Abstract
The kidney contains a network of lymphatic vessels that clear fluid, small molecules, and cells from the renal interstitium. Through modulating immune responses and via crosstalk with surrounding renal cells, lymphatic vessels have been implicated in the progression and maintenance of kidney disease. In this Review, we provide an overview of the development, structure, and function of lymphatic vessels in the healthy adult kidney. We then highlight the contributions of lymphatic vessels to multiple forms of renal pathology, emphasizing CKD, transplant rejection, and polycystic kidney disease and discuss strategies to target renal lymphatics using genetic and pharmacologic approaches. Overall, we argue the case for lymphatics playing a fundamental role in renal physiology and pathology and treatments modulating these vessels having therapeutic potential across the spectrum of kidney disease.
Keywords: cell signaling, endothelial cells, kidney development, pathophysiology of renal disease and progression, renal cell biology, kidney disease
Lymphatic vessels serve as a conduit for the clearance of tissue fluid, cells, and small molecules, within a protein-rich fluid termed lymph, from the interstitial compartment of vertebrate organs. This fluid enters the lymphatic system via lymphatic capillaries within tissues, traveling down a hierarchic network of collecting vessels before reaching lymph nodes which drain to large ducts, eventually returning lymph to the venous circulation. Through the identification of molecular markers of lymphatics, advances in imaging technologies, and novel genetic tools to visualize and manipulate their function, the roles of lymphatic vessels have expanded to include cholesterol transport,1 clearance of cerebrospinal fluid,2 electrolyte homeostasis,3 and regulation of peripheral tolerance and innate and adaptive immunity.4,5
The kidney possesses a lymphatic system that has been implicated in the progression and maintenance of kidney disease.6–8 However, in spite of an increasing understanding of lymphatic biology in other organs (reviewed in9–11), the kidney's lymphatics have received relatively little attention. In this Review, we first outline how lymphatics form during kidney development and describe their structure and function in adult kidneys. We then combine our current understanding of kidney lymphatics with parallels drawn from lymphatic biology in other organs to discuss their potential contribution to and therapeutic implications for several renal diseases.
Development, Architecture, and Function of Kidney Lymphatics
Development
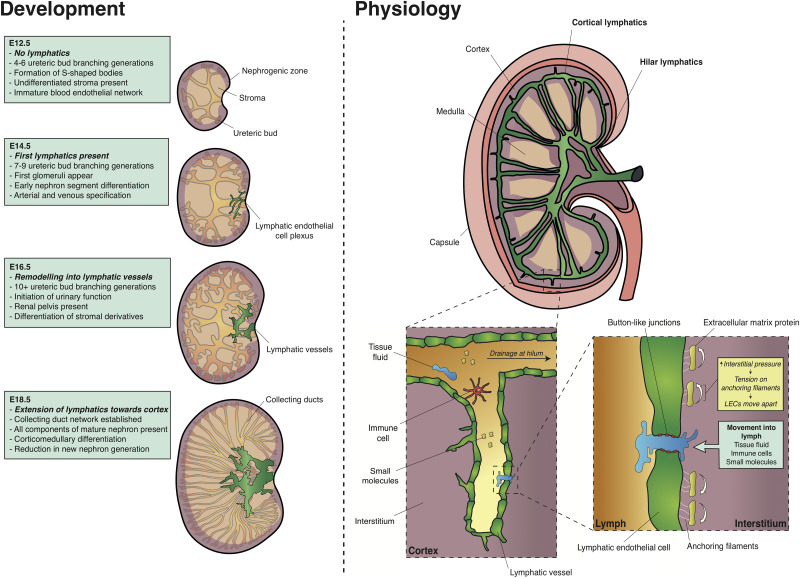
A combination of immunohistochemical studies12–15 and, more recently, three-dimensional (3D) imaging coupled with quantitative analysis have characterized lymphatic development in mouse and human embryonic kidneys (Figure 1).16 In mouse, the metanephros, the precursor to the adult kidney, begins to form around embryonic day (E) 10.5.17 At early stages of its development,18 the embryonic kidney is devoid of lymphatics. It is not until E14.5, after considerable development of the blood vasculature,19,20 that lymphatic endothelial cells (LECs) are detectable as a cellular plexus using immunohistochemistry for lymphatic markers (Table 1) such as Prospero homeobox protein 1 (PROX1) or vascular endothelial growth factor (VEGF) receptor 3 (VEGFR-3). The developing kidney lymphatics wrap around the base of the nascent kidney pelvis and are suggested to be continuous with an extrarenal network supplying the ureter, adrenal gland, and gonad.12,14 Between E15.5 and E18.5, the hilar lymphatics rapidly remodel and expand, forming lumenized vessels that extend alongside arterioles into the renal cortex. During this time, a complex collecting duct network is established;21 fully differentiated cell types within the kidney emerge such as the mature glomerulus, segments of the nephron,22 perivascular, and mesangial cells23 and renal excretory function is initiated.17,24 By the end of mouse gestation, lymphatics are present in both the hilum and the cortex. A similar pattern of lymphatic vessels is established by the end of the first trimester in humans.16
Figure 1.
Development, structure, and function of kidney lymphatics. During early nephrogenesis at E12.5, the kidney is devoid of lymphatics. Thereafter, kidney lymphatic development proceeds in three distinct phases: first, the appearance of a plexus of LECs in the kidney at E14.5; then, the remodeling of LECs into a patent vascular network by E16.5; and finally, the extension of these vessels toward the renal cortex at E18.5. All stages are presented in the context of important morphologic and differentiation events during renal development. The specification of the blood vasculature,19,20 ureteric bud branching and nephron generation,21 stages of nephron differentiation,18,22 the appearance of stromal derivatives such as pericytes and mesangial cells,23 and initiation of urinary function17,24 are taken from the indicated references. In the adult kidney, lymphatics reside in the cortical interstitium and drain to large lymphatic vessels in the hilum. The renal medulla is devoid of lymphatics. Drainage begins in the cortical interstitium. Increased pressure in this compartment causes ECM-bound anchoring filaments to force LECs apart, thus allowing tissue fluid, immune cells (such as dendritic cells and neutrophils), and small molecules (such as soluble antigen and antibodies) to enter lymphatic capillaries.
Table 1.
Molecular markers of lymphatic endothelium in the adult kidney
| LEC Marker | Molecular Function | Non-LEC Expression in the Kidneya |
|---|---|---|
| PROX1 | Transcription factor involved in the maintenance of lymphatic identity25 | Thick ascending limbs of the loop of Henle restricted to the inner medulla26 and reported in ascending vasa recta27 |
| LYVE-1 | Membrane glycoprotein and receptor for hyaluronan facilitating DC entry into lymphatic vessels28 | Some endothelial cells in the glomerulus12 |
| VEGFR-3 | Receptor tyrosine kinase required for LEC tip function and vessel sprouting29 | Ascending vasa recta27 and cortical peritubular capillaries30 |
| PDPN | Membrane glycoprotein maintaining separation between blood and lymphatic endothelium31 | Podocytes32 |
| Neuropilin 2 (NRP2) | Transmembrane protein interacting with VEGFR-3 promoting VEGF-C–mediated lymphatic sprouting33 | Blood endothelial cells34 b |
Determined from studies of mouse or rat adult kidney.
Derived from single-cell RNA sequencing, because no published immunostaining is available for NRP2.
Macroarchitecture
In the mature adult kidney, lymph drainage begins in the cortical interstitium, with blind-ended lymphatic capillaries draining into arcades running with arcuate arteries at the corticomedullary junction (Figure 1).35,36 The cortical lymphatics then follow the interlobar blood vessels, descending toward the renal pelvis. Finally, the lymphatics drain out of the kidney through hilar lymphatic vessels, located adjacent to the major renal arteries and veins as they enter and exit the kidney.
