Abstract
Ameloblastic fibrosarcoma (AFS) now designated as odontogenic sarcoma is a malignant odontogenic tumor characteristically composed of a benign epithelium and a malignant mesenchymal component. It can arise de novo without any preexisting lesion or it can result from the malignant transformation of ameloblastic fibroma (AF). Hereby, we report an extremely rare case of odontogenic sarcoma which was transformed from AF over a period of about 2 years. This is the first case to be reported after it has been reclassified as odontogenic sarcoma. A systematic review was also done to evaluate the studies that reported AFS arising de novo and AFS arising from AF. The objective of this study is to systematically review the studies that reported AFS arising de novo and AFS arising from AF. Articles that reported AFS arising de novo and AFS arising from AF were collected from PubMed, Medline, Embase, Cochrane, Google search and manual search. The results of the systematic review showed that six studies (46.1%) reported AFS arised de novo with no previous history of AF. Seven studies (53.84%) reported that amelobastic fibrosarcoma arised from AF. A rare case of odontogenic sarcoma transformed from AF is reported here. This is the first case report to be published on odontogenic sarcoma after the World Health Organization reclassification. AF once diagnosed should be treated immediately without any delay to avoid the chances of its malignant transformation into odontogenic sarcoma.
Keywords: Ameloblastic fibroma, ameloblastic fibrosarcoma, malignant transformation, odontogenic sarcoma
INTRODUCTION
Odontogenic tumors (OTs) constitute a rare group of heterogeneous diseases that are derived from epithelial, ectomesenchymal and mesenchymal elements of the tooth-forming apparatus. The tumors range from nonneoplastic tissue proliferations to malignant tumors with metastatic potential.[1] Malignant OTs are classified as odontogenic carcinomas and odontogenic sarcomas, of which ameloblastic fibrosarcoma (AFS) is the most common. Heath[2] first described AFS in 1887, and the World Health Organization (WHO) defined AFS as “a neoplasm with a similar structure to AF, but in which the ectomesenchymal component shows the features of a sarcoma.” Thus, only the mesenchymal component shows the signs of malignancy, whereas the epithelial component is normal.[3] The WHO's classification of OTs designated AFS a distinctive neoplasm since the inaugural “Blue Book” edition published in 1972[4] has been designated as odontogenic sarcoma in the recent WHO classification of OTs in 2017.
Odontogenic sarcoma (here, AFS) can be classified as primary, i.e., arising de novo without any preexisting lesion and secondary as a result of malignant transformation of AF. AFS sometimes shows dentin and enamel deposition because of the inductive phenomenon in some cases. In cases, when the inductive process resulted in the deposition of dentin, the lesions were called ameloblastic fibrodentinosarcomas; when dentin and enamel were identified concurrently, the term ameloblastic fibro-odontosarcoma was used.[5] All these are now termed as odontogenic sarcomas. We hereby report an extremely rare case of odontogenic sarcoma which was transformed from ameloblastic fibroma (AF). This is the first case to be reported after the WHO reclassified it as odontogenic sarcoma. A systematic review was also done to evaluate the studies that reported AFS arising de novo and AFS arising from AF.
CASE PRESENTATION
A 31-year-old female patient reported to a local hospital in Chennai, India, on December 2017 with a chief complaint of swelling in the right lower back tooth region. A solitary swelling was noticed measuring 2 cm × 2 cm in the right body of the mandible which was tender on palpation. Computed tomography (CT) examination showed a multiloculated lytic lesion in the right posterior mandible and had an incisional biopsy done but did not report back to the hospital.
Then after a month, the patient consulted in another hospital when the swelling increased to the size of 3 cm × 2 cm and was obliterating the right buccal vestibule in the right posterior mandible. Incisional biopsy suggested a mixed tumor with epithelial and connective tissue components. The epithelial components were arranged in narrow strands, cords and islands of proliferating odontogenic epithelium with peripheral ameloblast-like cells and central stellate reticulum-like cells in a richly cellular mesenchymal tissue resembling dental papilla and were diagnosed with AF. The patient did not undergo any further treatment.
The patient after 2 years in March 2019 noticed a sudden increase in the swelling and experienced pain in the right posterior mandible region and reported to a private hospital in Amritsar, Punjab, India. An incision biopsy report had suggested spindle-cell sarcoma.
