Abstract
FRET (fluorescence resonance energy transfer) between far-upstream(−100) and downstream(+14) cyanine dyes showed extensive bending/wrapping of λPR promoter DNA on E. coli RNA polymerase (RNAP) in closed and open complexes (CC, OC). Here we determine the kinetics and mechanism of DNA bending/wrapping by FRET and of formation of RNAP contacts with −100 and +14 DNA by single-dye protein-induced fluorescence enhancements (PIFE). FRET/PIFE kinetics exhibit two phases: rapidly-reversible steps forming a CC ensemble ({CC} of four intermediates (initial (RPC), early (I1E), mid- (I1M), late (I1L)), followed by conversion of {CC} to OC via I1L. FRET and PIFE are first observed for I1E, not RPc. FRET/PIFE together reveal large-scale bending/wrapping of upstream and downstream DNA as RPC advances to I1E, reducing −100/+14 distance to ~75Å and making RNAP-DNA contacts at −100 and +14. We propose that far-upstream DNA wraps on the upper β’-clamp while downstream DNA contacts the top of the β-pincer in I1E. Converting I1E to I1M (~1s time-scale) reduces FRET efficiency with little change in −100/+14PIFE, interpreted as clamp-opening that moves far-upstream DNA (on β’) away from downstream DNA (on β) to increase the −100/+14 distance by ~14Å. FRET increases greatly in converting I1M to I1L, indicating bending of downstream duplex DNA into the clamp and clamp-closing to reduce the −100/+14 distance by ~21Å. In the subsequent rate-determining DNA-opening step, in which the clamp may also open, I1L converts to the initial unstable OC (I2). Implications for facilitation of CC-to-OC isomerization by upstream DNA and upstream-binding, DNA-bending transcription activators are discussed.
Graphical Abstract
Introduction
The rate of open complex (OC) formation by E. coli RNA polymerase σ70 holoenzyme (RNAP; R) at promoter DNA (P) is an important determinant of the rate of transcription initiation.1, 2. Hence the kinetics and equilibria of the steps of OC formation are highly regulated by promoter sequence, transcription factors, ligands, and solution variables. Monitored by abortive initiation and filter binding assays that detect only long-lived OC, the kinetics of OC formation are single-exponential in excess RNAP and are well-described by the two-step minimal Mechanism 1.1-7 (See accompanying Glossary for a list of the symbols and acronyms used here.)
| Mechanism 1 |
In Mechanism 1, KCC is the composite equilibrium constant for reversible formation of an ensemble of early and advanced closed complexes (called the CC ensemble, symbolized as {CC}) from promoter DNA.4 Single-exponential kinetics indicates that {CC}rapidly equilibrates with R and P on the time scale of its conversion to OC5, 8. Evidence for three likely members of {CC} is provided by the different downstream boundaries of chemical and/or enzymatic CC footprints obtained at different promoters. These CC include the initial specific complex (RPC; downstream footprint boundary at approximately −5)9-15 and two more-advanced CC (designated I1E and I1L for “early” and “late”) with downstream footprint boundaries at approximately +2/+716 and at +20. 9, 10, 12, 15 (All positions are numbered relative to the +1 start site.) The isomerization rate constant kisom is the composite rate constant for conversion of {CC} to OC via I1L, the most advanced member of {CC}, and the subsequent DNA opening step.4
This interpretation of the parameters of Mechanism 1 differs subtly but significantly from that used originally. Most previous discussions of effects of changes in promoter sequence or length, transcription factors and solution variables on KCC and kisom have not considered changes in the composition of the equilibrated {CC} ensemble with these variables. In general, any shift in the proportions of the different members of the equilibrated {CC} ensemble will change both KCC and kisom (see ref. 4 and SI Eqs. S19-22). The large effects of upstream truncation of promoter DNA on kisom 17, 18 are well-explained in this way (see ref. 4 and Discussion).
The initial closed complex RPC is stabilized by some combination of specific interactions of the flexibly-tethered αCTDs with the promoter UP element (a 20 bp region containing A and T tracts immediately upstream of the −35 region)19 and of regions 2 and 4 of the σ70 subunit with the promoter −10 and −35 elements.20 After RPC formation, the downstream duplex must bend by at least 90° into the open clamp in order for 13-14 bp, including the −10 and discriminator regions and the transcription start site, to be opened (melted) by RNAP using binding free energy 3-5, 21 Bacteriophage T7 RNAP also bends the downstream duplex in the process of DNA opening 22-25 .
CryoEM studies indicate that ATP-independent promoter opening by eukaryotic RNA polymerases involves a similar set of conformational changes. In the assembly of the Pol II pre-initiation complex (Pol II PIC), the upstream DNA is bent by 90° by binding to the TATA element of transcription factors TBP (TATA-binding protein) together with TFIIA, TFIIB, and TAFs (TBP-associated factors).26-31 After Pol II PIC assembly, cryoEM reveals three closed complexes, designated CC1, CC2, and CCdist, which appear to be on-pathway intermediates in OC formation.32 In CC1, the downstream promoter is positioned above the closed clamp of RNAP. In CC2 the clamp domain is open and in CCdist the downstream duplex is bent into the open clamp. In the conversion of CCdist to the final OC, the clamp closes and the DNA is opened.32
For E. coli RNAP, the isomerization of {CC} to OC is greatly facilitated by the presence of far-upstream DNA. For full-length (FL) λPR promoter DNA, real-time HO footprinting of {CC} revealed interactions with RNAP up to at least position −82.16 Upstream truncation of λPR and lacUV5 promoters at positions between −63 (UT-63) and −42 (UT-42) reduces the isomerization rate constant kisom (Mechanism 1) for conversion of {CC} to OC by one to two orders of magnitude.17,18 For the UT-47 λPR truncation variant, DNase footprinting of {CC} early in the time course of OC formation revealed downstream boundaries of partial protection of the template and nontemplate strands of the downstream duplex at +2 and +7, compared to +20 for {CC} at FL λPR.16 This indicates that the {CC} population distribution is much less advanced for UT-47 than for FL λPR. To explain these profound differences in OC-formation kinetics and {CC} footprints for FL and UT-47 λPR, we previously proposed that bending and wrapping of FL λPR upstream DNA in an earlier closed complex (I1E) facilitates bending of the downstream duplex into the clamp/cleft in the most advanced member of the {CC} ensemble (I1L), in which the duplex is poised to be opened by RNAP.4, 16 The absence of upstream wrapping in UT-47 was proposed to greatly disfavor conversion of I1E to I1L, thereby reducing kisom.4 Tests of these proposals are provided by the FRET and PIFE kinetics studies reported here.
Equilibrium FRET studies on low temperature (2 °C; closed) and higher temperature (19 °C; open) complexes revealed that far-upstream DNA in both complexes is highly bent and wrapped on RNAP.33 DNA backbone footprinting of RNAP- λPR complexes revealed far-upstream protection to approximately −82 ({CC})16 and −65 (OC),16, 34 interpreted as upstream bending and wrapping, and AFM compaction measurements indicated extensive upstream wrapping in OC.35 Upstream modulators of initiation that bend and in some cases wrap DNA like CAP36, IHF37-39 and HU40-42 may therefore affect kisom by altering the distribution of wrapped and unwrapped intermediates in the {CC} ensemble and/or the trajectory of bending and wrapping of upstream DNA on RNAP in these intermediates.
In addition to FRET kinetic studies of changes of DNA end-to-end distance from bending and wrapping on RNAP, we report the kinetics of Cy3 and Cy5 fluorescence enhancements (PIFE)43-45 induced by contacts between RNAP and these dyes at the −100 and +14 positions of promoter DNA. The PIFE kinetics complement the FRET kinetics by reporting on the development of these RNAP-promoter contacts in {CC} intermediates of OC formation. PIFE effects using Cy3 at positions near the TSS were previously used to study aspects of the kinetics of OC formation, initiation, and escape.46-48 Cy3 PIFE kinetic measurements were also used to study the cooperative interaction between CarD and RbpA transcription factor in OC formation for Mycobacterium tuberculosis σA RNAP holoenzyme. 49-51
Here we use FRET and PIFE in kinetic assays of OC formation to investigate large DNA conformational changes and changes in interactions of upstream and downstream DNA with RNAP as the initial CC advances and converts to OC. Analysis of the FRET and PIFE kinetic data provides compelling evidence for a sequential, five step mechanism with four closed complexes (the initial closed complex (RPC) and three more advanced members of {CC} designated I1E, I1M, I1L) on the pathway to open complex formation.
| Mechanism 2 |
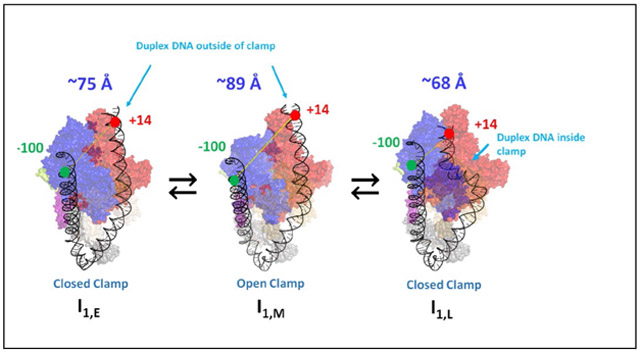
Illustrations of DNA bending and wrapping in the three I1 intermediates and attendant conformational changes in the RNAP clamp, deduced from the research reported here, are provided in the graphical abstract and in the corresponding section of Discussion.
Rate and equilibrium constants for reversible formation of the members of {CC} (RPC, I1E, I1M, I1L) are obtained, as well as the DNA opening rate constant k5 and intrinsic FRET and PIFE signatures of these closed intermediates to compare with those of OC and free P. FRET signatures of the intermediates provide information about the progression of upstream wrapping and downstream bending as {CC} advances. PIFE signatures of the intermediates provide information about the strength of contacts between RNAP and far-upstream (−100) and downstream (+14) regions of duplex DNA in early vs late CC and OC. The placement of the downstream duplex and the status of the RNAP clamp in the different CC intermediates deduced from the FRET and PIFE studies reported here relate well to cryoEM observations and proposals for pol II32 and E. coli RNAP intermediates.47, 52 We also predict the time-evolution of the populations of individual early and late CC and the final OC. Rate and equilibrium constants obtained from FRET and PIFE analyses of OC formation at the λPR promoter are compared with previous determinations of the composite quantities KCC and kisom for λPR by filter binding assays.
These studies provide novel insights into the time-evolution of the {CC} ensemble and how wrapping of upstream DNA facilitates isomerization in OC formation. They provide strong support for the proposal that increases in the isomerization rate constant kisom when upstream DNA or upstream-binding transcription factors are present, as well as from changes in promoter sequence, result from changes in the {CC} population distribution to favor the advanced closed complex I1L, in which the start site region of duplex promoter DNA is poised for DNA-opening in the RNAP clamp/cleft, and not primarily from increases in the rate constant of the subsequent DNA-opening step.4, 53, 54
Materials and Methods
Buffers:
Storage Buffer (SB) for RNA polymerase holoenzyme is 50% glycerol (v/v), 10 mM Tris base (pH 7.5 at 4 °C), 0.1 M NaCl, 0.1 mM DTT, and 0.1 mM Na2EDTA. Fluorescence buffer (FB) for FRET and PIFE kinetics experiments is 40.2 mM Tris, pH 8, 2 mM NaCl, 0.12 M KCl, 10mM MgCl2, 2 μM DTT, 2 mM Na2EDTA, 0.05 mg/ml BSA, 0-2% glycerol and 0.02% Tween. Permanganate footprinting buffer (PFB) is 40 mM Tris (pH 8 at 19 °C), 10 mM MgCl2, 0.12 M NaCl and 100 μg/ml BSA. Urea loading buffer (ULB), used to resuspend footprinting samples, is 8 M urea, 0.5 X TBE (45mM Tris-borate and 1 mM Na2EDTA), 0.05% xylene cyanol (w/v) and 0.05% bromophenol blue (w/v).
Preparation of E. coli RNA Polymerase (RNAP) Holoenzyme and Labeled λPR Promoter DNA
RNAP core enzyme was overexpressed and purified as described previously, using E. coli BL21(λDE3) transformed with pVS10.33, 55 σ70 was overexpressed and purified using E. coli M5219 transformed with plasmid pMRG8, as described previously.33 RNAP core enzyme and σ70 were incubated at a 1:2 molar ratio for 1 hour at 37°C in SB to reconstitute RNAP holoenzyme, then stored at −20°C until use. Other experiments were performed with preparations of WT RNAP holoenzyme.54, 56 Holoenzyme activities, determined from promoter binding assays at high promoter concentration,6 were in the range 50-90%. No significant differences between different RNAP preparations were observed in experiments reported here.
