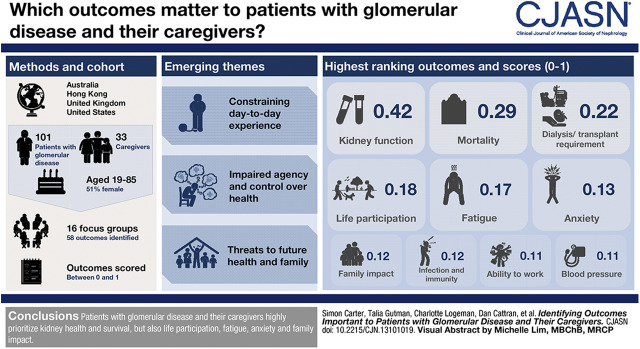
Visual Abstract
Keywords: Focus Groups, Caregivers, renal dialysis, blood pressure, Decision Making, Shared, Data Accuracy, Blood Pressure Determination, Family Relations, Anxiety, Fatigue, Infections
Abstract
Background and objectives
Shared decision making in patients with glomerular disease remains challenging because outcomes important to patients remain largely unknown. We aimed to identify and prioritize outcomes important to patients and caregivers and to describe reasons for their choices.
Design, setting, participants, & measurements
We purposively sampled adult patients with glomerular disease and their caregivers from Australia, Hong Kong, the United Kingdom, and the United States. Participants identified, discussed, and ranked outcomes in focus groups using the nominal group technique; a relative importance score (between zero and one) was calculated. Qualitative data were analyzed thematically.
Results
Across 16 focus groups, 134 participants (range, 19–85 years old; 51% women), including 101 patients and 33 caregivers, identified 58 outcomes. The ten highest-ranked outcomes were kidney function (importance score of 0.42), mortality (0.29), need for dialysis or transplant (0.22), life participation (0.18), fatigue (0.17), anxiety (0.13), family impact (0.12), infection and immunity (0.12), ability to work (0.11), and BP (0.11). Three themes explained the reasons for these rankings: constraining day-to-day experience, impaired agency and control over health, and threats to future health and family.
Conclusions
Patients with glomerular disease and their caregivers highly prioritize kidney health and survival, but they also prioritize life participation, fatigue, anxiety, and family impact.
Introduction
The management of glomerular disease can be challenging because of the heterogeneity and unpredictability of the disease course. Moreover, there is uncertainty about what outcomes of the disease and its treatment are most important to patients. Patients with glomerular disease may experience kidney failure (1,2), bone disease (3), cancer (4–6), infertility (7,8), fatigue, swelling (9–11), impaired psychosocial wellbeing (9,11,12), and reduced life expectancy (13–15). In weighing treatment options, decision makers require comprehensive information about all relevant harms and benefits. Many of these outcomes, particularly patient-reported outcomes, are highly relevant yet under-reported in trials in glomerular disease, which limits informed decision making (16–18).
Instead, trials frequently report biochemical or clinical outcomes selected by researchers with little or no patient involvement (19–21). Scant attention has been paid to patient-reported outcomes that reflect how patients feel and function (22,23), even though symptoms, such as swelling, fatigue, and depression, are often identified by patients as major concerns (10,11,24,25). Life participation, defined as the ability to do meaningful activities in life, has been identified as an important outcome for people receiving peritoneal dialysis or living with a kidney transplant (26,27). Patients may also experience distressing treatment-related side effects, such as weight gain, mood swings, and Cushingoid appearance from corticosteroids, and these are often omitted from trial reports (28,29).
Patient-centered outcomes in many glomerular diseases have not been identified, and their relative importance is unknown. The aims of this study were to identify and prioritize outcomes important to patients with glomerular disease and their caregivers, as well as to describe the reasons for their choices. This may guide the selection of outcomes for research in glomerular disease and strengthen the patient-centered evidence base for decision making.
Materials and Methods
Participant Selection and Recruitment
We recruited patients aged 18 years old or older and their caregivers (family member or support person involved in caring for the patient) from six centers in Australia (Brisbane, Melbourne, and Sydney), four centers in Hong Kong, three centers in the United Kingdom (London, Sheffield, and York), and one center in the United States (Los Angeles). Patients were English speaking (English or Spanish speaking in the United States). We used purposive sampling to ensure diversity on the basis of demographic and clinical characteristics (e.g., age, sex, ethnicity, glomerular disease, and KRT) because differences were likely in priorities, values and goals on the basis of these characteristics. This approach can help to elicit breadth and differences of opinion. We recorded participant characteristics to target recruitment for subsequent groups.
Patients with primary and secondary glomerular disease were eligible and nominated by their nephrologist. We excluded patients with conditions that have substantially different core clinical features (e.g., deafness and liver cirrhosis) and treatments (e.g., antiviral medications and no immunosuppression). These include pure postinfectious nephritis; hepatitis B, hepatitis C, and HIV-associated nephropathy; collagenopathies; amyloidosis; diabetic and hypertensive nephropathies; and storage diseases.
Informed consent was obtained from all participants. Participants were reimbursed United States $50 or the equivalent in local currency for travel expenses. Ethics approval was obtained for all participating sites.
Data Collection
We conducted a nominal group technique embedded in focus groups; patients and caregivers self-reported all characteristics. The nominal group technique is a consensus method used in health care priority research (30–34). It uses a moderated, structured discussion to help participants generate ideas (e.g., outcomes) followed by a ranking exercise that allows them to privately assign priorities to outcomes, thereby reducing the influence of dominant individuals or perceived social acceptability (35). The 2-hour groups were conducted from March to July 2018, and they involved (1) discussion about their experiences of glomerular disease and interventions; (2) identification of outcomes that were then compiled (supplemented by outcomes from trials and previous groups); (3) individual ranking of the outcomes identified (one being most important); and (4) discussion of the reasons for their choices. The question guide is provided in Supplemental File 1. One facilitator (A.T., L.R., S.A.C., or T.G.) conducted the groups in a place external to clinical settings. All facilitators (A.T., L.R., S.A.C., and T.G.) were trained qualitative researchers with experience moderating focus groups. A cofacilitator (C.L., L.D., S.A.C., or T.G.) noted participant dynamics and nonverbal communication. All groups were audio recorded and transcribed verbatim. We convened subsequent groups until no new outcomes or themes emerged (i.e., data saturation).
Data Analyses
Nominal Group Ranking.
A relative importance score was calculated for each outcome that incorporated the rank assigned and the frequency with which the outcome was given a rank (Supplemental File 2). Values approaching one indicate a highly prioritized outcome on the basis of higher ranks and more frequent nominations, whereas values approaching zero indicate infrequently and/or poorly ranked outcomes. Confidence intervals were calculated for each importance score using bootstrapping. We performed prespecified subgroup analysis by patient/caregiver role, age, sex, country, disease stage, and type (36). Data were analyzed using R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Qualitative Analyses.
