | 18 | Rosiglitazone can be used as a well tolerated and safe alternative to insulin | 15087762 |
| Rosiglitazone | 2005 | Pietruck et al. (57) | Prospective cohort study/observational study | Kidney transplant recipients | 22 | Rosiglitazone improves fasting blood glucose without changing tacrolimus or cyclosporin levels | 15773972 |
| Repaglinide | 2006 | Türk et al. (58) | Observational study | Kidney transplant recipients | 23 | Repaglinide can be a safe option, specifically in white patients | 16539642 |
| Gliquidone | 2008 | Tuerk et al. (59) | Retrospective database study | Kidney transplant recipients | 47 | Gliquidone improves fasting blood glucose, similar efficacy as rosiglitazone | 18793545 |
| Metformin | 2008 | Kurian et al. (60) | Retrospective chart review | Kidney transplant recipients | 32 | Metformin is safe for an average of 16 mo after transplant | 19095596 |
| Metformin | 2014 | Stephen et al. (61) | Retrospective cohort study | Kidney transplant recipients | 46,914 | Metformin is not associated with negative allograft or patient survival effects | 25613554 |
| Thiazolidinediones | 2008 | Kurian et al. (60) | Retrospective chart review | Kidney transplant recipients | 46 | Thiazolidinediones are safe for an average of 37 mo after transplant | 19095596 |
| Sitagliptin | 2011 | Lane et al. (62) | Pilot study | Kidney transplant recipients | 15 | Sitagliptin can significantly reduce HbA1c without changes in tacrolimus, sirolimus, and eGFR | 22067216 |
| Sitagliptin | 2014 | Boerner et al. (63) | Retrospective analysis | Kidney transplant recipients | 22 | Sitagliptin is safe for treatment in kidney transplant recipients | 24817885 |
| Vildagliptin | 2013 | Werzowa et al. (64) | Randomized, placebo-controlled clinical trial | Kidney transplant recipients | 48 | Vildagliptin significantly decreases HbA1c and 3-mo 2-h plasma glucose | 23380864 |
| Vildagliptin | 2013 | Gueler et al. (65) | Retrospective study | Heart transplant recipients | 30 | Vildagliptin can significantly decrease average glucose levels and HbA1c | 23630415 |
| Pioglitazone | 2013 | Werzowa et al. (64) | Randomized, placebo-controlled clinical trial | Kidney transplant recipients | 48 | Pioglitazone significantly decreases HbA1c, improves fasting plasma glucose, and 3-mo 2-h plasma glucose | 23380864 |
| Liraglutide | 2013 | Pinelli et al. (66) | Patient series | Kidney transplant recipients | 5 | Liraglutide administered together with tacrolimus does not change tacrolimus levels | 24065848 |
| Liraglutide | 2018 | Liou et al. (67) | Retrospective analysis | Kidney transplant recipients | 7 | Liraglutide improved weight, glucose control, and eGFR | 30316386 |
| Empagliflozin | 2019 | Halden et al. (68) | Single-center, prospective, doubly blind study | Kidney transplant recipients | 49 | Empagliflozin decreases body weight and improves glycemic control | 30862658 |
| Empagliflozin | 2019 | Schwaiger et al. (69) | Prospective, interventional pilot study | Kidney transplant recipients | 14 | Empagliflozin improves β-cell sensitivity, decreases oral glucose insulin sensitivity, and decreases body weight | 30585690 |
PMID, PubMed reference number; HbA1c, hemoglobin A1c.
Sodium glucose cotransporter 2 inhibitors are newer on the market and of interest in the kidney transplant population because of their cardiovascular benefits, including decreased macrovascular incidents in patients with established cardiovascular disease. However, because their mechanism of action is to concentrate glucose in the urine, there is increased risk of urinary tract infections, especially in patients with immunosuppression. A small study in which 49 patients >1 year post-transplant with stable immunosuppression and GFR were placed on empagliflozin demonstrated only a modest reduction in hemoglobin A1c (−0.2%), with only one withdrawal for urosepsis (68). Sodium glucose cotransporter 2 inhibitors are a reasonable medication to consider for post-transplant diabetes mellitus in stable patients with transplants a year out from transplant when immunosuppression has likely been reduced. Further studies are needed to determine cardiovascular benefit in this patient population.