Cellular Architecture
Initially within organs, lymph enters lymphatic capillaries, which consist of a single, continuous layer of LECs. Unlike most blood vessels, lymphatic capillaries have a sparse, discontinuous basement membrane and lack supporting cells such as vascular smooth muscle cells, pericytes or fibroblasts.13 Instead, LECs lining lymphatic capillaries overlap, are held together by specialized button-like junctions37 and are physically connected to surrounding extracellular matrix (ECM) by fibrillin-rich anchoring proteins.38,39 As fluid leaks across blood capillaries, the ECM in the interstitium expands, causing anchoring proteins to pull on LECs. Consequently, button-like junctions between LECs open, allowing the constituents of lymph to enter lymphatics paracellularly (Figure 1).40 Solutes may alternatively enter lymphatics transcellularly via vesicle formation and transcytosis across LECs.41 Lymphatic capillaries drain lymph into functional units of precollecting and collecting vessels13 known as lymphangions. Within these larger caliber vessels, LECs are lined by continuous zipper-like junctions, are supported by smooth muscle and mural cells and contain valves to facilitate unidirectional lymph flow.37,42
Heterogeneity of Lymphatics and “Lymphatic-like” Vessels
Lymphatic vessels have a unique molecular signature distinguishable from that of blood endothelia.43 Some markers are expressed in all lymphatic vessels, such as PROX1 and VEGFR-3. However, there is heterogeneity in the molecular profile between lymphatic capillaries and precollecting and collecting vessels.9 Heterogeneity of the adult kidney’s blood vascular system is well recognized, with blood endothelial cells molecularly distinguishable between cortex, medulla, and glomerulus.44,45 Whether similar molecular diversity exists within kidney lymphatics remains unexplored.
Populations of hybrid kidney blood vessels expressing both blood and lymphatic endothelial markers have been identified.27,30 Peritubular capillaries—which facilitate the reabsorption of fluids and molecules from adjacent cortical tubular epithelium—express CD31 and VEGFR-3, but not the other lymphatic markers PROX1, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), or podoplanin (PDPN).30 Conversely, in a recent study, ascending vasa recta, which maintain the medullary osmotic gradient critical for urinary concentrating ability, were found to express PROX1 and VEGFR-3 but not LYVE-1 or PDPN.27 Unlike LECs, specialized functions of peritubular capillaries and ascending vasa recta are facilitated by endothelial fenestrations, which are identifiable in electron micrographs as transcellular channels of approximately 70 nm in diameter.46 Whether these vessels, which have been termed lymphatic-like, share other molecular or structural features with LECs is yet to be determined, but clearly multiple markers in parallel are required to reliably distinguish kidney lymphatics from other cell types in the kidney.
Function and Composition of Renal Lymph
Based on their anatomical location47 and uptake of radiolabeled albumin,48 kidney lymphatics are proposed to drain the interstitium of the renal cortex and hilum interstitium, but not the medulla. In the cortex, a mismatch between tubular reabsorption and the capacity for uptake by peritubular capillaries may raise cortical interstitial pressure49 and facilitate lymphatic clearance. Early functional studies indicate a functional interplay between kidney lymphatic flow, venous pressure50–54 and solute load55,56 that warrants further investigation.57–60
Rich in Igs and albumin,48 lymph, like plasma, also contains complement cascade components, coagulation factors, ECM proteases and their inhibitors and enzymes involved in cellular metabolism. Lymph is also enriched in nuclear histones, cytosolic enzymes, transcription factors, and ribosomal components61 likely derived from cellular apoptosis.62 Few studies have examined the composition of renal lymph. Sodium, chloride, potassium, and calcium content of lymph draining from the kidneys may have physiologic relevance.51,56 Analysis of rodent models of renal ischemia-perfusion injury have identified that renal lymph contains cytokines such as IL-1β and IL-6, TNF-α and monocyte chemoattractant protein 1,63 albeit at low quantities compared with blood draining from the kidney. Renal draining lymph nodes receive dendritic cells (DCs) and T and B lymphocytes from afferent renal lymphatics.64 These studies advocate roles for kidney lymphatics in the maintenance of peripheral tolerance and clearance of cellular debris in adult renal physiology. Renal lymph can also drain renin and angiotensin II,63,65,66 but the physiologic relevance of this route to the systematic circulation is unclear.
Roles of Lymphatics in Kidney Diseases
Lymphangiogenesis and Its Role in Chronic Renal Injury and Fibrosis
Structural changes to the vasculature are a prominent feature of CKD. Whereas peritubular capillaries undergo rarefaction, potentially triggering interstitial hypoxia and generating a profibrogenic environment within diseased kidney,67 renal lymphatics proliferate and sprout, giving rise to new vessels in a process termed lymphangiogenesis.68 In biopsies of IgA nephropathy, focal glomerulosclerosis, lupus, ANCA-related GN, diabetic kidney disease (DKD) and chronic interstitial nephritis acquired from patients with disease ranging from moderate CKD to ESKD, the cross-sectional area of lymphatics is significantly greater than in nondiseased kidneys;36,69 a finding replicated in multiple murine models of CKD.64,70–74
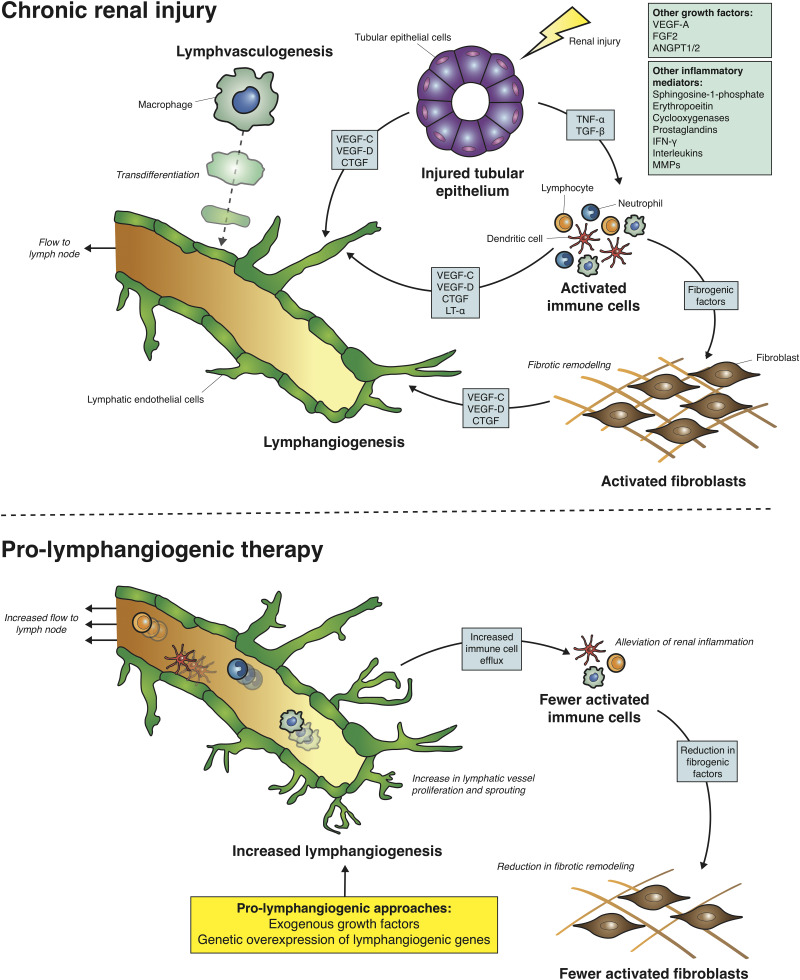
Lymphangiogenesis facilitates the clearance of inflammatory cells from the damaged tissue environment, which is a vital step in the resolution of inflammation and prevention of fibrotic remodeling. This function is illustrated by ligating lymphatics exiting the kidney in rats, leading to loss of renal function with tubulointerstitial fibrosis and mesangial expansion.75,76 However, the accumulation of DCs, T and B lymphocytes, and fibroblasts along with accompanying tubulointerstitial fibrosis in rodent or human CKD still occurs despite a lymphangiogenic response.36,64,77 An explanation for this may come from studies from other organs which indicate that lymphangiogenesis, when occurring as a response to chronic inflammation, results in leaky vessels with a reduced capacity for clearance.78 Thus, therapies to target lymphangiogenic pathways hold potential for restoring clearance function, modulating the inflammatory environment, and preventing fibrotic remodeling in CKD (Figure 2). Whether enhancing lymphangiogenesis may also exert beneficial effects through clearance of interstitial edema or inflammatory macromolecules within the kidney is not known.