Transcripts were imported into HyperRESEARCH (version 4.0.3; ResearchWare Inc., Randolph, MA) for thematic analysis and coding. A qualified translator who was the moderator for the Spanish-speaking groups translated them into English. S.A.C. reviewed transcripts line by line to identify the underlying reasons and values that led to participants’ rankings. These concepts were coded and analyzed inductively for each group, and then, they were compared between groups to generate initial subthemes and themes. The preliminary coding framework was discussed and reviewed by the research team (A.T., C.L., L.R., S.A.C., and T.G.) to ensure that all of the data were reflected in the themes (i.e., investigator triangulation).
Results
Participant Characteristics
We recruited 101 (75%) patients and 33 (25%) caregivers (total N=134) to participate in 16 focus groups held across Australia (six groups), Hong Kong (two groups), the United Kingdom (four groups), and the United States (four groups) (Table 1). Reasons for nonparticipation included prior work commitments, being unwell, and lack of interest. The groups were conducted in English (14 groups) and Spanish (2 groups) languages. Participants were aged 19–85 years old (mean of 51 years old), and 68 (51%) were women. Patients were diagnosed at a mean age of 39 years old (range, 2–85 years old). Most had CKD (66, 65%); however, 29 (29%) patients had received dialysis and/or transplant. Fifty (50%) had a kidney-limited glomerular disease, and 38 (38%) had a glomerular disease with systemic involvement. Seventy-three (72%) had received immunosuppressive therapy. Comorbid conditions are provided in Supplemental Table 1. Of the 33 caregivers, 21 (64%) were spouses, 4 (12%) were parents, 7 (21%) were other family members, and 1 (3%) was a friend.
Table 1.
Characteristics of adult patients with glomerular disease and their caregivers who prioritized outcomes using focus groups with nominal group technique
| Characteristic | Australia, n=50 | Hong Kong, n=22 | United Kingdom, n=29 | United States, n=33 | All, N=134 |
|---|---|---|---|---|---|
| Patient | 38 (76) | 16 (73) | 24 (83) | 23 (70) | 101 (75) |
| Caregiver or family | 12 (24) | 6 (27) | 5 (17) | 10 (30) | 33 (25) |
| Sex | |||||
| Men | 29 (58) | 13 (59) | 14 (48) | 10 (30) | 66 (49) |
| Women | 21 (42) | 9 (41) | 15 (52) | 23 (70) | 68 (51) |
| Age group, yr | |||||
| 18–39 | 14 (28) | 3 (14) | 4 (14) | 11 (33) | 32 (24) |
| 40–59 | 20 (40) | 15 (68) | 8 (28) | 14 (42) | 57 (43) |
| 60–79 | 16 (32) | 4 (18) | 16 (55) | 6 (18) | 42 (32) |
| >80 | — | — | 1 (3) | 1 (3) | 2 (2) |
| Ethnicity | |||||
| White/European | 34 (68) | — | 24 (83) | 3 (9) | 61 (46) |
| Asian (Central, South, East) | 13 (26) | 22 (100) | 1 (3) | 2 (6) | 38 (28) |
| Hispanic | — | — | 1 (3) | 22 (67) | 23 (17) |
| African/black | — | — | 2 (7) | 4 (12) | 6 (4) |
| Other | 3 (6) | — | 1 (3) | 2 (6) | 6 (4) |
| Educational attainmenta | |||||
| Primary school | 4 (11) | 4 (25) | 5 (21) | 8 (35) | 21 (21) |
| Secondary school (grade 10) | 5 (13) | 3 (19) | 1 (4) | 1 (4) | 10 (10) |
| Secondary school (grade 12) | 6 (16) | 3 (19) | 2 (8) | 5 (22) | 16 (16) |
| Certificate/diploma | 9 (24) | — | 7 (29) | 6 (26) | 22 (22) |
| University degree | 14 (37) | 6 (38) | 7 (29) | 3 (13) | 30 (30) |
| Employmenta | |||||
| Full time or part time | 22 (58) | 8 (50) | 6 (25) | 4 (17) | 40 (40) |
| Student | 1 (3) | — | — | 3 (13) | 4 (4) |
| Not employed | 4 (11) | 4 (25) | 3 (13) | 10 (43) | 21 (21) |
| Other/retired | 11 (29) | 4 (25) | 14 (58) | 5 (22) | 34 (34) |
| Type of glomerular diseasea | |||||
| Lupus nephritis | 6 (16) | 2 (13) | 6 (25) | 4 (17) | 18 (18) |
| Vasculitis | 6 (16) | — | 7 (29) | 5 (22) | 18 (18) |
| IgA nephropathy | 10 (26) | 5 (31) | 2 (8) | 1 (4) | 18 (18) |
| FSGS | 6 (16) | — | — | 4 (17) | 10 (10) |
| Membranous nephropathy | 3 (8) | 1 (6) | 1 (4) | 1 (4) | 6 (6) |
| Minimal change nephropathy | 2 (5) | — | 1 (4) | 2 (9) | 5 (5) |
| MPGN | 1 (3) | — | — | 5 (22) | 6 (6) |
| C3 glomerulopathy | 2 (5) | — | 3 (13) | — | 5 (5) |
| Anti-GBM disease | 1 (3) | — | — | — | 1 (1) |
| IgG4-related disease | 1 (3) | — | — | — | 1 (1) |
| Years since diagnosisa | |||||
| ≤2 | 11 (29) | 1 (6) | 8 (33) | 10 (43) | 30 (30) |
| 3–11 | 14 (37) | 3 (19) | 7 (29) | 7 (30) | 31 (31) |
| ≥12 | 13 (34) | 11 (69) | 6 (25) | 4 (17) | 34 (34) |
| Immunosuppression exposurea | |||||
| Any | 30 (79) | 9 (56) | 17 (71) | 17 (74) | 73 (74) |
| Corticosteroids | 26 (68) | 7 (44) | 14 (58) | 13 (57) | 60 (60) |
| Antiproliferative/calcineurin inhibitor | 20 (53) | 3 (19) | 12 (50) | 6 (26) | 41 (41) |
| Cyclophosphamide | 9 (24) | — | 6 (25) | 12 (52) | 27 (27) |
| Plasma exchange | 7 (18) | — | 5 (21) | 5 (22) | 17 (17) |
| Biologic agent | 2 (5) | — | 3 (13) | 2 (9) | 7 (7) |
| Stage of kidney diseasea | |||||
| CKD | 31 (82) | 4 (25) | 13 (54) | 18 (78) | 66 (65) |
| Hemodialysis | 3 (8) | 3 (19) | 3 (13) | 5 (22) | 14 (14) |
| Peritoneal dialysis | 2 (5) | 8 (50) | — | 3 (13) | 13 (13) |
| Living donor transplant | 1 (3) | 1 (6) | 1 (4) | 1 (4) | 4 (4) |
| Deceased donor transplant | 1 (3) | 5 (31) | 4 (17) | 1 (4) | 11 (11) |
MPGN, membranoproliferative GN; GBM, glomerular basement membrane.