The patient was then referred to our institution on July 11, 2019, for further management. CT scan revealed a lytic destructive lesion with cortical thinning and associated soft-tissue swelling involving the ramus and body of the mandible. Incisional biopsy was done under local anesthesia, and histopathology showed highly cellular connective tissue stroma with pleomorphic cells of variable size and shape [Figure 1] along with numerous mitotic figures. Nuclear features included vesicular nuclei, prominent nucleoli, hyperchromatism and pleomorphism suggestive of spindle-cell sarcoma. The surface epithelium was parakeratinized stratified squamous type.
Figure 1.
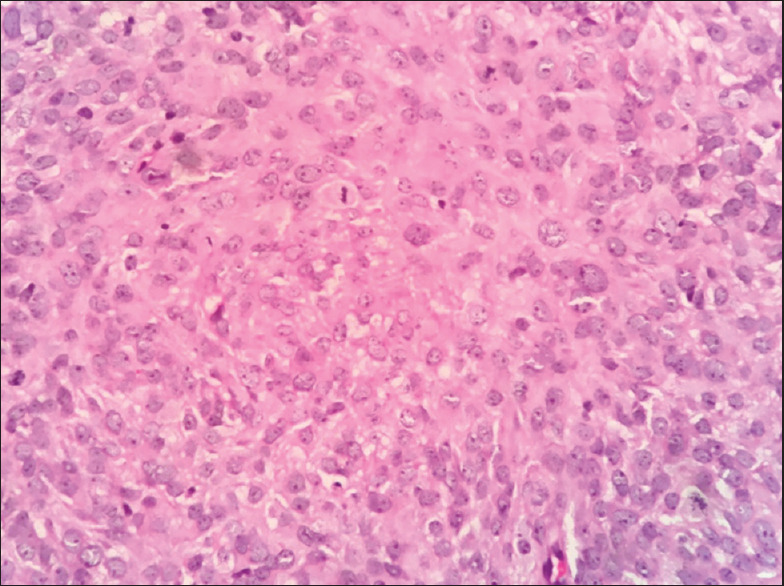
Highly cellular connective tissue stroma with round-to-spindle-shaped cells showing pleomorphism
Immunohistochemistry showed strong positivity of the tumor cells for vimentin [Figure 2] and negative for pan-cytokeratin [Figure 3], smooth muscle actin [Figure 4], S 100 [Figure 5], CD45 [Figure 6], desmin [Figure 7], FLI-1 [Figure 8], CD34 [Figure 9] and CD68 [Figure 10]. Hence, the diagnosis of fibrosarcoma was made correlating the findings. Hence, the surgeons were informed to proceed with the surgical treatment and to submit the entire specimen to rule out odontogenic sarcoma as the previous diagnosis was AF 2 years ago.
Figure 2.
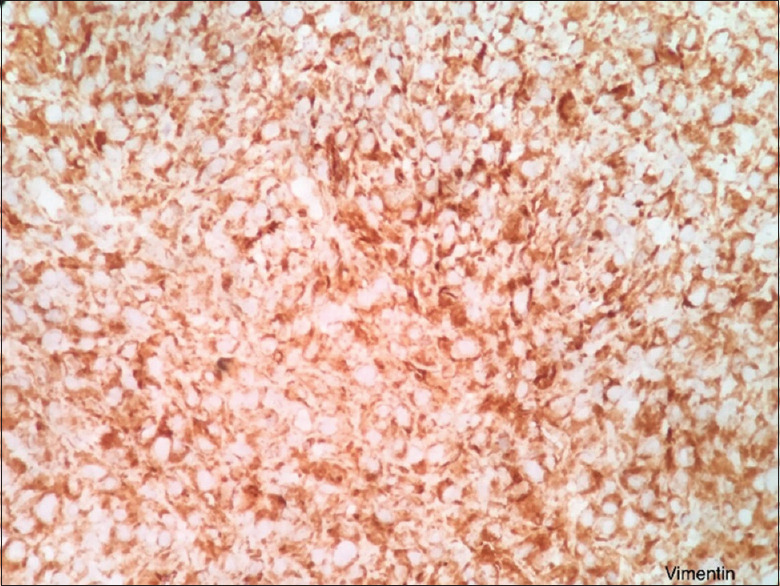
Vimentin is positive for tumor cells
Figure 3.
