Abstract
Context:
Candida is a yeast-like fungus, and it causes candidiasis. Since it is commonly encountered in many cases, the need of the hour is for rapid and reliable method for identification of these fungi in tissue sections.
Aim:
The aim of this study was to evaluate and compare the staining efficacy of calcofluor white (CFW) and acridine orange (AO) for the detection of Candida species in formalin-fixed paraffin-embedded tissue samples of oral squamous cell carcinoma (OSCC) using fluorescence microscopy.
Settings and Design:
Sample size comprised forty cases of OSCC.
Materials and Methods:
Before tissue sampling, a swab of the area was taken, it was immediately inoculated on Sabouraud's dextrose agar media and germ tube test was performed for positive cultures for species identification. Tissue sections were obtained from cases of OSCC from the formalin-fixed paraffin-embedded tissue blocks of the same cases in which microbiological assessment was done at the time of tissue sampling, were stained with CFW and AO stain, respectively, and were examined using a fluorescent microscope.
Statistical Analysis:
Descriptive statistics were expressed in numbers and percentage. Independent t-test (unpaired t-test) and Chi-square test were used. P ≤0.05 was taken to be statistically significant.
Results:
The mean number of microorganisms per high-power field stained by CFW and AO was 6.35 and 2.57, respectively, and a statistically significant difference (P ≤ 0.001) was observed. CFW compared to swab culture gave P = 0.018, which showed a statistically significant association.
Conclusions:
CFW is a better fluorescent stain when compared to AO to detect Candida species in tissue sections of OSCC cases.
Keywords: Acridine orange, calcofluor white, Candida, fluorescence
INTRODUCTION
Candida is a yeast-like fungus. It exists in three forms, namely pseudohyphae, yeast and chlamydospore. It reproduces by asexual budding and forms pseudohyphae.[1] These species grow rapidly at 25°C–37°C.[1] These yeasts are relatively common commensal organisms found in the oral cavity, gastrointestinal tract and vagina; however, it is predominantly found on the oral mucosa. The predominant species isolated being Candida albicans. It is said to be the most opportunistic infection in the world.[1] Numerous oral mucosal lesions are associated with Candida such as pseudomembranous candidiasis, erythematous candidiasis, chronic hyperplastic candidiasis and chronic atrophic candidiasis.[1]
The property of fluorescence is when some substances, when illuminated by light of a certain wavelength, will re-emit the light at a longer wavelength.[2] In fluorescence microscopy, the exciting radiation is usually in the ultraviolet wavelength (360 nm) or blue region (400 nm).[2]
The use of calcofluor white (CFW) in clinical mycology was first described by Hageage and Harrington[3] and has found extensive use in this area and in parasitology for the rapid detection of microorganisms. CFW is a nonspecific fluorochrome stain that binds to fungi. Acridine orange (AO) is an organic compound. AO is a nucleic acid-selective metachromatic stain. It is a N, N, N', N'- tetramethylacridine-3,6-diamine compound. It is also used as a fluorescent stain to demonstrate fungi in tissue sections, smears and fresh preparations, rapidly and simply.[4,5]
Thus, the purpose of this prospective study was to compare the staining efficacy of CFW stain and AO stain for the detection of Candida in formalin-fixed paraffin-embedded tissue sections of oral squamous cell carcinoma (OSCC).
MATERIALS AND METHODS
The sample for the present prospective microbiological study comprised forty cases of OSCC [Figure 1] from the Department of Oral Pathology and Microbiology. During the conduct of the study, all human ethical principles as per the WMA-Declaration of Helsinki and the guidelines of Good Clinical Practice (ICMR) were observed. The Institutional Ethical Committee approval was obtained.
Figure 1.