Patients only. May not sum to totals because some categories represent overlapping experience. Thirteen patients did not know their type of glomerular disease. One patient was missing for age; two patients had missing data for education and immunosuppression. Six patients had missing years since diagnosis; six patients had data missing for kidney disease stage.
Nominal Group Ranking
Overall, participants identified 58 different outcomes (Figure 1). Kidney function was the highest-ranked outcome, which was conceptualized as overall how well their kidneys work or stage of CKD as estimated by eGFR. The top ten outcomes for patients were kidney function (importance score of 0.40), mortality (importance score of 0.29), need for dialysis or transplant (importance score of 0.24), life participation (importance score of 0.18), fatigue (importance score of 0.17), infection and immunity (importance score of 0.12), anxiety (importance score of 0.12), family impact (importance score of 0.12), ability to work (importance score of 0.11), and BP (importance score of 0.10). The top ten outcomes for caregivers were kidney function (importance score of 0.47), mortality (importance score of 0.31), life participation (importance score of 0.19), need for dialysis or transplant (importance score of 0.18), fatigue (importance score of 0.18), cardiovascular disease (importance score of 0.15), anxiety (importance score of 0.15), BP (importance score of 0.13), family impact (importance score of 0.13), and relapse (importance score of 0.10) (Supplemental Table 2).
Figure 1.
Kidney health, mortality and patient-reported outcomes were the most highly prioritized out of 58 outcomes ranked by importance score (error bars represent 95% confidence interval).
When analyzed by sex, men and women had the same top five outcomes in similar order: kidney function, mortality, need for dialysis or transplant, life participation, and fatigue (Supplemental Table 3). By age, the top four outcomes were the same in participants aged <51 years old and those 51 years old or older (Supplemental Table 4). In a crosscountry comparison, mortality and kidney function were consistently in the top three ranked outcomes (Supplemental Table 5).
Patients with CKD shared seven of the top ten outcomes with patients who had experienced dialysis and/or transplant (Supplemental Table 6). Patients with kidney-limited glomerular disease also shared seven of the top ten outcomes with those who had glomerular involvement as part of a systemic disease (Supplemental Table 7). Kidney function and mortality were consistently present in the top three for CKD stage and disease subgroups; life participation and fatigue were within the top five. Patients with largely proteinuric, kidney-limited conditions had similar top priorities to other subgroups, however, remission, relapse, and fluid retention were ranked in the top ten (Supplemental Table 8). Proteinuria was not highly prioritized by any subgroup.
Qualitative Findings
Three themes explained the reasons for the identification and prioritization of outcomes: constraining day-to-day existence (five subthemes), impaired agency and control over health (four subthemes), and threats to future health and family (four subthemes). The subthemes are outlined below, and selected quotations are presented in Table 2. The thematic schema (Figure 2) demonstrates the links among the themes and prioritization of outcomes.
Table 2.
Selected illustrative quotations for the themes and subthemes
| Subthemes | Illustrative Quotations |
|---|---|
| Constraining day-to-day existence | |
| Permeating and confining daily living | “This sickness is just killing me, I couldn’t focus on doing—I’m running a business myself, I just can’t get focused on anything. This memory thing is bothering me as well, because I think I can’t focus on anything, I’m not able to remember anything.” —Patient (man), Hong Kong |
| Permeating and confining daily living | “I’m very anxious all the time. It’s actually created almost a mental problem within me, of anxiety. I think that’s probably my worst side effect of having kidney disease.” —Patient (woman), Australia |
| Permeating and confining daily living | “I put life participation because I know that looking from the outside, I know [his kidney disease] stops [him] from thinking bigger…Although that’s really big, there’s this life that has to happen at the same time.” —Caregiver (woman), Australia |
| Altered appearance eroding self-confidence | “People couldn’t recognize me. I walked past old colleagues and had to introduce myself again because they couldn’t believe I was the same person.” —Patient (woman), Australia |
| Altered appearance eroding self-confidence | “It has a knock-on effect on your confidence because you lose hair. You lose confidence, and that’s very important. Self-esteem.” —Patient (woman), United States |
| Altered appearance eroding self-confidence | “When you go out you look quite horrible, you feel quite horrible. Particularly when your steroid dose goes up really high and you get that real moon face. It’s just awful. How do you live with that?” —Patient (woman), Australia |
| Trauma of past events | “If you’ve not experienced [dialysis] you can’t possibly comprehend how difficult it is.” —Patient (woman), United Kingdom |
| Trauma of past events | “The reason why we all have slightly different views as to what is one, two, three is because those are the things which impacted us the most when we got diagnosed with that condition” —Caregiver (man), Australia |
| Trauma of past events | “Yeah, keeping it away, because I don’t want to go through the hell again…that was probably one of the worst nights of my life.” —Patient (man), Australia |
| Loss of valued social and work opportunities | “I was going to do my job. But I couldn’t do it, just too exhausted. I knew that I couldn’t fulfill the role that I was doing, so very hard for me to say that I couldn’t go back to work, very hard. I found that really quite emotional time then.” —Patient (woman), United Kingdom |
| Loss of valued social and work opportunities | “I think it’s like people look at you and think oh, there’s nothing wrong with you. You’re not sick…You’re tired again, what’s wrong with you? Oh, you’re sick again, what’s wrong with you? They just don’t get it.” —Patient (woman), Australia |
| Loss of valued social and work opportunities | “I lost my job. It was huge for me. I was doing a lot of hours there as well, and I was constantly tired, but I loved it. I wasn’t as tired as I am now, but yeah, that really, really hurt, that they did that to me.” —Patient (woman), Australia |
| Undermining family roles and relationships | “I picked death, because now I’m fine, but there was a moment, when I saw how my children and grandchildren were affected by my condition, that I thought it would be better if I died. They would have to accept it if I died.” —Patient (woman), United States |
| Undermining family roles and relationships | “My husband actually has a man cave now and he doesn’t even live in my house. He said ‘I can’t live with you.’” —Patient (woman) Australia |
| Undermining family roles and relationships | “When I get sick I can’t help anybody. I can’t even help myself. And when she sees me being sick, that makes her more anxious, and that puts pressure on her. Then my father-in-law not being well, he then gets anxious. It’s just a cycle that keeps going round and round, so it does make it hard.”—Patient (woman), Australia |
| Impaired agency and control over health | |
| Demoralizing loss of freedom | “I can’t do anything except take medicine. I can only follow the instructions, taking low salts, low protein diets. There’s nothing more I can do. In other words, I can’t control. It seems I can’t control the whole thing.” —Patient (man), Hong Kong |