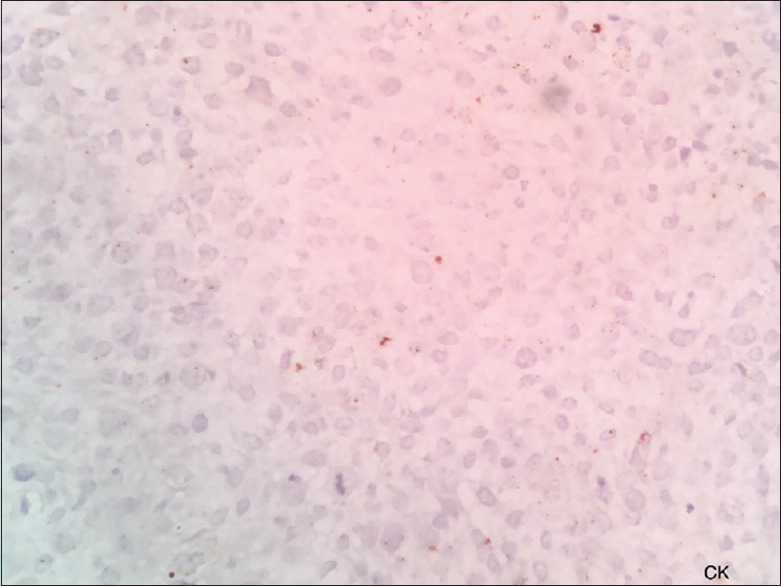
Pan-cytokeratin is negative for tumor cells
Figure 4.
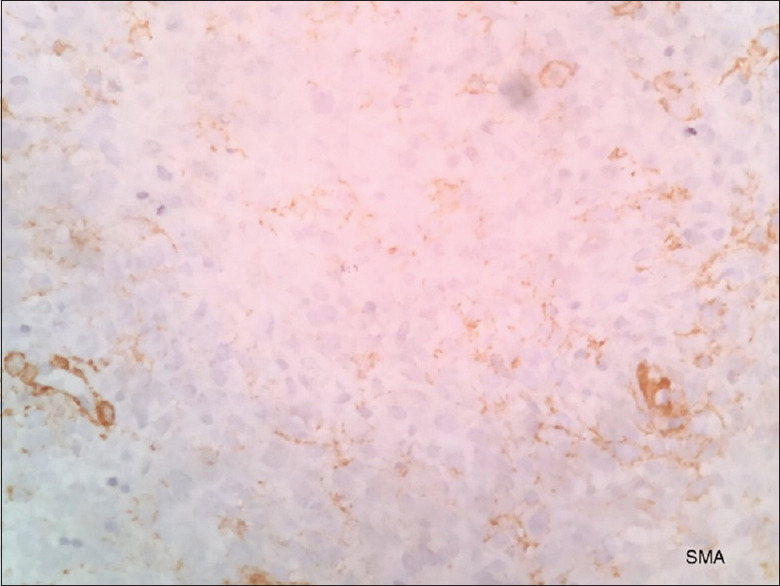
Smooth muscle actin is negative for tumor cells
Figure 5.
S100 is negative for tumor cells
Figure 6.
CD45 is negative for tumor cells
Figure 7.
Desmin is negative for tumor cells
Figure 8.
FLI-1 is negative for tumor cells
Figure 9.
CD34 is negative for tumor cells
Figure 10.
CD68 is negative for tumor cells
Then, the patient underwent right hemimandibulectomy, and the specimen was subjected for histopathological examination. The sections showed highly cellular connective tissue stroma with pleomorphic cells of variable size and shape. The ovoid-to-spindle-shaped tumor cells exhibited vesicular nuclei and prominent nucleoli [Figure 11]. Nuclei exhibited varying degrees of hyperchromatism and pleomorphism along with numerous mitotic figures. Few tumor giant cells [Figure 12] along with the clustering of pleomorphic cells in a streaming pattern were also evident. Among the highly cellular and pleomorphic connective tissue stroma, few ameloblastic follicles with peripherally arranged tall columnar cells with reversal of polarity and central stellate reticulum-like areas were evident [Figure 13]. There was evidence of an area of transition of ameloblastic island with primitive mesenchyme resembling AF [Figures 14-16] to pleomorphic stroma. These findings confirmed the diagnosis of odontogenic sarcoma. A proliferative marker Ki67 was also done, and the report was negative for tumor cells. The patient is disease-free for 3 months.
