Abstract
The kidney is a complex organ responsible for maintaining multiple aspects of homeostasis in the human body. The combination of distinct, yet interrelated, molecular functions across different cell types make the delineation of factors associated with loss or decline in kidney function challenging. Consequently, there has been a paucity of new diagnostic markers and treatment options becoming available to clinicians and patients in managing kidney diseases. A systems biology approach to understanding the kidney leverages recent advances in computational technology and methods to integrate diverse sets of data. It has the potential to unravel the interplay of multiple genes, proteins, and molecular mechanisms that drive key functions in kidney health and disease. The emergence of large, detailed, multilevel biologic and clinical data from national databases, cohort studies, and trials now provide the critical pieces needed for meaningful application of systems biology approaches in nephrology. The purpose of this review is to provide an overview of the current state in the evolution of the field. Recent successes of systems biology to identify targeted therapies linked to mechanistic biomarkers in the kidney are described to emphasize the relevance to clinical care and the outlook for improving outcomes for patients with kidney diseases.
Keywords: kidney disease, transcriptional profiling, gene expression, kidney biopsy, Genomics, systems biology, bioinformatics, proteomics, precision medicine, humans, nephrology, human body, kidney diseases, kidney, urinary tract physiological phenomena, biomarkers, homeostasis, cohort studies, Kidney Genomics Series
Why Is a Systems Approach Needed to Tackle Kidney Diseases?
The kidney plays a central role in maintaining homeostasis in the human body. To successfully carry out these functions, the kidney contains numerous cell types arranged in the complex three-dimensional structure of the nephron to respond to a variety of hormonal, neuronal, inflammatory, and intra- and intercellular signals. The network of complex, intertwined regulatory functions across different cell types make the delineation of specific mechanism associated with loss or decline in kidney function challenging. Clinical trials for the development of diagnostic markers and novel therapies targeting kidney diseases have consequently been limited (1–3), resulting in few prevention and treatment options available to clinicians and patients.
Much of our knowledge of kidney physiology and pathophysiology has been gleaned from a reductionist approach, where careful experimentation has elucidated the effect of one molecular pathway on kidney health and disease, mostly in animal models. However, many kidney diseases, most notably CKD and AKI, comprise diseases of multiple etiologies. Thus, patients enrolled in clinical trials often have heterogeneous disease mechanisms activated, which has likely contributed to the low success rate of clinical trials of pathway-specific targets in nephrology. Biomarkers to further classify subgroups of patients, thus far, have focused more on clinical features rather than on molecular mechanisms that might indicate efficacy of putative therapies. Integrating a wide spectrum of information on the underlying disease mechanism using a systems biology approach has the potential to addresses some of these challenges. This strategy leverages our existing granular knowledge obtained through traditional reductionist approaches toward a holistic understanding of disease processes in a given patient.
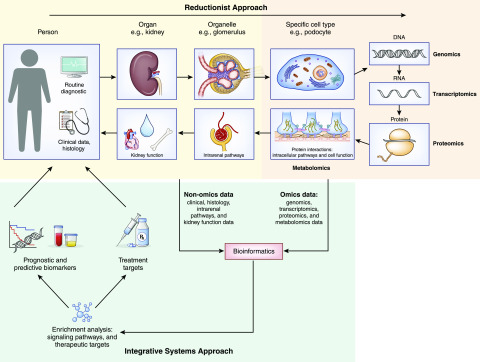
A global, or systems biology, approach takes advantage of recent developments in computational methods to integrate diverse types of data, such as molecular, tissue, and clinical parameters, to unravel the interplay of multiple genes, proteins, and molecular mechanisms (Figure 1) that drive discrete steps in kidney health and disease (4,5). A core element of this strategy is the integration of various data sources, including conventional clinical phenotypic patient data, clinicopathologic parameters, and comprehensive genome-scale data sets (also referred to as “omics”) through bio-informatics analytical workflows. Integrating such diverse data into analytical processes necessitate the blending of a variety of expertise including clinical, biologic, information technology, mathematical, statistical, and computational research in nephrology research teams. Sophisticated information technology infrastructure is required to manage and connect information across research and health care. Meanwhile, software tools for analysis and interpretation of data need “integrative workflows” that involve a combination of statistical, computational, and mathematical techniques (6,7). The purpose of this review is to provide an overview of the current state in the evolution of systems biology for the nephrologist. By highlighting the advances made through this approach toward developing targeted therapies linked to mechanistic biomarkers, the potential effect on clinical care and improving outcomes for patients with kidney diseases are discussed (8–10).
Figure 1.
Integrative systems approach to address challenges in kidney disease. A systems biology approach to the kidney adds to the reductionist approach by leveraging recent advances in computational technology and methods to integrate diverse sets of data.
What Is Systems Biology and How Does It Work?
The understanding of what systems biology encompasses has evolved and the definition of “systems biology” has reflected these changes over time (11). The National Centers for Systems Biology’s definition emphasizes the use of advanced computational modeling techniques and high-throughput technologies to integrate physical, computational, and experimental sciences in biomedical research (12). For the purposes of this review, we define systems biology as the use of computational modeling and mathematical techniques in developing an integrative picture of a biologic system derived from multiple types of data.
The types and complexity of data used in this approach have also evolved with advances in biologic and computational capabilities. Initially, linking one gene to one phenotype was considered systems biology; the example of HER2 and breast cancer is used later in this paper (13). Development of microarray technologies, single-nucleotide polymorphism arrays for genome-wide association studies, or Affymetrix microarrays for gene expression analysis, formed the first phase of “big data” that allowed researchers to examine thousands of genes or transcripts at one time. Development of multiple cell- and organ-scale molecular (omic) platforms, focusing on changes in fundamental cellular components like DNA, RNA, and proteins, have enabled the generation of substantial quantities of data that can comprehensively map the human genome, transcriptome, proteome, and metabolome (Figure 1). Combining the insights derived through each platform in healthy and disease states is now at the heart of systems biology approaches aiming to define the underlying biology of a complex organ like the kidney.