Figure 2.
Lymphatic expansion in chronic renal injury and its targeting using prolymphangiogenic therapies. In mouse models of chronic renal injury and in human CKD, lymphangiogenesis (the expansion of lymphatics via proliferation and sprouting of existing lymphatic endothelium) occurs and is considered the predominant mechanism of lymphatic expansion in disease. A number of cell types in the inflammatory milieu including injured tubular epithelium, activated T and B lymphocytes, neutrophils, DCs and activated fibroblasts secrete growth factors (VEGF-C, VEGF-D, CTGF) and inflammatory mediators (lymphotoxin-α [LT-α], TNF-α, TGF-β) which act directly or indirectly on lymphatic endothelium to support lymphangiogenesis. The boxes indicates other possible factors which have been implicated in lymphangiogenesis in other organs but have not been explored in the context of renal injury. Some studies suggest that lymphvasculogenesis; the transdifferentiation of other cell types such as macrophages, into LECs and their integration into lymphatic vessels, as an alternative mechanism of lymphatic expansion in chronic renal injury. The premise of prolymphangiogenic therapies, such as growth factors or genetic approaches in mice, is to augment the expansion of lymphatics to increase the clearance of the activated immune cells. A number of studies show that this alleviates renal inflammatory and reduces fibrotic remodeling in the kidney. ANGPT1/2, angiopoietin 1/2; FGF2, fibroblast growth factor 2; MMP, matrix metalloproteinase.
Cellular and Molecular Mechanisms of Lymphangiogenesis in the Diseased Kidney
Studies of lymphangiogenesis in development79 or pathology80 have identified a plethora of growth factors that promote or inhibit lymphatic vessel growth. Of those studied in the kidney, VEGF-C and VEGF-D are central for lymphangiogenesis in renal disease. These growth factors predominantly trigger lymphangiogenesis by activation of VEGFR-3, but VEGF-C can also act via VEGFR-2 to stimulate LEC and blood vessel proliferation and migration.81 VEGF-C is highly expressed by macrophages in the rat remnant kidney,71 murine unilateral ureteral obstruction (UUO),82 human biopsies of IgA nephropathy, DKD36 and chronic allograft rejection.83 These macrophages, which are likely derived from bone marrow and infiltrate the diseased kidney from the vasculature,84 may be modulated by TGF-β1 and TNF-α release70,85 from damaged renal cells86,87 or from activation of hypoxia-inducible factor 188 due to local renal hypoxia.67,89 Proximal tubular and collecting duct epithelium are also potential sources of VEGF-C upon renal injury,77,85 although the relative contributions of individual cell types to lymphangiogenesis are unknown. Expression of VEGF-D is increased in kidney lysates from a mouse model of UUO70 and immunostaining demonstrates injured tubular epithelium as a potential cellular source in cisplatin-induced nephrotoxicity and ischemia-reperfusion injury (IRI) in mice.74 Induction of VEGF-D in the tubular epithelium of otherwise healthy adult mice resulted in a four-fold expansion of the mean cross-sectional area of kidney lymphatics, demonstrating the potent effect of VEGF-D on renal lymphangiogenesis.90
Connective tissue growth factor (CTGF), an ECM-associated heparin-binding protein, has been identified as a contributor to renal lymphangiogenesis. CTGF is highly expressed by damaged tubular epithelium and interstitial cells (likely macrophages91 or fibroblasts92) in human kidneys with urinary obstruction or DKD.93 Following total knockout of CTGF in adult mice, UUO resulted in reduced lymphangiogenesis and VEGF-C mRNA levels compared with wild-type obstructed kidneys. In culture, CTGF induces VEGF-C production in immortalized mouse and human proximal tubular epithelial cell lines and binds directly to VEGF-C in a dose-dependent manner.93 Whether and how CTGF exhibits activity directly upon LECs has not yet been examined. Inflammatory mediators, secreted by a variety of cell types upon tissue injury, also have roles in lymphangiogenesis. Upon stimulation of LECs by the inflammatory milieu, PROX1 is activated and VEGFR-3 is upregulated downstream of NF-κΒ, increasing the responsiveness of lymphatics to VEGF-C and VEGF-D.94 One inflammatory mediator which stimulates renal lymphangiogenesis is lymphotoxin-α, with overexpression in mice proximal tubules leading to expansion of cortical lymphatics accompanied by T and B lymphocyte–rich infiltrates.95 However, it was not determined whether lymphotoxin-α stimulates LECs directly or acts indirectly through other renal cell types.
Lymphvasculogenesis in Renal Injury
In addition to lymphangiogenesis, some evidence suggests that a small proportion of LECs arise from differentiation of tissue-resident or circulating progenitors in a process termed lymphvasculogenesis.9–11 In sex-mismatched renal transplants, in which male recipients received female donor kidneys, immunohistochemical analysis demonstrated that 4.5% of PROX1+ PDPN+ lymphatics contained a single Y chromosome, indicating a host-derived contribution to graft lymphatics.96 From these experiments, it was proposed that bone marrow–derived macrophages can transdifferentiate into lymphatic endothelium in inflammatory contexts.97
In contrast, a study performing parabiosis between green fluorescent protein (GFP) transgenic and wild-type mice or adoptive transfer of GFP-expressing bone marrow in murine UUO64 showed low levels of co-expression of GFP and LYVE-1+ lymphatics occurred, although this was not quantified. It is not clear whether the efficiency of GFP or time point of the experiment contributed to the difference between the above studies. More extensive lineage tracing is required to validate these findings but, until then, a myeloid origin of LECs during renal injury cannot be ruled out.
Targeting Lymphangiogenesis in Chronic Renal Injury
Several strategies have been implemented to augment renal lymphangiogenesis in preclinical studies (Table 2). Daily intraperitoneal administration of a recombinant isoform of VEGF-C protein (VEGF-C156S), which binds preferentially to VEGFR-3 over VEGFR-298, led to an expansion of the periarterial renal lymphatic network but not blood microvasculature in murine UUO. VEGF-C156S also attenuated collagen I/III deposition, reduced proinflammatory macrophage number, and lowered total TGF-β1 in UUO kidneys compared with untreated controls.99 To what extent VEGF-C156S may exert these beneficial effects through VEGFR-3+ blood endothelial cells in the kidney27,30 is unclear.
Table 2.