Single-dye-labeled λPR fragments [Cy3 (−100), Cy5 (−100), Cy3 (+14) and Cy5 (+14)] for PIFE experiments and two-dye-labeled λPR promoter DNA fragments [Cy3 (+14) Cy5 (−100) and Cy3 (−100) Cy5 (+14)] for FRET experiments were prepared by PCR as described previously using dye-labeled primers.33 P-labeled λPR promoter DNA fragments for MnO4− footprinting experiments were prepared by PCR as described previously.53, 56 Sequences and lengths of the different primers and DNA constructs are described in the supporting information (Tables S1-2).
Each lane and reactive band of a given MnO4− footprint was boxed and the total intensity was quantified using ImaqeQuant TL. The fraction of promoter DNA modified at a given position was determined by dividing the intensity of each uncut and reactive band by the total intensities of all uncut and reactive bands within a lane, and background was subtracted to determine corrected intensity. These corrected intensities were plotted vs time and fit to a single exponential time course. Corrected intensities were normalized by the appropriate fitted plateau intensity and plotted as normalized reactivity vs time.
Kinetics of Open Complex Formation from Free RNAP and Promoter DNA by Stopped-Flow Fluorescence (FRET, PIFE):
Fluorescence-detected kinetic experiments were performed at 19°C in a Kintek SF-300X stopped flow fluorimeter (Kintek Corporation, PA) equipped with a 150 watt Hg-Xe lamp (Hamamatsu, Japan) by rapid-mixing of equal volumes (20 μL) of dye-labeled promoter DNA and RNAP stock solutions. Each mixing of aliquots of the same stock solutions is called a “shot”. Previous equilibrium FRET results on OC33 were obtained at 19°C because the fluorescence of cyanine dyes decreases strongly with increasing temperature.33, 57 This temperature is used for kinetics experiments as well because at 19°C the equilibrium constant for forming the CC ensemble from promoter DNA is near-maximal, the rate of isomerization of the CC ensemble to OC is sufficiently slow to separate this kinetic phase from the earlier CC phase, and the final OC is sufficiently stable that its formation is irreversible.5, 58
Stocks of dye-labeled promoter DNA and RNAP were prepared at twice the desired final concentration in FB. Most experiments were performed at either 1:1 or 0.5:1 mole ratio of RNAP to promoter DNA (50 nM final DNA concentration), in order to avoid a competitive binding mode observed previously on this promoter DNA fragment in RNAP excess.33 Control experiments (5-6 shots) were performed by mixing a DNA solution in FB with no RNAP to verify that the fluorescence signal was time-independent, without photobleaching or large instrumental drift. DNA and RNAP solutions were loaded in the stopped flow syringes and incubated for at least 10 minutes at 19°C before being mixed in a series of shots.
Dye-labeled λPR promoter constructs were excited in the observation cell at wavelengths of 515nm (Cy3) or 610nm (Cy5) for single dye Cy3/Cy5 PIFE experiments. In FRET experiments, Cy3 dye (FRET donor) was excited at 515 nm wavelength and fluorescence emission of Cy3 and Cy5 (FRET acceptor) were monitored as a function of time. Fluorescence emission signals were monitored using a 565-625 nm band pass filter for Cy3 and a 660 nm long-pass filter for Cy5 (Omega Optical, Brattleboro, VT). A monochromator excitation slit width of 1.56 mm or 3.14 mm was used. Fluorescence intensity was monitored from 10ms to 400s with 600 data points that typically were uniformly distributed on a log time scale.
In RNAP- DNA mixing experiments the initial 4 shots were typically discarded and the next 5 - 15 shots were collected and analyzed. FRET data collected from each shot were normalized as described in supplemental and then averaged to reduce noise at earlier time points for further analysis. A total of 15 FRET experiments (each ~10 shots) were performed with Cy5 acceptor at −100, and another 12 experiments were performed with Cy5 at +14. In PIFE experiments, individual shots were also normalized and averaged. For comparative analysis of PIFE effects at −100 and +14, fluorescence increases relative to the initial (10 ms) signal were determined for each shot and averaged. A total of 34 PIFE experiments were performed: 15 monitoring +14 PIFE (10 with Cy3+14, 5 with Cy5+14) and 19 monitoring −100 PIFE (11 with Cy3-100, 8 with Cy5-100). Different experiments used independent dilutions (and in some cases independent preparations) of RNAP and DNA solutions. Analyses of these data in terms of a sum of exponentials and in terms of the proposed five-step mechanism are described in Supplemental Methods. Curves shown in Results are averages of series of normalized shots obtained in single experiments, selected as high S/N examples but otherwise representative of the full sets of independent experiments.
Salt-Upshift Dissociation Kinetic Assays Monitored By FRET and +14 PIFE
Dye-labeled OC, prepared as described above, were rapidly mixed with KCl (50nM final OC concentration; 0.4 M final KCl concentration) in FB in the stopped-flow fluorimeter. Fluorescence kinetic data collected in each shot were normalized, and results from series of 10 or more shots were averaged as described above. FRET and PIFE curves shown in Results are representative of 4 independent FRET experiments and 5 independent PIFE experiments.
Illustrating the Bending and Wrapping of Promoter DNA in Open Complex Formation
The PYMOL Molecular Graphics System, Version 2.3.2 (Schrödinger, LLC) was used to replace part or all of the DNA in the crystal structure of a transcription initiation complex (4YLN) by segments of DNA duplex (−100 to +14) to illustrate a plausible path for bending and wrapping of upstream DNA and bending of downstream DNA in the closed intermediates in OC formation as well as the final OC. These proposals for the approximate location of upstream and downstream duplex DNA in the bent-wrapped CC intermediates were developed to be semi-quantitatively consistent with the FRET distances and PIFE contacts determined in this research.
Results
Fast Permanganate Footprinting Kinetics of OC Formation
To test whether opening of the λPR initiation bubble occurs as a single kinetic step and to validate the interpretation of the kinetics of open complex formation obtained previously from filter-binding data using Mechanism 1, fast permanganate (MnO4−) footprinting studies in the single-hit regime were used to determine rates of opening individual thymines in the λPR open region (−11 to +2) in open complex formation at 19°C. Results are shown in Fig 1 and SI. Fast MnO4− footprinting of thymines in the −10 and start site regions of the promoter was previously performed to characterize the kinetics of open complex formation at the T7A1 promoter14 and to quantify the size of the bubble and the extent of reactivity of individual thymines in the unstable intermediate open complex (I2) formed in the DNA-opening step at the λPR promoter.53 Although MnO4− reaction kinetics are moderately slow, requiring a reaction time of 50 ms even at high MnO4− concentration, this is fast relative to the time scale of the CC-to-OC isomerization at 19°C (1/kisom ~ 70 s).5 Since only stable OC are detected, the MnO4− kinetic assay is expected to be equivalent to the filter binding kinetic assay in which brief exposure of each sample to a competitor before assaying for binding eliminates contributions to the binding assay from any short-lived (closed or nonpromoter) complexes that were present.
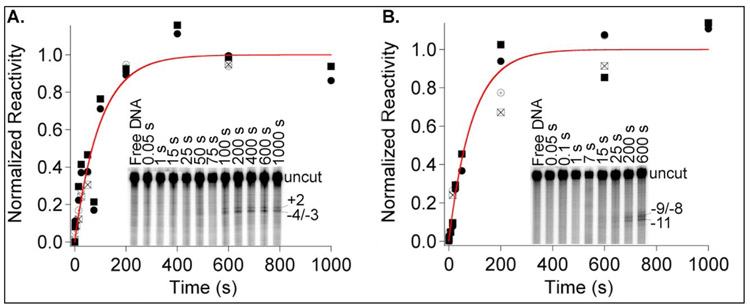
Figure 1. Kinetics of OC Formation Monitored by MnO4− Reactivity.
Fast (50 ms) MnO4− snapshots53 monitor the time course of opening individual thymines in OC formation after mixing excess RNAP (55 nM) with λPR promoter DNA (0.3 nM) at 19 °C. Representative gels are shown as insets. Kinetics of development of MnO4− reactivity are plotted for thymines on the nt strand (+2 (⊕, □***) and −4/−3 (☒***, □***) in panel A and for the t strand (−9/−8 (⊕, □***) and −11 (☒***, ) in panel B. Rate constants kobs for OC formation from these fits are the same within uncertainty (template strand kobs = 0.010 ± 0.002 s−1 ; non-template strand kobs = 0.011 ± 0.002 s−1). Times indicated are times after mixing RNAP with DNA at which the MnO4− snapshot was initiated. Gels were quantified and results normalized as described in Materials and Methods.
Gel lanes in the insets in Fig. 1 show the kinetics of development of MnO4− reactivity in the region of the initiation bubble on non-template (nt, panel A) and template (t, panel B) strands. In the first ~10 s, these MnO4− snapshots detect no reactive (open) thymines (Fig S1), indicating that only CC complexes are present. MnO4− reactivity of all observable thymines (−4/−3 and +2 on the nt strand; −11 and −9/−8 on the t strand) develops on a much slower timescale, becoming visible in the gel lanes only after 15 s and increasing to a plateau at times greater than 200 s. Single exponential global fits to the data for each strand (Figs. 1, S1) describe the entire reaction time-course and yield values of kobs, the observed first order rate constant for the formation of MnO4−-detected complexes at 55 nM excess RNAP, which are the same within the uncertainty (10-20%) for all thymines detected on both template and nontemplate strands.
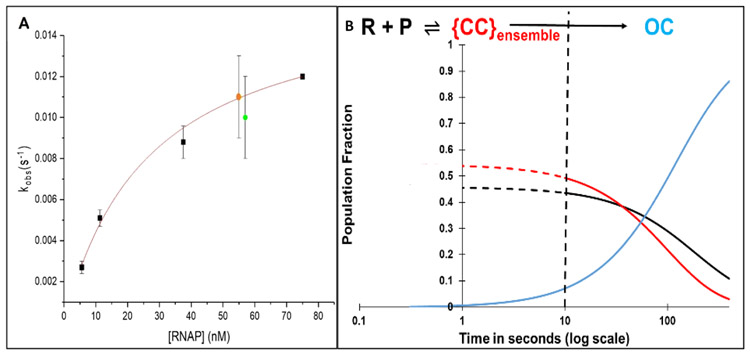
Rate constants kobs obtained from these MnO4− footprinting assays at 19 °C are compared with those obtained previously by filter binding5 at 20 °C in a plot vs [RNAP] in Figure 2A, and were fit to the expected hyperbolic functional form:1-7
| Eq. 1 |
where KCC is the equilibrium constant for forming the CC ensemble from RNAP and promoter DNA, and kisom is the CC-to-OC isomerization rate constant. Values of KCC and kisom obtained from this analysis (see Fig. 2 caption) agree within the uncertainty with those reported previously.5
Figure 2. Panel A: Dependence of Rate Constant kobs for λPR OC Formation on RNAP Concentration in RNAP Excess.
First order rate constants kobs (Eq. 1) for OC formation in excess RNAP are plotted as a function of RNAP concentration. ∎*** : kobs values from filter binding assays at 20°C.4, ● , ● : kobs values from MnO4− footprinting of template and non-template strands, respectively at 19 °C (Fig 1). A fit of these data to hyperbolic Eq. 1 gives a composite {CC} binding constant K{CC} = (5 ± 1) x 107 M−1 and an isomerization rate constant kisom = 0.014 ± 0.003 s−1 for conversion of {CC} to OC. Panel B: Simulated Time Evolution of {CC} and OC Formation for FRET/PIFE Conditions (50 nM λPR Promoter and RNAP). Fractional populations of open complexes (OC) ──, closed complexes {CC} ──, and free promoter DNA ──, predicted from KCC and kisom as a function of time (log scale) assuming that the {CC} population equilibrates with free promoter DNA by 10 s. Dashed curves (– – - and– – -) assume equilibration of {CC} and P occurs in 1 s . The dashed vertical line at 10 s marks the onset of OC formation from the equilibrium mixture of {CC} and free promoter DNA determined from this simulation.
Predicted Time Evolution of CC and OC Populations at RNAP and Promoter Concentrations of FRET and PIFE Experiments
The kinetic results (KCC, kisom) from the analysis in Fig. 2A, obtained in RNAP excess at a low promoter concentration (0.3 nM) allow prediction of two key aspects of the time course of open complex formation for the very different conditions of the fluorescence experiments (1:1 or limiting (e.g. 0.5:1) RNAP; high (50 nM) DNA concentrations). The extent of conversion of P to {CC} in the rapidly reversible first step of Mechanism 1 is predicted by KCC and the kinetics of conversion of this rapidly-reversible mixture of CC and P to OC is predicted by kisom. These predictions, given in Fig. 2B for 50 nM RNAP and promoter DNA on a logarithmic time scale, show that significant OC formation does not occur in the fluorescence kinetics experiments until approximately 10 s after mixing.