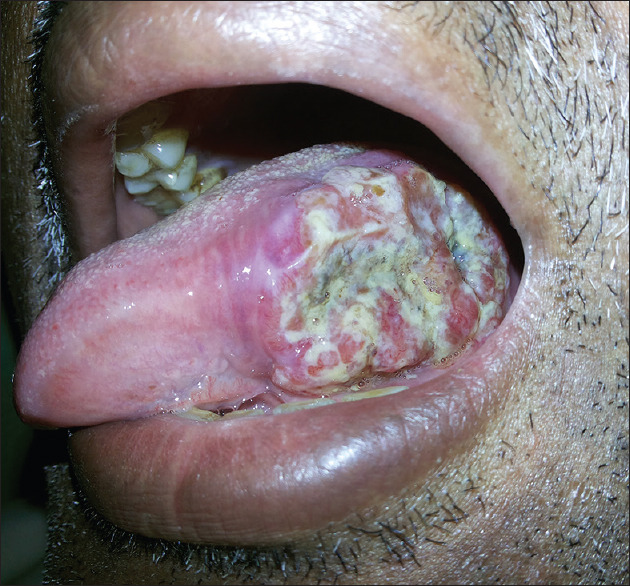
Clinical photograph of the lesional tissue
Before tissue sampling under aseptic conditions, with the informed consent of the patient, a swab of the respective area was taken using a disposable swab stick [Figure 2]. The area was gently rubbed with the swab stick. The swab sample collected was immediately inoculated on Sabouraud's dextrose agar (SDA) media. After getting positive cultures [Figure 3], a small inoculum was incubated in serum at 37°C for about 3 h and a few drops of the suspension were then examined under light microscope to observe germ tube (GT) formation for the detection of C.albicans or non-Candidaalbicans species, respectively (Raynaud's phenomenon).[6,7]
Figure 2.
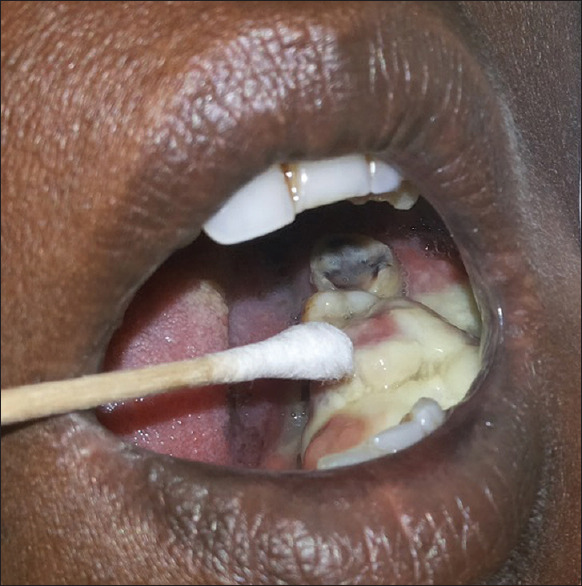
Swab culture from site of the lesion
Figure 3.

Swab culture inoculated on Sabouraud's dextrose agar media showing creamy white, convex colonies of Candida species after incubation
Tissue sections (5 μ thickness) were obtained from cases of OSCC from the formalin-fixed paraffin-embedded tissue blocks of the same cases in which microbiological assessment was done at the time of tissue sampling. These tissue sections were stained with both CFW and AO. Serial sections were obtained to avoid any sampling errors. Commercially available CFW stain (Sigma-Aldrich, USA) was placed for 1 min, and CFW stain containing Evan's blue as a counterstain was used which suppresses background staining.[3,8,9,10] Freshly prepared AO stain (HiMedia) was placed for 2 min.[5,11] The staining procedure was carried out under darkroom setting. Both slides were examined immediately using a Nikon Eclipse Ni epi-fluorescence microscope. Each slide was assessed on the following parameters for number of Candida microorganisms seen per high-power field, delineation and fluorescence. These slides were subsequently Graded (I–III):[4] Grade I = 1–10 microorganisms/high-power field and poor delineation and light fluorescence (+), Grade II = 11–20 microorganisms/high-power field and moderate delineation and light-moderate fluorescence (++) and Grade III = 21–30 microorganisms/high-power field and moderate delineation and light-moderate fluorescence (+++).[4] Data obtained were entered in Microsoft Excel worksheet and presented using descriptive statistics using SPSS software. (SPSS Inc. Version 17.0, Chicago, IL, USA).
RESULTS
The present prospective microbiological study was designed to evaluate and compare the staining efficacy of CFW and AO for the detection of Candida species in formalin-fixed paraffin-embedded tissue samples using fluorescence microscopy. The mean number of microorganisms per high-power field stained by CFW and AO was 6.35 and 2.57, respectively [Table 1]. There was a statistically significant difference (P ≤ 0.001) between the two fluorescence stains for the detection of Candida (Chi-square test).