In addition to omics data, there are increasing amounts of non-omic, large-scale data derived from clinical settings that are becoming available to kidney researchers. These include the digital transformation of traditional phenotype-defining clinical features, like histopathology. Digital pathology using whole-slide imaging (WSI) is poised to transform the practice of diagnostic pathology. WSI data sets can now be treated as a data type of equal complexity to genomic data and can be integrated with other data types (14). In nephrology, kidney biopsies are used routinely in clinical practice for the diagnosis and management of glomerular diseases. Therefore, the availability of tissue samples combined with digital pathology promises to generate comprehensive structural information (15). In large cohorts it has enabled unbiased capture of complex features of kidney structure and associated changes in disease (16). For example, in the Nephrotic Syndrome Study Network (NEPTUNE), careful and reproducible assessment of histologic features allowed identification of molecular pathways associated with increased interstitial fibrosis and progressive loss of kidney function (17). WSI data repositories are expanded in a series of efforts, including the recently initiated Kidney Precision Medicine Project (kpmp.org) for studying AKI and CKD while establishing a framework to safely obtain research kidney biopsies. Electronic health records (EHRs), electronic medical records, and epidemiologic data contain rich descriptions of health-related traits (18) and are an additional data type that can be leveraged via a system biology approach. Demographic information, such as socioeconomic factors, have been linked to CKD and its major risk factors (19), and can be incorporated into systems biology workflows. Integrating the clinical profiles, environmental exposures, and imaging data from organs and tissues with the molecular data sets is the next step in the evolution of systems biology.
The coalescence of these different data types with state of the art data integration technologies has the potential to advance our understanding of molecular mechanisms involved in kidney disease. One of the main thrusts is to move from a descriptive disease pattern (i.e., nephrotic syndrome) to a mechanistic disease definition (phospholipase A2 receptor [PLA2R]-positive membranous nephropathy) to identify and classify patients on the basis of characteristics relevant to disease prognosis and treatment. This is a critical step in the pursuit of personalized medicine—that is, “the right drug for the right patient at the right time.”
Systems Biology Using a Single Omic Data Type
There are now several techniques that systems biologists can use to organize omics data in a way that will uncover the molecular mechanisms involved in kidney diseases. For example, clustering algorithms using genome-wide mRNA (transcriptomics) data, can identify patients with similar expression profiles in specific tissues or cell-types in the kidney, irrespective of clinical disease status or classification. Grouping patients on the basis of these molecular signatures, rather than clinical features, helps identify common molecular pathways involved in organ or tissue damage across different kidney diseases. This type of molecular clustering has been prioritized in rare diseases such as nephrotic syndrome and ANCA vasculitis, where disease cause is wide-ranging in a relatively small patient population (20). Another useful tool applied to transcriptomics data is the development of individual, patient-level pathway activation scores. An activation score is calculated for each participant on the basis of the expression levels of every gene known to be regulated by a well established cellular pathway in a specific tissue compartment, such as the glomerulus. This score can then be used as a “read-out” for the activation or inhibition of that pathway in a specific disease (sub)type or in response to treatment exposures. Comparison across individuals on the basis of this score enables identification of common pathways, potentially implicated in the conditions and outcomes experienced by those participants. Although activation scores are not yet used in clinical practice, they hold great promise to help classify diseases. For example, as proof of concept, data-driven clustering of patients with FSGS and minimal change disease identified two subgroups of patients independent of traditional disease classification. One cluster displayed higher activation of the TNF pathway, and was associated with worse long-term outcomes, including loss of kidney function. Urinary biomarkers in the same pathway were then identified that could be used to identify patients with increased TNF activity (21). Similarly, JAK/STAT pathway activation was identified as a marker of kidney disease progression in FSGS (22) and across glomerular diseases (20). Although further elucidation and validation of these findings are needed, they illustrate the potential of systems biology in matching interventions to patients.
Systems Biology Connecting Multiple Omics Data Types
As our ability grows to generate different, comprehensive data sets from the same individuals with kidney disease, a plethora of computational methods to link multiple, heterogeneous data types have been developed (23). The most prevalent data integration methods are unsupervised, i.e., the analysis draws an inference from input data sets without any guidance by the investigator from existing classifications. To facilitate comparison across data sets, matrix factorization–based methods like joint non-negative matrix factorization (joint-NMF) and iCluster are dimensionality reduction methods that reduce number of variables under consideration by obtaining a set of principle variables. For example, using a few metagenes to describe the patterns of expression for >20,000 genes in a data set. Commonalities within the data, across samples, are used to group variables that behave similarly. One can also use the underlying similarity structure in network-based approaches like PAthway Recognition Algorithm using Data Integration on Genomic Models (PARADIGM) and similarity network fusion (SNF), where mathematical models are used to decipher the underlying data patterns to discover regulatory networks.