Hence the kinetics of OC formation are predicted to exhibit two phases. The first phase (0 – 10 s after mixing for the conditions simulated) is the step-wise formation of the ensemble of closed complex intermediates ({CC}) from free promoter DNA. Because the kinetics of OC formation in filter binding and MnO4− reactivity assays that detect only OC are single-exponential in excess RNAP, formation of the {CC} ensemble (Mechanism 1) and hence the individual steps of {CC} formation (Mechanism 2) are rapidly reversible.8 Equilibrium between {CC} and P is therefore established in the first 10 s of the reaction, quantified by the equilibrium constant KCC.
At 10 s, Fig. 2B predicts that approximately half of total promoter DNA is bound in closed complexes ({CC}) with RNAP while the other half is free (unbound) P. This {CC} ensemble (and free P) convert to OC in the second kinetic phase. The kinetics of the steps of evolution of {CC} in the transient first phase cannot be obtained from studies of OC formation like those in Fig 1, but are determined by fitting of FRET and PIFE kinetic data for these dye positions to the five-step Mechanism 2.
FRET-Detected Kinetics of DNA Bending and Wrapping in Closed and Open Complex Formation
Previous FRET studies of equilibrium populations of closed (2°C) and open (19°C) complexes of RNAP and doubly-labeled (Cy3 and Cy5) λPR promoter DNA revealed that upstream and downstream DNA regions are highly bent and upstream DNA is wrapped on RNAP in the low-temperature (2 °C) population of closed complexes and in the 19 °C open complex, reducing the distance between −100 and +14 positions (numbered by convention relative to the +1 start site) from >300 Å before binding RNAP to 60-70 Å in these complexes. To determine the time course and mechanism of these DNA deformations, FRET kinetics experiments were performed at 19° for Cy3(−100)Cy5(+14) and Cy3(+14)Cy5(−100). Representative FRET acceptor time-courses for both promoter fragments, plotted on a logarithmic time scale, are shown in Fig. 3.
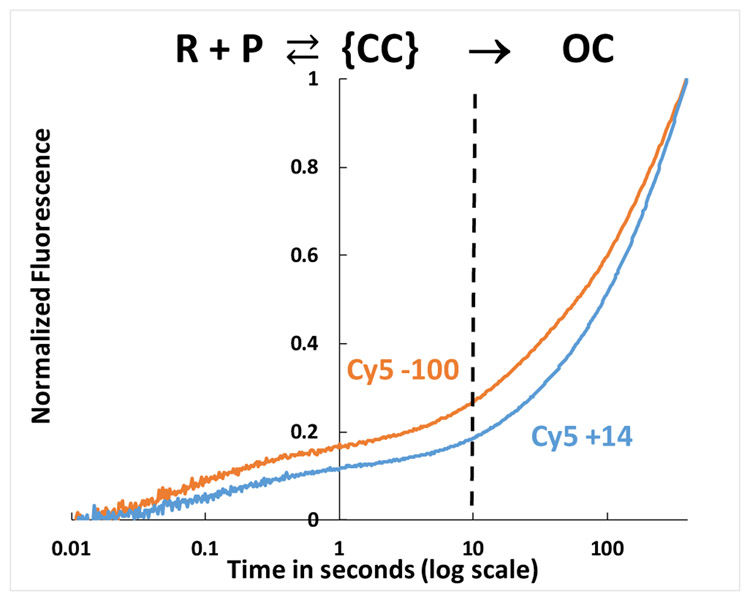
Figure 3. FRET-detected Bending and Wrapping of Promoter DNA by RNAP as {CC} Ensemble Advances and Forms the Stable OC.
Time course (log scale) of normalized FRET acceptor (Cy5) emission intensity after mixing Cy3-Cy5 dye-labelled λPR DNA (50 nM final) with E. coli RNAP (50 nM final) at 19°C and exciting FRET donor (Cy3) at 515nm. Both dye orientations are shown: Cy5(+14) Cy3(−100) ──, Cy3(+14) Cy5(−100) ──. The vertical dashed line at 10 s corresponds to the onset of OC formation at these conditions.
The FRET acceptor kinetics span a time range of more than four orders of magnitude, from ~20 ms to at least 400 s. As predicted in Fig. 2B, two kinetic phases are observed. The faster phase, accounting for approximately 15% of the total FRET increase, occurs in the first 10 s after mixing, and the slower second phase develops from 10 s to 400 s. By comparison with the simulations of Fig. 2B, these two kinetic phases clearly correspond to reversible formation of the {CC} ensemble from reactants, followed by conversion of this {CC} ensemble and free promoter to OC. The FRET inflection point in Fig. 3 in the time range 1-10 s, when ~50% of promoter DNA has been converted to the {CC} ensemble, is only ~15% of the long-time (400 s) value, at which ~80% of promoter DNA is in an OC (Fig 2B). This indicates that the {CC} ensemble is on average less bent/wrapped, with a larger dye-dye distance, than the final OC. The two high S/N experiments plotted in Fig. 3 are otherwise representative of the full set of 27 FRET experiments. In the time range from 0.5 s to 200 s the normalized FRET signal for Cy5+14 is generally smaller than for Cy5-100.
RNAP-Induced Fluorescence Enhancements (PIFE) at Both −100 and +14 Exhibit Similar Rates to FRET Acceptor Increases but Decrease Late in {CC}. Phase and in OC Formation
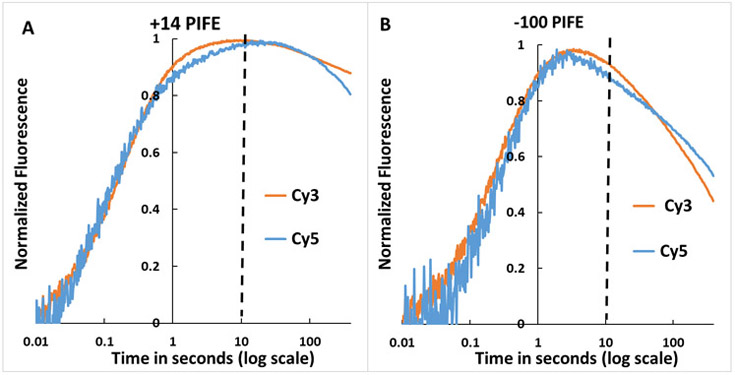
Single-dye kinetics experiments (Fig. 4) show large increases in Cy3 and Cy5 fluorescence (PIFE effects) in the time range 10 ms to 1 s at both −100 and +14 positions of λPR promoter DNA. −100 and +14 PIFE signals in Fig. 4 both develop on the same time scale as the FRET acceptor signal (t > 10 ms; Fig. 3). These +14 and −100 PIFE effects provide kinetic information regarding the involvement of downstream and upstream DNA in steps of OC formation. At each position, Fig. 4 shows that normalized PIFE effects of Cy3 and Cy5 are similar. PIFE effects for both dyes at both positions exhibit two kinetic phases, as in FRET assays (Fig. 3). In all four cases, PIFE effects are first detectable approximately 10-20 ms after mixing and increase to a maximum near the end of the {CC} phase. For +14 PIFE, a broad maximum in the time range 2 – 20 s is observed (Fig. 4A). For −100 PIFE the maximum is sharper and occurs earlier (1-3 s). For t > 3 s (−100 PIFE) and t > 20 s (+14 PIFE). PIFE signals decrease as the {CC} ensemble is completed and conversion to the stable OC begins, indicating that intrinsic −100 and +14 PIFE signal intensities of {CC} intermediates like I1E and I1M exceed those of the stable OC (see Discussion).
Figure 4. OC Formation Monitored by Single-Dye Cy3, Cy5 Fluorescence (PIFE) at Downstream (+14) and Far-Upstream (−100) Positions of λPR Promoter DNA.
Representative time courses (log scale) of normalized single-dye fluorescence (PIFE) of Cy3- or Cy5-labeled λPR DNA after mixing with RNAP (final concentrations 50 nM RNAP and λPR DNA) at 19 °C. +14 PIFE (Panel A) and −100 PIFE (Panel B) from Cy3 ── and Cy5 ──. Cy3 was excited at 515 nm and Cy5 at 610 nm. The vertical dashed line at 10 s is the predicted onset of OC formation (see Fig. 2B).
Position +14 is ~25 bp downstream of the footprint boundary for RPC (typically −5) but within the +20 downstream boundary of the footprint observed for more advanced CC and OC. 3, 4 Position −100 is 20-40 bp upstream of footprint boundaries of all RNAP-λPR promoter CC and OC investigated. Nevertheless, the PIFE results demonstrate that RNAP must contact45 these dye positions on promoter DNA in the step(s) that advance the {CC} ensemble by bending and wrapping upstream and downstream promoter DNA on RNAP. The Discussion considers the implications of these contacts for the operation of the RNAP-promoter biophysical machinery that uses binding free energy to bend the downstream duplex onto the top of the β pincer and then into the open clamp, before clamp-closing and opening of 13 base pairs including the transcription start site.
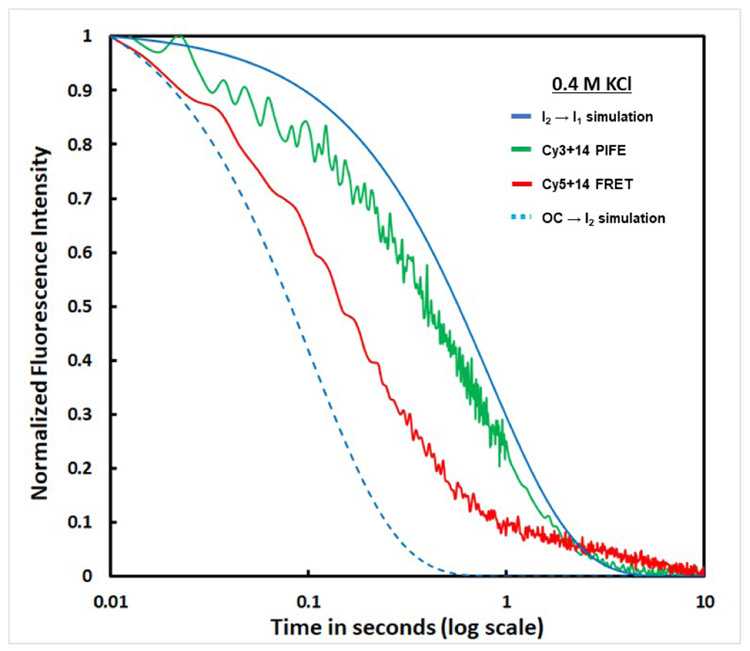
Unwrapping in Dissociation of OC by a Salt-Upshift
Rapid salt upshifts were used to investigate unwrapping in OC dissociation. Mixing of a stable RNAP-λPR promoter OC with high KCl or urea rapidly destabilizes it, forming the unstable but [KCl]-insensitive open-promoter intermediate I2, which undergoes DNA closing on a 1 s time scale.53, 54, 59, 60 Upshifts of doubly- and singly-labeled OC to 0.4 M KCl at 19°C result in rapid reductions in Cy5+14 acceptor FRET and Cy3 +14 PIFE. In Fig. 5 the kinetics of these fluorescence changes are compared with one another and with simulations of the two-step irreversible conversion of the stable OC at 19 °C to closed promoter complexes via the unstable open-promoter intermediate I2 and the DNA-closing step.53, 54, 59, 60 Figure 5 shows that the FRET and PIFE fluorescence changes occur prior to the DNA closing step of high-salt-induced dissociation. The FRET decrease occurs faster than the reduction in +14 PIFE. Both are slower than the conversion of RPo to I2 but faster than the conversion of I2 to closed-promoter complexes. The finding that the FRET decrease occurs faster than the reduction in +14 PIFE indicates that KCl-driven unwrapping of upstream DNA occurs before the release of downstream contacts. This sequence of steps in response to a large KCl-upshift differs from the mechanism of OC formation at lower KCl, where upstream DNA wrapping and formation of +14 contacts occur together as the {CC} ensemble advances.
Figure 5. Unwrapping (detected by FRET) and Release of Downstream Contacts (detected by +14 PIFE) in OC Dissociation after an Upshift to 0.4 M KCl at 19 °C.