Figure 11.
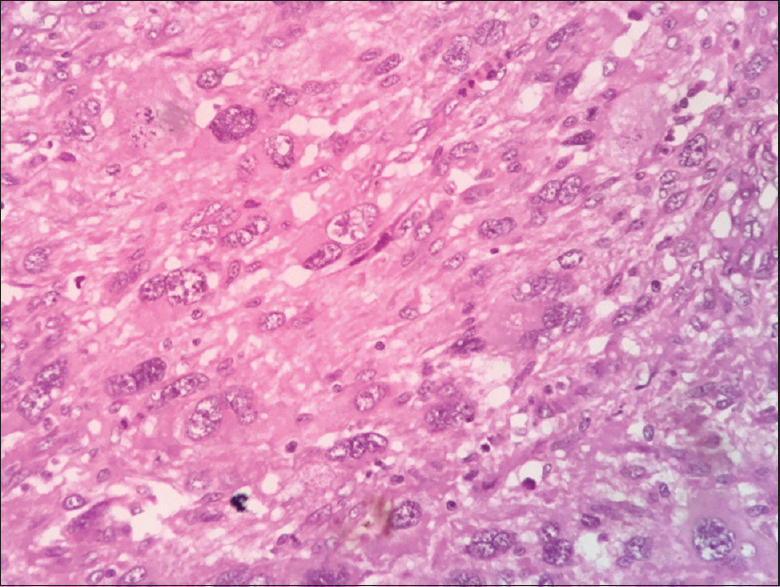
Cells with vesicular nuclei and prominent nucleoli
Figure 12.
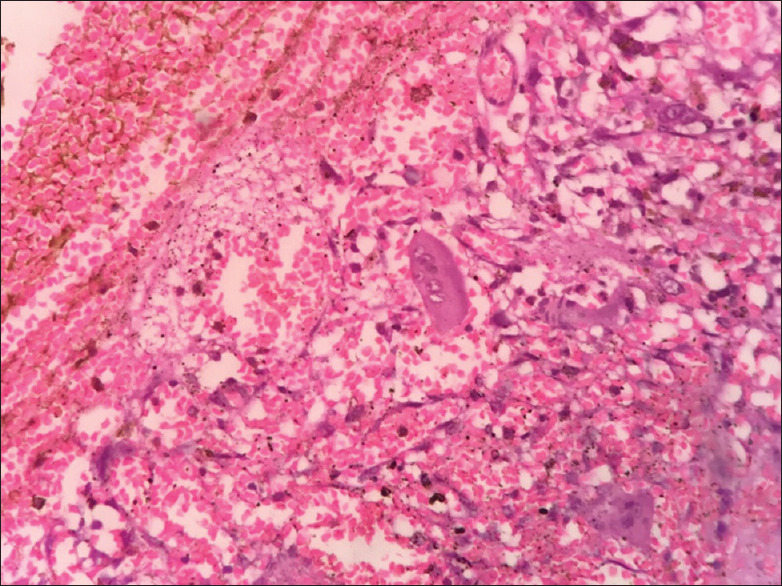
Tumor giant cells
Figure 13.
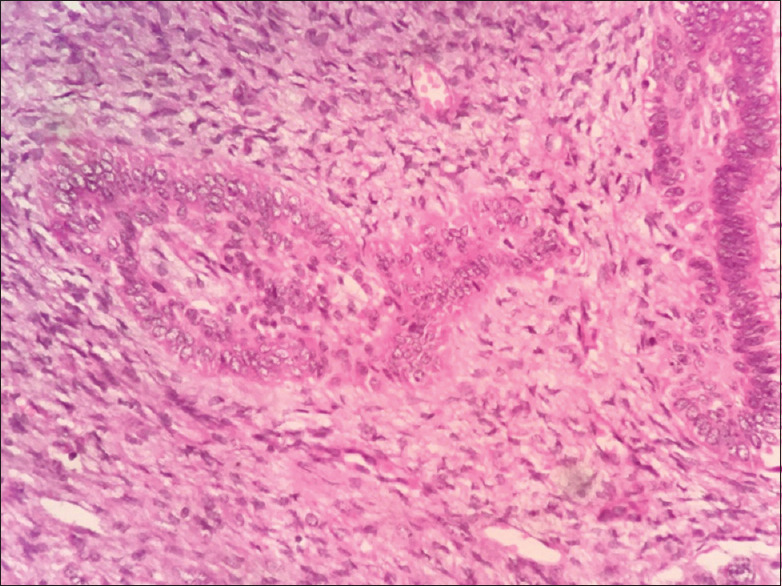
Highly cellular connective tissue stroma with ameloblastic follicles
Figure 14.