Preclinical strategies targeting lymphangiogenesis in chronic renal injury
| Strategy | Mechanism of Action and Model | Effect on Renal Lymphatics | Effect of Strategy on Model of CKD |
|---|---|---|---|
| VEGFC-156S | Mutated form of VEGF-C and selective agonist of VEGFR-3 to promote lymphangiogenesis.98 10 μg/d delivered IP over 14 d in murine UUO99 | Increase in cross-sectional area of LYVE-1+ total and perivascular renal lymphatics compared with control | Reduction in fibrotic remodeling (Sirius red stain) |
| Reduction in collagen I deposition (Western blot) | |||
| Reduction in infiltrating macrophages (IHC) | |||
| Reduction in TGF-β1 expression (Western blot) | |||
| Ksp-rtTA;TRE-Vegfd mice | Transgenic mice with doxycycline-dependent overexpression of VEGF-D from tubular epithelium to promote lymphangiogenesis. Tested in l-NAME–dependent hypertensive nephropathy for 2 wk with or without 3 wk high-salt diet100 | Increase in cross-sectional area of LYVE-1+ cortical renal lymphatics and branches per artery compared with control | Reduction in infiltrating macrophage and T lymphocytes with high-salt diet (flow cytometry) |
| Reduction in infiltrating macrophage and DCs without high-salt diet (flow cytometry) | |||
| Pod-rtTA;TRE-Vegfc mice | Transgenic mice with doxycycline-dependent overexpression of VEGF-C from podocytes. Diabetic nephropathy induced using 50 mg/kg STZ per day for 5 d.101 Doxycycline either given before or 4 wk after STZ injection | Effects attributed to glomerular VEGF activity, so renal lymphatics were not investigated | Reduction in urinary albumin-creatinine ratio (ELISA) |
| Reduction in mesangial matrix expansion (histology) | |||
| Reduction in collagen I (Sirius red stain) | |||
| IMC-3C5 | Anti–VEGFR-3 antibody delivered to inhibit lymphangiogenesis in rats with adriamycin nephropathy.102 At 6 wk after adriamycin treatment, 40 mg/kg body wt of IMC-C35 delivered three times per wk IP | Reduction in cross-sectional area of PDPN+ cortical lymphatics in both adriamycin-treated and non-adriamycin-treated kidneys compared with controls | No significant reduction in infiltrating macrophage or T lymphocytes (IHC) |
| No significant reduction in tubulointerstitial fibrosis (histology) | |||
| No significant reduction in collagen I (IHC and qPCR) | |||
| Lyve1-Cre;R26R-DTR mice | Transgenic mice expression DTR in LYVE-1+ cells and their progeny. Tested in murine UUO and IRI with 7 d follow-up after a single IP dose of 1.25 ng/kg body wt DT64 | Significant reduction in cross-sectional area of LYVE-1+ lymphatic vessels assessed at 3 d after DT administration | Reduction in DCs, macrophages, T and B lymphocytes, neutrophils, and NK cells in UUO (flow cytometry) |
| Reduction of inflammatory cytokines in UUO (qPCR) | |||
| Reduction in fibrosis (qPCR, IHC, and Sirius red) | |||
| LYVE-1 or VEGFR-3 soluble fusion proteins | Soluble LYVE-1 or VEGFR-3 proteins hypothesized to inhibit lymphangiogenesis through sequestering lymphangiogenic growth factors. Injected via tail vein before or after UUO or IRI induction in mice64 | Significant reduction in cross-sectional area of LYVE-1+ lymphatic vessels assessed at 7 d after UUO surgery | Reduction in DCs and T lymphocytes in UUO kidneys (flow cytometry) |
| Reduction of inflammatory cytokines in UUO (qPCR) | |||
| Reduction in fibrosis (qPCR, IHC, and Sirius red) |
IP, intraperitoneal; IHC, immunohistochemistry; l-NAME, nitro- l -arginine methyl ester; STZ, streptozocin; qPCR, quantitative PCR; DTR, diphtheria toxin receptor; DT, diphtheria toxin; NK, natural killer.
Another strategy to augment lymphangiogenesis in CKD has been the ectopic expression of pro-lymphangiogenic growth factors in transgenic mice. In adult mice with either salt-sensitive or nitro-l-arginine methyl ester–induced hypertension, tubular VEGF-D overexpression increased cortical lymphatic density while reducing renal macrophage and T lymphocyte or DC accumulation. This led to an decrease in systolic BP in both models,100 but the effect of VEGF-D overexpression on fibrotic remodeling in the hypertensive kidney was not explored. Moreover, a servo-control technique to maintain renal perfusion pressure was not applied, so it is not clear whether the mechanism of injury arises as a direct consequence of nitro-l-arginine methyl ester on the kidney or indirectly from hypertension. Another study used mice overexpressing VEGF-C from podocytes in streptozocin-induced DKD. Podocyte VEGF-C overexpression significantly reduced the hallmarks of early DKD, including albuminuria, mesangial expansion, and decreased glomerular collagen deposition.101 This effect was attributed to restoration of glomerular endothelial barrier function, but the authors did not examine enhanced renal lymphangiogenesis as a potential cause.
Two other rodent studies explored the hypothesis that inhibition of lymphangiogenesis might be beneficial in CKD. Rats, which had adriamycin delivered intravenously to trigger proteinuria, were treated with a monoclonal anti-VEGFR3 antibody (IMC-3C5)102 from 6 weeks after induction of nephropathy. At 12 weeks of follow-up, treatment with IMC-3C5 significantly reduced the mean cortical lymphatic vessel number in both healthy and adriamycin-treated kidneys, without altering leukocyte count, collagen deposition, or interstitial fibrosis in the injured kidneys. Although the authors concluded tubulointerstitial inflammation and fibrosis to be independent of lymphangiogenesis in adriamycin nephropathy, the late onset of treatment may influence the efficacy of IMC-3C5. Nevertheless, when interpreted in light of above studies, these results suggest that augmentation, rather than inhibition, of lymphangiogenesis is beneficial in CKD.
In another study,64 the kidneys of mice with LYVE-1+ cells ablated and then subjected to either UUO or IRI harbored lower numbers of infiltrating DCs, T and B lymphocytes, macrophages, neutrophils, natural killer cells and genes encoding for inflammatory cytokines and those associated with renal fibrosis 7 days after injury. Reduction of the inflammatory milieu was also observed in UUO and IRI mice administered soluble LYVE-1 or VEGFR-3 fusion proteins. The anti-inflammatory and antifibrotic effects of LYVE-1+ cell ablation and fusion protein delivery were attributed to inhibition of lymphangiogenesis, because both strategies reduced the density and decreased proliferation of LYVE-1+ lymphatics in diseased kidneys. To what extent these strategies target non-LECs or cells outside the kidney to exert their beneficial effects is unclear.
Contribution of Lymphatics to Adaptive Immunity in Renal Transplant Rejection
A lymphangiogenic response has been documented in rejected human renal allografts83,96,103–105 and rodent models of transplant nephropathy.106–108 In this context, lymphangiogenesis is associated with lymphocyte-rich infiltrates and correlates with allograft fibrosis and impaired graft function.83 A body of evidence points toward a fundamental role for lymphatics in the generation and maintenance of adaptive immune responses in renal transplants. Across organs, LECs maintain peripheral tolerance by actively modulating immune cell function, including T and B lymphocytes and DCs, by activating or inhibiting their maturation, driving the proliferation or apoptosis of these cells, generating chemokine gradients for their chemotaxis, or controlling their trafficking and efflux through lymph.4 In different contexts, it has emerged that LECs may themselves archive soluble antigen for presentation to DCs.109,110
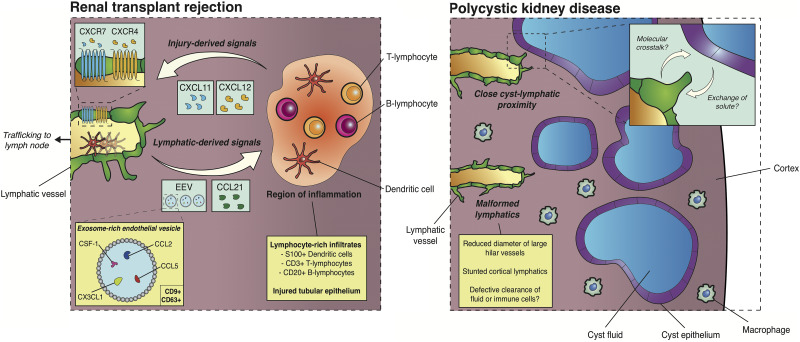
Injured cells within the tubulointerstitium in renal allograft rejection may guide the sprouting of lymphatic endothelium. One potential candidate is C-X-C motif chemokine receptor 7 (CXCR7), which is upregulated on kidney lymphatic vessels in acute allograft rejection in humans.103 Abundance of CXCR7+ lymphatics correlate with the serum creatinine level in these patients. At the same time, the mRNA level of the CXCR7 ligands, C-X-C motif chemokine ligand type 11 (CXCL11) and CXCL12, was significantly higher in the tubulointerstitium of human renal allografts with borderline lesions or undergoing acute rejection. The cellular sources of CXCL12 and CXCL11 were not identified in this study but, in renal ischemic injury, injured tubular epithelium was shown to secrete CXCL12.111 CXCL12 results in dose-dependent increases in migration of murine and human LECs in vitro and promotes tube formation in the latter.112 Thus, chemokines such as CXCL12, generated by inflamed regions, may guide lymphangiogenesis toward sites of injury in rejecting allografts (Figure 3).