Representative time courses (log scale) of reductions in Cy5+14 acceptor FRET ── and Cy3+14 PIFE ── after destabilizing the 19 °C OC with 0.4 M KCl. Blue curves are simulations based on 0.4 M KCl rate constants, interpolated to 19 °C from results at 10 °C and 37 °C.59 These predict the time course of conversion of the initial OC to the unstable open intermediate I2 (OC → I2, rate constant kOC → I2 ≈ 9.7 s−1;– –) and subsequent DNA-closing, designated as I2 → I1 with rate constant kI2 → I1 = ~1.2 s−1; ──
Analysis and Discussion
A Five-Step Mechanism of OC Formation with a Key Intermediate CC (I1M) Not Previously Observed by DNA Footprinting
OC formation, studied in RNAP excess by MnO4− footprinting and filter binding assays that only detect the stable OC (e.g. Fig. 1), occurs over two decades in time and exhibits single-exponential kinetics (Fig. S1). The RNAP concentration dependence of the kinetics is well described by two-step Mechanism 1 (parameters KCC, kisom). The single-exponential kinetics of OC formation in these experiments show that formation of the {CC} ensemble equilibrates rapidly (characterized by KCC) on the time scale of the conversion of {CC} to OC (characterized by kisom). Because of {CC} rapid equilibrium, no information is obtained in these kinetic studies about the number of CC intermediates or the kinetics of their interconversions.
On the other hand, four exponentials with exponential-decay time constants τi,obs (1 ≤ i ≤ 3) that span three decades (~ 60 ms to ~ 50 s; Table S3) are needed to fit the FRET (Fig. 3) and PIFE (Fig. 4) kinetic data for OC formation, as described in SI. This indicates the presence of significant populations of at least three intermediates in the {CC} ensemble with FRET and/or PIFE fluorescence signals which differ from free promoter DNA, from one another and from the final OC. Because the {CC} ensemble is in rapid equilibrium with reactants on the time scale of its isomerization to OC, each step of advancing this ensemble also rapidly equilibrates on the time scale of the next forward step (i.e. k−1 > k2, k−2 > k3, etc.). Consequently values of 1/τ1,obs , 1/τ2,obs , and 1/τ3,obs in the four-exponential fit can be interpreted as decay-to equilibrium rate constants61 for formation of three observable CC (designated I1E, I1M, I1L; see SI Eqs. S3-S6). We deduce that rapid reversible formation of the initial specific CC (RPC) from reactants is not directly detected by FRET, nor by −100 or +14 PIFE, because promoter DNA is not sufficiently bent in RPC to give FRET and because contacts between RNAP and the promoter in RPC do not extend to −100 or +14. The final (fourth) exponential is interpreted as irreversible conversion of the most advanced closed intermediate (I1L) to the stable 19 °C OC in several steps, which are not separable because they follow the rate-determining DNA opening step (I1L → I2, with rate constant k5).
Given the need for four exponentials to fit these data, it is not surprising that a five-step mechanism (Mechanism 2) including RPC, I1E, I1M, and I1L is needed to obtain good fits to all the FRET acceptor and PIFE kinetic data using a common set of rate constants (see below). FRET and +14PIFE kinetic data can be adequately fit using a four step mechanism, but significantly different sets of rate constants are obtained for FRET and PIFE datasets. −100 PIFE kinetic data are not adequately fit by a four step mechanism.
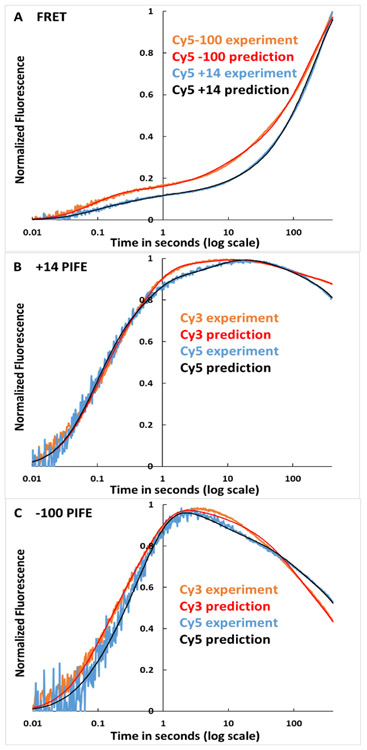
Fits of the 5-step mechanism to the FRET and PIFE kinetic data of Figs. 3 and 4 are shown in Panels A-C of Fig. 6, using the best-fit amplitudes and rate constants for these individual data sets listed in Table S4. Clearly Mechanism 2 fits the experimental data with only minor deviations. For comparison, Panels A-C of Fig. S2 show fits to the same FRET and PIFE time-courses using average FRET and PIFE signal amplitudes (Table S5) and average rate constants obtained from fitting all 61 data sets to Mechanism 2. Use of these average amplitudes and rate constants also provides good fits with approximately two-fold larger deviations (Table S5).
Figure 6. Comparison of Predicted and Observed FRET and PIFE Kinetics of OC Formation at λPR Promoter.
Panel A: FRET data (Fig. 3) for Cy5-100 ── and Cy5+14 ──. Panel B: +14 PIFE data (Fig 4A) for Cy3 ── and Cy5 ──. Panel C: −100 PIFE data (Fig. 4B) for Cy3 ── and Cy5 ──. Predictions use rate constants and amplitudes in Table S4, obtained from fitting these data sets to Eq. S8 for Mechanism 2. For comparison, fits in SI Fig. S2 use average rate constants (Table 3) and amplitudes (Table S5) for the entire data sets.
The following sections discuss the average FRET acceptor and PIFE amplitudes of the intermediate CC and OC in 5-step Mechanism 2, their implications for the large-scale conformational changes in promoter DNA and RNAP in these steps, the average rate and equilibrium constants of these steps, the simulated time-course of OC formation and the free energy vs progress diagram for this process.
FRET Evidence that Promoter DNA is Wrapped on RNAP in All Three I1 Intermediates and that Far-Upstream (−100) and Downstream (+14) DNA are Farther Apart in I1M than in I1E, I1L
Table 1 lists average Cy5 FRET acceptor signal amplitudes of {CC} intermediates for both Cy3(−100) Cy5(+14) and Cy3(+14)Cy5(−100), calculated from the average FRET acceptor fitting amplitudes in Table S5 and expressed relative to OC. Cy3 (FRET donor) fluorescence is dominated by PIFE and provides no FRET information. No Cy5+14 or Cy5-100 FRET is observed for the initial closed complex RPC, showing that the distance between −100 and +14 exceeds the FRET detection limit (~100 Å).
Table 1.
Relative FRET and PIFE Fluorescence Signals of RNAP-Promoter Complexes
| FRET Acceptor Amplitude (Between −100 and +14, Relative to OC)a |
+14 PIFE Intensity (Relative to P)b |
−100 PIFE Intensity (Relative to P)b |
||||
|---|---|---|---|---|---|---|
| Cy5+14 | Cy5−100 | Cy3 | Cy5 | Cy3 | Cy5 | |
| RPc | 0 ± 0.1 | 0 ± 0.1 | 1 ± 0.1 | 1 ± 0.1 | 1 ± 0.1 | 1 ± 0.1 |
| I1E | 0.34 ± 0.13 | 0.56 ± 0.26 | 1.66 ± 0.32 | 1.15 ± 0.08 | 1.20 ± 0.11 | 1.06 ± 0.03 |
| I1M | 0.13 ± 0.09 | 0.24 ± 0.13 | 1.53 ± 0.25 | 1.11 ± 0.06 | 1.19 ± 0.11 | 1.11 ± 0.04 |
| I1L | 0.71 ± 0.24 | 0.79 ± 0.15 | 1.42 ± 0.16 | 1.12 ± 0.06 | 1.04 ± 0.04 | 1.02 ± 0.02 |
| OC | 1 | 1 | 1.27 ± 0.10 | 1.07 ± 0.03 | 1.04 ± 0.04 | 1.02 ± 0.02 |
Average values of Ai/Aoc (Eq. S7) from fits of FRET kinetic data to Mechanism 2
Average values of relative PIFE intensities , calculated from fits of PIFE kinetic data to Mechanism 2 using Eq. S18.
The three more advanced I1 intermediates in the {CC} ensemble all exhibit significant FRET between −100 and +14, indicating that these positions, more than 300 Å apart in free promoter DNA, are less than ~100 Å apart in these intermediates. FRET amplitudes of the I1 intermediates range from ~ 10-80% that of OC (Table 1). These observations are consistent with previous equilibrium FRET results which indicated that promoter DNA in the 2 °C {CC} ensemble is highly wrapped on RNAP, with a somewhat smaller FRET efficiency (i.e. larger −100/+14 distance) than the 19 °C OC.33
These average FRET acceptor signal amplitudes of {CC} intermediates are analyzed to obtain relative FRET amplitudes, FRET efficiencies and distances between dyes at −100 and +14 in Table 2, using the previously-determined OC FRET efficiency (0.32) and the assumption that the Forster distance is the same for each intermediate as for OC (56 Å).33 FRET efficiencies of I1E , I1M and I1L are ~45%, ~18% and ~75% that of OC. Use of the previously-determined −100/+14 OC distance (63 Å)33 as a reference yields a −100/+14 distance for I1E of ~75 Å The −100/+14 distance increases by ~14 Å in conversion of I1E to I1M before decreasing by ~21 Å in conversion of I1M to I1L. The −100/ +14 distance in I1L (~68 Å) is ~5 Å greater than in the stable OC. Details of this analysis are provided in SI. Differences in −100 to +14 distance between these three CC intermediates and between them and the OC are better determined than their absolute values because of the ± 12% uncertainty in the OC distance33, but all distances are reasonable given the previous finding that the size of RNAP made a −100 to +14 distance of less than 50 Å (wrapped CC) or 60 Å (wrapped OC) unlikely, while the Forster distance for these dyes (56 Å) would make it difficult to detect −100 to +14 distances much greater than 100 Å.
Table 2.
FRET-Determined Distances between −100 and +14 Probe Positions on λPR Promoter DNA in CC Intermediates
| Species | FRET Acceptor Relative Amplitudea |
FRET Efficiencyb | Distance between −100 and +14b,c |
|---|---|---|---|
| I1E | 0.45 ± 0.27 | 0.14 ± 0.08 | 75 ± 9 Å |
| I1M | 0.18 ± 0.16 | 0.059 ± 0.052 | 89 ± 14 Å |
| I1L | 0.75 ± 0.30 | 0.24 ± 0.09 | 68 ± 6 Å |
| OC | 1 | 0.32 b | 63 Å c |
Average of Cy5−100 and Cy5+14 results ± 1 SD (Table 1) expressed relative to OC.
FRET efficiencies and dye-dye distances for I1E, I1M and I1L are calculated from the OC values listed here, assuming the Forster distance Ro is the same for I1 intermediates as for OC.
OC distance is from ref. 33; uncertainty is ± 12%, which does not affect relative distances but does increase the uncertainty in the absolute distances for the I1 intermediates.
Although the uncertainties in these distances (Tables S5, 1, 2) are appreciable, comparable in some cases to the differences between the different I1 intermediates, the trend is unambiguous. These uncertainties result from the imperfect reproducibility of the data for each position of the Cy5 acceptor and from the marginally-significant difference in FRET signals for the two placements of the dye probes (Fig. 3). Separate analyses of Cy5-100 and Cy5+14 FRET acceptor data sets (Tables S4, S5) reveal the same progression of FRET fitting amplitudes for these intermediates, with I1M exhibiting the smallest amplitude and therefore the greatest distance between −100 and +14 probe positions. The conversion of higher-FRET I1E to low-FRET E1M is the origin of the inflections in the Cy5+14 and Cy5-100 FRET acceptor time courses at about 1 s (Fig. 3).
These large changes in distance between −100 and +14 for the different intermediates, together with PIFE contact information at these positions, form the basis for a structural mechanism of OC formation, proposed below. They also provide a structural explanation of the very-large facilitation of isomerization in OC formation by upstream wrapping.
PIFE Indicates that RNAP-Promoter Contacts at +14 and Especially at −100, Absent in RPC, Are Stronger in I1 Intermediates than In OC
Table 1 also summarizes the RNAP-induced fluorescence enhancements (PIFE) determined for the different CC intermediates and OC, expressed relative to free promoter DNA (P). These are calculated as described in SI (Eqs. S9-18) from the average PIFE fitting amplitudes (Table S5), which span a ~10-fold range. Several general trends are clear from Table 1. Of most significance, PIFE intensities at −100 and +14 are larger for I1E and I1M intermediates than for OC for both Cy3 and Cy5, while RPC exhibits no detectable PIFE intensity. The −100 PIFE intensity of I1L is small, comparable to the −100 PIFE intensity of OC, while the +14 PIFE intensity of I1L is larger, comparable to the −100 PIFE intensities of I1E and I1M. These rank orders of PIFE intensities indicate that relatively strong contacts45 of RNAP with both far-upstream (−100) and downstream (+14) DNA are made in converting RPC to I1E, where −100/+14 FRET is also first observed. These relatively strong contacts persist in I1M, demonstrating that the decrease in FRET in converting I1E to I1M does not result from partial unwrapping of upstream or downstream DNA.