Data support the use of dipeptidyl peptidase-4 inhibitors in post-transplant diabetes mellitus to avoid hypoglycemic events. Sitagliptin has demonstrated improved fasting blood glucose levels in patients with kidney transplant (70), but it does require dose adjustment on the basis of kidney function. Linagliptin may be more beneficial in this patient population because it is not cleared by the kidneys and should provide the same benefit. Other incretin therapies, like the glucagon-like peptide-1 receptor agonists, also could provide benefit in patients with transplants by improving insulin resistance with the additional benefit of weight lost (67).
Immunosuppressant medications have a role in inducing post-transplant hyperglycemia and post-transplant diabetes mellitus as summarized in Table 2. Often, these effects are dose dependent, such as with glucocorticoids and tacrolimus (74,79). Minimization of these immunosuppressive agents in select populations can be a reasonable strategy to aid in diabetes management; however, this confers a higher risk of graft rejection. To balance the risk of post-transplant hyperglycemia or diabetes with rejection, immunosuppressant regimens need to be individualized on the basis of past clinical trials demonstrating the effect of each immunosuppressant agent and patient characteristics.
Table 2.
Summary of studies of immunosuppressant medications’ post-transplant diabetes mellitus effects
| Agent | Study Year | Study Authors | Type of Study | Population Type | No. of Participants | Outcomes | PMID |
|---|---|---|---|---|---|---|---|
| Tacrolimus | 1990 | Starzl et al. (71) | Experimental drug observational study | Kidney (with and without other organ[s]) transplant recipients | 36 | FK506 has similar side effects as cyclosporin but potentially to a lesser degree | 1693970 |
| Tacrolimus | 1991 | Shapiro et al. (72) | Observational study | Kidney transplant recipients | 65 | FK506 has similar profile as cyclosporin but may be superior to cyclosporin in its hyperuricemia, hypertension, and hypercholesterolemia side effects | 1703352 |
| Tacrolimus | 2001 | Duijnhoven et al. (73) | Prospective longitudinal study | Kidney transplant recipients | 18 | Insulin sensitivity index if abnormal or indeterminate can be a risk factor for future development of diabetes after transplant | 11181807 |
| Tacrolimus | 2002 | Boots et al. (74) | Prospective study | Kidney transplant recipients | 15 | Decreased tacrolimus trough results in improvement in β-cell secretion and withdrawing steroids results in decreased LDL, HDL, total lipid, triglycerides, and HbA1c | 11752041 |
| Tacrolimus | 2008 | Porrini et al. (75) | Multicenter, prospective study | Kidney transplant recipients | 154 | Within 1 yr of transplant, 20% of transplant recipients receiving tacrolimus develop diabetes | 18431233 |
| Tacrolimus versus cyclosporin | 1997 | Pirsch et al. (76) | Randomized, open label study | Kidney transplant recipients | 412 | Incidence of post-transplant diabetes much higher with tacrolimus (19.9%) than with cyclosporin (4.0%), but lower rate of rejection with tacrolimus compared with cyclosporin | 9112351 |
| Tacrolimus versus cyclosporin | 2007 | Vincenti et al. (77) | Open label, randomized, multicenter study | Kidney transplant recipients | 682 | Incidence of impaired fasting glucose significantly higher with tacrolimus than with cyclosporin at 6 mo | 17359512 |
| Tacrolimus versus cyclosporin | 2008 | Ghisdal et al. (78) | Single-center, retrospective analysis | Kidney transplant recipients | 54 | Conversion to cyclosporin from tacrolimus significantly decreases HbA1c and fasting plasma glucose, and it may lead to remission of diabetes after transplant | 17971033 |
| Early steroid withdrawal versus chronic low-dose steroid treatment | 2008 | Woodle et al. (79) | Prospective, multicenter, randomized, double-blind, placebo-controlled trial | Kidney transplant recipients | 386 | Early steroid withdrawal is associated with an increased rate of acute rejection but has comparable long-term graft function as chronic low-dose steroid treatment | 18936569 |
| Sirolimus | 2008 | Johnston et al. (80) | Retrospective data analysis | Kidney transplant recipients | 20,124 | Sirolimus is associated with diabetes after transplantation | 18385422 |
| Sirolimus versus tacrolimus/MMF | 2011 | Flechner et al. (81) | Randomized trial | Kidney transplant recipients | 443 | Sirolimus-based regimens were not found to be superior in outcomes to tacrolimus/MMF | 21668635 |
| Sirolimus versus cyclosporin versus steroids | 2011 | Gyurus et al. (82) | Retrospective analysis | Kidney transplant recipients | 514 | Sirolimus treatment can be a risk factor for development of diabetes | 21693238 |
| Sirolimus versus calcineurin inhibitors | 2013 | Veroux et al. (83) | Retrospective study | Kidney transplant recipients | 344 | After development of diabetes after transplantation, converting from calcineurin inhibitors to sirolimus significantly improves or resolves the diabetes without increased risk of graft rejection | 23762090 |
| Corticosteroids | 2015 | Pirsch et al. (39) | Double-blind, prospective, randomized, controlled trial | Kidney transplant recipients | 277 | Early corticosteroid withdrawal has limited benefits compared with chronic low-dose prednisone | 25881802 |
| Belatacept versus cyclosporin | 2011 | Rostaing et al. (84) | Post hoc analysis of randomized, controlled trial | Kidney transplant recipients | 336 | Numerically better graft and patient survival at 12 mo in patients with diabetes on belatacept versus cyclosporin | 21921152 |
PMID, PubMed reference number; HbA1c, hemoglobin A1c; MMF, mycophenolate mofetil.