The majority of these data integration strategies focus on specific aspects of the data (features), e.g., linking a specific gene within the genomics data set with the corresponding protein from proteomics data, rather than integrating data from different samples (e.g., blood, urine, and tissue samples). These feature-specific approaches focus on specific genes, proteins, or metabolites and use known biologic relationship among the data types. For example, for a specific gene, what is the relationship between gene copy number variation, RNA transcription levels, and protein abundance across individuals and samples? Each omic platform measures different aspects (variables) of this interdependent biologic process. The goal of the systems biology approach, in these examples, is to identify the interconnected crosscutting signals that link a biologic process to a particular condition and can describe this process, i.e., a network signature. For example, iCluster, a machine learning approach, was used to reveal distinct tumor subtypes (24,25) suggestive of different genetic pathways in colon cancer progression. This was achieved by connecting gene mutation, gene copy number, DNA methylation, and gene expression information derived from genomic, epigenomic, and transcriptomic data available for 189 tumor samples (24). In the case of CKD, such approaches could be used to determine the interconnected network signature involved in an established pathway, such as the JAK/STAT pathway, to identify inflammation-specific disease subtypes that might be responsive to targeted therapeutic interventions. However, these approaches have been limited primarily to omics data integration.
Systems Biology Integrating Omics and Non-Omic Data Types
The next challenge for systems biology is to link molecular data sets to clinical features in the absence of an a priori mapping structure. These strategies often use the patient as the anchor point to link the diverse data sets together via a participant-centric approach. These are highly flexible algorithms that can not only integrate omics data, but also include non-omic data types, such as clinical data, digital pathology, and even environmental exposures and demographic information. Person-centric integration methodologies, e.g., the frequently used SNF, construct individual networks mapping the similarities within each available data type for each person. Next, the network pattern seen in each data set are iteratively linked or “fused” together to build a consensus network mapping the patients on the basis of their similarity across all data types. The consensus network now represents all of the individual networks into a single network capturing the entire spectrum of heterogeneous, diverse data types, regardless of biologic relationship. This enables formation of distinct patient subgroups on the basis of all of the information sources available, as well as identifying key data points (i.e., set of genes or set of histology observations) contributing to the overall grouping. SNF methods were successfully applied to identifying and linking cancer subtypes to associated clinical outcome in five different cancers (26). With the emergence of long-term studies in kidney disease, the collected data types are not limited to omics data and include digital pathology and detailed clinical phenotyping and demographic information. Therefore, patient-centric approaches like SNF can be leveraged to delineate molecular mechanisms and pathways relevant to disease progression in different patient subgroups.
Translating Multiscalar Data into Clinical Practice with Artificial Intelligence
The future direction of system biology will involve the use of artificial intelligence (AI) approaches such as machine learning and deep learning. A specific strength of these computational processes are to extract from large-scale, multiscalar data sets clinically actionable knowledge, for example outcome predictions or risk stratifications. AI is especially well suited to tackle the challenges of scalability (e.g., going from cell level to a patient population), and high dimensionality (e.g., number of variables and data types describing each sample) of data. Natural language processing has enabled information to be automatically extracted and summarized from electronic medical records or from manually written doctor notes (27–30). In another example, AI has transformed medical image reading (31) in diagnosing metastatic breast cancer (32), melanoma (33), and several eye diseases (34). In the kidney, applications range from AI-based prediction of AKI events across diverse EHR systems (35) via noninvasive, AI-based GFR estimation from kidney ultrasound imaging (36), to AI-driven identification for patients at risk of rapid loss of kidney function via a combination of EHR-derived clinical parameters and a targeted biomarker panel (37).
Why Systems Biology in Nephrology Now?
The past decade has seen the development of multiple cohorts across a wide spectrum of kidney diseases with associated rich, multilevel data sets, including genome, transcriptome, proteome, digital pathology, and prospective long-term clinical characteristics and outcomes. These studies have also collected and stored a variety of biosamples, including blood, urine, kidney tissue, and DNA, which ensures that these cohorts will continue to contribute data as novel technologies and mathematical techniques are discovered.
As several of these studies have now prospectively ascertained clinically meaningful endpoints, the comprehensive information from these patient cohorts can now be used to address key questions asked of nephrologists by patients in clinical care settings: “Why do I have this disease?”, “What will this disease do to me?”, and “What treatment options do I have?”. Defining kidney diseases in mechanistic terms has the potential to provide new starting points for pathophysiology-driven disease definition and management.
Application of Systems Biology to Improve Clinical Care
Oncology has provided a framework for how systems biology approaches can be used to provide new answers to at least some of the fundamental challenges in nephrology. The following examples highlight the application of systems biology approaches to complex disease processes to develop new solutions for diagnosis and treatment.
HER2 in Breast Cancer
Personalized medicine in cancer started with the use of imatinib, targeting the 922 translocation (38), in chronic myeloid leukemia but is best described through examples in breast cancer. Multiple studies in breast cancer in the 1980s demonstrated alterations in the expression pattern of a single transmembrane protein, HER2, in breast cancer could explain some of the underlying biology in tumor progression. Tumors that were positive for HER2 were associated with more aggressive biology, leading to poorer outcomes for patients (13). A systems biology approach was able to associate a specific single genetic aberration with clinical outcome, leading to the development of anti-HER2 therapies via an mAb against HER2, trastuzumab. This drug has shown immense clinical benefit in treating HER2-positive, metastatic breast cancers (39) and targeted agents are considered the standard of care in oncology clinics (40), paving the way for 140 therapies targeting 95 different genetic aberrations to be approved by the US Food and Drug Administration (41).
Gene Signature Score Prognostic Marker
With the advent of array technologies, thousands of genes could be measured at the same time. Using a modest cohort of 65 surgical breast tumor specimens from 42 patients, Perou et al. demonstrated that tumors can be classified into subtypes on the basis of their gene expression patterns (42), and that these subtypes showed significantly different outcomes. This led to the discovery of a 70-gene signature that was able to show that women initially identified as high risk for recurrence on the basis of clinical and pathologic factors could be reclassified because of low genomic risk, and successfully forgo chemotherapy, which was replicated in a study of 6000 patients across 112 centers (43,44). These findings have revolutionized patient care practices, promoted more individualized treatment, and minimized harm related to unnecessary treatments and procedures leading to improved, cost-effective care (45).
