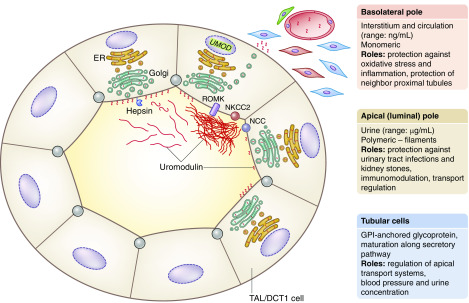
Uromodulin, initially named Tamm–Horsfall protein after its description by Igor Tamm and Frank L. Horsfall in 1950, is a zona pellucida matrix-type glycoprotein exclusively produced by the thick ascending limb (TAL) and to a minor extent, the distal convoluted tubule of the kidney. As a glycosylphosphatidylinositol-anchored protein containing 48 cysteine residues that is heavily glycosylated, uromodulin matures along the secretory pathway of the tubular cells. After it reaches the apical membrane of the cells, the protein undergoes proteolytic cleavage, with uromodulin monomers released into the urine to form high molecular weight polymeric filaments that organize in a gel-like matrix. The latter is the major constituent of hyaline casts (Figure 1). Uromodulin is encoded by the UMOD gene located on chromosome 16p12.3–16p13.11. UMOD is the most abundant transcript expressed in the human kidney, and uromodulin is by far the most abundant protein excreted in healthy urine (mean daily excretion rates of up to 100 mg/24 h in humans) (1).
Figure 1.
Biomarker value and potential roles of uromodulin. Uromodulin is produced in the cells lining the thick ascending limb (TAL) of the loop of Henle and to a minor extent, the initial part of distal convoluted tubule (DCT1). These segments are involved in the reabsorption of NaCl and divalent cations. The NaCl reabsorption is mediated by the apical cotransporters NKCC2 (TAL) and NCC (DCT1). The K+ channel ROMK is essential for the activity of NKCC2 and for the paracellular reabsorption of cations (including Ca2+ and Mg2+). In the tubular cells, uromodulin is processed along the secretory pathway (including the endoplasmic reticulum, ER, and the Golgi apparatus), and then, it is cleaved on the apical membrane by the serine protease hepsin. In the urine, the monomeric uromodulin molecules assemble into macromolecular filaments, forming a matrix gel. Uromodulin is also present in vesicles close to the basement membrane of TAL cells and detected in the blood at concentrations that are much lower than in the urine. On the basis of that processing, uromodulin plays potentially distinct roles in the tubular cells, in the urine, in the kidney interstitium, and in the circulation. Details are in the text. GPI, glycosylphosphatidylinositol; UMOD, uromodulin.
Biochemical studies and investigations in mouse models have suggested that uromodulin plays important roles in the kidney tubule and lumen, including regulation of salt handling and BP and capacity to concentrate the urine via regulation of transport systems operating in TAL cells and beyond; protection against kidney stones by reducing the aggregation of calcium crystals; defense against urinary tract infections mediated by high mannose binding to type 1 fimbriae of uropathogenic Escherichia coli; and a role in innate immunity by binding Igs, cytokines, and lymphokines (Figure 1). Furthermore, genetic studies indicated that rare (mostly missense) mutations in UMOD cause autosomal dominant tubulointerstitial kidney disease, a rare disorder causing CKD and progression to kidney failure, whereas common UMOD variants evidenced by genome-wide association studies are associated with kidney function and risk of CKD in the general population. Of interest, the top UMOD genome-wide association study risk variants are located in the promoter of the gene, and they directly increase the expression of uromodulin in the kidney and its excretion in urine (1,2).
Although most of the uromodulin is exported to the apical pole of the tubular cells to be released in the urine, a fraction of the monomeric protein is released through the basolateral plasma membrane into the interstitium and furthermore, to the circulation (3). The basolateral release of uromodulin might participate in a crosstalk between tubular segments, including the TAL and proximal tubules, protecting against acute ischemic injury and modulating the immune response (1,4).
The idea that uromodulin may be used as biomarker in healthy individuals and in patients with kidney diseases was triggered by its specific production by the kidney, and the possibility to measure it reliably using ELISA methods (1). Many cohort studies measured the levels of uromodulin in urine because this sample reflects the physiologic processing of the protein, contains high amounts of it, and is noninvasive. Both timed urinary excretion (uromodulin expressed in milligrams per 24 hours) and spot urine samples (uromodulin expressed in milligrams per gram of creatinine) have been used (5). These studies validated urinary uromodulin as a biomarker for tubular mass and tubular function (“functional tubular marker”) in the general population as well as in disease subgroups. In particular, higher levels of urinary uromodulin were associated with a lower risk of eGFR decline, a lower risk of incident CKD, a lower risk of postoperative AKI, and a lower risk of mortality in the aged population—all associations compatible with the functional tubular marker concept (reviewed in ref. 1).