Tacrolimus has been shown to potentially confer a lower risk of rejection compared with cyclosporin; however, the incidence of post-transplant diabetes mellitus was shown to be significantly higher with tacrolimus (76). Decreasing tacrolimus trough levels and switching a patient from tacrolimus to cyclosporin have both been shown to improve or even remit post-transplant dysglycemia and diabetes (74,77). Additionally, sirolimus has been shown to confer an independent risk toward developing post-transplant diabetes mellitus (81). Similar to tacrolimus, switching patients from sirolimus to cyclosporin after development of post-transplant diabetes mellitus has been shown to improve or even resolve diabetes without compromising graft function (83). Belatacept is an alternative to calcineurin inhibitors that may be used as the backbone of immunosuppressive therapy to avoid the toxic effects of tacrolimus. Belatacept has been shown to improve glucose tolerance when withdrawing tacrolimus potentially through CD86 blockade, which may be involved in insulin resistance (85).
Secondary hyperparathyroidism occurs in most patients with CKD and can persist after kidney transplant in up to 66% of patients, despite an initial decrease in PTH levels (86). Maintaining normal PTH post-transplant is critical because it affects bone health; additionally, it is associated with graft loss and all-cause mortality. Pihlstrøm et al. (87) found that, after a follow-up time of approximately 7 years, patients with kidney transplants and hyperparathyroidism (intact PTH >65 pg/ml) had an increased risk of all-cause mortality (hazard ratio, 1.04) and graft loss (hazard ratio, 1.06), indicating the importance of maintaining PTH levels post-transplant.
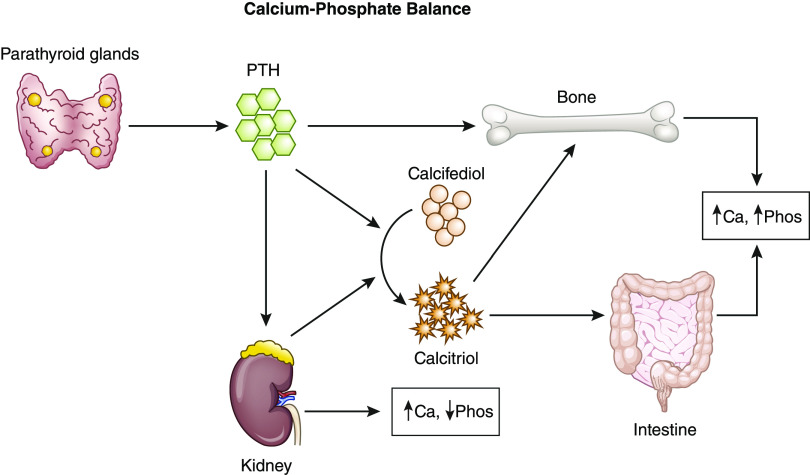
PTH helps to maintain the calcium-phosphate balance along with the kidneys, bones, and gastrointestinal tract (Figure 2). Vitamin D, vitamin D analogs, and calcimimetics are most commonly used for the medical management of hyperparathyroidism. Cinicalcet, a calcimimetic, has the most data for use after transplantation. There is only one randomized, controlled trial comparing medical management with parathyroidectomy in the post-transplant setting (88). Delos Santos et al. (89) provide an excellent overview of medical management, including data in patients with transplants, and it will not be included here.