Systems Biology Effect on Clinical Care of Kidney Disease
Identification of PLA2R in Primary Membranous Nephropathy
Salant and colleagues integrated proteomics data with clinical data to identify a protein antigen in serum samples from patients with idiopathic and secondary membranous nephropathy. Serum from patients with idiopathic membranous nephropathy contained antibodies binding to a protein band of glomerular cell lysates. Proteomic analysis of the protein band generated an extensive list of peptides, which were filtered using a systems biology approach toward a small list of candidate proteins leading to the discovery of PLA2R (46) as the main antigen in membranous nephropathy. A causal role of PLA2R for disease pathogenesis was further supported by the identification of a major risk allele associated with membranous nephropathy using a genomic approach (47). These independent lines of evidence from omics data sets implicating PLA2R in the pathophysiology of membranous nephropathy have, like HER2 in cancer, established a mechanistically homogenous subgroup of patients with membranous nephropathy. Antibodies against PLA2R are now an integral part of clinical practice for diagnosis and management of primary membranous nephropathy. The serum test is used to monitor disease activity and guide treatment decisions regarding immunosuppressive therapy withdrawal (48). Efforts are underway to combine the serum test with genetic testing for noninvasive disease diagnosis and monitoring. Clinical trials, like Membranous Nephropathy Trial of Rituximab, can now use the molecular-defined disease types to document the specificity of the interventions (49).
EGF in CKD
Impaired kidney function particularly in early stages of CKD remains difficult to accurately prognosticate with eGFR and urinary protein excretion as the only available tests in clinical practice. There have been numerous attempts to identify other prognostic markers for progression of CKD, with varying degrees of success. Many of these attempts have relied on using markers of acute tubular injury because tubulointerstitial fibrosis is the best-known predictor of kidney tissue outcomes. Unfortunately, some of these biomarkers are expressed in other tissues or are affected by eGFR, which makes interpretation difficult.
By analyzing the transcriptome from the tubulointerstitial compartment of >200 kidney biopsies from patients with various causes of CKD, a transcript set predicting eGFR decline was identified at the time of biopsy using an AI-based strategy (50). Among the tissue-level outcome predictors, EGF was found to be almost exclusively expressed in the distal tubular compartment of the kidney and excreted in urine. Levels of EGF measured in urine improved prediction of CKD progression compared with models that relied only on eGFR and urine protein. This biomarker has since been validated in >10,000 patients and has shown robust ability to improve prediction of CKD progression and prognosis (51–53).
Identification of JAK-STAT as a Drug Target for Diabetic Kidney Disease
Integration of clinical and tissue transcriptomics data of patients with diabetic kidney disease identified the JAK-STAT pathway as a potential molecular target for therapeutic intervention (54). Animal models supported the involvement of this pathway in mediating responses to important fibrotic factors such as TGFβ, leading to a phase 2 clinical trial of diabetic kidney disease where the JAK inhibitor, baricitinib, demonstrated a dose dependent decrease in albuminuria, the established biomarker for diabetic kidney disease (55). Target engagement biomarkers predicted by systems biology approach from human diabetic kidney diseases cohorts were able to detected the response to JAK inhibition after 14 days, preceding observable responses in albuminuria levels by 10 weeks.
A New Level of Resolution: Single-Cell Analyses Unveiling the Molecular Architecture of the Kidney
One of the key challenges in defining molecular mechanisms of kidney disease is the cellular heterogeneity of the kidney, second probably only to the human brain (56). Sustained efforts to overcome this challenge include microdissection of kidney tissue and biopsies for molecular analysis (57,58), development of profiling technologies for individual microdissected cells (59), and computational efforts to define cell type–specific signals from complex kidney biopsies signatures (60). The recent development of genome-wide single-cell RNA sequencing (scRNAseq) of cell populations harvested from kidney tissues and biopsies harbors unparalleled opportunities to dissect molecular mechanisms of kidney function and disease (61–63). This technology contributes another dimension of cell-level data and will test the ability of systems biology approaches to link signals derived from molecularly defined kidney cell populations with an individual phenotype. scRNAseq relies on cell separation techniques combined with omic technologies to generate a cell type–specific molecular profile (63). Thus far, scRNAseq has been successfully applied to mouse kidney (64), human kidney allografts (61), and organoids (62,65), and explored in lupus nephritis (66,67) and glomerular disease (62). Technologies are being developed to integrate the signals assigned to a kidney cell state into the tissue context of the healthy and diseased organs, with research networks dedicated to define the cellular compartments of the kidney in molecular terms (Human Cell Atlas, HCA.org [68]; and HubMap [69]) and in diseases states, with the Kidney Precision Medicine Project targeting AKI and CKD (kpmp.org), NEPTUNE proteinuric diseases (Neptune-study.org), and the Accelerating Medicines Partnership rheumatoid arthritis and lupus program (amp-ralupus.stanford.edu [67]). Generating and analyzing several thousand genes in >10,000 cells from an individual patient poses unprecedented challenges and strategies are still being developed to integrate the data deluge from single-cell data with phenotypic and histologic data types, among others. However, first compelling insights of the power of these strategies are starting to emerge, giving us a new window how the intrakidney immune defense is set up in a spatially orchestrated manner to counteract invading pathogens in the healthy kidney (70), or how specific tissue resident macrophage populations in lupus nephritis can be recovered from urinary single cell studies to monitor the immune state of the kidney in lupus (67).
