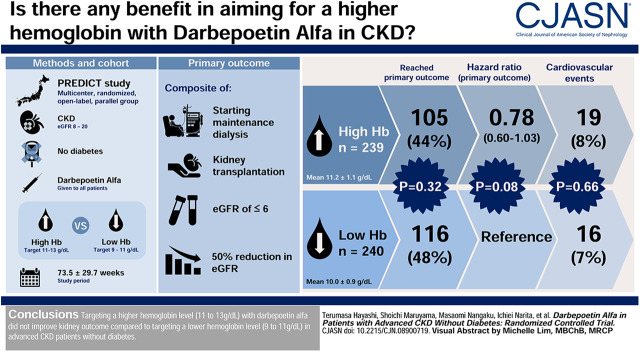
Visual Abstract
Keywords: chronic kidney disease; hemoglobin; renal outcome; non-diabetic; Darbepoetin alfa; Hematinics; renal dialysis; glomerular filtration rate; kidney transplantation; Proportional Hazards Models; Renal Insufficiency, Chronic; diabetes mellitus; Hemoglobins; Cardiovascular Diseases
Abstract
Background and objectives
Large, randomized, controlled trials targeting higher hemoglobin level with erythropoiesis-stimulating agents for Western patients with CKD showed harm. However, the effect of anemia correction using erythropoiesis-stimulating agents may differ between CKD subpopulations. The Prevention of ESKD by Darbepoetin Alfa in CKD Patients with Non-diabetic Kidney Disease study, a multicenter, randomized, open-label, parallel-group study, aimed to examine the effect of targeting hemoglobin levels of 11–13 g/dl using darbepoetin alfa with reference to a low-hemoglobin target of 9–11 g/dl on kidney outcome in patients with advanced CKD without diabetes in Japan.
Design, setting, participants, & measurements
We enrolled 491 patients with CKD without diabetes, and an eGFR of 8–20 ml/min per 1.73 m2. Of these 491 patients, 239 and 240 were ultimately assigned to the high- and low-hemoglobin groups, respectively (12 patients were excluded). The primary outcome was a kidney composite end point (starting maintenance dialysis, kidney transplantation, eGFR≤6 ml/min per 1.73 m2, and 50% reduction in eGFR).
Results
Mean hemoglobin levels were 11.2±1.1 and 10.0±0.9 g/dl in the high- and low-hemoglobin groups, respectively, during the mean study period of 73.5±29.7 weeks. The kidney composite end point occurred in 105 (44%) and 116 (48%) patients in the high- and low-hemoglobin groups, respectively (log-rank test; P=0.32). The adjusted Cox proportional hazards model showed that the hazard ratio for the high- versus low-hemoglobin group was 0.78 (95% confidence interval, 0.60 to 1.03; P=0.08). Cardiovascular events occurred in 19 (8%) and 16 (7%) patients in each group, respectively, with no significant between-group difference (log-rank test; P=0.66).
Conclusions
Targeting a higher hemoglobin level (11–13 g/dl) with darbepoetin alfa did not improve kidney outcome compared with targeting a lower hemoglobin level (9–11 g/dl) in patients with advanced CKD without diabetes.
Clinical Trial registry name and registration number
Prevention of ESKD by Darbepoetin Alfa in CKD Patients with Non-diabetic Kidney Disease (PREDICT), NCT01581073.
Introduction
Anemia is a common complication of CKD and is associated with cardiovascular morbidity and mortality (1–3). Studies on erythropoiesis-stimulating agent (ESA) therapy for anemia and its effects on various outcomes have been conflicting in the context of kidney protection, decrease of cardiovascular events, and improvement of life expectancy (4–10). A series of large, randomized, controlled trials (RCTs) conducted in the 2000s comparing the effect of normalizing hemoglobin level (>13 g/dl) with conservative hemoglobin level (10–11 g/dl) on overall, cardiovascular, and kidney outcomes showed that setting the target hemoglobin level >13 g/dl in predialysis patients with CKD might increase mortality, cardiovascular events (especially stroke in patients with diabetes), and ESKD (6–8). On the basis of these results, the 2012 Kidney Disease: Improving Global Outcomes Guidelines set an upper limit of hemoglobin target of 11.5 g/dl for predialysis patients with CKD (11). Study participants enrolled in the above RCTs had a high cardiovascular burden (e.g., approximately half of the participants had a history of cardiovascular events) and high-dose ESAs were used to achieve high target hemoglobin levels. Post hoc analyses of these RCTs suggested that poor outcomes might not have resulted from achieving higher hemoglobin level itself, but resulted from toxicities associated with high-dose ESAs, patient-related factors promoting ESA hyporesponsiveness, or a combination of both (12,13). Recently, the CKD-Japan Cohort (CKD-JAC) study demonstrated that the incidence of cardiovascular events and death in Japanese predialysis patients with CKD was much lower than that of Western patients with CKD (14). Thus, the effect of anemia correction using ESAs on clinical outcomes may differ between CKD subpopulations (e.g., among races or between dialysis and nondialysis, diabetic and nondiabetic, and elderly and younger).