Figure 2.
Calcium (Ca)-phosphate (Phos) balance. PTH, parathyroid hormone.
In studies of parathyroidectomy post-transplant, the benefits on the graft are not as clear, and there is potential for allograft function impairment in the year postparathyroidectomy (90). In the only randomized, controlled trial of parathyroidectomy versus medical management with cinacalcet post-transplant, parathyroidectomy was found to be superior at controlling calcium and PTH levels and increasing femoral neck bone mineral density; however, the long-term clinical benefits are not known (88). However, hyperparathyroidism is a clear indicator of poor transplant outcomes, and effective treatment prior to transplant is critical for post-transplant success. The method of management is not well studied and still up for discussion with larger randomized, controlled trials.
Obesity is the most common metabolic disorder affecting the transplant, and it should not be ignored post-transplant because weight gain can be common (91,92). Multiple studies have corroborated that bariatric surgery in general is safe and feasible in patients having undergone previous kidney transplantation as well as those with ESKD, and it seems to be more efficacious than supervised medical weight loss strategies (19,27,93,94). A Markov decision analytic model was used in a recent study performed by Choudhury et al. (27) in assessing which option is the best suited for kidney transplant candidates in reaching a BMI goal of <35 kg/m2 for listing. These analyses compared medically supervised weight loss with both sleeve gastrectomy and Roux-en-y gastric bypass arms, demonstrating both improved overall survival as well as transition to a transplanted state in the Roux-en-y gastric bypass arm (27). Clearly, more research is needed to fully identify the best strategies for weight loss in our kidney transplant population to support greater access to and improved results in obese candidates and recipients.
There are significant considerations when managing metabolic disorders after kidney transplantation given the crucial immunosuppressive balance with medications and the effect that procedures, such as parathyroidectomy and surgery, may have on graft outcomes. However, the close management of these metabolic disorders is necessary for long-term patient survival.
Metabolic disorders, including diabetes, hyperparathyroidism, and obesity, clearly have significant effect on the kidney transplant population during multiple phases of care—affecting patient access to the waitlist, the ability to successfully receive a transplant, and ultimately, the likelihood of experiencing a good outcome long term. Understanding the risks associated with these disorders and optimal management strategies is crucial to the ultimate successful outcome post-transplant.
Disclosures
Dr. Belfort de Aguiar, Dr. Callender, Dr. Cohen, Dr. Haakinson, and Dr. Korah have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Polonsky WH, Henry RR: Poor medication adherence in type 2 diabetes: Recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 10: 1299–1307, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevins TE, Nickerson PW, Dew MA: Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol 28: 2290–2301, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y: Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant 8: 593–599, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Baek CH, Kim H, Baek SD, Jang M, Kim W, Yang WS, Han DJ, Park SK: Outcomes of living donor kidney transplantation in diabetic patients: Age and sex matched comparison with non-diabetic patients. Korean J Intern Med (Korean Assoc Intern Med) 33: 356–366, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim WH, Wong G, Pilmore HL, McDonald SP, Chadban SJ: Long-term outcomes of kidney transplantation in people with type 2 diabetes: A population cohort study. Lancet Diabetes Endocrinol 5: 26–33, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Glicklich D, Mustafa MR: Obesity in kidney transplantation: Impact on transplant candidates, recipients, and donors. Cardiol Rev 27: 63–72, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Shin JI, Palta M, Djamali A, Astor BC: Higher pretransplantation hemoglobin A1c Is associated with greater risk of posttransplant diabetes mellitus. Kidney Int Rep 2: 1076–1087, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharif A, Baboolal K: Complications associated with new-onset diabetes after kidney transplantation. Nat Rev Nephrol 8: 34–42, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Jung M, Warren B, Grams M, Kwong YD, Shafi T, Coresh J, Rebholz CM, Selvin E: Performance of non-traditional hyperglycemia biomarkers by chronic kidney disease status in older adults with diabetes: Results from the Atherosclerosis Risk in Communities Study. J