Figure 3.
Context-specific lymphatic functions in transplant rejection and PKD. In chronically rejecting renal allografts from humans and rodent models, lymphatic vessels are found in close association with inflamed regions containing lymphocyte-rich infiltrates. Injured tubular epithelium and cells within infiltrates secrete guidance cues for lymphatic endothelium including CXCL11 and CXCL12 with cognate G protein–coupled receptors (CXCR4, CXCR7) expressed on lymphatic endothelium. Conversely, LECs secrete CCL21—a potent agent of chemotaxis for immune cell subtypes—and CD9+ CD63+ EEVs. EEVs contain immunomodulatory proteins (colony stimulating factor 1 [CSF-1]) and factors involves in leukocyte migration (CX3CL1, CCL2, CCL5). In the early stages of murine PKD, lymphatics are found in close association with cyst epithelium, potentially suggesting molecular crosstalk between lymphatic and cyst epithelium or the transport of cyst solute to lymph. Kidney lymphatics themselves are malformed, which may suggest that defects in the kidney lymphatic vasculature result in defective tissue fluid and immune cell clearance and contribute to cyst expansion and decline of renal function in PKD.
Increasing the efflux of immune cells from renal allografts is associated with poor graft function in rat renal transplant.113 One factor responsible for immune cell mobilization is CD9+ CD63+ exosome-rich endothelial vesicles, which are identified surrounding lymphatic vessels in chronic allograft nephropathy. Exposure of cultured LECs to TNF-α, which is released from damaged renal cells,86,87 increases the chemokine and growth factor content of exosome-rich endothelial vesicles (EEVs). These EEVs promote the transmigration of human DCs across LECs. A key chemokine supporting this process, released by LECs stimulated by TNF-α, is chemokine (C-C motif) ligand 21 (CCL21).114,115 CCL21 encourages chemotaxis of DCs toward lymphatic vessels,116 their transmigration across LECs114 and the egress of DCs through lymphatics and toward lymph nodes.117 In human chronic renal allograft rejection, CCL21-secreting LECs are found in close association with nodular infiltrates83 containing multiple leukocyte subtypes expressing the CCL21 receptor, chemokine (C-C motif) receptor 7,118 including T and B lymphocytes and DCs. Increased expression of CCL21 is associated with recurrent nephropathy in patients who have received renal transplants independent of their age, sex, or expression levels of chemokine (C-C motif) receptor 7 within the transplanted kidney.119 Thus, LEC-generated EEVs and chemokine gradients likely modulate immune tolerance in renal transplantation by mobilizing antigen-presenting cells through lymphatics and toward lymph nodes (Figure 3).
Therapies to inhibit lymphangiogenesis may represent a strategy to improve the outcome and survival of renal allografts. To our knowledge, the targeting of VEGFR-3/VEGF-C or other canonical lymphangiogenic pathways have not been tested in animal models of renal transplant, although it has been shown to be beneficial in cardiac graft survival in rat.120 It is emerging that existing clinical therapies that promote renal transplant survival, such as sirolimus (an inhibitor of mammalian target of rapamycin), may exert their effects directly on LECs by inhibiting lymphangiogenesis.121,122 Another therapeutic strategy tested in rat renal allografts is inhibition of Rho-associated protein kinase (ROCK). By treating transplant-recipient rats with lysozymes conjugated to a ROCK inhibitor (Y27632), glomerular and tubulointerstitial macrophage influx was decreased at 1 and 4 days post-transplantation, respectively, accompanied by a significant decrease in lymphatic vessel abundance. However, BP and proteins associated with fibrosis, including vimentin and procollagen-1α1, did not significantly change upon Y27632 treatment.106 Whether inhibition of mammalian target of rapamycin or ROCK directly exert antilymphangiogenic effects, or whether lymphatic abundance decreases as a secondary consequence of immunosuppression, is not clear.
Polycystic Kidney Disease
The hallmark of polycystic kidney disease (PKD), the most common form of which is autosomal dominant PKD and is caused by mutations in PKD1 or PKD2, is the formation and growth of multiple epithelial fluid-filled cysts within the kidney which drive inflammation and fibrosis, resulting in a progressive decline in renal function. Our understanding of the progression of PKD has largely focused on renal epithelial cell metabolism, fluid transport, survival and differentiation or molecular crosstalk.123 However, polycystin 1 and 2—encoded by PKD1 and PKD2, respectively—are also expressed within lymphatics.124 Zebrafish with a loss-of-function mutation in the pkd1a gene (lyc1), a duplicate gene encoding polycystin 1, have lymphatic defects where the main axial lymphatic vessel fails to form during development. In mice, knockout of Pkd1 or Pkd2 results in blood-filled lymph sacs, severe edema in the absence of structural heart defects, hemorrhaging, cutaneous lymphatic vessel defects, and early lethality.124 The lymphatic defects in the skin were replicated in mice with conditional knockout of Pkd1 using an endothelial Sox18-CreERT2 mouse line.125 Small interfering RNA–mediated knockdown of PKD1 or PKD2 in human LECs results in loss of cell number, filopodial abnormalities, disorganized adherens junctional complexes, and impaired capillary network formation and cell migration in wound-healing scratch assays. In this model and also in Pkd1-null mice, the orientation of the Golgi apparatus in individual LECs was found to be randomized. Together, these findings suggest that the polycystins cell-autonomously regulate LEC orientation and migration and are required for normal lymphatic development.
Using whole-mount immunofluorescence, optical clearing, and high-resolution 3D imaging,16,126 we examined the kidney lymphatics in mice homozygous for a p.R3277C allele (Pkd1RC), a slow progressing model of autosomal dominant PKD. We found that lymphatic vessels and corticomedullary cysts in this model sat in close proximity, suggestive of fluid transport41 or molecular crosstalk between LECs and cyst epithelium. We found complex lymphatic defects in homozygous Pkd1RC/RC mice, including a stunting of the lymphatic network relative to the volume of the kidney and a significant decrease in the diameter of large hilar lymphatics. The presence of these defects at E18.5; an early time point of cyst progression in this mouse model of PKD, suggests that these defects could arise directly due to loss of Pkd1 in LECs, rather than secondarily from structural changes to the kidney due to cyst expansion and compression. In either case, defective lymphatic function may contribute to cyst expansion through impaired clearance of cells or tissue fluid (Figure 3).
In a preclinical study, we targeted the lymphatics in PKD by delivering recombinant VEGF-C intraperitoneally in two rapidly progressing mouse models of PKD. In Pkd1nl/nl mice, which have hypomorphic Pkd1 alleles and renal vascular malformation,127 VEGF-C administration led to an expansion of renal lymphatics.30 Additionally, VEGF-C restored the defective architecture of VEGFR-3+ peritubular capillaries observed in Pkd1nl/nl mice. These changes were accompanied by reduced inflammation, decrease in cyst size and normalization of the kidney:body weight ratio. Our findings suggest targeting VEGFR-3+ endothelium, including lymphatics and lymphatic-like vessels, could reduce disease severity and progressive decline in renal function observed in cystic renal disease.