With the convergences of nanotechnology, molecular biology, and computational biology as applied to the kidney in its infancy, we should be prepared to find more novel insights into the molecular intricacies of human kidney disease.
Summary and Outlook
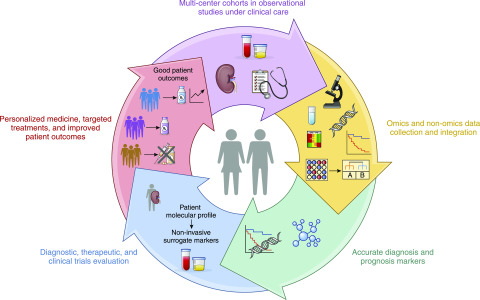
Systems biology is opening up paths forward for improved care for our patients with kidney disease (Figure 2). Integration of omics and nonomics data provide opportunities for identifying new molecular signatures indicative of disease progression, disease subtypes, and common shared disease pathways. These molecular signatures are the basis for developing specific biomarkers and related clinical assessments to help with diagnosis and prognosis. The molecular pathways can also help prioritize optimal therapeutic targets for molecular intervention and clinical trials.
Figure 2.
The systems biology process toward targeted treatments in nephrology. The emergence of large, detailed, multilevel biological and clinical data from national databases, cohorts studies and trials provide critical pieces needed to improve the pathway to targeted treatments in nephrology.
Challenges to the systems biology approach include the need to keep pace with our increasing capacity to capture the cellular and molecular complexity of the kidney. Researchers have to identify ways to link the data emerging from these novel technologies with other data types, most importantly clinical presentation and the variable outcomes seen across diseases. The identification of PLA2R-positive membranous nephropathy provides a compelling example of how a complex multiomics research effort led to an ELISA test now routinely used to manage patients. The feasibility and costs associated with translating the accumulation of large quantities of data into meaningful options for personalized medicine in the clinic is a frequently raised concern. However, requirements for making omics data publicly available, diverse bioinformatics, computational capabilities, and iteratively increasing knowledge about molecular mechanisms allow the same data sets to be queried in different ways by the global kidney research community to answer numerous questions. Further, judicious use of specimens and careful biobanking allow for measurements with multiple omics platforms from a single sample provided by an individual participating in a study.
Thus, systems biology provides the techniques and tools necessary to build a comprehensive and integrated picture of the kidney, both in health and disease. The foundations laid by successes in oncology, increasing availability of digitized clinical data, and the investments made in developing longitudinal studies provide the other key pieces needed for accelerated innovations in the prevention and treatment of kidney diseases. The rapid emergence of a multitude of targeted therapies from these efforts has already changed the field of clinical research in nephrology, with 20 clinical trials active in primary glomerular diseases alone (kidneyhealthgateway.com), allowing our patients for the first time to ask a core question in precision medicine: which is the best trial for me?
Disclosures
Dr. Kretzler reports receiving grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elpidera, European Union Innovative Medicine Initiative, Gilead Sciences, Goldfinch Bio, Janssen, JDRF, Merck, and Novo Nordisk, all outside of the submitted work. Dr. Kretzler also reports holding a United States patent on “Biomarkers for CKD progression,” encompassing urinary EGF as biomarker of CKD progression. Dr. Hamidi, Dr. Schaub, and Dr. Subramanian have nothing to disclose.
Funding
Dr. Kretzler is supported by grants from the National Institutes of Health (NIH) and receives nonfinancial support from the University of Michigan. Support for this work is through the George M. O’Brien Michigan Kidney Translational Core Center, funded by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant 2P30-DK-081943, awarded to Dr. Kretzler.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Inrig JK, Califf RM, Tasneem A, Vegunta RK, Molina C, Stanifer JW, Chiswell K, Patel UD: The landscape of clinical trials in nephrology: A systematic review of Clinicaltrials.gov. Am J Kidney Dis 63: 771–780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Herrington WG, Coresh J, Landray MJ, Levin A, Perkovic V, Pfeffer MA, Rossing P, Walsh M, Wanner C, Wheeler DC, Winkelmayer WC, McMurray JJV; KDIGO Controversies Conference on Challenges in the Conduct of Clinical Trials in Nephrology Conference Participants : Challenges in conducting clinical trials in nephrology: Conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 92: 297–305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler KJ, Conway PT: The new HHS kidney innovation accelerator. When innovation stalls, HHS says floor it! Clin J Am Soc Nephrol 13: 1747–1749, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He JC, Chuang PY, Ma’ayan A, Iyengar R: Systems biology of kidney diseases. Kidney Int 81: 22–39, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers D, Day PJ: Systems medicine: The application of systems biology approaches for modern medical research and drug development. Mol Biol Int 2015: 698169, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apweiler R, Beissbarth T, Berthold MR, Blüthgen N, Burmeister Y, Dammann O, Deutsch A, Feuerhake F, Franke A, Hasenauer J, Hoffmann S, Höfer T, Jansen PLM, Kaderali L, Klingmüller U, Koch I, Kohlbacher O, Kuepfer L, Lammert F, Maier D, Pfeifer N, Radde N, Rehm M, Roeder I, Saez-Rodriguez J, Sax U, Schmeck B, Schuppert A, Seilheimer B, Theis FJ, Vera J, Wolkenhauer O: Whither systems medicine? Expt Mol Med 50: e453, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Cabrero D, Abugessaisa I, Maier D, Teschendorff A, Merkenschlager M, Gisel A, Ballestar E, Bongcam-Rudloff E, Conesa A, Tegnér J: Data integration in the era of omics: Current and future challenges. BMC Syst Biol 8[Suppl 2]: I1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder S, Hamidi H, Kretzler M, Ju W: An integrative systems biology approach for precision medicine in diabetic kidney disease. Diabetes Obes Metab 20[Suppl 3]: 6–13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidi H, Kretzler M: Systems biology approaches to identify disease mechanisms and facilitate targeted therapy in the management of glomerular disease. Curr Opin Nephrol Hypertens 27: 433–439, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariani LH, Pendergraft WF 3rd, Kretzler M: Defining glomerular disease in mechanistic terms: Implementing an integrative biology approach in nephrology. Clin J Am Soc Nephrol 11: 2054–2060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleidgen S, Fernau S, Fleischer H, Schickhardt C, Oßa AK, Winkler EC: Applying systems biology to biomedical research and health care: A précising definition of systems medicine. BMC Health Serv Res 17: 761, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Centers for Systems Biology (NCSB). Bethesda, MD, National Institutes of Health, 2019, Available at: https://www.nigms.nih.gov/Research/SpecificAreas/SysBio/Pages/default.aspx. Accessed August 26, 2019.