Diabetes 10: 276–285, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johal S, Jackson-Spence F, Gillott H, Tahir S, Mytton J, Evison F, Stephenson B, Nath J, Sharif A: Pre-existing diabetes is a risk factor for increased rates of cellular rejection after kidney transplantation: An observational cohort study. Diabet Med 34: 1067–1073, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Chakkera HA, Weil EJ, Swanson CM, Dueck AC, Heilman RL, Reddy KS, Hamawi K, Khamash H, Moss AA, Mulligan DC, Katariya N, Knowler WC: Pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 34: 2141–2145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigo E, Fernández-Fresnedo G, Valero R, Ruiz JC, Piñera C, Palomar R, González-Cotorruelo J, Gómez-Alamillo C, Arias M: New-onset diabetes after kidney transplantation: Risk factors. J Am Soc Nephrol 17[Suppl 3]: S291–S295, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hucker A, Lawrence C, Sharma S, Farrington K: Adherence behavior in subjects on hemodialysis is not a clear predictor of posttransplantation adherence. Kidney Int Rep 4: 1122–1130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres A, Rodríguez AP, Concepción MT, García S, Rufino M, Martín B, Pérez L, Machado M, de Bonis E, Losada M, Hernández D, Lorenzo V: Parathyroid function in long-term renal transplant patients: Importance of pre-transplant PTH concentrations. Nephrol Dial Transplant 13[Suppl 3]: 94–97, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Coyne DW, Delos Santos R: Evaluating the safety and rationale for cinacalcet posttransplant hyperparathyroidism and hypercalcemia. Am J Transplant 14: 2446–2447, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Callender GG, Malinowski J, Javid M, Zhang Y, Huang H, Quinn CE, Carling T, Tomlin R, Smith JD, Kulkarni S: Parathyroidectomy prior to kidney transplant decreases graft failure. Surgery 161: 44–50, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Pondrom S: The AJT report: News and issues that affect organ and tissue transplantation. Am J Transplant 12: 1663–1664, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA: Obesity impacts access to kidney transplantation. J Am Soc Nephrol 19: 349–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Bahri S, Fakhry TK, Gonzalvo JP, Murr MM: Bariatric surgery as a bridge to renal transplantation in patients with end-stage renal disease. Obes Surg 27: 2951–2955, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Huang E, Shye M, Elashoff D, Mehrnia A, Bunnapradist S: Incidence of conversion to active waitlist status among temporarily inactive obese renal transplant candidates. Transplantation 98: 177–186, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Lesage J, Gill JS: Management of the obese kidney transplant candidate. Transplant Rev (Orlando) 31: 35–41, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Huizinga MM: Weight-loss pharmacotherapy: A brief review. Clin Diabetes 25: 135–140, 2007 [Google Scholar]
- 23.MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC: Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-Year follow-up. Am J Kidney Dis 55: 69–76, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dziodzio T, Biebl M, Öllinger R, Pratschke J, Denecke C: The role of bariatric surgery in abdominal organ transplantation—the next big challenge? Obes Surg 27: 2696–2706, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Jung AD, Dhar VK, Tadros JS, Schauer DP, Smith EP, Hanseman DJ, Cuffy MC, Alloway RR, Shields AR, Shah SA, Woodle ES, Diwan TS: Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients. Am J Transplant 18: 410–416, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Thomas IA, Gaynor JJ, Joseph T, De La Cruz-Munoz N, Sageshima J, Kupin W, Chen LJ, Ciancio G, Burke GW 3rd, Mattiazzi AD, Roth D, Guerra G: Roux-en-Y gastric bypass is an effective bridge to kidney transplantation: Results from a single center. Clin Transplant 32: e13232, 2018 [DOI] [PubMed] [Google Scholar]
- 27. Choudhury RA, Hoeltzel G, Prins K, Chow E, Moore HB, Lawson PJ, Yoeli D, Pratap A, Abt PL, Dumon KR, Conzen KD, Nydam TL: Sleeve gastrectomy compared with gastric bypass for morbidly obese patients with end stage renal disease: A decision analysis [published online ahead of print May 1, 2019]. J Gastrointest Surg doi:10.1007/s11605-019-04225-w. [DOI] [PubMed]