Contacts of RNAP with far upstream (−100) DNA appear to be largely disrupted in conversion of I1M to I1L and OC, while contacts of RNAP with downstream (+14) DNA appear similar in all three I1 intermediates and surprisingly are stronger in these intermediates than in the stable OC. In other trends, contacts of RNAP with +14 appear stronger than with −100, since the PIFE signal of each dye in each complex (I1 intermediates, stable OC) is stronger at +14 than at −100. Also, the effect of RNAP-DNA interaction on dye fluorescence is dye-specific, with larger Cy3 PIFE intensities than Cy5 PIFE intensities at each position.
FRET PIFE Insights into the Mechanism of Operation of the RNAP-Promoter Machine in OC Formation
a). Specific Contacts in RPC Direct RNAP to Bend and Wrap Both Upstream and Downstream DNA in One Step to Form High-FRET PIFE I1E
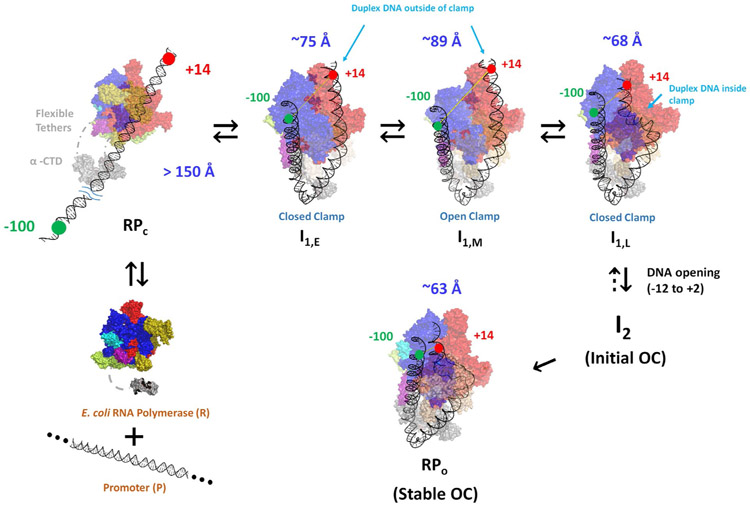
The development of −100/+14 FRET and −100 and +14 PIFE in conversion of RPC to I1E demonstrates that both upstream and downstream promoter DNA are bent and wrapped on RNAP in this step, making contacts between RNAP and both −100 and +14 positions of promoter DNA and reducing the −100/+14 distance to ~75 Å (Table 2). To interpret these concerted upstream and downstream effects, we propose that the contacts made in RPc between RNAP and −35 and/or UP elements of promoter DNA initiate strong upstream bending, resulting from or accompanied by contraction/folding of the flexible tethers linking α-CTD to α-NTD, as discussed below. Upstream bending allows far-upstream wrapping, which, as proposed previously, allows the −100 position of the promoter to be contacted by a mobile downstream element (DME) or other region at the downstream end of the RNAP clamp.3, 16 If only the upstream DNA but not the downstream DNA were bent and wrapped, the distance between −100 and +14 probe positions is estimated to be >130 Å, which is too large to exhibit FRET. Therefore bending and wrapping of upstream DNA must be accompanied by bending of the downstream duplex, presumably at the upstream end of the −10 region,5, 20, 21, 52, 62 reducing the distance between +14 and −100 positions on promoter DNA to ~75 Å to give the FRET efficiency for I1E calculated in Table 2. This downstream bending is not present in RPC, because downstream footprints of RPC complexes end at −5 while that of I1E extends to at least +2/+7.16 Hence the interactions of σ70 region 2 with the −10 region in RPC are not sufficient by themselves to induce the large-scale bending of the downstream duplex observed in I1E. If other interactions of the downstream duplex with RNAP are involved in the conversion of RPC to I1E, these might be with a RNAP element like the βSI1 sequence insertion or the NCD of σ70.
A plausible illustration of I1E is provided in Fig. 7. A −100/+14 dye-dye distance of ~75 Å is obtained by bending the upstream duplex around the α subunits, wrapping it on the outside of the β’ clamp of RNAP, and bending the downstream duplex at the upstream end of the −10 region to bring +14 in contact with the top of the β pincer and sequence insertion βSI1. This model is similar in its placement of the downstream duplex to the CC1 pol II intermediate observed by cryoEM.32 A recent cryoEM study of allosteric regulation of RNAP by TraR finds that this protein also interacts with the top of the β pincer and sequence insertion βSI163 , which should affect formation of I1E from RPC and could also affect conversion of I1M to I1L.
Figure 7. Illustrations of Bending and Wrapping of Promoter DNA in CC Intermediates and OC from FRET Distances and PIFE Contacts.
Proposed mechanism of OC formation by RNAP determined by FRET and PIFE probes at −100 and +14 on promoter DNA. Unbound reactants (unbent promoter DNA, free RNAP) are at lower left. The absence of FRET and PIFE in the initial CC (RPC; upper left) shows that promoter DNA is not yet bent and wrapped on RNAP. Promoter DNA in subsequent CC intermediates (I1E, I1M, I1L) and in OC is highly bent and wrapped, making RNAP-DNA contacts at both −100 and +14. To explain the FRET distances and PIFE effects, we propose that concerted upstream bending/wrapping of upstream duplex DNA around the α subunits and onto the upper β’ clamp and bending of downstream duplex DNA onto the top of the β clamp in I1E (top left-center) trigger clamp opening to form I1M (top right-center). Clamp-opening triggers descent of the downstream duplex into the clamp and clamp-closing to form I1L (top right), which opens the initiation bubble to form the initial unstable OC (I2). Stabilization of I2 by binding of RNAP mobile elements to the downstream duplex yields the stable OC (bottom right).
b). RNAP Clamp-Opening Moves Wrapped Upstream DNA Away from Downstream DNA to Form Low-FRET, High-PIFE I1M
The FRET efficiency of I1M is less than that of I1E (Table 2) while −100 and +14 PIFE signals of I1M are similar to those of I1E (Table 1). Analysis reveals that the conformational change that converts I1E to I1M increases the distance between the probes at −100 and +14 by ~14 Å (Table 2) without diminishing the PIFE contact of these positions with RNAP (Table 1). This increase in −100/+14 distance is readily explained if this step involves opening of the β’ clamp. Movement of the β’ clamp away from β in opening increases the distance between the tips of β and β’ pincers by ~14 Å 64, 65 . The time scale of clamp opening in the absence of promoter DNA (1 s)64, 65 is similar to that of the I1E to I1M conversion (Table 3). Fig. 7 therefore proposes that the far-upstream DNA is wrapped high on the outside of the upper portion of the β’ clamp, and that in clamp opening the −100 DNA moves ~14 Å away from +14 DNA on the top of the β subunit. This movement of the clamp presumably distorts the −10 region of the promoter DNA greatly.
Table 3.
Rate Constants and Equilibrium Constants for Formation of the Ensemble of Closed Complex Intermediates and DNA Opening at 19 °C
| Kinetic Step | Rate Constantsa | Equilibrium Constantb,c | ||
|---|---|---|---|---|
| 1 | R + P ⇌ RPc | k1 ≈ 0.3 nM−1s−1 | k−1 ≈ 44 s−1 | K1 ≈ 6.8 x 106 M−1 |
| 2 | RPc ⇌ I1,E | k2 ≈ 13 s−1 | k−2 ≈ 7.4 s−1 | K2 ≈ 1.8 |
| 3 | I1,E ⇌ I1,M | k3 ≈ 2.5 s−1 | k−3 ≈ 1.7 s−1 | K3 ≈ 1.5 |
| 4 | I1,M ⇌ I1,L | k4 ≈ 0.10 s−1 | k−4 ≈ 0.10 s−1 | K4 ≈ 1.0 |
| 5 | I1,L → OC | k5 ≈ 0.040 s−1 d | ||
Uncertainties in rate constants and in K1 are approximately ±30-50%, based on the quality of fits obtained with different sets of rate constants satisfying rapid equilibrium of each step .
These four equilibrium constants yield a predicted K{CC} = 5.5 x 107 M−1 from Eqs. S21-22, in agreement with the experimental K{CC} (Fig. 2A).
Sets of values of K2, K3 and K4 in the range 1.0 – 2.0 that satisfy the conditions in footnotes b and d provide good quality fits to the experimental FRET and PIFE kinetic data.
c). The Downstream Duplex Is Bent into the Open Clamp, which Closes to Form the High-FRET, Most Advanced CC, I1L.
The FRET efficiency of I1L is greater than that of I1E and much greater than that of I1M (Table 2), indicating that the upstream DNA has moved much closer to the downstream DNA in I1L. We interpret this as a very large-scale set of conformation changes in which the downstream duplex is bent into the open clamp, followed by closing of the clamp (Fig 7). The −100/+14 distance is ~7 Å less than in I1E and ~21 Å less than in I1M. A simple interpretation of these changes in distance is that bending the downstream duplex into the clamp brings +14 DNA ~7 Å closer to −100 DNA, and that closing the clamp brings −100 DNA ~14 Å closer to +14 DNA. The PIFE intensity at −100 is smaller for I1L than for I1M or I1E (Table 1) indicating less strong contacts with both these DNA positions in I1L, while +14 PIFE intensity is similar for all three I1 intermediates.
d). Comparison with Other Mechanistic Proposals.
A mechanism of open complex formation by E. coli σ70 RNAP was proposed based on fluorescence studies of interactions of designed short DNA oligomers with a −10 region or −35 and −10 regions (lacking upstream DNA), using RNAP drug complexes locked in closed and open clamp states. Probes of base flipping in the −10 element and of interactions of the +2 position in open complex formation were used in these studies.47 Evidence was obtained for an early intermediate (called a recognition complex) in which closed-clamp RNAP is bound to somewhat bent, base-flipped but otherwise closed promoter DNA. This appears to correspond to the I1E intermediate4 of Fig. 7 (without the upstream DNA) in which the RNAP is closed and the downstream DNA is sufficiently bent to contact the top of the β pincer. In the next proposed intermediate, called RPI1, the downstream duplex is more highly bent and the clamp of RNAP is open, similar to the I1M intermediate characterized here. No evidence was obtained for the key late closed intermediate I1L. The initiation bubble in the next proposed intermediate (designated RPI2, based on a crystal structure of a fork-junction complex with open-clamp RNAP), appears similar to the initial, unstable OC previously identified by MnO4− footprinting and kinetic analysis and called I2.53, 59, 64 Another possible I2 model is the open-clamp complex designated RPip observed by cryoEM with σ54 RNAP, stabilized by using a pre-melted heteroduplex DNA.52 Since we find the clamp closes in converting I1M to I1L, the proposal that the clamp is open in I2 indicates that the clamp opens when the initiation bubble opens in converting I1L to I2. If the strand backbones are held in the closed clamp in I1L, as seems likely, clamp opening may be connected to bubble opening in I1L → I2.
Closed-promoter intermediates formed by yeast pol II RNAP have been characterized by cryoEM. In its downstream interactions, the I1E intermediate proposed here (Fig. 7) is similar to the yeast pol II CC1 intermediate, in which the downstream duplex is bent and located above the closed clamp. In its downstream interactions, open-clamp I1M (Fig. 7) corresponds to the subsequent pol II CC2 complex. In the next step for both enzymes, the downstream duplex is bent into the open clamp, but the clamp is closed on the downstream duplex in E. coli σ70 RNAP intermediate I1L (Fig. 7) but the clamp is open in yeast pol II intermediate CCdist. Because the next step (DNA opening) for E. coli σ70 RNAP is rate-determining, no subsequent intermediate (like I2) is observable, but only the final OC. Presumably a similar situation exists for yeast pol II.