Future Directions
There is a rapidly increasing body of evidence supporting a role for lymphatic vessels across multiple forms of renal disease; however, several questions remain. Structural changes to renal lymphatics are not evident in murine or human AKI.36,74 However, the possibility that renal lymphatics are altered at the molecular or functional level in acute nephropathy, and whether these can be exploited to prevent the transition from AKI to CKD, warrants further study. The mechanisms of action of pro- or anti-lymphangiogenic factors to prevent fibrotic remodeling in preclinical models of CKD, whether through clearing inflammatory cells, modulating factors secreted by LECs, effects on the blood vasculature through VEGFR-2 activation, or consequences on other cells in the fibrotic environment are yet to be elucidated. Strategies to target lymphatics or modulate lymph egress128,129 could be delivered in synergy with pre-existing or emerging approaches, such as antifibrotic drugs in CKD, immunosuppressive agents in renal transplant or epithelial-centric medication in PKD.
New advances are rapidly transforming our understanding of lymphatics in health and disease. Among these, the concept of lymphatic heterogeneity—that every organ possesses a unique lymphatic vascular bed with organ-specific functions has not been approached in the kidney. We used 3D imaging to identify a population of highly dynamic LEC clusters present during mammalian renal development.16 In other organs, these clusters represent tissue-specific progenitors, which may impart molecular and functional heterogeneity in the adult lymphatic vasculature.9–11 Another emerging area, demonstrated by recent studies in animal models and humans, is that lymphatics in skin and muscle may be key players in the regulation of tissue fluid and sodium homeostasis,130,131 revealing an unprecedented relationship between extrarenal lymphatics and kidney disease or salt-sensitive hypertension. Ultimately, for the renal lymphatic field to move forward, novel technologies to visualize,132,133 ablate,134–136 and genetically manipulate137 lymphatic endothelium need to be translated to renal research to advance our understanding of this understudied system in renal development, physiology, and disease.
Disclosures
Dr. Long reports a patent JP6261617B2 (agents that induce lymphangiogenesis used in the treatment of cystic kidney disease) issued January 2018. Mr. Jafree has nothing to disclose.
Funding
Mr. Jafree and Dr. Long are jointly funded by UCL Great Ormond Street Institute of Child Health and the National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre Child Health Research PhD Studentship and Doctoral Training Support Fund, the UCL MB/PhD program, Kidney Research UK Project (Paed_RP_10_2018) and Innovation (IN_012_2019) grants, and Rosetrees Trust small project grant PGS19-2/10174. Dr. Long’s laboratory is further funded by Medical Research Council grant MR/P018629/1, Diabetes UK grants 13/0004763 and 15/0005283, and Kidney Research UK grant RP36/2015.
Acknowledgments
The authors would like to acknowledge Professors Peter Scambler and Christiana Ruhrberg (University College London [UCL]), Paul Riley (University of Oxford), Adrian Woolf (University of Manchester), and Norman Rosenblum (The Hospital for Sick Children) for useful discussions about this work.
Mr. Jafree wrote the original draft of the manuscript and both authors reviewed the manuscript after which revisions were made and approved by both authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Huang L-H, Elvington A, Randolph GJ: The role of the lymphatic system in cholesterol transport. Front Pharmacol 6: 182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J: Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127: 3210–3219, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titze J, Luft FC: Speculations on salt and the genesis of arterial hypertension. Kidney Int 91: 1324–1335, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Card CM, Yu SS, Swartz MA: Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest 124: 943–952, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betterman KL, Harvey NL: The lymphatic vasculature: Development and role in shaping immunity. Immunol Rev 271: 276–292, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Seeger H, Bonani M, Segerer S: The role of lymphatics in renal inflammation. Nephrol Dial Transplant 27: 2634–2641, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Yazdani S, Navis G, Hillebrands J-L, van Goor H, van den Born J: Lymphangiogenesis in renal diseases: Passive bystander or active participant? Expert Rev Mol Med 16: e15, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Russell PS, Hong J, Windsor JA, Itkin M, Phillips ARJ: Renal lymphatics: Anatomy, physiology, and clinical implications. Front Physiol 10: 251, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulvmar MH, Mäkinen T: Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res 111: 310–321, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrova TV, Koh GY: Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med 215: 35–49, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong BW, Zecchin A, García-Caballero M, Carmeliet P: Emerging concepts in organ-specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell 45: 289–301, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Lee H-W, Qin YX, Kim YM, Park EY, Hwang JS, Huo GH, et al.: Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res 343: 429–444, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Tanabe M, Shimizu A, Masuda Y, Kataoka M, Ishikawa A, Wakamatsu K, et al.: Development of lymphatic vasculature and morphological characterization in rat kidney. Clin Exp Nephrol 16: 833–842, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Munro DAD, Hohenstein P, Coate TM, Davies JA: Refuting the hypothesis that semaphorin-3f/neuropilin-2 exclude blood vessels from the cap mesenchyme in the developing kidney. Dev Dyn 246: 1047–1056, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH: Fetal anatomy of peripheral lymphatic vessels: A D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm). J Anat 216: 671–682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafree DJ, Moulding D, Kolatsi-Joannou M, Perretta Tejedor N, Price KL, Milmoe NJ, et al.: Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. eLife 8: e48183, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon AP: Development of the mammalian kidney. Curr Top Dev Biol 117: 31–64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunskill EW, Park JS, Chung E, Chen F, Magella B, Potter SS: Single cell dissection of early kidney development: Multilineage priming. Development 141: 3093–3101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munro DAD, Hohenstein P, Davies JA: Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci Rep 7: 3273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel E, Azizoglu DB, Ryan AR, Walji TA, Chaney CP, Sutton GI, et al.: Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21: 617–634, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, et al.: Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell 29: 188–202, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Combes AN, Phipson B, Lawlor KT, Dorison A, Patrick R, Zappia L,et al. : Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk [published correction appears in Development 146: dev182162, 2019]. Development 146: dev178673, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Sequeira-Lopez MLS, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA: The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol 308: R138–R149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caubit X, Lye CM, Martin E, Coré N, Long DA, Vola C, et al.: Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135: 3301–3310, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, et al.: The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28: 2175–2187, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y-M, Kim WY, Nam SA, Choi AR, Kim H, Kim YK, et al.: Role of Prox1 in the transforming ascending thin limb of henle’s loop during mouse kidney development. PLoS One 10: e0127429, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenig-Kozlovsky Y, Scott RP, Onay T, Carota IA, Thomson BR, Gil HJ, et al.: Ascending vasa recta are angiopoietin/Tie2-dependent lymphatic-like vessels. J Am Soc Nephrol 29: 1097–1107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson LA, Banerji S, Lawrance W, Gileadi U, Prota G, Holder KA, et al.: Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat Immunol 18: 762–770, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Ulvmar MH, Stanczuk L, Martinez-Corral I, Frye M, Alitalo K, et al.: Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nat Commun 9: 1296, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang JL, Woolf AS, Kolatsi-Joannou M, Baluk P, Sandford RN, Peters DJ, et al.: Vascular endothelial growth factor C for polycystic kidney diseases. J Am Soc Nephrol 27: 69–77, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi R, Russo E, Bachmann SB, Proulx ST, Sesartic M, Smaadahl N, et al.: Postnatal deletion of podoplanin in lymphatic endothelium results in blood filling of the lymphatic system and impairs dendritic cell migration to lymph nodes. Arterioscler Thromb Vasc Biol 37: 108–117, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, et al.: Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 151: 1141–1152, 1997 [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, et al.: Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 188: 115–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, et al.: Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa Y, Akasaka Y, Kiguchi H, Akishima-Fukasawa Y, Hasegawa T, Ito K, et al.: The human renal lymphatics under normal and pathological conditions. Histopathology 49: 265–273, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, et al.: Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 75: 828–838, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al.: Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204: 2349–2362, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leak LV, Burke JF: Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol 36: 129–149, 1968 [PMC free article] [PubMed] [Google Scholar]