- 28.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D: Importance of perioperative glycemic control in general surgery: A report from the Surgical Care and Outcomes Assessment Program. Ann Surg 257: 8–14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE: Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33: 1783–1788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes V, Ferreira F, Guerra J, Bugalho MJ: New-onset diabetes after kidney transplantation: Incidence and associated factors. World J Diabetes 9: 132–137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas MC, Mathew TH, Russ GR, Rao MM, Moran J: Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: A pilot study. Transplantation 72: 1321–1324, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Shivaswamy V, Boerner B, Larsen J: Post-transplant diabetes mellitus: Causes, treatment, and impact on outcomes. Endocr Rev 37: 37–61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wojtusciszyn A, Mourad G, Bringer J, Renard E: Continuous glucose monitoring after kidney transplantation in non-diabetic patients: Early hyperglycaemia is frequent and may herald post-transplantation diabetes mellitus and graft failure. Diabetes Metab 39: 404–410, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Pasti K, Szabo AJ, Prokai A, Meszaros K, Peko N, Solyom R, Sallay P, Reusz G, Rusai K: Continuous glucose monitoring system (CGMS) in kidney-transplanted children. Pediatr Transplant 17: 454–460, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Hecking M, Haidinger M, Döller D, Werzowa J, Tura A, Zhang J, Tekoglu H, Pleiner J, Wrba T, Rasoul-Rockenschaub S, Mühlbacher F, Schmaldienst S, Druml W, Hörl WH, Krebs M, Wolzt M, Pacini G, Port FK, Säemann MD: Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol 23: 739–749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coan KE, Schlinkert AB, Beck BR, Haakinson DJ, Castro JC, Schlinkert RT, Cook CB: Perioperative management of patients with diabetes undergoing ambulatory elective surgery. J Diabetes Sci Technol 7: 983–989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coan KE, Schlinkert AB, Beck BR, Haakinson DJ, Castro JC, Apsey HA, Schlinkert RT, Cook CB: Clinical inertia during postoperative management of diabetes mellitus: Relationship between hyperglycemia and insulin therapy intensification. J Diabetes Sci Technol 7: 880–887, 2013. [DOI] [PMC free article] [PubMed]
- 39.Pirsch JD, Henning AK, First MR, Fitzsimmons W, Gaber AO, Reisfield R, Shihab F, Woodle ES: New-onset diabetes after transplantation: Results from a double-blind early corticosteroid withdrawal trial. Am J Transplant 15: 1982–1990, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Rickels MR: A role for transplant endocrinologists—it’s about time. Endocr Pract 21: 697–699, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Newland DM, Edwards AR, Hall RC, Maxwell PR: Positive impact of a pilot pharmacist-run diabetes pharmacotherapy clinic in solid-organ transplant recipients. Diabetes Spectr 31: 167–176, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood A, Hakim DN, Hakim NS: Consequences of recipient obesity on postoperative outcomes in a renal transplant: A systematic review and meta-analysis. Exp Clin Transplant 14: 121–128, 2016 [PubMed] [Google Scholar]
- 43.Hanevold CD, Ho PL, Talley L, Mitsnefes MM: Obesity and renal transplant outcome: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 115: 352–356, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ: Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 250: 1014–1020, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Oberholzer J, Giulianotti P, Danielson KK, Spaggiari M, Bejarano-Pineda L, Bianco F, Tzvetanov I, Ayloo S, Jeon H, Garcia-Roca R, Thielke J, Tang I, Akkina S, Becker B, Kinzer K, Patel A, Benedetti E: Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant 13: 721–728, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzvetanov I, Bejarano-Pineda L, Giulianotti PC, Jeon H, Garcia-Roca R, Bianco F, Oberholzer J, Benedetti E: State of the art of robotic surgery in organ transplantation. World J Surg 37: 2791–2799, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Roca R, Garcia-Aroz S, Tzvetanov I, Jeon H, Oberholzer J, Benedetti E: Single center experience with robotic kidney transplantation for recipients with BMI of 40 kg/m2 or greater: A comparison with the UNOS registry. Transplantation 101: 191–196, 2017 [DOI] [PubMed] [Google Scholar]
- 48.de Oliveira CM, Moura AE, Gonçalves L, Pinheiro LS, Pinheiro FM Jr., Esmeraldo RM: Post-transplantation weight gain: Prevalence and the impact of steroid-free therapy. Transplant Proc 46: 1735–1740, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, Gill J: The survival benefit of kidney transplantation in obese patients. Am J Transplant 13: 2083–2090, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Steigerwalt S: Management of hypertension in diabetic patients with chronic kidney disease. Diabetes Spectr 21: 30–36, 2008 [Google Scholar]