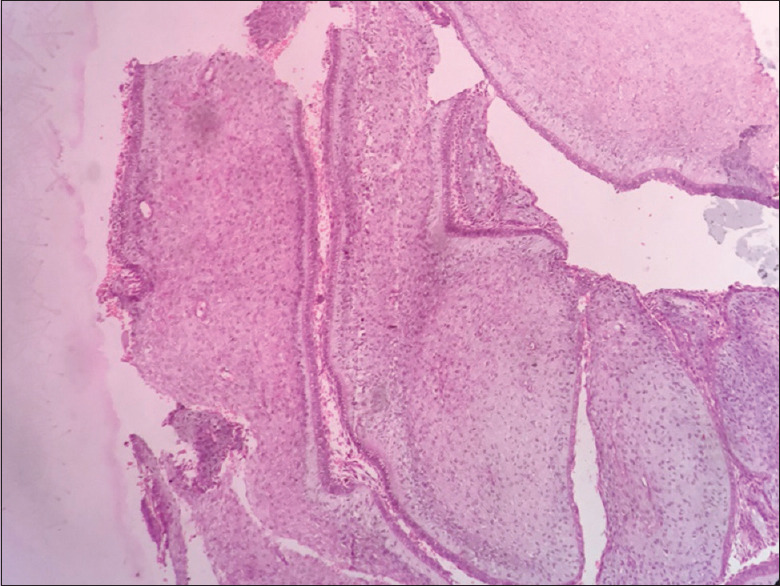
Cellular fibrous connective tissue with ameloblast like cells
Figure 16.
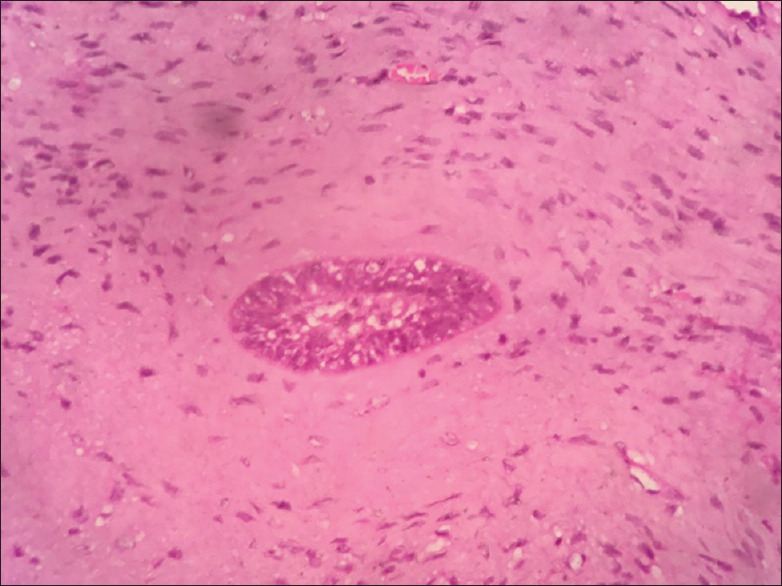
Higher magnification of ameloblastic fibromalike areas
Figure 15.