Table 1.
Mean numbers of microorganisms per high-power field seen in tissue sections stained with calcofluor white and acridine orange in oral squamous cell carcinoma cases
| Number of microorganisms | CFW (n=40) | AO (n=40) | P (independent t-test) |
|---|---|---|---|
| Mean | 6.35 | 2.57 | <0.001* |
| SD | 5.58 | 3.43 |
*P<0.05 is statistically significant. The mean number of microorganisms per high-power field stained by CFW and AO was 6.35 and 2.57, respectively. There is a statistically significant difference (P<0.001) between the two stains. SD: Standard deviation, CFW: Calcofluor white, AO: Acridine orange
Using CFW stain, most of the cases were positive (n = 27) and the remaining were negative (n = 13). Grade I Candida species was seen in 40% (n = 16) of the samples, whereas Grade II and Grade III Candida species was seen in 25% (n = 10) and 2.5% (n = 1) of the samples, respectively [Graph 1]. For AO fluorescence stain, we found that in our study, out of all the cases (n = 40), most of the cases were positive (n = 21) and the remaining were negative (n = 19). Grade I Candida species was seen in 50% (n = 20) of the samples and Grade II Candida species was seen in 2.5% (n = 1) [Graph 1].
Graph 1.

Comparison of grades of Candida species in slides stained with calcofluor white and acridine orange in oral squamous cell carcinoma cases (color online only). Association between grades of Candida species in tissue sections stained with calcofluor white and acridine orange
On SDA swab culture out of all cases of OSCC (n = 40), most of the cases (n = 20) had shown growth of white, creamy convex colonies of Candida species seen on SDA culture media. GT test was then performed to identify the species of Candida and was designated as C.albicans (n = 15) or non-Candida albicans (n = 05) on negative GT test, respectively.
Furthermore, total cases (n = 40) of both fluorescent methods were compared with that of the swab culture method. In CFW fluorescent microscopy, 27 out of 40 cases were positive and remaining cases were negative. Out of the 27 positive CFW cases, 17 cases were positive on swab culture and the remaining 10 cases were negative, respectively. Out of the 13 negative cases, 3 cases were positive and 10 cases were negative on swab culture, respectively.
Similarly, AO when compared with swab culture out of the 21 positive cases by AO fluorescent microscopy, 12 were positive by swab culture and the rest were negative. Out of the negative cases by AO fluorescent microscopy, 8 were positive on swab culture and the remaining 11 cases were negative. respectively.
DISCUSSION
Candida species can be classified broadly into albicans and non-albicans, respectively. The predominant species isolated being C.albicans. C.albicans has the potential to infect virtually any tissue within the body causing candidiasis. It is said to be the most opportunistic infection in the world.[1] In numerous states of impaired local and/or general health, C.albicans may assume a pathogenic role giving rise to acute and chronic clinical manifestations such as atrophic glossitis, leukoplakic lesions, thrush, angular stomatitis and others.[12]
The pathogenicity of C.albicans depends on two major factors. One is the immune status of the host and another is related to the virulence factors of this pathogen. The virulence of C.albicans is determined by how well it can successfully colonize the host. The role of C.albicans in oral mucosal carcinogenesis was proposed by Cawson and Binnie in 1980.[13,14] According to recent studies,[8,9] Candida species plays an important role in the progression of OSCC.[15] Due to a strong association of candidiasis and OSCC reported in the literature,[15] the sample in our study consisted of forty cases of formalin-fixed paraffin-embedded tissue samples of OSCC.
In our study findings, C.albicans species was most commonly seen, which was in accordance with the studies done by Krogh et al.[16] and Kumar et al.[8] In both the studies, C.albicans was the most commonly encountered Candida species.
In our study, Candida species was identified based on morphology in tissue sections stained with CFW and AO. CFW staining when observed under a fluorescent microscope revealed the Candida species as green in color [Figure 4]. In our study, 67.5% cases of OSCC, Candida species was observed using fluorescence CFW technique, which is comparable to the study done by Jahanshahi and Shirani in 2016 in which they found 74% cases of Candida with CFW stain in OSCC.[9]
Figure 4.