| Demoralizing loss of freedom | “When you’ve been in there a few times, you kind of feel constrained or imprisoned. You just want to be able to walk out and do something else.” —Patient (man), Australia |
| Demoralizing loss of freedom | “I say ‘what choice?’ They say, you have it or else you die…I think well, I better have it then.” —Patient (woman), United Kingdom |
| Fear of unexpected bodily harms | “Straight into hospital…dialysis for another 4 mo after I came out. But then, it just stopped. Stopped the dialysis for 2.5 yr. But it was a big surprise, because I didn’t feel sick. I felt fine. I was working like a madman, next day you’re in hospital and they’re saying that you’re really, really sick. I don’t feel sick.” —Patient (man), Australia |
| Fear of unexpected bodily harms | “They said 13% for him…to me that’s like my battery is low on my phone. You think he shouldn’t be able to, I would think he’d be in bed at that point, but then you’re working. They put these numbers out there” —Caregiver (woman), United States |
| Fear of unexpected bodily harms | “I didn’t think it was that serious. Got my blood test done, went to the doctor, the doctor said that this is stage four kidney disease. There were no symptoms. I’m still fine, I’m not on dialysis yet, but I’m currently running at 10%. It was a big shock.” —Patient (woman), Australia |
| Gaps in care | “Prednisolone is the killer, because that’s how I broke my back. I wasn’t told by the specialist or the GP when I was on Prednisolone, and then I did the weightlifting. I cracked my L2 and L5. Later on they told me oh, that could affect your bone. It’s too late.” —Patient (man), Australia |
| Gaps in care | “They didn’t say okay, you can’t have babies. Thanks for letting me know, you know?” —Patient (man), United Kingdom |
| Gaps in care | “We manage disease, but we don’t actually make people healthy…the pillars of health are diet, sleep, movement and exercise and stress management, and that if you get those things right, the body has an amazing capacity to heal itself if you nourish all of those things.” —Caregiver (woman), Australia |
| Managing triggers and driving factors | “I picked kidney function as number 1, because all the other conditions come from kidney failure, and if your kidneys are working, you won’t have any of that.” —Patient (woman), United Kingdom |
| Managing triggers and driving factors | “In my case stress, anxiety and depression. I have anger issues and if I keep them under control my medical condition will get better. Because if I’m able to control those, I’ll be able to control my medical condition. In the second place, my ability to work, my finances, if I’m able to control that, I’ll have a positive response to my treatment. Death is the least important to me.” —Patient (man), United States |
| Managing triggers and driving factors | “Dialysis and death doesn’t really worry me because it’s something I can’t control. Anxiety and stress. Time to dialysis and transplant is uncontrollable…The stress of worrying about it is more important…It’s the stress and anxiety of not being able to control something.” —Patient (man), Australia |
| Threats to future self and family | |
| Adaptability to diverging expectations | “[Anxiety, cognitive function] Your life changes completely when you get all this crap. Completely changes. Changes you. I don’t feel like I am the same person. My brain doesn’t work anymore.” —Patient (woman), United Kingdom |
| Adaptability to diverging expectations | “The dialysis word is a very scary word…I went you know what, we can live with this. It’s not something that’s going to define my life completely, there are still going to be options.” —Patient (woman), United Kingdom |
| Adaptability to diverging expectations | “[Life participation] It was more like, you’re not going to go back to that. You need to learn how to go around and come back. To me, the first couple years I was angry. This is really an inconvenience. That’s why my first word was frustrated.” —Patient (woman), United States |
| Endangering life goals | “My mum’s a teacher and she’s been teaching for 45 yr, and I would love to be able to do that. I think that’s why it’s different. It’s not a usual activity for me, it’s something else.” —Patient (woman), Australia |
| Endangering life goals | “We found out when I was 30 wk pregnant…I was hospitalized. Sorry, no more children. That’s the end. That was a big impact for us.” —Patient (woman), Australia |
| Endangering life goals | “You can’t work, so your income isn’t what you envisaged it was going to be…when you thought you were contributing to your pension. All of a sudden it’s wiped out.” —Patient (man), United Kingdom |
| Inevitable, irreversible consequences | “Eventually you’re going to end up with dialysis or transplant. Everything else fits in around that. My end result is this.” —Patient (man), Australia |
| Inevitable, irreversible consequences | “Dialysis in 1 yr time, probably a kidney transplant in future. That will be my story.” —Patient (man), Australia |
| Inevitable, irreversible consequences | “I’m unlucky…The doctor told me that the kidney wouldn’t get well by itself. It’ll just get worse and worse. I feel very worried about that.” —Patient (woman), Hong Kong |
| Uncertainty and unpredictable hazards | “You definitely need to know whether it’s going to get back to that remission again, or you’re just going to continue on having these ups and downs all the time.” —Patient (man), Australia |
| Uncertainty and unpredictable hazards | “When you’re on dialysis, anything could happen.” —Patient (woman), United States |
| Uncertainty and unpredictable hazards | “Predictability. I’m looking at it from my perspective as a mother and a caregiver. It affects the whole thing, like her future, her health status, financial-wise, whatever.” —Caregiver (woman), Australia |
Figure 2.
Thematic schema indicating three themes that underpin the prioritization of major outcome groups.
Constraining Day-to-Day Existence.
Permeating and Confining Daily Living.
Symptoms described as “relentless” and all “consuming” (e.g., anxiety) were highly prioritized because they restricted daily activities. Some outcomes were “exhausting” (e.g., fatigue, cognitive function) and impaired their ability to perform basic daily tasks because “it’s a struggle.” Patients were frustrated by their “very restrictive lifestyle.”
Altered Appearance Eroding Self-Confidence.
For some patients, “horrible” and “embarrassing” changes to their appearance were of high priority because they lost “confidence…self-esteem.” This caused “anxiety and stress,” which impaired social functioning and work. Some lost a sense of self—“people couldn’t recognize me…couldn’t believe I was the same person” and “I was bloated and looked like a monster.”
Trauma of Past Events.
“Terrifying” outcomes were ranked highly because they were “scary and sudden” or “very hurtful” (e.g., infection, loss of kidney, or loss of cognitive function). Recurrent, “damaging” outcomes (e.g., dialysis or relapse) were also prioritized highly because participants wanted to avoid going “through the hell again.” Outcomes that occurred around the time of traumatic events (for example, at diagnosis or near-death experiences) were seen as important (e.g., infection, swelling, and hospitalization).
Loss of Valued Social and Work Opportunities.
Symptoms that threatened patients’ ability to work and their financial means (e.g., fatigue and cognitive function) were “stressful” and highly prioritized. Participants valued life participation and ability to work because they feared being limited in these areas, and this was compounded by a lack of understanding and empathy from friends, colleagues, and employers due to their “silent” glomerular disease.