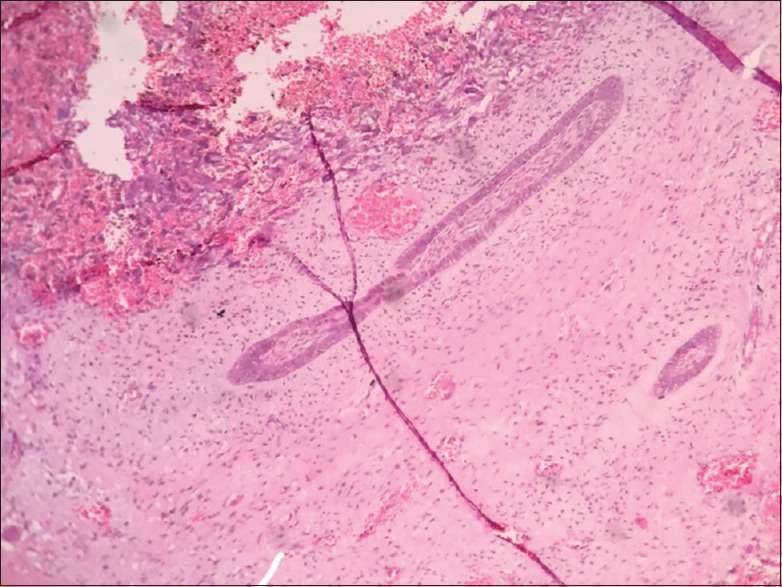
Ameloblastic fibroma-like areas
A systematic review was also done to evaluate the studies that reported AFS arising de novo and AFS arising from AF.
METHODOLOGY
The search strategy was in agreement with the Cochrane guidelines for systematic reviews. The articles relevant to the search strategy were identified from the search databases of PubMed, Embase, Medline, Cochrane, Google search and manual search till the year 2019. An Internet search was also done to obtain the relevant articles of our interest. The article search included only those from English literature. The title of the articles and abstracts were reviewed. The full text of selected articles was retrieved and further analyzed. Search terminologies used were AF, AFS, ameloblastic dentinosacoma, ameloblastic odontosarcoma and odontogenic sarcoma. A total of 13 articles were selected for the systematic review.
RESULTS
The selection and exclusion of the reviewed studies are summarized in Tables 1 and 2. The search strategy identified 13 studies. The results of the systematic review showed that six studies (46.1%) reported AFS arised de novo with no previous history of AF [Table 1]. Seven studies (53.84%) reported that amelobastic fibrosarcoma arised from AF [Table 2].
Table 1.
Systematic review of ameloblastic fibrosarcoma (de novo)
| Study | Author | Patient | AFS clinical feature | Treatment |
|---|---|---|---|---|
| AFS: A case report and literature review | Servato et al.[5] | Mandible | Large firm swelling, pain and dysphagia for 4 months | En bloc surgical resection |
| Ameloblastic fibro-odontosarcoma of the mandible in a pediatric patient | Chen et al.[6] | 4-year-old male, right mandible | Multilocular radiolucency, swelling for 4 months | Right hemimandibular resection |
| AFS of the mandible: A case report and a review of the literature | Al Shetawi et al.[7] | 27-year-old male, left mandible | Acute onset of progressive left mandibular swelling and mild pain for 2 months | Composite resection of the mandible |
| AFS of the mandible: A case report and brief review of the literature | Loya-Solis et al.[1] | 22-year-old female, left mandible | 2 months asymptomatic swelling in the left mandible, exophytic growth | Left hemimandibular resection |
| Aggressive atypical ameloblastic fibrodentinoma: Report of a case | Giraddi and Garg[8] | 17-year-old female, right body of the mandible | Expansile swelling | Radical resection |
| AFS of the mandible: Treatment, long-term follow-up, and subsequent reconstruction of a case | Noordhoek et al.[9] | 36-year-old female, left mandible | 4 months swelling |
AFS: Ameloblastic fibrosarcoma
Table 2.
Systematic review of ameloblastic fibroma to ameloblastic fibrosarcoma
| Study | Author | Patient | Fibroma treatment and duration of malignant transformation | AFS clinical feature | Treatment |
|---|---|---|---|---|---|
| A delayed presentation of AFS in an African patient | Chauke et al.[10] | 24-year-old female, mandible | Enucleation, 13 months | Aggressive expansion for 4 months | Surgical resection |
| AFS of the mandible evolving from a prior AF after 2 years: An unusual finding | Bertoni et al.[11] | 27-year-old male, mandible | 2 years | Asymptomatic swelling | Segmental resection |
| AFS of the mandible with distant metastases | Pourdanesh et al.[12] | 34-year-old female | 12 months | Recurrent rapidly growing, metastasize to the lung | |
| Epithelial dysplasia in AFS arising from recurrent AF in a 26-year-old Iranian male | Mohsenifar et al.[13] | 26-year-old Iranian male, left mandible | Enucleation, 3 years | Swelling, pain and paresthesia for 1 month | Hemi mandibulectomy |
| AFS of the mandible: A case report and mini review | Hu et al.[14] | 22-year-old Chinese male, left mandible | Mandibulectomy, 5 months | Swelling | Resection |
| AFS of the mandible: Report of 2 chemosensitive pediatric cases | Demoor- Goldschmidt et al.[3] | 13-year-old male, left mandible | Enucleation, 9 months | Swelling fast growing | |
| Rapid sarcoma to us transformation of an AF of the mandible: Case report and literature review | Kousar et al.[15] | 20-year-old female, posterior mandible | 6 months | Swelling for 3 weeks | Patient died within 2 weeks |
AFS: Ameloblastic fibrosarcoma, AF: Ameloblastic fibroma
Due to the heterogeneity of the reviewed studies, a meta-analysis could not be performed. However, the collected data were tabulated and analyzed as a systematic review.