- 51.Gaede P, Vedel P, Larsen N, Jensen GV, Parving H-H, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383–393, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Pepe J, Cipriani C, Sonato C, Raimo O, Biamonte F, Minisola S: Cardiovascular manifestations of primary hyperparathyroidism: A narrative review. Eur J Endocrinol 177: R297–R308, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y: Obesity and hypertension. Exp Ther Med 12: 2395–2399, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole EH, Johnston O, Rose CL, Gill JS: Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 3: 814–821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sagedal S, Asberg A, Hartmann A, Bergan S, Berg KJ: Glipizide treatment of post-transplant diabetes does not interfere with cyclosporine pharmacokinetics in renal allograft recipients. Clin Transplant 12: 553–556, 1998 [PubMed] [Google Scholar]
- 56.Baldwin D Jr., Duffin KE: Rosiglitazone treatment of diabetes mellitus after solid organ transplantation. Transplantation 77: 1009–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Pietruck F, Kribben A, Van TN, Patschan D, Herget-Rosenthal S, Janssen O, Mann K, Philipp T, Witzke O: Rosiglitazone is a safe and effective treatment option of new-onset diabetes mellitus after renal transplantation. Transpl Int 18: 483–486, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Türk T, Pietruck F, Dolff S, Kribben A, Janssen OE, Mann K, Philipp T, Heemann U, Witzke O: Repaglinide in the management of new-onset diabetes mellitus after renal transplantation. Am J Transplant 6: 842–846, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Tuerk TR, Bandur S, Nuernberger J, Kribben A, Mann K, Philipp T, Heemann U, Viklicky O, Witzke O: Gliquidone therapy of new-onset diabetes mellitus after kidney transplantation. Clin Nephrol 70: 26–32, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Kurian B, Joshi R, Helmuth A: Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr Pract 14: 979–984, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK: Metformin use in kidney transplant recipients in the United States: An observational study. Am J Nephrol 40: 546–553, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Lane JT, Odegaard DE, Haire CE, Collier DS, Wrenshall LE, Stevens RB: Sitagliptin therapy in kidney transplant recipients with new-onset diabetes after transplantation. Transplantation 92: e56–e57, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Boerner BP, Miles CD, Shivaswamy V: Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. Int J Endocrinol 2014: 617638, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werzowa J, Hecking M, Haidinger M, Lechner F, Döller D, Pacini G, Stemer G, Pleiner J, Frantal S, Säemann MD: Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: A randomized, placebo-controlled clinical trial. Transplantation 95: 456–462, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Gueler I, Mueller S, Helmschrott M, Oeing CU, Erbel C, Frankenstein L, Gleißner C, Ruhparwar A, Ehlermann P, Dengler TJ, Katus HA, Doesch AO: Effects of vildagliptin (Galvus®) therapy in patients with type 2 diabetes mellitus after heart transplantation. Drug Des Devel Ther 7: 297–303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinelli NR, Patel A, Salinitri FD: Coadministration of liraglutide with tacrolimus in kidney transplant recipients: A case series. Diabetes Care 36: e171–e172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liou JH, Liu YM, Chen CH: Managament of diabetes mellitus with glucagonlike peptide-1 agonist liraglutide in renal transplant recipients: A retrospective study. Transplant Proc 50: 2502–2505, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, Bollerslev J, Hartmann A, Åsberg A, Jenssen T: Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 42: 1067–1074, 2019 [DOI] [PubMed] [Google Scholar]
- 69.Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, Pacini G, Wrba T, Antlanger M, Schmaldienst S, Werzowa J, Säemann MD, Hecking M: Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant 19: 907–919, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strøm Halden TA, Åsberg A, Vik K, Hartmann A, Jenssen T: Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant 29: 926–933, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Starzl TE, Fung J, Jordan M, Shapiro R, Tzakis A, McCauley J, Johnston J, Iwaki Y, Jain A, Alessiani M, Todo S: Kidney transplantation under FK 506. JAMA 264: 63–67, 1990 [PMC free article] [PubMed] [Google Scholar]
- 72.Shapiro R, Jordan M, Fung J, McCauley J, Johnston J, Iwaki Y, Tzakis A, Hakala T, Todo S, Starzl TE: Kidney transplantation under FK 506 immunosuppression. Transplant Proc 23: 920–923, 1991 [PMC free article] [PubMed] [Google Scholar]