In this Prevention of ESKD by Darbepoetin Alfa in CKD Patients with Non-diabetic Kidney Disease (PREDICT) trial, we hypothesized that targeting a higher hemoglobin level (11–13 g/dl) using darbepoetin alfa, compared with targeting a lower hemoglobin level (9–11 g/dl), would prevent ESKD in patients with advanced CKD without diabetes in Japan.
Materials and Methods
Study Design
This was a multicenter, randomized, open-label, parallel-group study conducted on patients with anemia and advanced CKD, without diabetes. The study design and other details of the study protocol have been published elsewhere (15). The protocol was approved by the institutional review board of each participating center, and the trial was conducted under the health insurance system of Japan, and in accordance with the principles of the Declaration of Helsinki; Ethical Guidelines on Clinical Studies of the Ministry of Health, Labor, and Welfare of Japan; and International Conference on Harmonization Good Clinical Practice guidelines. This study was also performed according to the Consolidated Standards of Reporting Trials 2010 Statement (16). Written, informed consent was provided by each participant. The trial was designed, implemented, and overseen by the PREDICT Executive Committee, together with representatives of Translational Research Center for Medical Innovation, Kobe, Japan, a third-party organization that is independent of the investigators’ institutions and was responsible for randomization, allocation, and onsite monitoring in addition to data collection, data management, and data analysis. The study was registered at Clinicaltrials.gov (identifier NCT01581073).
Study Population
We enrolled 491 patients with CKD without diabetes, aged 20–85 years, with an eGFR of 8–20 ml/min per 1.73 m2 (calculated with the Japanese Equation [17]) and with hemoglobin <10 g/dl at 74 sites in Japan. The registration period was between December 2011 and June 2014, and the observation period ended in June 2016, 2 years after the enrollment of the last patient. Major exclusion criteria were an anticipated need for maintenance dialysis treatment or kidney transplantation within 6 months from registration, iron deficiency defined as transferrin saturation (TSAT) <20% and serum ferritin <100 ng/ml (18), uncontrolled hypertension (systolic BP >180 mm Hg or diastolic BP >110 mm Hg), congestive heart failure (New York Heart Association class 3 or 4), malignancy, hematologic disorders, chronic inflammation, infection, and active bleeding. Furthermore, patients with myocardial infarction within 6 months, symptomatic stroke, and pulmonary thrombosis or embolism within 1 year were excluded.
Randomization and Masking
Patients were enrolled through a web-based registration and follow-up system provided by the Translational Research Informatics Center, Kobe, Japan. The system automatically evaluated the eligibility of each patient and randomly assigned participants to either a high- or low-hemoglobin group (1:1 allocation), stratified by pretreatment hemoglobin, proteinuria, and eGFR levels, and prior use of ESAs. The study was open label, with both patients and physicians aware of treatment assignment.
Intervention
Darbepoetin alfa was recommended to be administered along its product information (30 μg every 2 weeks for the initial dose and 60–240 μg/mo for the maintenance dose, administered subcutaneously or intravenously) (19). In the high-hemoglobin group, a maximum dosage of 240 μg/mo of darbepoetin alfa was permitted to achieve the target hemoglobin level. In the low-hemoglobin group, darbepoetin was reduced or stopped when hemoglobin exceeded 10.0 g/dl. Darbepoetin alfa dose, hemoglobin concentration, serum TSAT, and ferritin level, as well as clinical and laboratory variables, were monitored every 12 weeks.
In both groups, the use of antihypertensive agents to achieve a BP of <130/80 mm Hg was encouraged without changing the dosage of renin-angiotensin-system blockers during the study period. In addition, iron supplementation (intravenous or oral) was recommended at the discretion of the investigators to maintain serum ferritin ≥100 ng/ml or TSAT level ≥20%, in accordance with the 2008 Japanese Society for Dialysis Therapy guidelines for anemia of CKD (18).
End Points
The primary end point was time to the kidney composite event of initiation of maintenance dialysis treatment, kidney transplantation, eGFR reduction of ≤6 ml/min per 1.73 m2, or ≥50% reduction in eGFR from baseline. The secondary end points included a composite cardiovascular event (cardiovascular death, nonfatal myocardial infarction, stroke, hospitalization for heart failure, hospitalization for angina, or amputation of lower extremities), all-cause death, initiation of maintenance dialysis treatment, 50% reduction in eGFR from baseline, a composite of kidney events or death from any cause, onset of stroke, myocardial infarction, and malignancy.