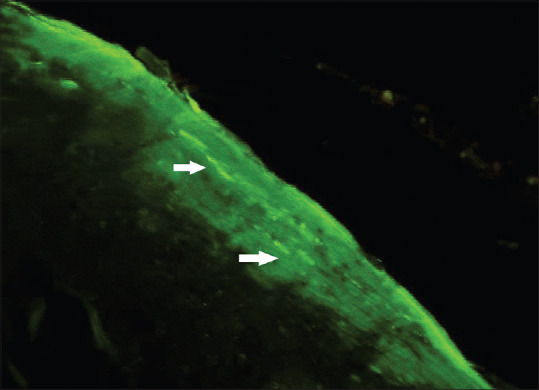
Photomicrograph calcofluor white stain (×100) showing green-colored brightly fluorescent Candida hyphae (white arrows) in oral squamous cell carcinoma tissue sections
CFW has been compared to various routinely used staining techniques by other authors. Lynch and Gibson[17] concluded that CFW is better since it is more rapid, easy and cheaper. Kumar et al.[8] compared CFW and PAS stain and concluded that CFW detects more Candida microorganisms. Dass et al.[18] compared CFW with KOH and SDA culture techniques, and they concluded that CFW is better compared to both KOH and culture techniques.
Similarly, the tissue sections were stained with AO and were evaluated. Candida species on AO staining when observed under a fluorescence microscope appear yellow-green [Figure 5] in color.[19] Few authors like Pickett et al.[19] in 1960 and Chick[5] in 1961 used AO to stain various fungal species in tissue sections including Candida species. Both stated that AO can be widely used in histopathology as well as on smears and fresh preparations to demonstrate fungi rapidly and simply. In oral candidiasis cases, Naik et al.[4] in 2014 used AO stain and compared it to PAS stain in oral exfoliative smears. They found that the PAS-stained smears were more reliable for the detection of Candida species.
Figure 5.
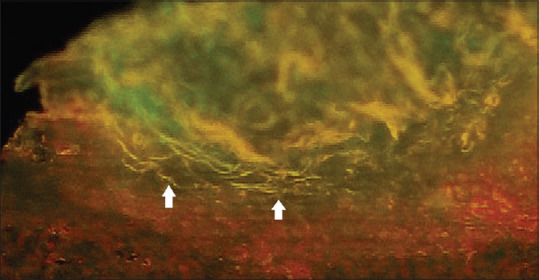
Photomicrograph acridine orange stain (×100) showing yellow-colored brightly fluorescent Candida hyphae (white arrows) in oral squamous cell carcinoma tissue sections
A comparative evaluation of CFW and AO fluorescence staining was done based on the grades[4] (number of microorganisms per high-power field, delineation and fluorescence). The association between grades of Candida species in tissue sections stained with CFW and AO was evaluated. Chi-square test (P = 0.019) revealed that there is a statistically significant association of both the group samples. The mean values of the number of microorganisms seen in both techniques were compared as well. The mean number of microorganisms per high-power field stained by CFW and AO was 6.35 and 2.57, respectively. On statistical analysis, it revealed P < 0.001. Thus, there is a statistically significant difference (P ≤ 0.001) between the two fluorescence stains with CFW detecting more microorganisms per field [Table 1].
Further statistical analysis was done to compare both the fluorescent stains individually, to the standard procedures done in clinical mycology for Candida detection (swab culture on SDA media). Sensitivity, specificity, positive predictive value and negative predictive value were evaluated and compared. CFW compared to swab culture gave a χ2 = 5.584, with P = 0.018, which shows that there is a statistically significant association between CFW fluorescent microscopy and swab culture technique. The sensitivity and specificity were 85% and 50%, respectively, which was in accordance with the study done in 2015 by Dass et al.[18] on nail scrapings and clippings, wherein the sensitivity of CFW microscopy was 89.83% and specificity was 60.44%. The positive predictive value and negative predictive value were 63% and 76.9%, respectively [Table 2].
Table 2.