DISCUSSION
MOTs constitute about <10% of the total OTs, and odontogenic sarcoma accounts for <5% of all sarcomas of the oral and maxillofacial regions reported so far in the literature. There were about 100 cases of AFS reported in English literature.[5] Here, we have reported an extremely rare case of odontogenic sarcoma which was transformed from AF. The mandible is the most common site compared to the maxilla (79% vs. 21% of cases).[3] Odontogenic sarcoma occurs mostly in the younger population, although it has been reported in ages ranging from 3 to 89 years.[16] Even in our case, the posterior mandible is the affected site and seen in the third decade of life. Radiographically, odontogenic sarcoma usually appears as an expansile destructive radiolucency with ill-defined margins.[17]
Histologically, the tumor is usually defined as a low-to-intermediate-grade malignancy and clinically shows locally aggressive behavior. The tumor shows a high recurrence rate of approximately 37%,[3] but metastasis is rare. To diagnose AFS histopathologically, columnar and/or cuboidal benign ameloblastic epithelial cells are seen arranged in budding and branching cords, admixed with islands and knots. All these components are included in a highly cellular malignant connective stromal component, with cells showing variable degrees of anaplasia.
It is critical to differentiate odontogenic sarcoma, especially low-grade variant, from AF, and recently, several immunohistochemical studies with Ki67, Bcl-2, PCNA, c-KIT and P53 have been performed, suggesting that proliferating markers in association to histopathological features could be useful for identifying the malignant tumor. In our case, Ki67 was negative which indicates that this tumor is less aggressive. In the literature, about 50% of all AFSs are described as malignant transformations of AFs2; the other 50% are described as having arisen de novo.[18]
The systematic review showed 46.1% of studies reported AFS arised de novo with no previous history of AF. Nearly 53.84% of studies reported that AFS arised from AF. The conversion of AF to odontogenic sarcoma can occur at the varied time from 4 months to 3 years. In our case, also AFS seems to be the malignant transformation of AF diagnosed 2 years ago.
Interestingly, loss of heterozygosis (LOH) is evident in AFS. LOH from the short arms of chromosomes 3 and 9 at the 3p24.3, 9p22-p21 and 9p22 loci is evident in AFS; the allelic loss fraction was reported to be 74.6 compared with that of AF.9 These findings suggest that genetic changes may cause an AF to become malignant.[9]
CONCLUSION
This is an extremely rare case of odontogenic sarcoma which was transformed from AF and is the first case report to be published after WHO reclassification.