- 13.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Griffin J, Treanor D: Digital pathology in clinical use: Where are we now and what is holding us back? Histopathology 70: 134–145, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Barisoni L, Gimpel C, Kain R, Laurinavicius A, Bueno G, Zeng C, Liu Z, Schaefer F, Kretzler M, Holzman LB, Hewitt SM: Digital pathology imaging as a novel platform for standardization and globalization of quantitative nephropathology. Clin Kidney J 10: 176–187, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, Palmer M, Rosenberg A, Gasim A, Liensziewski C, Merlino L, Chien HP, Chang A, Meehan SM, Gaut J, Song P, Holzman L, Gibson D, Kretzler M, Gillespie BW, Hewitt SM: Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 29: 671–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani LH, Martini S, Barisoni L, Canetta PA, Troost JP, Hodgin JB, Palmer M, Rosenberg AZ, Lemley KV, Chien HP, Zee J, Smith A, Appel GB, Trachtman H, Hewitt SM, Kretzler M, Bagnasco SM: Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transplant 33: 310–318, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López de Maturana E, Alonso L, Alarcón P, Martín-Antoniano IA, Pineda S, Piorno L, Calle ML, Malats N: Challenges in the integration of omics and non-omics data. Genes (Basel) 10: E238, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain MP, Goyder EC, Rigby JE, El Nahas M: CKD and poverty: A growing global challenge. Am J Kidney Dis 53: 166–174, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Eddy S, Nair V, Mariani LH, Eichinger FH, Hartman J, Huang H, Parikh H, Taroni JN, Lindenmeyer MT, Ju W, Greene CS, Grayson PC, Godfrey B, Cohen CD, Sampson MG, Lafayette RA, Krischer J, Merkel PA, Kretzler M: Inflammatory and JAK-STAT pathways as shared molecular targets for ANCA-associated vasculitis and nephrotic syndrome. bioRxiv, 427898, 2018 [Google Scholar]
- 21.Mariani LH, Eddy S, Martini S, Eichinger F, Godfrey B, Nair V, Adler SG, Appel GB, Athavale A, Barisoni L, Brown E, Cattran DC, Dell KM, Derebail V, Fervenza FC, Fornoni A, Gadegbeku CA, Gibson KL, Gipson D, Greenbaum LA, Hingorani SR, Hlandunewich MA, Hogan J, Holzman LB, Jefferson JA, Kaskel FJ, Kopp JB, Lafayette RA, Lemley KV, Lieske JC, Lin J-J, Myers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sambandam K, Sedor JR, Sethna CB, Srivastava T, Trachtman H, Tran C, Wang C-s, Kretzler M: Redefining nephrotic syndrome in molecular terms: Outcome-associated molecular clusters and patient stratification with noninvasive surrogate biomarkers. bioRxiv, 427880, 2018 [Google Scholar]
- 22.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA: JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 94: 795–808, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Chaudhary K, Garmire LX: More is Better: Recent progress in multi-omics data integration methods. Front Genet 8: 84, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo Q, Wang S, Seshan VE, Olshen AB, Schultz N, Sander C, Powers RS, Ladanyi M, Shen R: Pattern discovery and cancer gene identification in integrated cancer genomic data. Proc Natl Acad Sci U S A 110: 4245–4250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen R, Olshen AB, Ladanyi M: Integrative clustering of multiple genomic data types using a joint latent variable model with application to breast and lung cancer subtype analysis. Bioinformatics 25: 2906–2912, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Mezlini AM, Demir F, Fiume M, Tu Z, Brudno M, Haibe-Kains B, Goldenberg A: Similarity network fusion for aggregating data types on a genomic scale. Nat Methods 11: 333–337, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Meystre S, Haug PJ: Natural language processing to extract medical problems from electronic clinical documents: Performance evaluation. J Biomed Inform 39: 589–599, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Miotto R, Li L, Kidd BA, Dudley JT: Deep patient: An unsupervised Representation to predict the future of patients from the electronic health records. Sci Rep 6: 26094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne JD, Wyatt M, Westfall AO, Willig J, Bethard S, Gordon G: Efficient identification of nationally mandated reportable cancer cases using natural language processing and machine learning. J Am Med Inform Assoc 23: 1077–1084, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bedi G, Carrillo F, Cecchi GA, Slezak DF, Sigman M, Mota NB, Ribeiro S, Javitt DC, Copelli M, Corcoran CM: Automated analysis of free speech predicts psychosis onset in high-risk youths. npj Schizophrenia 1: 15030, 2015. [DOI] [PMC free article] [PubMed]