Statistical Analyses
Sample size calculations were on the basis of event rates in the high-hemoglobin group of a subanalysis of our previous study (20), in which the event-free rate of the low-hemoglobin group was assumed to be 35% over 2 years of observation, and the hazard ratio (HR) of the high-hemoglobin group was 0.70 with reference to the low-hemoglobin group. First, we determined the required sample size to be 220 per group to provide 80% statistical power with a two-sided type 1 error of 0.05 for detecting a significant difference between treatment groups using the log-rank test, assuming a 15% loss to follow-up or withdrawal of consent. However, after an interim assessment for sample size re-estimation conducted in October 2013, when the total number of patients enrolled reached 359, the sample size was amended from 220 to 238 for each group, judging from the results of the average hemoglobin levels reached at 16 weeks, the patient dropout rate until the interim assessment, and the HR for the kidney composite end point calculated using the data of our previous study patients with similar demographics to those in this study.
The primary efficacy analysis set was the full analysis set, which included all patients satisfying the following conditions: (1) fulfilled all entry criteria, (2) took the study drug at least once, and (3) were followed up at least once after randomization (15). Kaplan–Meier cumulative incidence curves were compared using a two-sided log-rank test. Estimated HRs and 95% confidence intervals (95% CIs) were obtained with the use of Cox proportional hazards models adjusted for age, sex, and prespecified baseline variables (eGFR, hemoglobin, proteinuria, and systolic BP). Analyses were censored at the date of death (n=24) and at the end of observation period without kidney events (n=234). We checked the proportional hazards assumption using Schoenfeld Residual and no violation was observed. The proportion of all adverse events in each group was compared using Fisher exact test.
All of the test statistics used in the analyses were the results of two-sided tests with a significance level of 5%. Data are reported as number (frequency), means±SD, medians (interquartile range), or proportion with 95% CI as appropriate. All analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
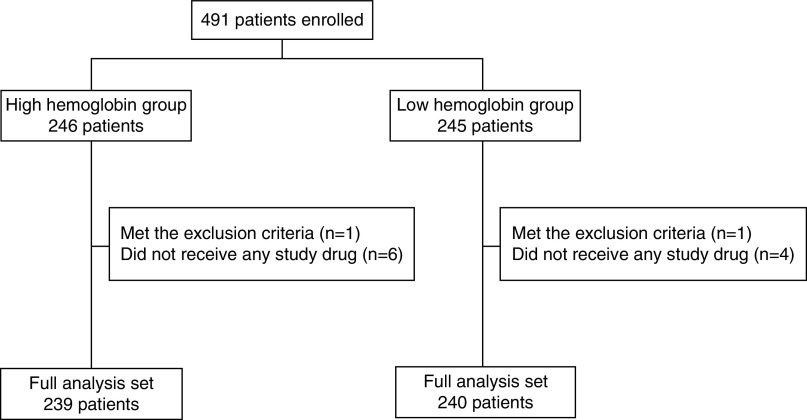
A total of 491 patients were randomly assigned to one of two groups as follows: 246 patients for the high-hemoglobin group and 245 patients for the low-hemoglobin group. We excluded 12 patients who met the exclusion criteria or did not adhere to the study protocol. Thus, the full analysis set comprised 479 patients; 239 and 240 patients in the high- and low-hemoglobin groups, respectively (Figure 1). The mean age of the overall subjects was 70±11 years and 60% of the patients were men. The mean baseline eGFR was 14±3 ml/min per 1.73 m2, and hemoglobin level was 9.3±0.6 g/dl. There were no clinically meaningful imbalances in baseline characteristics between the two groups (Table 1).
Figure 1.
Disposition of study participant. A total of 491 patients were randomly assigned to one of two groups as follows: 246 patients for the high-hemoglobin group and 245 patients for the low-hemoglobin group. After excluding 12 patients who met the exclusion criteria or did not adhere to the study protocol (seven patients in the high-hemoglobin group and five patients in the low-hemoglobin group), the analysis set of this study comprised 479 patients; 239 and 240 patients in the high- and low-hemoglobin groups, respectively.
Table 1.