Rate and Equilibrium Constants for Forming the Different Intermediates and their Use to Predict Time-Dependent Populations and the Free Energy vs. Progress Diagram
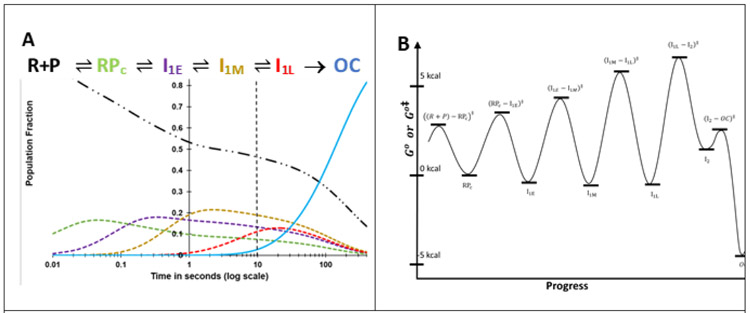
A prediction of the time-evolution of populations of free promoter DNA (P), all four closed intermediates and OC for the reactant concentrations and conditions investigated here is shown in Fig. 8A. This prediction is based on the rate constants of Table 3, determined by fitting FRET and PIFE kinetic data to Mechanism 2 as described in SI Methods. Uncertainties in these rate constants, estimated from the fitting, are approximately ± 30-50%. Variations in rate constants within this range which are consistent with KCC, kisom and rapid equilibrium constraints (see Table 3 footnotes and SI) provide fits of similar quality to the FRET and PIFE data (Figs. 3, 4) and yield very similar FRET acceptor amplitudes and distances to those in Tables 1 and 2. Differences in FRET amplitudes for these different fits are small in comparison to the uncertainties cited in Tables 1-2.
Figure 8. A) Time Evolution of Populations of Unbound Promoter DNA, Intermediates in the {CC} Ensemble and the Stable λPR Promoter OC.
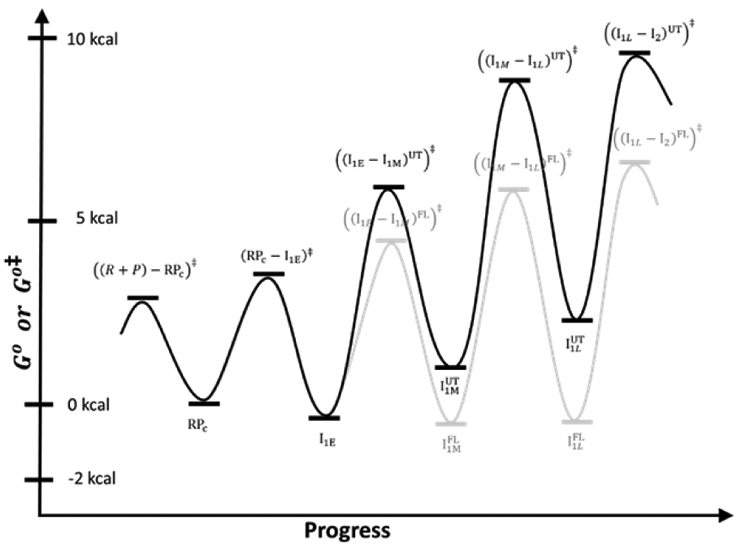
Simulations of population fractions of reactant, intermediates and product vs time (0.01 s to 400 s) for 5-step Mechanism 2 at 50 nM final concentrations of RNAP and promoter DNA, 19 ° C, using rate constants (Table 3) from analysis of FRET and PIFE kinetic data. Free promoter DNA — .. –; closed complex intermediates RPC – – –, I1,E – – – , I1M– – – , I1,L – – –, OC ──. The dashed vertical line at 10 s marks the onset of OC formation from the equilibrium mixture of {CC} and free promoter DNA predicted from filter binding and MnO4− kinetic data (Fig. 2B). B) Standard Free Energy (G°) vs. Progress Diagram for OC Formation with FL λPR Promoter DNA. G° values for CC intermediates and the stable OC are obtained from the equilibrium constants of Table 3 (ΔG° = −RT lnKi) and are expressed relative to RPC, which is arbitrarily assigned G° = 0 kcal. The G° value for I2 is obtained from K5 = k5/k−5, where k−5 = 1.2 s−1 (see Fig. 5, there designated kI2 → I1). Activation free energies Go‡ relative to the same reference are calculated from the relationship ΔGo‡ = − RTln(k/k max) where kmax is the (maximum) rate constant for the hypothetical situation ΔGo‡ = 0.4 For purposes of illustration, we choose kmax= 5 x 103 s−1 for all steps.
a) Free Promoter DNA (P): ):
Because the equilibrium constant KCC for forming the {CC} ensemble from reactants is modest (~5 x 107M−1; Fig 2A), more than half of total promoter DNA remains unbound, in rapid equilibrium with RPC, in the first 10 s of the reaction (Fig 2B) at the reactant concentrations studied here (50 nM). In these first 10 s, the {CC} ensemble forms as shown in Figs. 2B and 8A. After 10 s, formation of the very stable OC from the most advanced CC (I1L) results in additional conversion of P to RPC and I1 species.
b) Initial Specific CC Intermediate (RPC):
No FRET or PIFE signal is expected for RPC because footprinting indicates that the downstream promoter DNA is not bent and that the PIFE probe positions (−100, +14) are not in contact with RNAP.7, 9-12 Nevertheless, fitting to Mechanism 2 in which the subsequent intermediates are observable provides estimates of the equilibrium and rate constants for RPC formation. The second order rate constant k1 ≈ 0.3 nM−1 s−1 (Table 3) for RPC formation is in the middle of the range reported with other promoters (0.1 to 1 nM−1 s−1)50, 51. This k1 is about 5% of the diffusion-collision limit, most simply interpreted as that only about 5% of collisions of R with P result in RPC formation. The dissociation rate constant k−1 ≈ 44 s−1 (Table 3) is somewhat larger and the equilibrium constant K1 ≈ 6.8 x 106 M−1 (Table 3) is somewhat smaller than those reported for other promoters.50, 51
About 10% of promoter DNA is predicted to form RPC in the first 10-20 ms of the reaction at the concentrations simulated (50 nM R, P). No FRET or PIFE signal is observed in this time range (Figs. 3, 4), and indeed none is expected for RPC because footprinting indicates that the downstream promoter DNA is not bent and that the PIFE probe positions (−100, +14) are not in contact with RNAP. 8-12 Table 3 values of k1 and k−1 provide an estimate of 1/τ0 ≈ 70 s−1 for this step (τ0 ≈ 14 ms, Table S3), consistent with the lag before fluorescence effects are observed. RPC is predicted to increase to a maximum (−15% of total promoter DNA) at −40 ms and to remain the primary CC up to −100 ms.
c) Wrapped CC Intermediate I1E:
The rate constant for conversion of RPC to I1E (−13 s−1) is less than that for dissociation of RPC (~ 44 s−1), allowing RPC to equilibrate with R and P on the time scale of its conversion to I1E. I1E is only marginally if at all more stable than RPC (K2 ≈ 1.8 (Table 3)), showing that favorable contacts of these wrapped regions of promoter DNA with RNAP are just sufficiently favorable to overcome the costs of DNA-bending. Starting at 30 ms, the population of I1E (Fig. 8A) increases to a broad maximum (about 20% of total promoter DNA) at 300 ms and then decreases gradually at longer times as more advanced CC and OC form.
d) Open-clamp Wrapped CC Intermediate I1M:
The rate constant for conversion of I1E to I1M (~ 2.5 s−1) is less than that for reversal of I1E to RPC (~ 7 s−1), allowing I1E to equilibrate with RPC on the time scale of its conversion to I1M. I1M is only marginally if at all more stable than I1E (K3 ≈ 1.5; Table 3). The population of I1M increases from ~100 ms to a broad maximum near 2 s (Fig. 8A) and then decreases gradually at longer times as I1L_and OC form. As in the previous step (RPC → I1E), the cost of large conformational changes in conversion of I1E to I1M must be compensated by favorable interactions to result in a marginally favorable ΔG° for this step.
e) Closed-clamp Late CC Intermediate I1L:
The rate constant for conversion of I1M to I1L (~0.1 s−1) is much smaller than that for reversal of I1M to I1E (~2 s−1), allowing equilibration of I1M and I1L on the time scale of conversion of I1M to I1L. The population of I1L increases from ~1 s to a broad maximum (~15% of total promoter DNA) at ~25 s and decreases gradually at longer times as I1L slowly converts to OC in the DNA-opening step (Fig. 8A). I1M and I1L are of similar stability (K4 ≈ 1.0).
f) Stable Wrapped OC:
The FRET amplitude of the end-product 19 °C OC is somewhat larger than that of I1L, indicating that opening the bubble to form the initial unstable OC (I2) and subsequent formation of stabilizing downstream interactions in the conversion of I2 to the stable 19 °C OC reduce the distance between +14 and −100. Intrinsic PIFE signals at −100 and +14 in the stable OC are similar to those of I1L and smaller than those of the earlier I1 intermediates (Table 1). Only overall changes in FRET and PIFE in converting I1L to the stable OC are observed. While no information is obtained about the individual steps converting I1L to I2 and then to the stable OC, previous quantitative kinetic studies of the steps of OC dissociation induced by KCl-upshift (as in Fig. 5) can be used to obtain this information.
g) Free Energy vs. Progress Diagram
Fig. 8B shows a standard free energy (G°) vs. progress diagram for Mechanism 2, based on the best-estimates of rate and equilibrium constants in Table 3. RPC is taken as the point of reference and is set equal to zero. Activation free energies Go‡ relative to the same reference are calculated from the relationship ΔGo‡ = − RTln(k/k max) where kmax is the (maximum) rate constant for the hypothetical situation ΔGo‡ = 0.4*** For purposes of illustration, we choose kmax = 5 x 103 s−1 for all steps. The rapid-equilibrium condition for each step is indicated by the lower barrier for reversal of each intermediate, relative to going forward to the next intermediate. The highest Go‡ value is for the transition state for the rate-determining DNA opening step that converts I1L to I2. Barriers surrounding I2 show the opposite pattern to those for previous intermediates, with a much lower barrier for forward conversion of I2 to the stable OC than for reversal of I2 to I1L.
Fig. 8B reinforces the finding (Table 3) that the steps starting with RPC and forming the more advanced I1 intermediates are all only marginally favorable for this promoter and conditions, with equilibrium constants Ki between 1 and 2. Hence bending and wrapping upstream and downstream promoter DNA on RNAP is only marginally favorable, and all I1 species are present at significant levels when the equilibrium {CC} distribution is established. This finding is consistent with HO footprinting results which showed that far-upstream contacts are substantially weaker than downstream contacts, and with the absence of a far-upstream DNase footprint, explicable because weak protein-DNA contacts can be displaced by binding of DNase.16
How Far-Upstream DNA Facilitates Conversion of {CC} to OC: Insights from the Five-Step Mechanism
a) Upstream-DNA Greatly Increases the Isomerization Rate Constant kisom at the λPR Promoter with only a Modest Effect on KCC.
The presence of upstream DNA (−40 to −100) in the FL λPR promoter affects the kinetics of OC formation similarly to a strong, upstream-binding Type II transcription factor.17, 18 The isomerization rate constant of the FL λPR promoter under the conditions investigated here is ~50 times larger than that of a λPR promoter truncated upstream at position −47 (UT-47)18 Similarly, kisomFL for the lacUV5 promoter is 10- to 30-fold larger than kisomUT for UT-42, UT-45, and UT-63.17 KCC is either unaffected or increased by upstream truncation.17, 18 These effects of the presence or absence of upstream DNA on kisom of λPR and lacUV5 promoters are as large as or larger than effects of promoter sequence changes or addition of upstream-binding factors.
From Eq. S20-21, upstream DNA could increase kisom by increasing the fraction (fI1L) of the {CC} ensemble that is I11 and/or by increasing the intrinsic DNA opening rate constant k5. Several lines of evidence indicate that profound differences in fI1L are the origin of the much greater kisomFL as compared to kisomUT.4 Real-time footprinting of {CC} ensembles during OC formation revealed that downstream contacts of RNAP with FL λPR extend to +20 as compared to +2/+7 (partial protection) for λPR UT-4716 and to −5 for RPC at other promoters. To interpret these results we proposed that the UT-47 {CC} ensemble is composed primarily of RPC and the subsequent CC intermediate (I1E), with relatively small concentrations of the more advanced CC (I1L and probably also I1M) that are a major part of the FL {CC} ensemble4 We deduce that formation of these advanced CC from I1E is favored by the presence of upstream DNA for the FL promoter and greatly disfavored for the UT promoter variants. Insights from Mechanism 2 and Fig. 7 help clarify why wrapping of FL upstream DNA is needed to advance the {CC} ensemble beyond I1E and form a significant population of I1L, the only CC intermediate that can undergo the DNA opening step.
b) Predicted Large Differences in the Fraction of Advanced CC (fI1L) between FL and UT-47 λPR Promoter DNA.