Sensitivity, specificity, positive predictive value and negative predictive value of the calcofluor white fluorescent staining and acridine orange fluorescent staining with swab culture
| CFW fluorescent staining percentage | AO fluorescent staining percentage | |
|---|---|---|
| Sensitivity | 85 | 60 |
| Specificity | 50 | 55 |
| Positive predictive value | 63 | 57.1 |
| Negative predictive value | 76.9 | 57.9 |
CFW has a higher sensitivity (85% vs. 60%), higher positive predictive value (63% vs. 57.1%) and higher negative predictive value (76.9% vs. 57.9%) as compared to AO in detecting Candida species. CFW: Calcofluor white, AO: Acridine orange
AO when compared to swab culture showed no statistically significant association between them. (Chi-square value=0.902; P = 0.342). The sensitivity and specificity were 60% and 55%, respectively, whereas the positive predictive value and negative predictive value were 57.1% and 57.9%, respectively [Table 2].
A comparison of sensitivity, specificity, positive predictive value and negative predictive value (swab culture) between the two fluorescence staining techniques was done. It revealed that CFW has a higher sensitivity (85% vs. 60%), higher positive predictive value (63% vs. 57.1%) and higher negative predictive value (76.9% vs. 57.9%) as compared to AO in detecting Candida species [Table 2]. These results were in accordance with the study done by Kumar et al.[8] and Jahanshahi and Shirani[9] where CFW was compared with PAS staining.
Every staining technique has its advantages and disadvantages. Fluorescence stains have proven to be promising in identification of fungal species both in cytopathology and histopathology. Fluorescence staining techniques are rapid, easy to perform and have a high sensitivity, positive and negative predictive values as is evident for CFW in our study. In addition to this, fluorescence staining techniques can also be incorporated in various routinely done staining procedures. For example, PAP staining with CFW, KOH wet mount with CFW, which enhances the identification of microorganisms, stated by Monheit et al.[20] and Kumar et al.[8] However, fluorescence staining has few limitations. There is a requirement of a fluorescence microscope facility. There is a learning curve in fluorescence staining and identification of Candida species. The cost as compared to the routinely done laboratory procedures is more. Another limitation is that if the fluorescence intensity of fungal hyphae matches with that of surrounding debris and epithelial cells, then it is difficult to identify the micro-organisms. The tissue sections need to be evaluated as soon as they are stained, as with time, the amount of fluorescence reduces. Thus, further research and more studies are warranted to conclude with satisfaction that these techniques can be used more routinely in the laboratory with more definitive results.
CONCLUSIONS
CFW is a better fluorescent stain when compared to AO to detect Candida species in tissue sections of OSCC cases. Thus, CFW can be used to identify Candida hyphae in OSCC cases rapidly and simply. However, fluorescence staining requires special equipment, a fluorescence microscope and darkroom, and there exists a learning curve to identify the structures under the fluorescence light. CFW when used with Evan's blue has an added advantage that it suppresses background staining and highlights the structures containing cellulose and chitin. These stains are versatile and can be used with tissue sections, direct smears and other clinical specimens. The observations of the present study could be helpful to explore the potential use of CFW and AO as special stains in identification of Candida species mainly on archival tissue samples for research or the condition where patient/source is unavailable. It also highlights about the implication of CFW (which was found to show higher efficacy) in various other sampling methods such as frozen sections and exfoliative cytology. To the best of our knowledge, this is the first study to evaluate and compare CFW and AO stains for the detection of Candida in formalin-fixed paraffin-embedded tissue sections of OSCC.