Baseline characteristics of the patients
| Characteristic | Total (n=479) | High Hemoglobin (n=239) | Low Hemoglobin (n=240) | |
|---|---|---|---|---|
| Male sex | n (%) | 285 (60) | 142 (59) | 143 (60) |
| Age, yr | 70±11 | 71±11 | 70±12 | |
| Body mass index, kg/m2 | 22±3 | 22±3 | 22±3 | |
| Smoking | ||||
| Current/ever | n (%) | 70 (14) | 31 (13) | 39 (16) |
| Never | n (%) | 367 (77) | 185 (77) | 182 (76) |
| Unknown | n (%) | 42 (9) | 23 (10) | 19 (8) |
| Cause | ||||
| Chronic GN | n (%) | 149 (31) | 71 (30) | 78 (32) |
| Hypertensive nephrosclerosis | n (%) | 245 (51) | 128 (54) | 117 (49) |
| Polycystic kidney disease | n (%) | 38 (8) | 22 (9) | 16 (7) |
| Other | n (%) | 47 (10) | 18 (7) | 29 (12) |
| Past history of cardiovascular disease | ||||
| Myocardial infarction | n (%) | 25 (5) | 14 (6) | 11 (5) |
| Heart failure | n (%) | 52 (11) | 33 (14) | 19 (8) |
| Stroke | n (%) | 47 (10) | 23 (10) | 24 (10) |
| Peripheral artery disease | n (%) | 13 (3) | 6 (3) | 7 (3) |
| Darbepoetin alfa naïve | n (%) | 336 (70) | 167 (70) | 169 (70) |
| Systolic BP, mm Hg | 133±16 | 132±17 | 133±15 | |
| Diastolic BP, mm Hg | 72±11 | 72±12 | 73±11 | |
| eGFR, ml/min per 1.73 m2 | 14±3 | 14±3 | 14±3 | |
| CKD | ||||
| Stage 4 | n (%) | 165 (34) | 85 (36) | 80 (33) |
| Stage 5 | n (%) | 314 (66) | 154 (64) | 160 (67) |
| Hemoglobin, g/dl | 9.3±0.6 | 9.4±0.6 | 9.3±0.6 | |
| Ferritin, ng/ml | 148 (95–234) | 142 (91–225) | 152 (100–256) | |
| TSAT, % | 33±12 | 33±12 | 33±11 | |
| Uric acid, mg/dl | 7.2±1.8 | 7.2±1.9 | 7.2±1.7 | |
| Albumin, g/dl | 3.8±0.4 | 3.8±0.4 | 3.8±0.4 | |
| Urinary protein-to-creatinine ratio, g/g | 1.16 (0.47–2.29) | 1.13 (0.45–2.29) | 1.25 (0.48–2.28) | |
| C-reactive protein, mg/dl | 0.09 (0.03–0.20) | 0.08 (0.03–0.20) | 0.10 (0.03–0.20) | |
| Iron supplementation | n (%) | 137 (29) | 73 (31) | 64 (27) |
| Antihypertensive medication | n (%) | 399 (83) | 200 (84) | 199 (83) |
| ACEI | n (%) | 53 (11) | 25 (11) | 28 (12) |
| ARB | n (%) | 306 (64) | 165 (69) | 141 (59) |
| Lipid-lowering agents | n (%) | 179 (37) | 95 (40) | 84 (35) |
| Spherical carbonaceous adsorbent | n (%) | 99 (21) | 49 (21) | 50 (21) |
Data are expressed as number (frequency), mean±SD, or median (interquartile range), as appropriate. TSAT, transferrin saturation; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Intervention and Hemoglobin Values
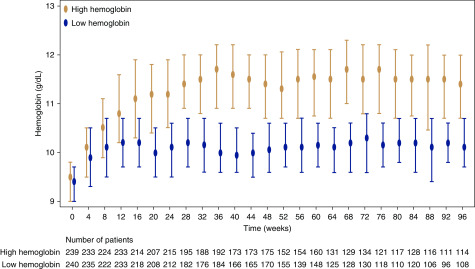
The hemoglobin value at each time-point is shown in Figure 2. In the high-hemoglobin group, the hemoglobin level sharply increased after randomization and reached 11 g/dl at 16 weeks. Thereafter, it was maintained at >11 g/dl throughout the study period. In the low-hemoglobin group, the hemoglobin level was maintained at about 10 g/dl, from 8 weeks after randomization to the end of the study. The monthly (4-week) averaged and body weight–adjusted darbepoetin alfa doses gradually increased during the study period in the high-hemoglobin group. According to the TSAT and serum ferritin levels, and proportion of iron supplementation in two groups, iron seemed to be adequately supplemented during the study period to avoid iron deficiency (Supplemental Figure 1).
Figure 2.
Hemoglobin levels in two groups had been maintained appropriately at each target level. Values shown are medians with interquartile ranges. In the high-hemoglobin group, the hemoglobin level sharply increased after randomization and reached 11 g/dl at 16 weeks. Thereafter, it was maintained at >11 g/dl throughout the study period. In the low-hemoglobin group, the hemoglobin level was maintained at about 10 g/dl from 8 weeks after randomization to the end of the study.