Undermining Family Roles and Relationships.
Outcomes that caused patients to feel that they were a “burden” on others (e.g., need for dialysis or transplant, fatigue, or mobility) were prioritized highly because of the “toll” caused by anxiety, guilt, and depression. Mortality, need for dialysis or transplant, and ability to work were “feared” and highly ranked if they jeopardized patients’ abilities to provide and care for their family. Outcomes were important if they threatened their relationship with their partner, fertility, or ability to fulfill parental responsibilities (e.g., mood swings, restless legs, or anxiety).
Impaired Agency and Control over Health.
Demoralizing Loss of Freedom.
Patients felt “depressed” and anxious by “untreatable” outcomes that they “can’t control” and ranked them highly. Patients felt “constrained or imprisoned” by time-consuming and inflexible outcomes for which there were no other options (e.g., need for dialysis or transplant and hospitalization). Some participants gave lower priority to “inevitable” outcomes, such as dialysis or death, because they could not alter them.
Fear of Unexpected Bodily Harms.
Patients were scared of “silent surprises” from outcomes that came “out of nowhere” because they “felt fine” (e.g., kidney function or proteinuria). This was “confronting” and caused anxiety. Patients were “never quite sure” what was happening, which compounded their sense of not “knowing” about their disease.
Gaps in Care.
Missed opportunities to prevent disease and inadequate or dismissive counseling by health care providers drove some patients to give high priority to outcomes such as kidney function, proteinuria, and bone health. Life participation, depression, anxiety, and ability to work were highly prioritized by patients who felt that their concerns in these areas were not addressed, and they similarly prioritized outcomes that “nourished” them (e.g., sleep, strength, and physical functioning). Patients were fearful and felt a sense of betrayal around adverse treatment outcomes of which they previously unaware (e.g., fertility and diabetes), and they ranked these higher.
Managing Triggers and Driving Factors.
Patients valued outcomes that were seen as a “root cause” or “key driver” of other important and “inter-related” outcomes (e.g., kidney function, proteinuria, relapse, or infection), especially if modifying them might prevent a “cascade of events.” Control over an outcome “increased certainty” and reduced anxiety; a lack of control meant that anxiety was more highly prioritized because it exacerbated and complicated the management of other outcomes (e.g., relapse or depression). Biochemical and clinical outcomes were valued if they increased the patient’s ability to monitor and manage the disease (e.g., proteinuria, kidney function, or BP).
Threats to Future Self and Family.
Adaptability to Diverging Expectations.
Patients wanted to “return to their lives” and ranked outcomes higher the more that their disease or treatment changed those outcomes (e.g., life participation and ability to work). Patients highly prioritized outcomes that threatened their identity (e.g., cognitive function and anxiety) because they felt that they were not “the same person” and did not want their disease to “define” them. Acceptance of some outcomes (e.g., need for dialysis or transplant and mortality) led to a lower priority because they were “built into” their lives and made a part of their “story.”
Endangering Life Goals.
Outcomes were highly ranked if they compromised “envisaged” goals or key roles during future stages of life (e.g., fertility, life participation, or ability to work). Patients highly prioritized more immediate “obstacles” to life goals (e.g., need for dialysis or transplant) or if they irreversibly “wiped out” future potential (e.g., fertility). Mortality, in particular, was highly ranked for all of these reasons.
Inevitable, Irreversible Consequences.
Patients highly prioritized kidney function and outcomes that “kept the damage at bay” (e.g., remission) because “scarring” meant that their kidneys could not “regenerate.” They were terrified of being “locked in to a certain path” and just waiting for “inevitable” and “grim” consequences (e.g., need for dialysis or transplant). Need for dialysis and transplant was seen as the “ultimate issue” that “everything else fits around” and a precursor to death.
Uncertainty from Unpredictable Hazards.
Patients ranked outcomes higher if they increased uncertainty where “anything could happen” or anxiety around “what the future holds” (e.g., relapse, cancer, and need for dialysis or transplant). Other patients gave those outcomes negatively affected by uncertainty or anxiety a higher priority (e.g., ability to work, finances, and life participation). Patients valued outcomes that increased “stability” and “predictability” in their lives (e.g., remission).
Discussion
Overall, patients with glomerular disease and their caregivers highly prioritized kidney function, an outcome reflecting disease progression and loss of kidney function, followed by mortality and need for dialysis or transplant. The patient-reported outcomes of life participation, fatigue, anxiety, and effect on family were also consistently and highly ranked. These outcomes were given higher priority because they led to extensive and distressing effects on patients’ current or future lifestyles, were unpredictable and difficult to control, and caused or exacerbated other important outcomes, such as depression, ability to work, and finances.
Kidney function was of the utmost importance to patients with glomerular disease and their caregivers. For patients, being able to know and monitor changes in their kidney function meant that they could better understand their condition, and this strengthened their sense of having agency in their health care. They feared the potential for asymptomatic yet irreversible deterioration in kidney function. Our results suggest that patients perceive kidney function to be a more important outcome than need for dialysis or transplant because this reflects their goal of preserving kidney function and an overall healthy life while avoiding the need for dialysis or transplant. Need for dialysis or transplant remains an important but perhaps less highly prioritized outcome for patients who have already commenced KRT.
Across all subgroups, kidney function and mortality were ranked within the top three, and need for dialysis or transplant was in the top seven. The top-ranked outcomes were generally concordant by country, age, sex, and patient/caregiver role. Mortality was ranked first in the United Kingdom and the United States; kidney function was the top outcome in Australia and Hong Kong. These differences potentially relate to patient perception of value within their health care system, practice patterns, or systems of care. For disease subgroups (stage and type), the top outcomes were generally consistent, but there were some expected differences in rankings. Fluid retention and relapse/remission were generally not highly ranked except by those patients with a typically proteinuric, kidney-limited disease. Proteinuria was not highly prioritized by any subgroup, including by patients with predominantly proteinuric conditions. These disparities in outcomes between subgroups were anticipated and reflect the divergent “second tier” priorities for patients with different types of glomerular disorders.
Notably, the patient-reported outcomes of life participation, fatigue, anxiety, and family impact were highly prioritized by patients and caregivers. Patients with active glomerular disease have a poor health-related quality of life, and they often have anxiety and depression (24,37–40). Fatigue is a frequent, under-recognized, and highly disabling symptom in patients with vasculitis (10,41), but it is also of concern in those with kidney-limited glomerular disease or nephrotic conditions, and it is worst in those who are on dialysis (37,40,42). Swelling has previously been shown to have a strong negative association with health-related quality of life in predominantly proteinuric glomerular diseases (37).