Even though odontogenic sarcoma is considered a low-grade tumor based on the fact that metastasis is rare, the patient should be treated with wider resection surgery. The postoperative treatment should include the combination of chemotherapy and radiotherapy. AF once diagnosed should be treated immediately without any delay to avoid the chances of its malignant transformation into odontogenic sarcoma. Long-term follow-up of the patient is essential in cases of both AF and odontogenic sarcoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Loya-Solis A, González-Colunga KJ, Pérez-Rodríguez CM, Ramírez-Ochoa NS, Ceceñas-Falcón L, Barboza-Quintana O. Ameloblastic fibrosarcoma of the mandible: A case report and brief review of the literature. Case Rep Pathol. 2015;2015:1–5. doi: 10.1155/2015/245026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath C. Five cases of tumor of jaws treated by excision. Brit M J. 1887;1:777–9. [Google Scholar]
- 3.Demoor-Goldschmidt C, Minard-Colin V, Cassagneau E, Supiot S, Oberlin O, D'hautuille C, et al. Ameloblastic fibrosarcoma of the mandible: Report of 2 chemosensitive pediatric cases. J Pediatr Hematol Oncol. 2012;34:e72–6. doi: 10.1097/MPH.0b013e31821ba989. [DOI] [PubMed] [Google Scholar]
- 4.Pindborg JJ, Kramer IR, Torloni H. Histological Typing of Odontogenic Tumors, Jaw Cysts, and Allied Lesions. 1st ed. Berlin: Springer Verlag; 1971. pp. 36–7. [Google Scholar]
- 5.Servato JP, Faria PR, Ribeiro CV, Cardoso SV, Faria PR, Dias FL, et al. Ameloblastic fibrosarcoma: A case report and literature review. Braz Dent J. 2017;28:262–72. doi: 10.1590/0103-6440201701050. [DOI] [PubMed] [Google Scholar]
- 6.Chen SJ, Zheng XW, Lin X, Liu H. Ameloblastic fibro-odontosarcoma of the mandible in a pediatric patient. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:419–21. doi: 10.1016/j.anorl.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Al Shetawi AH, Alpert EH, Buchbinder D, Urken ML. Ameloblastic fibrosarcoma of the mandible: A case report and a review of the literature. J Oral Maxillofac Surg. 2015;73:1661e1–7. doi: 10.1016/j.joms.2015.03.066. [DOI] [PubMed] [Google Scholar]
- 8.Giraddi GB, Garg V. Aggressive atypical ameloblastic fibrodentinoma: Report of a case. Contemp Clin Dent. 2012;3:97–102. doi: 10.4103/0976-237X.94557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noordhoek R, Pizer ME, Laskin DM. Ameloblastic fibrosarcoma of the mandible: Treatment, long-term follow-up, and subsequent reconstruction of a case. J Oral Maxillofac Surg. 2012;70:2930–5. doi: 10.1016/j.joms.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Chauke NY, Sofianos C, Liakos D, Ndobe E. A delayed presentation of ameloblastic fibrosarcoma in an African patient. BMJ Case Rep. 2017;2017:1–3. doi: 10.1136/bcr-2017-220436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoni F, Del Corso G, Bacchini P, Marchetti C, Tarsitano A. Ameloblastic fibrosarcoma of the mandible evolving from a prior ameloblastic fibroma after two years: An unusual finding. Int J Surg Pathol. 2016;24:656–9. doi: 10.1177/1066896916646448. [DOI] [PubMed] [Google Scholar]
- 12.Pourdanesh F, Mohamadi M, Moshref M, Soltaninia O. Ameloblastic fibrosarcoma of the mandible with distant metastases. J Oral Maxillofac Surg. 2015;73:2067e1–7. doi: 10.1016/j.joms.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Mohsenifar Z, Behrad S, Abbas FM. Epithelial dysplasia in ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma in a 26-year-old Iranian man. Am J Case Rep. 2015;16:548–53. doi: 10.12659/AJCR.892284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu YY, Deng MH, Yuan LL, Niu YM. Ameloblastic fibrosarcoma of the mandible: A case report and mini review. Exp Ther Med. 2014;8:1463–6. doi: 10.3892/etm.2014.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kousar A, Hosein MM, Ahmed Z, Minhas K. Rapid sarcomatous transformation of an ameloblastic fibroma of the mandible: Case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e80–5. doi: 10.1016/j.tripleo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Bregni RC, Taylor AM, García AM. Ameloblastic fibrosarcoma of the mandible: Report of two cases and review of the literature. J Oral Pathol Med. 2001;30:316–20. doi: 10.1034/j.1600-0714.2001.300510.x. [DOI] [PubMed] [Google Scholar]
- 17.Khalili M, Aminishakib P. Case report ameloblastic fibrosarcoma of the upper jaw: Report of a rare case with long – Term follow – Up. 2013 doi: 10.4103/1735-3327.111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvão CF, Gomes CC, Diniz MG, Vargas PA, de Paula AM, Mosqueda-Taylor A, et al. Loss of heterozygosity (LOH) in tumour suppressor genes in benign and malignant mixed odontogenic tumours. J Oral Pathol Med. 2012;41:389–93. doi: 10.1111/j.1600-0714.2011.01115.x. [DOI] [PubMed] [Google Scholar]