Primary Kidney Composite Outcome and Components
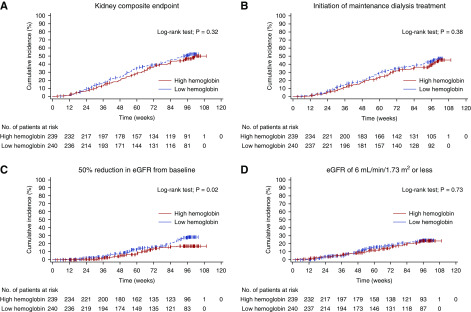
The kidney composite end point occurred in 105 (44%) patients in the high-hemoglobin group and in 116 (48%) patients in the low-hemoglobin group, with no statistically significant between-group difference (log-rank test; P=0.32) during the mean study period of 73.5±29.7 weeks (Figure 3). The adjusted Cox proportional hazards model showed that the HR for the high-hemoglobin group versus low-hemoglobin group was 0.78 (95% CI, 0.60 to 1.03; P=0.08) (Table 2). The incidence of the initiation of maintenance dialysis treatment and an eGFR reduction of 6.0 ml/min per 1.73 m2 or less was not significantly different between both groups (log-rank test; P=0.38 and 0.73, respectively). However, the number of patients with an eGFR reduction of 50% or more was significantly smaller in the high-hemoglobin group than in the low-hemoglobin group (n=30 versus 49; log-rank test; P=0.02), and the adjusted HR was 0.53 (95% CI, 0.33 to 0.85; P=0.008) (Figure 3, Table 2).
Figure 3.
Targeting a higher hemoglobin level had no significant effect on kidney outcome compared with targeting a lower hemoglobin level. (A) The primary kidney composite end point. (B) Initiation of maintenance dialysis. (C) A 50% reduction in eGFR from baseline. (D) eGFR of ≤6 ml/min per 1.73 m2. Only one and two patients underwent kidney transplantation in the high- and low-hemoglobin groups, respectively.
Table 2.
Primary and secondary end points and components
| End Point | High Hemoglobin (n=239), n (%) | Low Hemoglobin (n=240), n (%) | Hazard Ratio (95% CI) |
|---|---|---|---|
| Primary kidney composite end pointa | 105 (44) | 116 (48) | 0.78 (0.60 to 1.03) |
| Maintenance dialysis treatment | 91 (38) | 100 (42) | 0.79 (0.59 to 1.05) |
| 50% reduction in eGFR | 30 (13) | 49 (20) | 0.53 (0.33 to 0.85) |
| eGFR of ≤6 ml/min per 1.73 m2 | 43 (18) | 45 (19) | 0.81 (0.53 to 1.25) |
| Secondary end points | |||
| Cardiovascular composite end point | 19 (8) | 16 (7) | 1.07 (0.55 to 2.10) |
| Death | 14 (6) | 11 (5) | 1.28 (0.56 to 2.90) |
Patients may have had multiple kidney events of different types. The primary kidney composite end point reflects only the first occurrence of any of the components. 95% CI, 95% confidence interval.
Cardiovascular Events and All-Cause Mortality
Cardiovascular events occurred in 19 (8%) and 16 (7%) patients assigned to the high-hemoglobin and low-hemoglobin groups, respectively (adjusted HR, 1.07; 95% CI, 0.55 to 2.10 P=0.84). All-cause death occurred in 14 patients (6%) in the high-hemoglobin group and 11 patients (5%) in the low-hemoglobin group (adjusted HR, 1.28; 95% CI, 0.56 to 2.90; P=0.56) (Table 2). There was also no statistically significant difference in other secondary end points (data not shown).
Adverse Events
The incidence of adverse events was similar in both groups (Table 3). The number of patients with severe adverse events was not significantly different between the two groups. No thromboembolic events were reported. Malignant neoplasms were noted in four patients in each group.
Table 3.
Adverse events
| Adverse Events | High Hemoglobin (n=240), n (%) | Low Hemoglobin (n=241), n (%) | P Value |
|---|---|---|---|
| Any events | 41 | 38 | 0.71 |
| Any serious adverse event | 24 | 16 | 0.19 |
| Thrombovascular event | 0 | 0 | — |
| Pneumonia | 5 | 1 | 0.12 |
| Malignant neoplasm | 4 | 4 | 1.00 |
| Femur fracture | 3 | 1 | 0.37 |
The analysis includes 481 patients who received at least one dose of darbepoetin alfa.