- 73.Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP: Influence of tacrolimus on glucose metabolism before and after renal transplantation: A prospective study. J Am Soc Nephrol 12: 583–588, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Boots JM, van Duijnhoven EM, Christiaans MH, Wolffenbuttel BH, van Hooff JP: Glucose metabolism in renal transplant recipients on tacrolimus: The effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol 13: 221–227, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Porrini E, Moreno JM, Osuna A, Benitez R, Lampreabe I, Diaz JM, Silva I, Domínguez R, Gonzalez-Cotorruelo J, Bayes B, Lauzurica R, Ibernon M, Moreso F, Delgado P, Torres A: Prediabetes in patients receiving tacrolimus in the first year after kidney transplantation: A prospective and multicenter study. Transplantation 85: 1133–1138, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS: A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 63: 977–983, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N; DIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring Versus Tacrolimus) Investigators: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Ghisdal L, Bouchta NB, Broeders N, Crenier L, Hoang AD, Abramowicz D, Wissing KM: Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: A single-centre experience in renal transplanted patients and review of the literature. Transpl Int 21: 146–151, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P; Astellas Corticosteroid Withdrawal Study Group: A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Johnston O, Rose CL, Webster AC, Gill JS: Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 19: 1411–1418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre C, Russ G, Steinberg S, Wissing KM, Tai SS: The ORION study: Comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 11: 1633–1644, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Gyurus E, Kaposztas Z, Kahan BD: Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: A long-term analysis of various treatment regimens. Transplant Proc 43: 1583–1592, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Veroux M, Tallarita T, Corona D, Sinagra N, Giaquinta A, Zerbo D, Guerrieri C, D’Assoro A, Cimino S, Veroux P: Conversion to sirolimus therapy in kidney transplant recipients with new onset diabetes mellitus after transplantation. Clin Dev Immunol 2013: 496974, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rostaing L, Neumayer HH, Reyes-Acevedo R, Bresnahan B, Florman S, Vitko S, Heifets M, Xing J, Thomas D, Vincenti F: Belatacept-versus cyclosporine-based immunosuppression in renal transplant recipients with pre-existing diabetes. Clin J Am Soc Nephrol 6: 2696–2704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Graav GN, van der Zwan M, Baan CC, Janssen JAMJL, Hesselink DA: Improved glucose tolerance in a kidney transplant recipient with type 2 diabetes mellitus after switching from tacrolimus to belatacept: A case report and review of potential mechanisms. Transplant Direct 4: e350, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bleskestad IH, Bergrem H, Leivestad T, Gøransson LG: Intact parathyroid hormone levels in renal transplant patients with normal transplant function. Clin Transplant 25: E566–E570, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Pihlstrøm H, Dahle DO, Mjøen G, Pilz S, März W, Abedini S, Holme I, Fellström B, Jardine AG, Holdaas H: Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation 99: 351–359, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gómez-Vaquero C, Polo C, Revuelta I, Francos J, Torras J, García-Barrasa A, Bestard O, Grinyó JM: A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol 27: 2487–2494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delos Santos R, Rossi A, Coyne D, Maw TT: Management of post-transplant hyperparathyroidism and bone disease. Drugs 79: 501–513, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseng PY, Yang WC, Yang CY, Tarng DC: Long-term outcomes of parathyroidectomy in kidney transplant recipients with persistent hyperparathyroidism. Kidney Blood Press Res 40: 386–394, 2015 [DOI] [PubMed] [Google Scholar]
- 91.Kramer H, Luke A: Obesity and kidney disease: A big dilemma. Curr Opin Nephrol Hypertens 16: 237–241, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Hossain M, Woywodt A, Augustine T, Sharma V: Obesity and listing for renal transplantation: Weighing the evidence for a growing problem. Clin Kidney J 10: 703–708, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Modanlou KA, Muthyala U, Xiao H, Schnitzler MA, Salvalaggio PR, Brennan DC, Abbott KC, Graff RJ, Lentine KL: Bariatric surgery among kidney transplant candidates and recipients: Analysis of the United States renal data system and literature review. Transplantation 87: 1167–1173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Freeman CM, Woodle ES, Shi J, Alexander JW, Leggett PL, Shah SA, Paterno F, Cuffy MC, Govil A, Mogilishetty G, Alloway RR, Hanseman D, Cardi M, Diwan TS: Addressing morbid obesity as a barrier to renal transplantation with laparoscopic sleeve gastrectomy. Am J Transplant 15: 1360–1368, 2015 [DOI] [PubMed] [Google Scholar]