Yet, measurement of urine uromodulin requires adequate processing and storage of samples. Also, the urinary excretion of uromodulin is subject to large fluctuations both within and between individuals with the influence of diet (e.g., salt intake), hydration state, and medications (1). As discussed above, low levels of circulating uromodulin (nanograms per milliliter range; i.e., approximately 100–1000 times lower than urinary levels) can be detected in the general population. The circulating levels of uromodulin correlate with urinary uromodulin excretion and with creatinine clearance or eGFR in patients with CKD. Circulating uromodulin levels increase after kidney transplantation proportional to the recovery of graft function (3). In a large cohort (n=3057) of patients undergoing coronary angiography, higher serum uromodulin levels were associated with a favorable metabolic profile, a lower prevalence of comorbidities, and a lower risk of 10-year mortality independently of other cardiovascular risk factors, including eGFR. Such associations may suggest that serum uromodulin may have a beneficial role (6).
In this issue of CJASN, Steubl et al. (7) demonstrate that higher serum uromodulin levels were associated with lower mortality, major adverse cardiovascular events (MACEs), and ESKD risk in a white CKD population from Germany. The German Chronic Kidney Disease (GCKD) study is a multicentric, observational, prospective cohort initiated in 2010 including patients with prevalent or incident CKD under the care of nephrologists in 158 outpatient sites in Germany. Inclusion criteria were age between 18 and 74 years old, white race, and CKD Kidney Disease Improving Global Outcomes stage G3 (30–60 ml/min per 1.73 m2) or A3 (albuminuria >300 mg/g or proteinuria >500 mg/g). Patients were excluded if they had a transplant, suffered from heart failure New York Heart Association stage IV, or had an active malignancy in the previous 24 months. Serum uromodulin was measured in 5143 of 5217 (98.5%) participants. They were mainly men (60%) aged 60±12 years old with prevalent cardiovascular risk factors, such as diabetes (36%), hypertension (96%), and coronary heart disease or stroke history (30%). Most had eGFR<60 ml/min per 1.73 m2 (78%) and/or overt albuminuria (58%). Those with higher uromodulin levels had lower prevalence of cardiovascular risk factors, higher eGFR, and lower albuminuria. During the 4-year median follow-up, there were 335 deaths, 417 MACEs, and 229 kidney failures. In multiadjusted models, each SD higher serum uromodulin concentration was associated with a lower risk of mortality by 20% (4%–36%) and a lower risk of kidney failure by 39% (19%–54%). Regarding MACEs, only the highest quartile of serum uromodulin showed a reduced hazard of 47% (10%–55%) compared with the lowest quartile without strong evidence of an association when looking at the increase in SD (7).
The current GCKD study has many strengths: the large sample size allows for sufficient event numbers, the lost to follow-up (2%) and missing data numbers are low, and the statistical analyses are sound and reinforced by the use of restricted cubic splines and competitive risk models. The authors were able to adjust for multiple cardiovascular and demographic risk factors and for albuminuria, reducing residual confounding bias. They additionally split the cohort into a trained cohort and a tested cohort with good concordance numbers. Finally, serum uromodulin level measurements were robust and centralized. The limitations included the lack of stratification (age, diabetes, and hypertension) by inclusion site and the lack of information about the site characteristics as well as about screenings and exclusions. The fact that the GCKD study cohort includes a population seen by a nephrologist limits the relevance of the findings. In addition, eGFR defining eligibility was estimated using locally measured creatinine with different assays, not necessarily isotope dilution mass spectrometry, although the Chronic Kidney Disease Epidemiology Collaboration equation was then used. The mean eGFR is not very low (49 ml/min per 1.73 m2) for a CKD population: because the participants are relatively aged (mean: 60±12 years old), it would be interesting to know how many of them had an eGFR<45 ml/min per 1.73 m2. When looking at spline figures, the risk seems to decrease mainly at a cutoff value for serum uromodulin at 100 ng/ml. At higher levels (>200 ng/m), we can see that 95% confidence intervals are greater, leading to more uncertainty for kidney failure and mortality. In addition, the hazard ratios for kidney failure seem to follow a J curve rather than a straight line at higher levels.