The future perspective demands more number of research studies in the field of fluorescence stains to better adjudge the applicability and scope in the field of oral pathology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sivapathasundaram B, Gururaj N. Mycotic infections of the oral cavity. In: Rajendran R, Sivapathasundaram B, editors. Shafer's Textbook of Oral Pathology. 6th ed. India: Elsevier; 2009. pp. 363–7. [Google Scholar]
- 2.Bancroft J, Floyd A. Light Microscopy. In: Suvarna S, Layton C, Bancroft J, editors. Bancroft's Theory and Practice of Histological Technique. 7th ed. United Kingdom: Churchill Livingstone Elsevier; 2013. pp. 63–4. [Google Scholar]
- 3.Hageage GJ, Harrington BJ. Use of calcofluor white in clinical mycology. Lab Med. 1984;15:109–12. [Google Scholar]
- 4.Naik KL, Shetty P, Shroff SE, Karnekar VK, Prasad KM, Madathil LP. Detection of Candida species by acridine orange fluorescent dye in exfoliative smears of oral candidiasis. S J Oral Sci. 2014;1:41–66. [Google Scholar]
- 5.Chick EW. Acridine orange fluorescent stain for fungi. Arch Dermatol. 1961;83:305–9. doi: 10.1001/archderm.1961.01580080135015. [DOI] [PubMed] [Google Scholar]
- 6.Ananthanarayan R, Paniker CK. Textbook of Microbiology. 7th ed. India: Orient Longman Pvt Ltd; 2005. p. 617. [Google Scholar]
- 7.Marsh PD, Martin MV, Lewis MA, Williams DW. Oral Microbiology. 5th ed. United Kingdom Elsevier: 2009. pp. 173–75. [Google Scholar]
- 8.Kumar RS, Ganvir S, Hazarey V. Candida and calcofluor white: Study in precancer and cancer. J Oral Maxillofac Pathol. 2009;13:2–8. doi: 10.4103/0973-029X.44575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahanshahi G, Shirani S. Detection of Candida albicans in oral squamous cell carcinoma by fluorescence staining technique. Dent Res J (Isfahan) 2015;12:115–20. [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington BJ, Hageage GJ. Calcofluor white: A review of its uses and applications in clinical mycology and parasitology. Lab Med. 2003;34:361–7. [Google Scholar]
- 11.Chick EW, Behar VS. A simple fluorescent method for the detection of superficial fungi in skin and hair. A combined stain with acridine orange and potassium hydroxide. J Invest Dermatol. 1961;37:103–5. doi: 10.1038/jid.1961.90. [DOI] [PubMed] [Google Scholar]
- 12.Hornstein OP, Grässel R, Schirner E. Prevalence rates of candidosis in leukoplakias and carcinomas of the oral cavity. Arch Dermatol Res. 1979;266:99–102. doi: 10.1007/BF00412869. [DOI] [PubMed] [Google Scholar]
- 13.Cawson RA, Binnie WH. Candidal leukoplakia and carcinoma: A possible relationship. In: Mackenzie IA, Dabelsteen E, Squier C, editors. Oral Pre-Malignancy. Iowa: University of Iowa Press; 1980. pp. 59–66. [Google Scholar]
- 14.Cawson RA. Chronic oral candidiasis and leukoplakia. Oral Surg Oral Med Oral Pathol. 1966;22:582–91. doi: 10.1016/0030-4220(66)90161-7. [DOI] [PubMed] [Google Scholar]
- 15.Berkovits C, Tóth A, Szenzenstein J, Deák T, Urbán E, Gácser A, et al. Analysis of oral yeast microflora in patients with oral squamous cell carcinoma. Springerplus. 2016;5:1257. doi: 10.1186/s40064-016-2926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krogh P, Holmstrup P, Thorn JJ, Vedtofte P, Pindborg JJ. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol. 1987;63:48–54. doi: 10.1016/0030-4220(87)90339-2. [DOI] [PubMed] [Google Scholar]
- 17.Lynch DP, Gibson DK. The use of Calcofluor white in the histopathologic diagnosis of oral candidiasis. Oral Surg Oral Med Oral Pathol. 1987;63:698–703. doi: 10.1016/0030-4220(87)90373-2. [DOI] [PubMed] [Google Scholar]
- 18.Dass SM, Vinayaraj EV, Pavavni K, Pallam A, Rao MS. Comparison of KOH, calcofluor white and fungal culture for diagnosing fungal onychomycosis in an urban teaching hospital. Indian J Microbiol Res. 2015;2:148–53. [Google Scholar]
- 19.Pickett JP, Bishop CM, Chick EW, Baker RD. A simple fluorescent stain for fungi. Selective staining of fungi by means of a fluorescent method for mucin. Am J Clin Pathol. 1960;34:197–202. doi: 10.1093/ajcp/34.3.197. [DOI] [PubMed] [Google Scholar]
- 20.Monheit JG, Brown G, Kott MM, Schmidt WA, Moore DG. Calcofluor white detection of fungi in cytopathology. Am J Clin Pathol. 1986;85:222–5. doi: 10.1093/ajcp/85.2.222. [DOI] [PubMed] [Google Scholar]