Systematic reviews in membranous nephropathy, IgA nephropathy, and renal vasculitis show that the top three outcomes prioritized by patients and caregivers (kidney function, mortality, and need for dialysis or transplant) are among those most frequently reported (16–18). However, the disparity between the length of a clinical trial and the time to kidney failure or mortality in many glomerular diseases contributes to under-reporting of these critically important outcomes. Recent data suggest that short-term decline in GFR could be used as a surrogate end point for disease progression in trials (43). Our results provide support for the use of GFR slope as a surrogate trial end point from patient and caregiver perspectives.
Despite their importance to patients, patient-reported outcomes have not been routinely reported in trials in glomerular disease to date (44,45). Trials in oncology show that reporting health-related quality of life provides better information on the tradeoff between patient experience and survival, thereby improving communication and decision making and quality of life (46,47). Recent trials in glomerular disease have assessed quality of life using generic instruments, although this can lead to disease-specific outcomes (e.g., swelling) being incompletely captured (48,49). The prospective Cure Glomerulonephropathy cohort plans to collect patient-reported data on both generic and disease-specific outcomes (50). Glomerular disease-specific measures for patients with systemic ANCA-associated vasculitis and the FSGS symptom diary/effect questionnaire are starting to be validated (11,51). However, the importance of these outcomes to patients and caregivers highlights the need to develop instruments that can be used in specific diseases but also across a range of glomerular diseases.
Our study involved patients and caregivers from four countries who spoke two languages and had diverse demographic and clinical characteristics. Quantitative and qualitative methods were used to elicit patient priorities for outcomes and understand the reasons for their prioritization. However, there were some limitations. It is possible that the priorities and experiences of patients with specific (especially rarer) types of glomerular disease may have been missed. Moreover, the small sample for some subgroups limited the ability to make valid comparisons. Patients who were reluctant or unable to participate in a group setting may not have attended the focus groups. Finally, we did not include patients from low- and middle-income countries, and thus, the transferability of the findings beyond our setting remains uncertain.
Trials in glomerular diseases have specific challenges that necessitate a consensus-based collaborative approach (44,52). This study, as part of the Standardized Outcomes in Nephrology–Glomerular Disease initiative, will inform the development of a core outcome set for trials in glomerular disease on the basis of the shared priorities of patients, caregivers, and health professionals (36). Validated measures for each outcome will then be identified using a similar consensus-driven methodology. Although the top-ranked outcomes were concordant across the different subgroups of glomerular disease, future work is required to establish consensus on important disease-specific outcomes and their measures.
Patients and caregivers gave highest priority to the outcomes of kidney function, mortality, and need for dialysis or transplant. Importantly, they also highly prioritized patient-reported outcomes, such as life participation and fatigue, that are less well reported. Involving patients and caregivers in establishing outcomes to be reported in research can strengthen a patient-centered evidence base that supports shared decision making and better outcomes for patients with glomerular disease.
Disclosures
Dr. Caster reports consulting fees from Retrophin and being a site principal investigator for clinical trials from Aurinia, Calliditas, Retrophin, and Mallinckrodt outside the submitted work. Dr. Floege reports personal fees from Calliditas, Retrophin, Omeros, and Visterra during the conduct of the study. Dr. Hladunewich reports other from Pfizer, personal fees from Ontario Renal Network, personal fees from Uptodate, grants from CureGlomerulonephropathy, grants from Neptune, grants from Ionis, grants from Chemocentryx, and grants from Calliditas outside the submitted work. Dr. Hogan reports personal fees from Retrophin, personal fees from Dimerix, personal fees from Zyversa, personal fees from GSK, other from Calliditas, other from Omeros, and other from Achillion outside the submitted work. Dr. Johnson reports grants and personal fees from Baxter Healthcare, Fresenius Medical Care, personal fees from AstraZeneca, AWAK, and Ono, grants from the National Health and Medicla Research Council of Australia, and other from Amgen outside the submitted work. Dr. Lafayette reports grants and personal fees from Mallinckrodt, Riegel, Aurinia, Retrophin, and Calliditas, grants from Roche, and personal fees from Relypsa outside the submitted work. Dr. Rovin reports personal fees from Aurinia, personal fees from Calliditas, personal fees from Chemocentryx, personal fees from Retrophin, personal fees from Novartis, personal fees from Omeros, personal fees from Morphosys, personal fees from EMD Serono, personal fees from Bristol Myers Squibb, personal fees from Janssen, personal fees from AstraZeneca, nonfinancial support from the Lupus Foundation of America, and grants from the National Institutes of Health outside the submitted work. Dr. Wilkie reports speakers honoraria for Baxter and Fresenius and consultancy work for Triomed. All remaining authors have nothing to disclose.
Funding
This project is supported by National Health and Medical Research Council Program grant 1092957. Dr. Carter is supported by National Health and Medical Research Council Postgraduate Scholarship grant 1168994. Dr. Caster and Dr. Cattran are supported by National Institutes of Health grant K08DK102542. Dr. Cho is supported by National Health and Medical Research Council Early Career Fellowship grant 1126256. Ms. Gutman is supported by National Health and Medical Research Council Postgraduate Scholarship grant 1169149. Dr. Johnson is supported by National Health and Medical Research Council Practitioner Fellowship grant 1117534. Dr. Shen is supported by National Institutes of Health grant K23DK103972. Dr. Teixeira-Pinto is supported by National Health and Medical Research Council Career Development Fellowship grant 1106716. Dr. Viecelli received support from National Health and Medical Research Council Medical Postgraduate Scholarship grant 1114539.
Supplementary Material
Acknowledgments
We thank the patients and caregivers who generously offered their time and insights for this study.
Preliminary results from this study were presented in an abstract at the American Society of Nephrology Kidney Week, November 5–10, 2019.
The funding bodies did not have a role in the design, collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13101019/-/DCSupplemental.
Supplemental File 1. Run sheet.
Supplemental File 2. Relative importance score.
Supplemental Table 1. Comorbid patient conditions.
Supplemental Table 2. Top ten outcomes of patients and caregivers.
Supplemental Table 3. Top ten outcomes by sex.
Supplemental Table 4. Top ten outcomes by age.
Supplemental Table 5. Top ten outcomes by country.
Supplemental Table 6. Top ten outcomes by stage of kidney disease.
Supplemental Table 7. Top ten outcomes by glomerular disease subgroup.
Supplemental Table 8. Top ten outcomes for predominantly proteinuric kidney-limited disease.