- 39.Gerli R, Solito R, Weber E, Aglianó M: Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology 33: 148–157, 2000 [PubMed] [Google Scholar]
- 40.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG: Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14: 159–172, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Triacca V, Güç E, Kilarski WW, Pisano M, Swartz MA: Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ Res 120: 1440–1452, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Kunert C, Baish JW, Liao S, Padera TP, Munn LL: Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci U S A 112: 10938–10943, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M: Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A 99: 16069–16074, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lake BB, Chen S, Hoshi M, Plongthongkum N, Salamon D, Knoten A, et al.: A single-nucleus RNA-sequencing pipeline to decipher the molecular anatomy and pathophysiology of human kidneys. Nat Commun 10: 2832, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV,et al.: Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J Am Soc Nephrol 31: 118–138, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, et al.: The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev Cell 23: 1203–1218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntosh GH, Morris B: The lymphatics of the kidney and the formation of renal lymph. J Physiol 214: 365–376, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tenstad O, Heyeraas KJ, Wiig H, Aukland K: Drainage of plasma proteins from the renal medullary interstitium in rats. J Physiol 536: 533–539, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell RD: Renal lymph flow and composition during acetazolamide and furosemide diuresis. Lymphology 17: 10–14, 1984 [PubMed] [Google Scholar]
- 50.Haddy FJ, Scott J, Fleishman M, Emanuel D: Effect of change in renal venous pressure upon renal vascular resistance, urine and lymph flow rates. Am J Physiol 195: 97–110, 1958 [DOI] [PubMed] [Google Scholar]
- 51.Lebrie SJ, Mayerson HS: Influence of elevated venous pressure on flow and composition of renal lymph. Am J Physiol 198: 1037–1040, 1960 [DOI] [PubMed] [Google Scholar]
- 52.Bell RD: Changes in postglomerular hemodynamics alters the composition of canine renal lymph. Microcirc Endothelium Lymphatics 2: 477–485, 1985 [PubMed] [Google Scholar]
- 53.Vogel G, Gärtner K, Ulbrich M: The flow rate and macromolecule content of hilar lymph from the rabbit’s kidney under conditions of renal venous pressure elevation and restriction of renal function - studies on the origin of renal lymph. Lymphology 7: 136–143, 1974 [PubMed] [Google Scholar]
- 54.LeBrie SJ, Mayerson HS: Influence of elevated venous pressure on flow and composition of renal lymph. Am J Physiol 198: 1037–1040, 1960 [DOI] [PubMed] [Google Scholar]
- 55.LeBrie SJ: Renal lymph and osmotic diuresis. Am J Physiol 215: 116–123, 1968 [DOI] [PubMed] [Google Scholar]
- 56.O’Morchoe CC, O’Morchoe PJ, Heney NM: Renal hilar lymph. Effects of diuresis on flow and composition in dogs. Circ Res 26: 469–479, 1970 [DOI] [PubMed] [Google Scholar]
- 57.LeBrie SJ, Gotshall RW: Vasopressin-induced increase in renal lymph flow and natriuresis in dogs. Clin Sci Mol Med 46: 603–612, 1974 [DOI] [PubMed] [Google Scholar]
- 58.Papp M: Effects of angiotensin and noradrenaline on flow and composition of the renal lymph. Z Gesamte Exp Med 142: 216–221, 1967 [DOI] [PubMed] [Google Scholar]
- 59.Stowe NT, Hook JB: Effect of furosemide on renal hilar lymph flow. Arch Int Pharmacodyn Ther 224: 299–309, 1976 [PubMed] [Google Scholar]
- 60.Bell RD, Wainer BS: Effects of bradykinin on renal lymph flow and composition. Lymphology 16: 38–42, 1983 [PubMed] [Google Scholar]
- 61.Clement CC, Aphkhazava D, Nieves E, Callaway M, Olszewski W, Rotzschke O, et al.: Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J Proteomics 78: 172–187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen KC, D’Alessandro A, Clement CC, Santambrogio L: Lymph formation, composition and circulation: A proteomics perspective. Int Immunol 27: 219–227, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Bivol LM, Iversen BM, Hultström M, Wallace PW, Reed RK, Wiig H, et al.: Unilateral renal ischaemia in rats induces a rapid secretion of inflammatory markers to renal lymph and increased capillary permeability. J Physiol 594: 1709–1726, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pei G, Yao Y, Yang Q, Wang M, Wang Y, Wu J,et al. : Lymphangiogenesis in kidney and lymph node mediates renal inflammation and fibrosis. Sci Adv 5: eaaw5075, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bailie MD, Rector FC Jr., Seldin DW: Angiotensin II in arterial and renal venous plasma and renal lymph in the dog. J Clin Invest 50: 119–126, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Morchoe CC, O’Morchoe PJ, Albertine KH, Jarosz HM: Concentration of renin in the renal interstitium, as reflected in lymph. Ren Physiol 4: 199–206, 1981 [DOI] [PubMed] [Google Scholar]
- 67.Long DA, Norman JT, Fine LG: Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8: 244–250, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Tammela T, Alitalo K: Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140: 460–476, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, et al.: The contribution of B cells to renal interstitial inflammation. Am J Pathol 170: 457–468, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, et al.: Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 83: 50–62, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, et al.: Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: Aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels [published correction appears in J Am Soc Nephrol 14: following table of contents, 2003]. J Am Soc Nephrol 14: 1981–1989, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Uchiyama T, Takata S, Ishikawa H, Sawa Y: Altered dynamics in the renal lymphatic circulation of type 1 and type 2 diabetic mice. Acta Histochem Cytochem 46: 97–104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kneedler SC, Phillips LE, Hudson KR, Beckman KM, Lopez Gelston CA, Rutkowski JM, et al.: Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am J Physiol Renal Physiol 312: F861–F869, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarjou A, Black LM, Bolisetty S, Traylor AM, Bowhay SA, Zhang MZ, et al.: Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab Invest 99: 1376–1388, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang T, Guan G, Liu G, Sun J, Chen B, Li X, et al.: Disturbance of lymph circulation develops renal fibrosis in rats with or without contralateral nephrectomy. Nephrology (Carlton) 13: 128–138, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Cheng J, Wang J, Liu YT, Zhang T, Sun A, Wang W, et al.: Renal lymphatic ligation aggravates renal dysfunction through induction of tubular epithelial cell apoptosis in mononephrectomized rats. Clin Nephrol 79: 124–131, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Yazdani S, Poosti F, Kramer AB, Mirković K, Kwakernaak AJ, Hovingh M, et al.: Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis. PLoS One 7: e50209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwager S, Detmar M: Inflammation and lymphatic function. Front Immunol 10: 308, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng W, Aspelund A, Alitalo K: Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124: 878–887, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H, Kataru RP, Koh GY: Inflammation-associated lymphangiogenesis: A double-edged sword? J Clin Invest 124: 936–942, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmökel HG, et al.: Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 21: 1003–1012, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Guo Y-C, Zhang M, Wang FX, Pei GC, Sun F, Zhang Y, et al.: Macrophages regulate unilateral ureteral obstruction-induced renal lymphangiogenesis through C-C motif chemokine receptor 2-dependent phosphatidylinositol 3-kinase-AKT-mechanistic target of rapamycin signaling and hypoxia-inducible factor-1α/vascular endothelial growth factor-C expression. Am J Pathol 187: 1736–1749, 2017 [DOI] [PubMed] [Google Scholar]