- 31.Mandal S, Greenblatt AB, An J: Imaging intelligence: AI is transforming medical imaging across the imaging spectrum. IEEE Pulse 9: 16–24, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak JAWM, Hermsen M, Manson QF, Balkenhol M, Geessink O, Stathonikos N, van Dijk MC, Bult P, Beca F, Beck AH, Wang D, Khosla A, Gargeya R, Irshad H, Zhong A, Dou Q, Li Q, Chen H, Lin HJ, Heng PA, Haß C, Bruni E, Wong Q, Halici U, Öner MÜ, Cetin-Atalay R, Berseth M, Khvatkov V, Vylegzhanin A, Kraus O, Shaban M, Rajpoot N, Awan R, Sirinukunwattana K, Qaiser T, Tsang YW, Tellez D, Annuscheit J, Hufnagl P, Valkonen M, Kartasalo K, Latonen L, Ruusuvuori P, Liimatainen K, Albarqouni S, Mungal B, George A, Demirci S, Navab N, Watanabe S, Seno S, Takenaka Y, Matsuda H, Ahmady Phoulady H, Kovalev V, Kalinovsky A, Liauchuk V, Bueno G, Fernandez-Carrobles MM, Serrano I, Deniz O, Racoceanu D, Venâncio R; the CAMELYON16 Consortium : Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318: 2199–2210, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haenssle HA, Fink C, Schneiderbauer R, Toberer F, Buhl T, Blum A, Kalloo A, Hassen ABH, Thomas L, Enk A, Uhlmann L; Reader study level-I and level-II Groups : Man against machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol 29: 1836–1842, 2018 [DOI] [PubMed] [Google Scholar]
- 34.De Fauw J, Ledsam JR, Romera-Paredes B, Nikolov S, Tomasev N, Blackwell S, Askham H, Glorot X, O’Donoghue B, Visentin D, van den Driessche G, Lakshminarayanan B, Meyer C, Mackinder F, Bouton S, Ayoub K, Chopra R, King D, Karthikesalingam A, Hughes CO, Raine R, Hughes J, Sim DA, Egan C, Tufail A, Montgomery H, Hassabis D, Rees G, Back T, Khaw PT, Suleyman M, Cornebise J, Keane PA, Ronneberger O: Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med 24: 1342–1350, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, Meyer C, Ravuri S, Protsyuk I, Connell A, Hughes CO, Karthikesalingam A, Cornebise J, Montgomery H, Rees G, Laing C, Baker CR, Peterson K, Reeves R, Hassabis D, King D, Suleyman M, Back T, Nielson C, Ledsam JR, Mohamed S: A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572: 116–119, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuo C-C, Chang C-M, Liu K-T, Lin W-K, Chiang H-Y, Chung C-W, Ho M-R, Sun P-R, Yang R-L, Chen K-T: Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. npj Digital Medicine 2: 29, 2019. [DOI] [PMC free article] [PubMed]
- 37.Nadkarni GN, Fleming F, McCullough JR, Chauhan K, Verghese DA, He JC, Quackenbush J, Bonventre JV, Murphy B, Parikh CR, Donovan M, Coca SG: Prediction of rapid kidney function decline using machine learning combining blood biomarkers and electronic health record data. bioRxiv, 587774, 2019 [Google Scholar]
- 38.Capdeville R, Buchdunger E, Zimmermann J, Matter A: Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 1: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Garraway LA, Lander ES: Lessons from the cancer genome. Cell 153: 17–37, 2013 [DOI] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration : Table of Pharmacogenomic Biomarkers in Drug Labeling, 2018. Available at: https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling. Accessed July 30, 2019
- 42.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D: Molecular portraits of human breast tumours. Nature 406: 747–752, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga J-Y, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M; MINDACT Investigators : 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375: 717–729, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Esserman LJ, Yau C, Thompson CK, van ’t Veer LJ, Borowsky AD, Hoadley KA, Tobin NP, Nordenskjöld B, Fornander T, Stål O, Benz CC, Lindström LS: Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol 3: 1503–1510, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Retèl VP, Joore MA, Drukker CA, Bueno-de-Mesquita JM, Knauer M, van Tinteren H, Linn SC, van Harten WH: Prospective cost-effectiveness analysis of genomic profiling in breast cancer. Eur J Cancer 49: 3773–3779, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Beck LH Jr., Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HAF, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJH, den Heijer M, Kiemeney LALM, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JFM, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Beck LH, Jr.: PLA2R and THSD7A: Disparate paths to the same disease? J Am Soc Nephrol 28: 2579–2589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC; MENTOR Investigators : Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381: 36–46, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY-C, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang H-Y, Brosius FC, Gadegbeku CA, Kretzler M; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein J, Bascands JL, Buffin-Meyer B, Schanstra JP: Epidermal growth factor and kidney disease: A long-lasting story. Kidney Int 89: 985–987, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Segarra-Medrano A, Carnicer-Caceres C, Valtierra-Carmeno N, Agraz-Pamplona I, Ramos-Terrades N, Jatem Escalante E, Ostos-Roldan E: Value of urinary levels of interleukin-6, epidermal growth factor, monocyte chemoattractant protein type1 and transforming growth factor β1 in predicting the extent of fibrosis lesions in kidney biopsies of patients with IgA nephropathy. Nefrologia 37: 531–538, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Azukaitis K, Ju W, Kirchner M, Nair V, Smith M, Fang Z, Thurn-Valsassina D, Bayazit A, Niemirska A, Canpolat N, Bulut IK, Yalcinkaya F, Paripovic D, Harambat J, Cakar N, Alpay H, Lugani F, Mencarelli F, Civilibal M, Erdogan H, Gellermann J, Vidal E, Tabel Y, Gimpel C, Ertan P, Yavascan O, Melk A, Querfeld U, Wühl E, Kretzler M, Schaefer F; 4C Study; ESCAPE Trial Group : Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int 96: 214–221, 2019 [DOI] [PubMed] [Google Scholar]