Fractional populations of the different CC as a function of time in OC formation with full-length (FL) λPR promoter DNA are shown in Fig. 8A. After equilibration ( at > 10 s), the {CC} ensemble formed from FL λPR promoter DNA is relatively advanced (33% I1L, 33% I1M) with smaller percentages of RPC (12%) and I1E (22%). This population distribution is consistent with real-time footprinting of the {CC} ensemble, which shows strong protection downstream to +20, characteristic of I1L but not of I1E or RPC.16 By contrast, for UT-47 λPR , the analysis given in SI predicts that I1L and perhaps also I1M are greatly destabilized (Fig. 9). The predicted population distribution in the equilibrated {CC} ensemble for UT-47 is approximately 60% I1E , 33% RPC, 6% I1M and only 0.6 % I1L. This small population of I1L in the equilibratedUT-47 {CC} ensemble is the likely origin of its small isomerization rate constant.
Figure 9. Proposed Standard Free Energies (G°***) vs. Progress Diagram for OC Formation with UT-47 λPR Promoter DNA and Comparison with FL λPR Promoter DNA.
Standard free energies (G°) for CC intermediates formed by UT-47 λPR promoter DNA are obtained from equilibrium constants as described in SI and are expressed relative to RPC, which is arbitrarily assigned G° = 0 kcal. Effects of truncation on the steps converting I1E to I1L are assumed to be entirely on the forward rate constant of these steps. Activation free energies Go‡ relative to the same RPC reference are calculated from the relationship ΔGo‡ = − RTln(k/kmax) where kmax, the (maximum) rate constant for the hypothetical situation ΔGo‡ = 0, is arbitrarily assigned the value kmax = 5 x 103 s−1 for all steps. Diagram from Fig. 8B for the full-length (FL) promoter is shown for comparison.
c) Proposed Structural Basis of these Very Different {CC} Population Distributions.
For FL λPR the kinetic data and above analysis show that one or both steps of conversion of I1E to I1L is/are greatly favored by the interactions of bent-wrapped upstream DNA with RNAP, as previously proposed.4
If conversion of I1E to IM is the step that is much more favorable for FL λPR than for UT-47, then bending and wrapping of upstream DNA on RNAP favors clamp opening. This might involve compaction or folding of the α-CTD tethers to bend and wrap the upstream DNA in I1E, causing the assembly of UP element DNA, α-CTD, and compacted tethers to interact with the body of RNAP. A structural analogy would be the lac repression complex, in which the flexible tethers connecting the DNA binding domains (DBD) to the core repressor fold into helices, interact with operator DNA, and cause the DNA-DBD assembly to interact with the repressor core.66-69
If conversion of I1M to I1L is the step that is much more favorable for FL λPR than for UT-47, then upstream wrapping favors the entry and descent of the downstream duplex into the open clamp. A molecular explanation for this, proposed previously,3, 16 is that upstream wrapping could allow far-upstream DNA to contact downstream mobile elements (DME) of RNAP and move them away from the downstream clamp/cleft. This proposal and the interaction of far upstream DNA with DME would explain the significant −100 PIFE signals of I1E and I1M (Table 1), and the reduction in −100 PIFE in I1L and OC when the DME move to interact with the downstream duplex in the stable OC.4
We think it likely that both these steps (I1E → I1M and I1M → I1L) are affected by contacts of the upstream-wrapped DNA with the outside of the clamp and with the DME at the downstream end of the clamp (Fig. 7), and therefore that both conversions are facilitated by upstream wrapping. An example of this is shown in the comparison of free energy vs. progress diagrams for UT-47 and FL λPR in Fig. 9 (details provided in SI). Fig. 9 predicts that the much smaller kisom for UT-47 results from the relative instability of late CC intermediates , in contrast to the situation for FL λPR where late CC intermediates are more stable than earlier ones .
Conclusions
The FRET and PIFE fluorescence kinetics studies reported here reveal for the first time when upstream and downstream promoter DNA are bent and wrapped on RNAP in the mechanism of OC formation, as well as why upstream DNA (−40 to −100) is necessary for efficient isomerization of the {CC} ensemble to OC. Information in the promoter sequence (presumably primarily the UP-element, −35 and −10 regions), read by interactions with the α-CTD and σ70 regions 4 and 2 in the initial CC (RPC), directs concerted bending and wrapping of both upstream and downstream DNA in conversion of RPC to the more advanced CC, designated I1E, in which the distance between −100 and +14 positions on promoter DNA is reduced from >300 Å before binding of RNAP to ~75 Å. Interactions between promoter DNA and RNAP in I1E, which we propose are primarily between the upstream-wrapped DNA and the hinge of the clamp, result in opening of the clamp (on a ~1 s timescale for the conditions investigated) to form the intermediate I1M. Clamp opening moves the far-upstream DNA, which we propose is wrapped high on the back of the β’ clamp, ~ 14 Å further away from the downstream duplex, which we propose is bound on the top of the β clamp in I1E and I1M. Subsequently, on a ~10 s timescale, the downstream duplex is bent into the clamp, which closes to reduce the distance between −100 and +14 positions to ~68 Å in I1L, the most advanced CC. Opening of the initiation bubble by strand binding free energy appears to occur in a single kinetic step at this promoter to form the initial unstable OC (I2), which is rapidly stabilized by downstream interactions with DME. Each step of forming and advancing the {CC} ensemble rapidly equilibrates on the time scale of the next step. The DNA opening step is rate-determining for OC formation because its forward rate constant (k5 = 0.04 s−1) is smaller than those of previous steps and because it is effectively irreversible since stabilization of I2 by downstream interactions is more rapid than DNA-closing for the promoter and conditions investigated (Fig. 8B).
Accession Codes
P0A7Z4, subunit α; P0A8V2, subunit β; P0A8T7, subunit β’; P0A800, subunit ω; P00579 subunit σ.
Contents of SI:
The SI text describes the methods used in the following analyses. 1) the normalization of fluorescence kinetic data; 2) fitting methods, including the analysis of FRET and PIFE kinetics as a sum of four exponentials and fitting these data to five-step Mechanism 2; 3) consistency checks on the rate constants of Mechanism 2; 4) conversion of fluorescence fitting amplitudes to relative fluorescence intensities; 5) estimation of distance between upstream (−100) and downstream (+14) DNA in wrapped RNAP-promoter complexes from FRET amplitudes; 6) interpretation of rate and equilibrium constants of the classic two-step Mechanism 1 (kisom, KCC, ka) using Mechanism 2; and 7) calculations for free energy vs. progress diagram and {CC} population distribution for formation at UT-47 λPR. SI figures include: 1) the MnO4− footprinting data of Fig. 1 plotted on a logarithmic time scale; 2) comparison of the FRET and PIFE data of Figs. 3-4 with the prediction based on the average rate constants and signal amplitudes of Tables 1 and 3. SI Tables include 1) promoter and 2) primer sequences used in PCR synthesis of the different dye-labeled promoters investigated here; 3) comparison of rate constants (1/τi,obs) from the four-exponential fit with predictions from the rate constants of Mechanism 2; 4) individual rate constants and signal amplitudes obtained from analysis of all FRET and PIFE experiments; and 5) average FRET acceptor and PIFE fitting amplitudes of CC intermediates and the stable OC.
Supplementary Material
Acknowledgments:
We thank Profs. Eric Galburt, Ken Johnson, Tim Lohman, Oleg Tsodikov, and James Weisshaar, Dr. Wayne Kontur, and the referees for their very helpful comments on the manuscript. We also thank previous undergraduates Emily Lingemann, Miranda Mecha, Yurun Zhang, Priya Chittur, Wen Fu, Rahul Vivek, and Hertina Kan for their contributions to this research and gratefully acknowledge support of NIH grant GM R35-118100.
Glossary of terms
- αCTD
C-terminal domain of the α subunit of RNAP
- {CC}
Ensemble of CC, rapidly-reversible on time scale of open complex formation
- CC
Closed complex (a complex of RNAP with duplex promoter DNA)
- CC1
Closed Complex intermediate for Pol II, with downstream promoter positioned above the closed clamp
- CC2
Closed Complex intermediate for Pol II, with open clamp domain
- CCdist
Closed complex intermediate for Pol II, with downstream duplex bent into the clamp
- Cy3/5 (N)
Dyes used in FRET and PIFE experiments, attached to the promoter DNA at position N relative to the transcription start site
- DME
Downstream mobile elements of RNA polymerase
- FL
Full length λPR promoter
- FRET
Forster Resonance Energy Transfer
- I1E
”Early” closed complex intermediate after RPC in {CC} ensemble (Mechanism 2)
- I1L
”Late” closed complex intermediate in {CC} ensemble (Mechanism 2)
- I1M
“Middle” closed complex intermediate in {CC} ensemble (Mechanism 2)
- I2
Initial (unstable) open complex intermediate in stable-open-complex formation
- KCC
Equilibrium constant for the converting free promoter DNA and RNAP to the closed complex ensemble ({CC})
- Kisom
Forward rate constant for the isomerization of the closed complex ensemble ({CC}) to the open-promoter complex (OC)
- Kobs
Observed rate constant
- OC
Open promoter complex (a complex of RNAP with promoter DNA in which ~13 base pairs have been opened to form an initiation “bubble”)
- P
λPR promoter DNA
- PIC
pol II pre-initiation complex
- PIFE
Protein Induced Fluorescence Enhancement
- Pol II
Eukaryotic RNA Polymerase II
- RNAP or R
E. coli RNA Polymerase holoenzyme (composed of α2ββ’ω core enzyme + σ70 specificity subunit)
- RPC
Initial specific closed complex intermediate in {CC} ensemble
- UT-n
Variant of the λPR promoter in which the upstream DNA is truncated at position n. (e.g. UT-47 is truncated at position −47 relative to the transcription start site)
References
- [1].McClure WR (1985) Mechanism and control of transcription initiation in prokaryotes, Annual Review of Biochemistry 54, 171–204. [DOI] [PubMed] [Google Scholar]
- [2].Record MT Jr., Reznikoff WS, Craig ML, Mcquade KL and Schlax PJ. (1996) Escherichia coli RNA Polymerase (E sigma70), Promoters, and the Kinetics of the Steps of Transcription Initiation., Second Edition of Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology, 792–821. [Google Scholar]
- [3].Saecker RM, Record MT Jr., and DeHaseth PL (2011) Mechanism of bacterial transcription initiation: RNA polymerase-promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis, Journal of Molecular Biology 412, 754–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ruff EF, Record MT Jr., and Artsimovitch I (2015) Initial events in bacterial transcription initiation, Biomolecules 5, 1035–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saecker RM, Tsodikov OV, Mcquade KL, Schlax PE Jr, Capp MW, and Record MT Jr. (2002) Kinetic studies and structural models of the association of E. coli σ70 RNA polymerase with the λPR promoter: large scale conformational changes in forming the kinetically significant intermediates, Journal of Molecular Biology 319, 649–671. [DOI] [PubMed] [Google Scholar]
- [6].Roe J-H, Burgess RR, and Record MT Jr. (1984) Kinetics and mechanism of the interaction of Escherichia coli RNA polymerase with the λPR promoter, Journal of Molecular Biology 176, 495–522. [DOI] [PubMed] [Google Scholar]
- [7].Buc H, and McClure WR (1985) Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps, Biochemistry 24, 2712–2723. [DOI] [PubMed] [Google Scholar]
- [8].Tsodikov OV, and Record MT Jr. (1999) General method of analysis of kinetic equations for multistep reversible mechanisms in the single-exponential regime: Application to kinetics of open complex formation between E sigma(70) RNA polymerase and lambda P-R promoter DNA, Biophysical Journal 76, 1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cowing DW, Mecsas J, Record MT Jr., and Gross CA (1989) Intermediates in the formation of the open complex by RNA polymerase holoenzyme containing the sigma factor sigma 32 at the groE promoter, Journal of Molecular Biology 1210, 521–530. [DOI] [PubMed] [Google Scholar]
- [10].Mecsas J, Cowing DW, and Gross CA (1991) Development of RNA polymerase-promoter contacts during open complex formation, Journal of Molecular Biology 220, 585–597. [DOI] [PubMed] [Google Scholar]
- [11].Kovacic R (1987) The 0 degree C closed complexes between Escherichia coli RNA polymerase and two promoters, T7-A3 and lacUV5, Journal of Biological Chemistry 262, 13654–13661. [PubMed] [Google Scholar]