Discussion
The relationship between ESA therapy for anemia of kidney disease and kidney outcome in predialysis patients with CKD has been investigated in various small- and large-scale RCTs and observational studies over the past two decades (4–8,21,22). Particularly, in the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta study, targeting hemoglobin levels of 13–15 g/dl resulted in more frequent dialysis initiations than targeting hemoglobin levels of 10.5–11.5 g/dl (7). Contrary, Tsubakihara et al. (20) reported targeting higher (11–13 g/dl) hemoglobin levels using darbepoetin alfa had beneficial effect on kidney outcome compared with targeting lower (9–11 g/dl) hemoglobin levels using epoetin alfa, without an increase in cardiovascular events in Japanese predialysis patients with CKD. In the subanalysis of that study, we found that the cumulative kidney survival of patients with CKD stage 5 was significantly higher in the higher hemoglobin group than in the lower hemoglobin group (23). However, our study failed to show the superiority of targeting high-hemoglobin levels compared with targeting low-hemoglobin levels in terms of the kidney composite end point in patients with advanced CKD without diabetes.
The reason for a nonsignificant difference in the primary kidney composite end point may be partly attributable to the unexpected small difference in the maintenance hemoglobin level between the high- and low-hemoglobin groups. According to the previous studies in which beneficial effects of targeting higher hemoglobin level on kidney outcome were observed, the difference in hemoglobin levels between the two groups may be needed around 2 g/dl (4,20). Moreover, it is possible that the darbepoetin alfa dose was relatively small to increase hemoglobin level up to 12 or 13 g/dl in patients with advanced CKD. The physicians at each site all knew that recent large-scale RCTs showed unfavorable effects of high dose ESAs on cardiovascular outcome (6,8). These results might affect the physician’s discretion and they might treat the patients’ hemoglobin levels close to 11 g/dl. Furthermore, this study had been conducted under the national health insurance system in Japan. So, the darbepoetin alfa dose was finally determined on the basis of national health insurance coverage (recommended starting dose, 30 μg/2 wk and maintenance dose, 60–240 μg/mo). Generally, ESA doses used in Japan seems to be lower than those used in Western countries. A nationwide cohort study conducted on more than 5000 patients with predialysis CKD in the real-world clinical setting in Japan showed that hemoglobin levels had been maintained between 10.0 and 10.6 g/dl for about 3 years using darbepoetin alfa, with the mean dose of 17.1 μg/wk (22). Furthermore, previous reports revealed that the erythropoietin-resistance index was about two times higher in Western patients with CKD than in Japanese patients with CKD (24–27).
Although we could not show the beneficial effects of targeting higher hemoglobin level using darbepoetin alfa on the kidney outcome, a 22% reduction of the kidney composite end point, favoring the high-hemoglobin group (although not significant), may have clinically important implication for treatment of anemia of kidney disease in clinical practice. Of note, the number of patients with ≥50% reduction in eGFR from baseline, one of the components of the primary kidney composite end point, was significantly smaller in the high-hemoglobin group than in the low-hemoglobin group. In recent years, as the percent change in eGFR from baseline has been recognized as a good surrogate marker of kidney outcome (28–30), targeting hemoglobin levels >11 g/dl may be advantageous for preserving kidney function in patients with advanced CKD without diabetes.
Furthermore, there was the absence of a significant difference in cardiovascular events and mortality between the two groups in our study. No thromboembolic events were reported in both groups. The study excluded patients with diabetes and CKD; however, as the study participants were in advanced CKD stages, targeting higher hemoglobin levels using ESAs in such patients who had a high-cardiovascular risk could have promoted cardiovascular events (31). Our results could suggest that Japanese patients with CKD have a considerably lower cardiovascular burden than Western patients with CKD, as shown in the CKD-JAC study (14). Although this study could not answer the question about the effect of different target hemoglobin levels on cardiovascular events and mortality as they were not the primary outcome, targeting a higher hemoglobin level using ESAs may be reasonable and relatively safe in the Japanese CKD population. Also, our results may support the target maintenance hemoglobin level of 11–13 g/dl in nondialysis patients, as stated in the Japanese Society for Dialysis Therapy’s 2015 guidelines for anemia of CKD (32).