Altogether, the study by Steubl et al. (7) confirms the independent associations of higher serum uromodulin levels with lower risks of mortality and MACEs in aged populations and with lower risk of incident kidney failure in a middle-aged Chinese cohort (8). Of note, no association between serum uromodulin and cardiovascular disease events or all-cause mortality was observed in the latter study (8). These results raise interesting issues regarding biomarkers in general and uromodulin in particular. It should be pointed that serum uromodulin is simply associated with these risks independently of eGFR, CKD, and other cardiovascular risk factors. As discussed above, the production of uromodulin primarily reflects the activity of the TAL segment and is essentially directed to the urine. Arguably, any defect in the TAL cells may affect the production and/or intracellular trafficking of uromodulin, causing a decrease in the uromodulin levels in urine and/or blood. The latter hypothesis assumes that the apical (major) and basolateral (minor) releases are similarly affected (Figure 1). Thus far, a moderate correlation between urinary and serum levels of uromodulin (normalized for eGFR) has been reported in a subcohort of the Cardiovascular Health Study (9). Furthermore, genome-wide association studies have shown that the same UMOD promoter variant (rs12917707) was associated with urinary uromodulin and serum uromodulin (6). We should also understand how the activity of TAL cells or the levels of circulating uromodulin could be protective against CKD or cardiovascular events. Possible avenues include regulation of BP, influence on mineral metabolism, modulation of innate immunity or erythropoietin production in the interstitial compartment, or protection of other tubular segments (e.g., neighboring proximal tubules) (10). Direct protective effects of systemic, monomeric uromodulin may include protection against inflammation (6) and oxidative stress (10). Future studies should explore the specific value of uromodulin as a biomarker in the serum or urine because these two compartments probably stage different properties of the protein. We would then learn whether circulating uromodulin could have direct beneficial effects, be a marker of tubular health, or both.
Disclosures
Dr. Devuyst and Dr. Ponte have nothing to disclose.
Funding
Dr. Devuyst is supported by European Reference Network for Rare Kidney Diseases project 739532, the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung Kidney.CH program, and Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung grant 310030-189044. Dr. Ponte is funded by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung grants PMPDP3_186203 and PMPDP3_171352.
Acknowledgments
We thank Dr. Eric Olinger for fruitful discussion and help in preparing the figure.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Association of Serum Uromodulin with Death, Cardiovascular Events, and Kidney Failure in CKD,” on pages 616–624.
References
- 1.Devuyst O, Olinger E, Rampoldi L: Uromodulin: From physiology to rare and complex kidney disorders. Nat Rev Nephrol 13: 525–544, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Devuyst O, Pattaro C: The UMOD locus: Insights into the pathogenesis and prognosis of kidney disease. J Am Soc Nephrol 29: 713–726, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, Block M, Kaden J, Schlumberger W: Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 33: 284–295, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Achkar TM, Dagher PC: Tubular cross talk in acute kidney injury: A story of sense and sensibility. Am J Physiol Renal Physiol 308: F1317–F1323, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M: Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol 11: 70–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado GE, Kleber ME, Scharnagl H, Krämer BK, März W, Scherberich JE: Serum uromodulin and mortality risk in patients undergoing coronary angiography. J Am Soc Nephrol 28: 2201–2210, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steubl D, Schneider MP, Meiselbach H, Nadal J, Schmid MC, Saritas T, Krane V, Sommerer C, Baid-Agrawal S, Voelkl J, Kotsis F, Köttgen A, Eckardt KU, Scherberich JE; GCKD study investigators: Association of serum uromodulin with death, cardiovascular events and kidney failure in CKD. Clin J Am Soc Nephrol 15: 616–624, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv L, Wang J, Gao B, Wu L, Wang F, Cui Z, He K, Zhang L, Chen M, Zhao MH: Serum uromodulin and progression of kidney disease in patients with chronic kidney disease. J Transl Med 16: 316, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steubl D, Buzkova P, Ix JH, Devarajan P, Bennett MR, Chaves PHM, Shlipak MG, Bansal N, Sarnak MJ, Garimella PS: Association of serum and urinary uromodulin and their correlates in older adults—the Cardiovascular Health Study [published online ahead of print December 17, 2019]. Nephrology (Carlton) doi: 10.1111/nep.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaFavers KA, Macedo E, Garimella PS, Lima C, Khan S, Myslinski J, McClintick J, Witzmann FA, Winfree S, Phillips CL, Hato T, Dagher PC, Wu XR, El-Achkar TM, Micanovic R: Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med 11: eaaw3639, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]