- [12].Schickor P, Metzger W, Werel W, Lederer H, and Heumann H (1990) Topography of intermediates in transcription initiation of E.coli, EMBO Journal 9, 2215–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hofer B, Muller D, and Koster H (1985) The pathway of E. coli RNA polymerase-promoter complex formation as visualized by footprinting, Nucleic Acids Research 13, 5995–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rogozina A, Zaychikov E, Buckle M, Heumann H, and Sclavi B (2009) DNA melting by RNA polymerase at the T7A1 promoter precedes the rate-limiting step at 37 degrees C and results in the accumulation of an off-pathway intermediate, Nucleic Acids Research 37, 5390–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sclavi B, Zaychikov E, Rogozina A, Walther F, Buckle M, and Heumann H (2005) Real-time characterization of intermediates in the pathway to open complex formation by Escherichia coli RNA polymerase at the T7A1 promoter, Proceedings of the National Academy of Sciences 102, 4706–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Davis CA, Bingman CA, Landick R, Record MT Jr., and Saecker RM (2007) Real-time footprinting of DNA in the first kinetically significant intermediate in open complex formation by Escherichia coli RNA polymerase, Proceedings of the National Academy of Sciences 104, 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ross W, and Gourse RL (2005) Sequence-independent upstream DNA–αCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association, Proceedings of the National Academy of Sciences 102, 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davis CA, Capp MW, Record MT Jr., and Saecker RM (2005) The effects of upstream DNA on open complex formation by Escherichia coli RNA polymerase, Proceedings of the National Academy of Sciences 102, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Estrem ST, Gaal T, Ross W, and Gourse RL (1998) Identification of an UP element consensus sequence for bacterial promoters, Proceedings of the National Academy of Sciences 95, 9761–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murakami KS, Masuda S, Campbell EA, Muzzin O, and Darst SA (2002) Structural basis of transcription initiation: a RNA polymerase holoenzyme-DNA complex, Science 296, 1285–1290. [DOI] [PubMed] [Google Scholar]
- [21].Vassylyev DG, Sekine S-I, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, and Yokoyama S (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution, Nature 417, 712–719. [DOI] [PubMed] [Google Scholar]
- [22].Turingan RS, Liu CH, Hawkins ME, and Martin CT (2007) Structural confirmation of a bent and open model for the initiation complex of T7 RNA polymerase, Biochemistry 46, 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cijvari A, and Martin CT (2000) Evidence for DNA bending at the T7 RNA polymerase promoter, Journal of Molecidar Biology 295, 1173–1184. [DOI] [PubMed] [Google Scholar]
- [24].Tang GQ, and Patel SS (2006) T7 RNA polymerase-induced bending of promoter DNA is coupled to DNA opening, Biochemistry 45, 4936–4946. [DOI] [PubMed] [Google Scholar]
- [25].Tang GQ, and Patel SS (2006) Rapid binding of T7 RNA polymerase is followed by simultaneous bending and opening of the promoter DNA, Biochemistry 45, 4947–4956. [DOI] [PubMed] [Google Scholar]
- [26].Blair RH, Goodrich JA, and Kugel JF (2012) Single molecule FRET shows uniformity in TBP-induced DNA bending and heterogeneity in bending kinetics, Biochemistry 51, 7444–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Louder RK, He Y, López-Bianco JR, Fang J, Chacón P, and Nogales E (2016) Structure of promoter-bound TFIID and insight into human PIC assembly, Nature 531, 604–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schilbach S, Hantsche M, Tegunov D, Dienemann C, Wigge C, Urlaub H, and Cramer P (2017) Structures of transcription pre-initiation complex with TFIIH and Mediator, Nature 551, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].He Y, Fang J, Taatjes DJ, and Nogales E (2013) Structural visualization of key steps in human transcription initiation, Nature 495, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].He Y, Yan C, Fang J, Inouye C, Tjian R, Ivanov L, and Nogales E (2016) Near-atomic resolution visualization of human transcription promoter opening, Nature 533, 359–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Plaschka C, Hantsche M, Dienemann C, Burzinski C, Plitzko J, and Cramer P (2016) Transcription initiation complex structures elucidate DNA opening, Nature 533, 353–358. [DOI] [PubMed] [Google Scholar]
- [32].Dienemann C, Schwalb B, Schilbach S, and Cramer P (2019) Promoter distortion and opening in the RNA polymerase II cleft, Molecular Cell 73, 97–106. [DOI] [PubMed] [Google Scholar]
- [33].Sreenivasan R, Heitkamp S, Chhabra M, Saecker RM, Lingeman E, Poulos MA, Mccaslin D, Capp MW, Artsimovitch I, and Record MT Jr. (2016) Fluorescence resonance energy transfer characterization of DNA wrapping in closed and open Escherichia coli RNA polymerase-λPR promoter complexes, Biochemistry 55, 2174–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Craig ML, Suh WC, and Record MT Jr. (1995) HO. and DNase I probing of E sigma 70 RNA polymerase--lambda PR promoter open complexes: Mg2+ binding and its structural consequences at the transcription start site, Biochemistry 34, 15624–15632. [DOI] [PubMed] [Google Scholar]
- [35].Rivetti C, Guthold M, and Bustamante C (1999) Wrapping of DNA around the E. coli RNA polymerase open promoter complex, EMBO Journal 18, 4464–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu B, Hong C, Huang RK, Yu Z, and Steitz TA (2017) Structural basis of bacterial transcription activation, Science 358, 947–951. [DOI] [PubMed] [Google Scholar]
- [37].Rice PA, Yang S-W, Mizuuchi K, and Nash HA (1996) Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn, Cell 87, 1295–1306. [DOI] [PubMed] [Google Scholar]
- [38].Holbrook JA, Tsodikov OV, Saecker RM, and Record MT Jr. (2001) Specific and nonspecific interactions of integration host factor with DNA: Thermodynamic evidence for disruption of multiple IHF surface salt-bridges coupled to DNA binding, Journal of Molecular Biology 310, 379–401. [DOI] [PubMed] [Google Scholar]
- [39].VanderMeulen K, Saecker RM, and Record MT Jr. (2008) Formation of a wrapped DNA-protein interface: Experimental characterization and analysis of the large contributions of ions and water to the thermodynamics of binding IHF to H ' DNA, Journal of Molecular Biology 377, 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Swinger KK, Lemberg KM, Zhang Y, and Rice PA (2003) Flexible DNA bending in HU-DNA cocrystal structures, EMBO Journal 22, 3749–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Koh J, Saecker RM, and Record MT Jr. (2008) DNA Binding Mode Transitions of Escherichia coli HU alpha beta: Evidence for Formation of a Bent DNA - Protein Complex on Intact, Linear Duplex DNA, Journal of Molecular Biology 383, 324–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koh J, Shkel I, Saecker RM, and Record MT Jr. (2011) Nonspecific DNA Binding and Bending by HU alpha beta: Interfaces of the Three Binding Modes Characterized by Salt-Dependent Thermodynamics, Journal of Molecular Biology 410, 241–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hwang H, and Myong S (2014) Protein induced fluorescence enhancement (PIFE) for probing protein-nucleic acid interactions, Chemical Society Reviews 43, 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lerner E, Ploetz E, Hohlbein J, Cordes T, and Weiss S (2016) A Quantitative Theoretical Framework For Protein-Induced Fluorescence Enhancement–Förster-Type Resonance Energy Transfer (PIFE-FRET), Journal of Physical Chemistry B 120, 6401–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nguyen B, Ciuba MA, Kozlov AG, Levitus M, and Lohman TM (2019) Protein Environment and DNA Orientation Affect Protein induced Cy3 Fluorescence Enhancement (PIFE), Biophysical Journal 117, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Heyduk E, and Heyduk T (2018) DNA template sequence control of bacterial RNA polymerase escape from the promoter, Nucleic Acids Research 46, 4469–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Feklistov A, Bae B, Hauver J, Lass-Napiorkowska A, Kalesse M, Glaus F, Altmann KH, Heyduk T, Landick R, and Darst SA (2017) RNA polymerase motions during promoter melting, Science 356, 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ko J, and Heyduk T (2014) Kinetics of promoter escape by bacterial RNA polymerase: effects of promoter contacts and transcription bubble collapse, Biochemical Journal 463, 135–144. [DOI] [PubMed] [Google Scholar]
- [49].Rammohan J, Ruiz Manzano A, Gamer AL, Prusa J, Stallings CL, and Galburt EA (2016) Cooperative stabilization of Mycobacterium tuberculosis rrnA P3 promoter open complexes by RbpA and CarD, Nucleic Acids Research 44, 7304–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hubin EA, Fay A, Xu C, Bean JM, Saecker RM, Glickman MS, Darst SA, and Campbell EA (2017) Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA, Elife 6, e22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jensen D, Manzano AR, Rammohan J, Stallings CL, and Galburt EA (2019) CarD and RbpA modify the kinetics of initial transcription and slow promoter escape of the Mycobacterium tuberculosis RNA polymerase, Nucleic Acids Research 47, 6685–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Glyde R, Ye F, Jovanovic M, Kotta-Loizou I, Buck M, and Zhang X (2018) Structures of bacterial RNA polymerase complexes reveal the mechanism of DNA loading and transcription initiation, Molecular Cell 70, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Giles TJ, Kontur WS, Capp MW, Saecker RM, and Record MT Jr. (2010) One-step DNA melting in the RNA polymerase cleft opens the initiation bubble to form an unstable open complex, Proceedings of the National Academy of Sciences 107, 10418–10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kontur WS, Capp MW, Giles TJ, Saecker RM, and Record MT Jr. (2010) Probing DNA binding, DNA opening, and assembly of a downstream clamp/jaw in Escherichia cob RNA polymerase– λPR promoter complexes using salt and the physiological anion glutamate, Biochemistry 49, 4361–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Svetlov V, and Artsimovitch I (2015) Purification of bacterial RNA polymerase: tools and protocols, Methods in Molecular Biology 1276, 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Drennan A, Kraemer M, Capp M, Gries T, Ruff E, Sheppard C, Wigneshweraraj S, Artsimovitch I, and Record MT Jr (2012) Key roles of the downstream mobile jaw of Escherichia cob RNA polymerase in transcription initiation, Biochemistry 51, 9447–9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sanborn ME, Connolly BK, Gurunathan K, and Levitus M (2007) Fluorescence properties and photophysics of the sulfoindocyanine Cy3 linked covalently to DNA, Journal of Physical Chemistry B 111, 11064–11074. [DOI] [PubMed] [Google Scholar]
- [58].Roe J-H, Burgess RR, and Record MT Jr. (1985) Temperature dependence of the rate constants of the Escherichia cob RNA polymerase-λPR promoter interaction: Assignment of the kinetic steps corresponding to protein conformational change and DNA opening, Journal of Molecular Biology 184, 441–453. [DOI] [PubMed] [Google Scholar]
- [59].Kontur WS, Saecker RM, Capp MW, and Record MT Jr. (2008) Late steps in the formation of E. cob RNA polymerase-lambda P R promoter open complexes: characterization of conformational changes by rapid [perturbant] upshift experiments, Journal of Molecular Biology 376, 1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ruff EF, Drennan AC, Capp MW, Poulos MA, Artsimovitch I, and Record MT Jr. (2015) E. cob RNA Polymerase Determinants of Open Complex Lifetime and Structure, Journal of Molecidar Biology 427, 2435–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bemasconi CF (1976) Relaxation Kinetics, Academic Press Inc, New York, 40–75 [Google Scholar]
- [62].Zhang Y, Feng Y, Chatteijee S, Tuske S, Ho MX, Arnold E, and Ebright RH (2012) Structural basis of transcription initiation, Science 338, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen J, Gopalkrishnan S, Chiu C, Chen AY, Campbell EA, Gourse RL, Ross W, and Darst SA (2019) E. cob TraR allostericaby regulates transcription initiation by altering RNA polymerase conformation and dynamics, Elife 8, e49375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chakraborty A, Wang D, Ebright YW, Korlann Y, Kortkhonjia E, Kim T, Chowdhury S, Wigneshweraraj S, Irschik H, Jansen R, Nixon BT, Knight J, Weiss S, and Ebright RH (2012) Opening and closing of the bacterial RNA polymerase clamp, Science 337, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Duchi D, Mazumder A, Malinen AM, Ebright RH, and Kapanidis AN (2018) The RNA polymerase clamp interconverts dynamically among three states and is stabilized in a partly closed state by ppGpp, Nucleic Acids Research 46, 7284–7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sengupta R, Capp MW, Shkel IA, and Record MT Jr. (2017) The mechanism and high-free-energy transition state of lac repressor-lac operator interaction, Nucleic Acids Research 45, 12671–12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bell CE, and Lewis M (2000) A closer view of the conformation of the Lac repressor bound to operator, Nature Structural Biology. 7, 209–214. [DOI] [PubMed] [Google Scholar]
- [68].Spronk C, Folkers GE, Noordman A, Wechselberger R, Van Den Brink N, Boelens R, and Kaptein R (1999) Hinge-helix formation and DNA bending in various lac repressor-operator complexes, EMBO Journal. 18, 6472–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kalodimos CG, Biris N, Bonvin A, Levandoski MM, Guennuegues M, Boelens R, and Kaptein R (2004) Structure and flexibility adaptation in nonspecific and specific protein-DNA complexes, Science 305, 386–389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.