Our study has several potential limitations. First, the study population was restricted to patients with advanced CKD without diabetes, so our results may not be generalizable to all patients with CKD. Second, as our sample size calculation was on the basis of a previous study that compared kidney function between the high-hemoglobin group treated with darbepoetin alfa and the low-hemoglobin group treated with epoetin alfa, the difference in event rate between the two groups might have been affected not only by the different hemoglobin levels, but also by the different types of ESAs. So, we might have overestimated the difference in event rate in our study. Third, we prespecified the initiation of maintenance dialysis treatment as a component of the kidney composite end point. The timing of dialysis initiation is highly dependent on the physician’s discretion, without any standardized criteria, and this may have biased the assessment of this timing. Furthermore, given that the entry criteria of eGFR allowed patients with an eGFR of 8 ml/min per 1.73 m2 to be enrolled, the component of kidney composite end point, eGFR reduction to ≤6 ml/min per 1.73 m2, seems perplexing. So, we carefully judged the patient as achieving this end point if the patient had three consecutive times of eGFR reduction to ≤6 ml/min per 1.73 m2. Fourth, because of the study protocol, we did not have detailed information about the route, frequency, and dose of iron supplementation. However, according to serum ferritin level and TSAT, our patients were unlikely to be iron deficient. Fifth, we censored data from the time-to-event analysis for patients who died before achieving the kidney composite end point, and this may involve a competing risk bias; however, this is unlikely because the number of patients who died was small and did not significantly differ between the two groups. Lastly, we did not monitor adherence to diet and medication or other lifestyle modifications. The smaller number of patients with ≥50% reduction in eGFR from baseline observed in the high-hemoglobin group may have been induced not only by the difference in hemoglobin levels, but also by such factors.
In conclusion, targeting a higher hemoglobin level with darbepoetin alfa did not improve kidney outcome compared with targeting a lower hemoglobin level in patients with advanced CKD without diabetes. Further prospective studies to confirm the effect of targeting a higher hemoglobin level in ESA treatment on clinical outcomes in CKD subpopulations are necessary.
Disclosures
Dr. Akizawa reports advisory roles/consultancies (Astellas, Bayer, Fuso Pharmaceutical, GlaxoSmithKline, Japan Tabaco, Kyowa Hakko Kirin, Nipro, Ono Pharmaceutical, and Torii Pharmaceutical). Dr. Imai reports advisory roles/consultancies (Bayer). Dr. Nangaku reports advisory roles/consultancies (Chugai and Kyowa Hakko Kirin). Dr. Akizawa reports fees from Astellas and Kyowa Hakko Kirin. Dr. Akizawa reports honoraria from Bayer, Chugai, Fuso Pharmaceutical, Kissei Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and Torii Pharmaceutical. Dr. Hayashi reports honoraria from Chugai and Kyowa Hakko Kirin). Dr. Hirakata reports honoraria from Chugai, Japan Tabaco, Kyowa Hakko Kirin, and Torii). Dr. Imai reports honoraria from Boehringer Ingelheim, Chugai, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Mitsubishi Tanabe, Sanwa Kagaku, and Takeda. Dr. Morita reports honoraria from Chugai and Kyowa Hakko Kirin. Dr. Nangaku reports honoraria from Chugai and Kyowa Hakko Kirin. Dr. Narita reports honoraria from Daiichi Sankyo, Mitsubishi Tanabe, Mochida, MSD, Novartis, Otsuka, and Sanofi. Dr. Tsubakihara reports honoraria from Chugai. The Translational Research Center for Medical Innovation has received a research grant that was not specific for this study from Kyowa Hakko Kirin. Kyowa Hakko Kirin was not involved in designing, data interpretation, and manuscript writing for this study.
Funding
Dr. Maruyama is supported by Kyowa Hakko Kirin, Otsuka, Dainippon Sumitomo, and Mochida grants. Dr. Nangaku is supported by Kyowa Hakko Kirin and Chugai grants. Dr. Narita is supported by KUREHA, Chugai, Astellas, Kyowa Hakko Kirin, Genzyme Japan, Daiichi Sankyo, Takeda, and Terumo grants. Dr. Imai is supported by Mitsubishi Tanabe and AstraZeneca grants.
Supplementary Material
Acknowledgments
The authors would like to express our deepest gratitude to the patients, investigators, and staff at the study sites (presented in the Supplemental Appendix) for their contribution to the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08900719/-/DCSupplemental.
Supplemental Figure 1. Darbepoetin alfa dose and iron parameters during the study period. (A) Darbepoetin alfa dose, (B) transferrin saturation, (C) serum ferritin, and (D) proportion of iron supplementation. Values shown are medians with interquartile ranges for darbepoetin alfa dose, transferrin saturation, and serum ferritin, and proportion with 95% confidence interval for iron supplementation. Darbepoetin alfa doses are described as monthly (4-week) averaged and body weight–adjusted dose calculated by the quarterly total dose. TSAT, transferrin saturation.