- 54.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuttle KR, Brosius FC 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk ME, Cardillo TE, Duffin KL, Haas JV, Macias WL, Nunes FP, Janes JM: JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 33: 1950–1959, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, Turner JD, Meednu N, Mizoguchi F, Gutierrez-Arcelus M, Lieb DJ, Keegan J, Muskat K, Hillman J, Rozo C, Ricker E, Eisenhaure TM, Li S, Browne EP, Chicoine A, Sutherby D, Noma A, Nusbaum C, Kelly S, Pernis AB, Ivashkiv LB, Goodman SM, Robinson WH, Utz PJ, Lederer JA, Gravallese EM, Boyce BF, Hacohen N, Pitzalis C, Gregersen PK, Firestein GS, Raychaudhuri S, Moreland LW, Holers VM, Bykerk VP, Filer A, Boyle DL, Brenner MB, Anolik JH; Accelerating Medicines Partnership RA/SLE Network : Methods for high-dimensional analysis of cells dissociated from cryopreserved synovial tissue. Arthritis Res Ther 20: 139, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen CD, Frach K, Schlöndorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Datta S, Malhotra L, Dickerson R, Chaffee S, Sen CK, Roy S: Laser capture microdissection: Big data from small samples. Histol Histopathol 30: 1255–1269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kretzler M, Schröppel B, Merkle M, Huber S, Mundel P, Horster M, Schlöndorff D: Detection of multiple vascular endothelial growth factor splice isoforms in single glomerular podocytes. Kidney Int Suppl 67: S159–S161, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, Lee YS, Zhu Q, Kehata M, Li M, Jiang S, Rastaldi MP, Cohen CD, Troyanskaya OG, Kretzler M: Defining cell-type specificity at the transcriptional level in human disease. Genome Res 23: 1862–1873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD: Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harder JL, Menon R, Otto EA, Zhou J, Eddy S, Wys NL, O’Connor C, Luo J, Nair V, Cebrian C, Spence JR, Bitzer M, Troyanskaya OG, Hodgin JB, Wiggins RC, Freedman BS, Kretzler M; European Renal cDNA Bank (ERCB); Nephrotic Syndrome Study Network (NEPTUNE) : Organoid single cell profiling identifies a transcriptional signature of glomerular disease. JCI Insight 4: 122697, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H, Humphreys BD: The promise of single-cell RNA sequencing for kidney disease investigation. Kidney Int 92: 1334–1342, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K: Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen HJ, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH: Evaluation of variability in human kidney organoids. Nat Methods 16: 79–87, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Der E, Ranabothu S, Suryawanshi H, Akat KM, Clancy R, Morozov P, Kustagi M, Czuppa M, Izmirly P, Belmont HM, Wang T, Jordan N, Bornkamp N, Nwaukoni J, Martinez J, Goilav B, Buyon JP, Tuschl T, Putterman C: Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight 2: e93009, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, Chicoine A, Eisenhaure TM, Jonsson AH, Li S, Lieb DJ, Zhang F, Slowikowski K, Browne EP, Noma A, Sutherby D, Steelman S, Smilek DE, Tosta P, Apruzzese W, Massarotti E, Dall’Era M, Park M, Kamen DL, Furie RA, Payan-Schober F, Pendergraft WF 3rd, McInnis EA, Buyon JP, Petri MA, Putterman C, Kalunian KC, Woodle ES, Lederer JA, Hildeman DA, Nusbaum C, Raychaudhuri S, Kretzler M, Anolik JH, Brenner MB, Wofsy D, Hacohen N, Diamond B; Accelerating Medicines Partnership in SLE network : The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol 20: 902–914, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, Clevers H, Deplancke B, Dunham I, Eberwine J, Eils R, Enard W, Farmer A, Fugger L, Göttgens B, Hacohen N, Haniffa M, Hemberg M, Kim S, Klenerman P, Kriegstein A, Lein E, Linnarsson S, Lundberg E, Lundeberg J, Majumder P, Marioni JC, Merad M, Mhlanga M, Nawijn M, Netea M, Nolan G, Pe’er D, Phillipakis A, Ponting CP, Quake S, Reik W, Rozenblatt-Rosen O, Sanes J, Satija R, Schumacher TN, Shalek A, Shapiro E, Sharma P, Shin JW, Stegle O, Stratton M, Stubbington MJT, Theis FJ, Uhlen M, van Oudenaarden A, Wagner A, Watt F, Weissman J, Wold B, Xavier R, Yosef N; Human Cell Atlas Meeting Participants : The Human Cell Atlas. eLife 6: e27041, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu BC; HuBMAP Consortium : The human body at cellular resolution: The NIH Human Biomolecular Atlas Program. Nature 574: 187–192, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, Richoz N, Frazer GL, Staniforth JUL, Vieira Braga FA, Botting RA, Popescu DM, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Polanski K, Efremova M, Green K, Del Castillo Velasco-Herrera M, Guzzo C, Collord G, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Lindsay S, Bajenoff M, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Behjati S, Clatworthy MR: Spatiotemporal immune zonation of the human kidney. Science 365: 1461–1466, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]