- 83.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al.: Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al.: Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115: 2363–2372, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, et al.: Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 81: 865–879, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Sureshbabu A, Muhsin SA, Choi ME: TGF-β signaling in the kidney: Profibrotic and protective effects. Am J Physiol Renal Physiol 310: F596–F606, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ernandez T, Mayadas TN: Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int 76: 262–276, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Ahn G-O, Seita J, Hong BJ, Kim YE, Bok S, Lee CJ, et al.: Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci U S A 111: 2698–2703, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haase VH: Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol 24: 537–541, 2013 [DOI] [PubMed] [Google Scholar]
- 90.Lammoglia GM, Van Zandt CE, Galvan DX, Orozco JL, Dellinger MT, Rutkowski JM: Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am J Physiol Heart Circ Physiol 311: H384–H394, 2016 [DOI] [PubMed] [Google Scholar]
- 91.Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, et al.: Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol 25: 1008–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, et al.: Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J 17: 1220–1227, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Kinashi H, Falke LL, Nguyen TQ, Bovenschen N, Aten J, Leask A, et al.: Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int 92: 850–863, 2017 [DOI] [PubMed] [Google Scholar]
- 94.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, et al.: Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood 115: 418–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mounzer RH, Svendsen OS, Baluk P, Bergman CM, Padera TP, Wiig H, et al.: Lymphotoxin-alpha contributes to lymphangiogenesis. Blood 116: 2173–2182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, et al.: Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 12: 230–234, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Kerjaschki D: The lymphatic vasculature revisited. J Clin Invest 124: 874–877, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, et al.: A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem 273: 6599–6602, 1998 [DOI] [PubMed] [Google Scholar]
- 99.Hasegawa S, Nakano T, Torisu K, Tsuchimoto A, Eriguchi M, Haruyama N, et al.: Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab Invest 97: 1439–1452, 2017 [DOI] [PubMed] [Google Scholar]
- 100.Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER, et al.: Enhancing renal lymphatic expansion prevents hypertension in mice. Circ Res 122: 1094–1101, 2018 [DOI] [PubMed] [Google Scholar]
- 101.Onions KL, Gamez M, Buckner NR, Baker SL, Betteridge KB, Desideri S, et al.: VEGFC reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes 68: 172–187, 2019 [DOI] [PubMed] [Google Scholar]
- 102.Yazdani S, Hijmans RS, Poosti F, Dam W, Navis G, van Goor H, et al.: Targeting tubulointerstitial remodeling in proteinuric nephropathy in rats. Dis Model Mech 8: 919–930, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neusser MA, Kraus AK, Regele H, Cohen CD, Fehr T, Kerjaschki D, et al.: The chemokine receptor CXCR7 is expressed on lymphatic endothelial cells during renal allograft rejection. Kidney Int 77: 801–808, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Stuht S, Gwinner W, Franz I, Schwarz A, Jonigk D, Kreipe H, et al.: Lymphatic neoangiogenesis in human renal allografts: Results from sequential protocol biopsies. Am J Transplant 7: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Adair A, Mitchell DR, Kipari T, Qi F, Bellamy CO, Robertson F, et al.: Peritubular capillary rarefaction and lymphangiogenesis in chronic allograft failure. Transplantation 83: 1542–1550, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Poosti F, Yazdani S, Dolman ME, Kok RJ, Chen C, Ding G, et al.: Targeted inhibition of renal Rho kinase reduces macrophage infiltration and lymphangiogenesis in acute renal allograft rejection. Eur J Pharmacol 694: 111–119, 2012 [DOI] [PubMed] [Google Scholar]
- 107.Vass DG, Shrestha B, Haylor J, Hughes J, Marson L: Inflammatory lymphangiogenesis in a rat transplant model of interstitial fibrosis and tubular atrophy. Transpl Int 25: 792–800, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Rienstra H, Katta K, Celie JW, van Goor H, Navis G, van den Born J, et al.: Differential expression of proteoglycans in tissue remodeling and lymphangiogenesis after experimental renal transplantation in rats. PLoS One 5: e9095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamburini BA, Burchill MA, Kedl RM: Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun 5: 3989, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kedl RM, Lindsay RS, Finlon JM, Lucas ED, Friedman RS, Tamburini BAJ: Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Commun 8: 2034, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C: Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 112.Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X, et al.: The CXCL12-CXCR4 chemokine pathway: A novel axis regulates lymphangiogenesis. Clin Cancer Res 18: 5387–5398, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Talsma DT, Katta K, Boersema M, Adepu S, Naggi A, Torri G, et al.: Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin. PLoS One 12: e0180206, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vaahtomeri K, Brown M, Hauschild R, De Vries I, Leithner AF, Mehling M, et al.: Locally triggered release of the chemokine CCL21 promotes dendritic cell transmigration across lymphatic endothelia. Cell Rep 19: 902–909, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson LA, Jackson DG: Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol 22: 839–849, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, et al.: DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 208: 2141–2153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschké M, et al.: Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep 14: 1723–1734, 2016 [DOI] [PubMed] [Google Scholar]
- 118.Förster R, Davalos-Misslitz AC, Rot A: CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol 8: 362–371, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Zhou HL, Wang YT, Gao T, Wang WG, Wang YS: Distribution and expression of fibroblast-specific protein chemokine CCL21 and chemokine receptor CCR7 in renal allografts. Transplant Proc 45: 538–545, 2013 [DOI] [PubMed] [Google Scholar]
- 120.Nykänen AI, Sandelin H, Krebs R, Keränen MA, Tuuminen R, Kärpänen T, et al.: Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation 121: 1413–1422, 2010 [DOI] [PubMed] [Google Scholar]
- 121.Huber S, Bruns CJ, Schmid G, Hermann PC, Conrad C, Niess H, et al.: Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int 71: 771–777, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Palin NK, Savikko J, Koskinen PK: Sirolimus inhibits lymphangiogenesis in rat renal allografts, a novel mechanism to prevent chronic kidney allograft injury. Transpl Int 26: 195–205, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE: Polycystic kidney disease. Nat Rev Dis Primers 4: 50, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Outeda P, Huso DL, Fisher SA, Halushka MK, Kim H, Qian F, et al.: Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep 7: 634–644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coxam B, Sabine A, Bower NI, Smith KA, Pichol-Thievend C, Skoczylas R, et al.: Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep 7: 623–633, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jafree DJ, Long DA, Scambler PJ, Moulding D: Tissue clearing and deep imaging of the kidney using confocal and two-photon microscopy. Methods Mol Biol 2067: 103–126, 2020 [DOI] [PubMed] [Google Scholar]
- 127.Ogunlade O, Connell JJ, Huang JL, Zhang E, Lythgoe MF, Long DA, et al.: In vivo three-dimensional photoacoustic imaging of the renal vasculature in preclinical rodent models. Am J Physiol Renal Physiol 314: F1145–F1153, 2018 [DOI] [PubMed] [Google Scholar]
- 128.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al.: VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21: 181–195, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, et al.: Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res 95: 204–209, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, et al.: High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler Thromb Vasc Biol 38: 2054–2064, 2018 [DOI] [PubMed] [Google Scholar]
- 131.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, et al.: Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bianchi R, Teijeira A, Proulx ST, Christiansen AJ, Seidel CD, Rülicke T, et al.: A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research. PLoS One 10: e0122976, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hägerling R, Pollmann C, Kremer L, Andresen V, Kiefer F: Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochem Soc Trans 39: 1674–1681, 2011 [DOI] [PubMed] [Google Scholar]
- 134.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, et al.: CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21: 1380–1391, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al.: Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560: 185–191, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tammela T, Saaristo A, Holopainen T, Ylä-Herttuala S, Andersson LC, Virolainen S, et al.: Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med 3: 69ra11, 2011 [DOI] [PubMed] [Google Scholar]
- 137.Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, et al.: Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1: e85096, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]