References
- 1.Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, Itabashi M, Takei T, Uchida K, Nitta K: Prognosis in IgA nephropathy: 30-Year analysis of 1,012 patients at a single center in Japan. PLoS One 9: e91756, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetmore JB, Guo H, Liu J, Collins AJ, Gilbertson DT: The incidence, prevalence, and outcomes of glomerulonephritis derived from a large retrospective analysis. Kidney Int 90: 853–860, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Jefferson JA: Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol 13: 1264–1275, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faurschou M, Mellemkjaer L, Voss A, Keller KK, Hansen IT, Baslund B: Prolonged risk of specific malignancies following cyclophosphamide therapy among patients with granulomatosis with polyangiitis. Rheumatology (Oxford) 54: 1345–1350, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Heaf JG, Hansen A, Laier GH: Quantification of cancer risk in glomerulonephritis. BMC Nephrol 19: 27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heijl C, Harper L, Flossmann O, Stücker I, Scott DG, Watts RA, Höglund P, Westman K, Mahr A; European Vasculitis Study Group (EUVAS): Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: Follow-up data from European Vasculitis Study Group clinical trials. Ann Rheum Dis 70: 1415–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Blom K, Odutayo A, Bramham K, Hladunewich MA: Pregnancy and glomerular disease: A systematic review of the literature with management guidelines. Clin J Am Soc Nephrol 12: 1862–1872, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huong DL, Amoura Z, Duhaut P, Sbai A, Costedoat N, Wechsler B, Piette JC: Risk of ovarian failure and fertility after intravenous cyclophosphamide. A study in 84 patients. J Rheumatol 29: 2571–2576, 2002 [PubMed] [Google Scholar]
- 9.Grootscholten C, Snoek FJ, Bijl M, van Houwelingen HC, Derksen RH, Berden JH; Dutch Working Party of SLE: Health-related quality of life and treatment burden in patients with proliferative lupus nephritis treated with cyclophosphamide or azathioprine/ methylprednisolone in a randomized controlled trial. J Rheumatol 34: 1699–1707, 2007 [PubMed] [Google Scholar]
- 10.Herlyn K, Hellmich B, Seo P, Merkel PA: Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 62: 1639–1645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathias SD, Vallow S, Gipson DS, Thorneloe KS, Sprecher D: Development of focal segmental glomerulosclerosis patient-reported outcome measures: Symptom diary and symptom impact questionnaire. Am J Kidney Dis 70: 532–540, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Cattran DC: Toward quantitating the burden of glomerulonephritis in the United States. Kidney Int 90: 732–734, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Knoop T, Vikse BE, Svarstad E, Leh S, Reisæter AV, Bjørneklett R: Mortality in patients with IgA nephropathy. Am J Kidney Dis 62: 883–890, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, Kim S, Chin HJ: Mortality and renal outcome of primary glomerulonephritis in Korea: Observation in 1,943 biopsied cases. Am J Nephrol 37: 74–83, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM: Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 27: 3248–3254, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Schieppati A, Chen X, Cai G, Zamora J, Giuliano GA, Braun N, Perna A: Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev 10: CD004293, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, Molony DA, Schena FP, Strippoli GF: Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 8: CD003965, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Walters G, Willis NS, Craig JC: Interventions for renal vasculitis in adults. Cochrane Database Syst Rev 9: CD003232, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Price A, Albarqouni L, Kirkpatrick J, Clarke M, Liew SM, Roberts N, Burls A: Patient and public involvement in the design of clinical trials: An overview of systematic reviews. J Eval Clin Pract 24: 240–253, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Sautenet B, Tong A, Chapman JR, Warrens AN, Rosenbloom D, Wong G, Gill J, Budde K, Rostaing L, Marson L, Josephson MA, Reese PP, Pruett TL, Evangelidis N, Craig JC: Range and consistency of outcomes reported in randomized trials conducted in kidney transplant recipients: A systematic review. Transplantation 102: 2065–2071, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Sautenet B, Tong A, Williams G, Hemmelgarn BR, Manns B, Wheeler DC, Tugwell P, van Biesen W, Winkelmayer WC, Crowe S, Harris T, Evangelidis N, Hawley CM, Pollock C, Johnson DW, Polkinghorne KR, Howard K, Gallagher MP, Kerr PG, McDonald SP, Ju A, Craig JC: Scope and consistency of outcomes reported in randomized trials conducted in adults receiving hemodialysis: A systematic review. Am J Kidney Dis 72: 62–74, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Howell M, Wong G, Turner RM, Tan HT, Tong A, Craig JC, Howard K: The consistency and reporting of quality-of-life outcomes in trials of immunosuppressive agents in kidney transplantation: A systematic review and meta-analysis. Am J Kidney Dis 67: 762–774, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O’Neill R, Kennedy DL: Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 10[Suppl 2]: S125–S137, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Daleboudt GM, Berger SP, Broadbent E, Kaptein AA: Health-related quality of life in patients with systemic lupus erythematosus and proliferative lupus nephritis. Psychol Health Med 16: 393–404, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Neogi T, Jick S: A cohort study of comorbidity in patients with granulomatosis with polyangiitis. Rheumatology (Oxford) 57: 291–299, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Tong A, Gill J, Budde K, Marson L, Reese PP, Rosenbloom D, Rostaing L, Wong G, Josephson MA, Pruett TL, Warrens AN, Craig JC, Sautenet B, Evangelidis N, Ralph AF, Hanson CS, Shen JI, Howard K, Meyer K, Perrone RD, Weiner DE, Fung S, Ma MKM, Rose C, Ryan J, Chen LX, Howell M, Larkins N, Kim S, Thangaraju S, Ju A, Chapman JR; SONG-Tx Investigators: Toward establishing core outcome domains for trials in kidney transplantation: Report of the standardized outcomes in nephrology-kidney transplantation consensus workshops. Transplantation 101: 1887–1896, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies SJ: Reaching consensus on the important outcomes for peritoneal dialysis patients. Kidney Int 96: 545–546, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Tse KCTC, Tang CS, Lio WI, Lam MF, Chan TM: Quality of life comparison between corticosteroid- and-mycofenolate mofetil and corticosteroid- and-oral cyclophosphamide in the treatment of severe lupus nephritis. Lupus 15: 371–379, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mathias SD, Berry P, De Vries J, Askanase A, Pascoe K, Colwell HH, Chang DJ: Development of the Systemic Lupus Erythematosus Steroid Questionnaire (SSQ): A novel patient-reported outcome tool to assess the impact of oral steroid treatment. Health Qual Life Outcomes 15: 43, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van