References
- 1.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 28: 53–61, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, Greenwood R, Feldman HI, Port FK, Held PJ: Anaemia in haemodialysis patients of five European countries: Association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Walker AM, Schneider G, Yeaw J, Nordstrom B, Robbins S, Pettitt D: Anemia as a predictor of cardiovascular events in patients with elevated serum creatinine. J Am Soc Nephrol 17: 2293–2298, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O: Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 77: 176–185, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC: Treating anemia early in renal failure patients slows the decline of renal function: A randomized controlled trial. Kidney Int 66: 753–760, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Akizawa T, Pisoni RL, Akiba T, Saito A, Fukuhara S, Asano Y, Hasegawa T, Port FK, Kurokawa K: Japanese haemodialysis anaemia management practices and outcomes (1999-2006): Results from the DOPPS. Nephrol Dial Transplant 23: 3643–3653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for anemia in chronic kidney disease. Kidney Int 2: 279–335, 2012 [Google Scholar]
- 12.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA; Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators: Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, Matsuo S, Imai E, Makino H, Hishida A; CKD-JAC Investigators: Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int 91: 227–234, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Imai E, Maruyama S, Nangaku M, Hirakata H, Hayashi T, Narita I, Kono H, Nakatani E, Morita S, Tsubakihara Y, Akizawa T: Rationale and study design of a randomized controlled trial to assess the effects of maintaining hemoglobin levels using darbepoetin alfa on prevention of development of end-stage kidney disease in non-diabetic CKD patients (PREDICT Trial). Clin Exp Nephrol 20: 71–76, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D; CONSORT Group: CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152: 726–732, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T: 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 14: 240–275, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Kirin Kyowa: Product information of darbepoetin alfa. Available at: https://medical.kyowakirin.co.jp/druginfo/qa/nsp/index.html. Accessed December 9, 2019
- 20.Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, Saito A, Akiba T, Hirakata H, Akizawa T: High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial 16: 529–540, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Uemura Y, Kumagai M, Kimpara M, Kanno H, Ohashi Y; MIRACLE-CKD Study Group: Effect of achieved hemoglobin level on renal outcome in non-dialysis chronic kidney disease (CKD) patients receiving epoetin beta pegol: MIRcerA CLinical Evidence on Renal Survival in CKD patients with renal anemia (MIRACLE-CKD Study). Clin Exp Nephrol 23: 349–361, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T, Nangaku M, Imai E, Tsubakihara Y, Kamai M, Wada M, Asada S, Akizawa T: Safety and effectiveness of long-term use of darbepoetin alfa in non-dialysis patients with chronic kidney disease: A post-marketing surveillance study in Japan. Clin Exp Nephrol 23: 231–243, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsubakihara Y, Akizawa T, Iwasaki M, Shimazaki R: High hemoglobin levels maintained by an erythropoiesis-stimulating agent improve renal survival in patients with severe renal impairment. Ther Apher Dial 19: 457–465, 2015 [DOI] [PubMed] [Google Scholar]
- 24.López-Gómez JM, Portolés JM, Aljama P: Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl 111: S75–S81, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Mercadal L, Coudert M, Vassault A, Pieroni L, Debure A, Ouziala M, Depreneuf H, Fumeron C, Servais A, Bassilios N, Bécart J, Assogba U, Allouache M, Bouali B, Luong N, Dousseaux MP, Tezenas-du Montcel S, Deray G: L-carnitine treatment in incident hemodialysis patients: The multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin J Am Soc Nephrol 7: 1836–1842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locatelli F, Andrulli S, Viganò SM, Concetti M, Urbini S, Giacchino F, Broccoli R, Aucella F, Cossu M, Conti P, Fattori L, Punzo G, Angelini D, Peruzzini M, Di Giulio S, Piroddi M, Galli F, Del Vecchio L: Evaluation of the impact of a new synthetic vitamin E-bonded membrane on the hypo-responsiveness to the erythropoietin therapy in hemodialysis patients: A multicenter study. Blood Purif 43: 338–345, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Eriguchi R, Taniguchi M, Ninomiya T, Hirakata H, Fujimi S, Tsuruya K, Kitazono T: Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: The Q-Cohort study. J Nephrol 28: 217–225, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Kanda E, Usui T, Kashihara N, Iseki C, Iseki K, Nangaku M: Importance of glomerular filtration rate change as surrogate endpoint for the future incidence of end-stage renal disease in general Japanese population: Community-based cohort study. Clin Exp Nephrol 22: 318–327, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF: Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 153: 23–33, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, Hattori M, Suzuki T, Morita S, Ashida A, Ito Y, Kuragano T, Komatsu Y, Sakai K, Tsubakihara Y, Tsuruya K, Hayashi T, Hirakata H, Honda H: 2015 Japanese society for dialysis therapy: Guidelines for renal anemia in chronic kidney disease. Ren Replace Ther 3: 36, 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.