de Ven AH, Delbecq AL: The nominal group as a research instrument for exploratory health studies. Am J Public Health 62: 337–342, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corner J, Wright D, Hopkinson J, Gunaratnam Y, McDonald JW, Foster C: The research priorities of patients attending UK cancer treatment centres: Findings from a modified nominal group study. Br J Cancer 96: 875–881, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manera KE, Johnson DW, Craig JC, Shen JI, Ruiz L, Wang AY, Yip T, Fung SKS, Tong M, Lee A, Cho Y, Viecelli AK, Sautenet B, Teixeira-Pinto A, Brown EA, Brunier G, Dong J, Dunning T, Mehrotra R, Naicker S, Pecoits-Filho R, Perl J, Wilkie M, Tong A: Patient and caregiver priorities for outcomes in peritoneal dialysis: Multinational nominal group technique study. Clin J Am Soc Nephrol 14: 74–83, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson T, Hewlett S, Richards P, Morris M, Calnan M: Utilizing qualitative data from nominal groups: Exploring the influences on treatment outcome prioritization with rheumatoid arthritis patients. J Health Psychol 17: 132–142, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Wortley S, Tong A, Howard K: Preferences for engagement in health technology assessment decision-making: A nominal group technique with members of the public. BMJ Open 6: e010265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black N: Consensus development methods. In: Qualitative Research in Health Care, 3rd Ed., edited by Pope C, Mays N, Malden, MA, Blackwell, 2006 [Google Scholar]
- 36.Carter SA, Lightstone L, Cattran D, Bagga A, Barbour SJ, Barratt J, Boletis J, Caster D, Coppo R, Fervenza FC, Floege J, Hladunewich M, Hogan JJ, Kitching AR, Lafayette R, Malvar A, Radhakrishnan J, Rovin BH, Zhang H, Gutman T, Howell M, Logeman C, Shen JI, Teixeira-Pinto A, Alexander SI, Cho Y, Craig JC, Harris D, Johnson DW, Kerr PG, Ryan J, Viecelli AK, Wang AY, Wilkie M, Scholes-Robertson N, Tong A; SONG-GD Initiative: Standardized Outcomes in Nephrology-Glomerular Disease (SONG-GD): Establishing a core outcome set for trials in patients with glomerular disease. Kidney Int 95: 1280–1283, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canetta PA, Troost JP, Mahoney S, Kogon AJ, Carlozzi N, Bartosh SM, Cai Y, Davis TK, Fernandez H, Fornoni A, Gbadegesin RA, Herreshoff E, Mahan JD, Nachman PH, Selewski DT, Sethna CB, Srivastava T, Tuttle KR, Wang CS, Falk RJ, Gharavi AG, Gillespie BW, Greenbaum LA, Holzman LB, Kretzler M, Robinson BM, Smoyer WE, Guay-Woodford LM, Reeve B, Gipson DS; CureGN Consortium: Health-related quality of life in glomerular disease. Kidney Int 95: 1209–1224, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Middleton JP, Vehaskari VM, Hogan SL, Vento S, Flynn PA, Powell LM, McMahan JL, Siegel N, Friedman AL: Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int 79: 678–685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolly M, Toloza S, Goker B, Clarke AE, Navarra SV, Wallace D, Weisman M, Mok CC: Disease-specific quality of life in patients with lupus nephritis. Lupus 27: 257–264, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Libório AB, Santos JP, Minete NF, Diógenes CA, Soares AP, Queiroz AL, Barreto DM: Proteinuria is associated with quality of life and depression in adults with primary glomerulopathy and preserved renal function. PLoS One 7: e37763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mozaffarian N, Lobosco S, Lu P, Roughley A, Alperovich G: Satisfaction with control of systemic lupus erythematosus and lupus nephritis: Physician and patient perspectives. Patient Prefer Adherence 10: 2051–2061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urquhart-Secord R, Craig JC, Hemmelgarn B, Tam-Tham H, Manns B, Howell M, Polkinghorne KR, Kerr PG, Harris DC, Thompson S, Schick-Makaroff K, Wheeler DC, van Biesen W, Winkelmayer WC, Johnson DW, Howard K, Evangelidis N, Tong A: Patient and caregiver priorities for outcomes in hemodialysis: An international nominal group technique study. Am J Kidney Dis 68: 444–454, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, Simon AL, Ying J, Beck GJ, Wanner C, Floege J, Li PK, Perkovic V, Vonesh EF, Greene T: GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30: 1735–1745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baigent C, Herrington WG, Coresh J, Landray MJ, Levin A, Perkovic V, Pfeffer MA, Rossing P, Walsh M, Wanner C, Wheeler DC, Winkelmayer WC, McMurray JJV; KDIGO Controversies Conference on Challenges in the Conduct of Clinical Trials in Nephrology Conference Participants: Challenges in conducting clinical trials in nephrology: Conclusions from a kidney disease-improving global outcomes (KDIGO) controversies conference. Kidney Int 92: 297–305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig JC, Tong A, Strippoli GF: New approaches to trials in glomerulonephritis. Nephrol Dial Transplant 32[Suppl 1]: i1–i6, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Dutton SJ, Ferry DR, Blazeby JM, Abbas H, Dahle-Smith A, Mansoor W, Thompson J, Harrison M, Chatterjee A, Falk S, Garcia-Alonso A, Fyfe DW, Hubner RA, Gamble T, Peachey L, Davoudianfar M, Pearson SR, Julier P, Jankowski J, Kerr R, Petty RD: Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 15: 894–904, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, Swinson D, Falk S, Chau I, Cunningham D, Kareclas P, Cook N, Blazeby JM, Dunn JA; COUGAR-02 Investigators: Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol 15: 78–86, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC; MENTOR Investigators: Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381: 36–46, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Li X, Liu Z, Wang L, Wang R, Ding G, Shi W, Fu P, He Y, Cheng G, Wu S, Chen B, Du J, Ye Z, Tao Y, Huo B, Li H, Chen J: Tacrolimus monotherapy after intravenous methylprednisolone in adults with minimal change nephrotic syndrome. J Am Soc Nephrol 28: 1286–1295, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariani LH, Bomback AS, Canetta PA, Flessner MF, Helmuth M, Hladunewich MA, Hogan JJ, Kiryluk K, Nachman PH, Nast CC, Rheault MN, Rizk DV, Trachtman H, Wenderfer SE, Bowers C, Hill-Callahan P, Marasa M, Poulton CJ, Revell A, Vento S, Barisoni L, Cattran D, D’Agati V, Jennette JC, Klein JB, Laurin LP, Twombley K, Falk RJ, Gharavi AG, Gillespie BW, Gipson DS, Greenbaum LA, Holzman LB, Kretzler M, Robinson B, Smoyer WE, Guay-Woodford LM; CureGN Consortium: CureGN study rationale, design, and methods: Establishing a large prospective observational study of glomerular disease. Am J Kidney Dis 73: 218–229, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robson JC, Dawson J, Doll H, Cronholm PF, Milman N, Kellom K, Ashdown S, Easley E, Gebhart D, Lanier G, Mills J, Peck J, Luqmani RA, Shea J, Tomasson G, Merkel PA: Validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire. Ann Rheum Dis 77: 1157–1164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leaf DE, Appel GB, Radhakrishnan J: Glomerular disease: Why is there a dearth of high quality clinical trials? Kidney Int 78: 337–342, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.