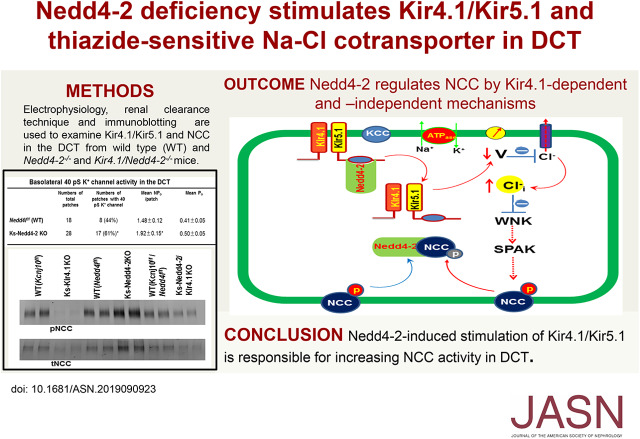
Significance Statement
The potassium channel Kir4.1 forms the Kir4.1/Kir5.1 heterotetramer in the basolateral membrane of the distal convoluted tubule (DCT) and plays an important role in regulating the thiazide-sensitive NaCl cotransporter (NCC). Deletion of the ubiquitin ligase Nedd4-2 has been shown to increase the expression of NCC and to cause salt-sensitive hypertension. The authors demonstrated that kidney-specific deletion of Nedd4-2 in mice also stimulates Kir4.1/Kir5.1 activity in the DCT and hyperpolarizes the DCT membrane. They also found that NCC activity/expression is largely inhibited in double-knockout mice deficient in both Kir4.1 and Nedd4-2 and that NCC activity/expression is higher in these double-knockout mice compared with mice lacking only Kir4.1. These findings suggest that Nedd4-2 regulates NCC expression through modulation of basolateral Kir4.1/Kir5.1 activity and through Kir4.1-independent regulation of NCC retrieval.
Keywords: potassium channels, renal tubular epithelial cells, Cell Signaling, Cell and Transport Physiology, hypokalemia
Visual Abstract
Abstract
Background
The potassium channel Kir4.1 forms the Kir4.1/Kir5.1 heterotetramer in the basolateral membrane of the distal convoluted tubule (DCT) and plays an important role in the regulation of the thiazide-sensitive NaCl cotransporter (NCC). Kidney-specific deletion of the ubiquitin ligase Nedd4-2 increases expression of NCC, and coexpression of Nedd4-2 inhibits Kir4.1/Kir5.1 in vitro. Whether Nedd4-2 regulates NCC expression in part by regulating Kir4.1/Kir5.1 channel activity in the DCT is unknown.
Methods
We used electrophysiology studies, immunoblotting, immunostaining, and renal clearance to examine Kir4.1/Kir5.1 activity in the DCT and NCC expression/activity in wild-type mice and mice with kidney-specific knockout of Nedd4-2, Kir4.1, or both.
Results
Deletion of Nedd4-2 increased the activity/expression of Kir4.1 in the DCT and also, hyperpolarized the DCT membrane. Expression of phosphorylated NCC/total NCC and thiazide-induced natriuresis were significantly increased in the Nedd4-2 knockout mice, but these mice were normokalemic. Double-knockout mice lacking both Kir4.1/Kir5.1 and Nedd4-2 in the kidney exhibited increased expression of the epithelial sodium channel α-subunit, largely abolished basolateral potassium ion conductance (to a degree similar to that of kidney-specific Kir4.1 knockout mice), and depolarization of the DCT membrane. Compared with wild-type mice, the double-knockout mice displayed inhibited expression of phosphorylated NCC and total NCC and had significantly blunted thiazide-induced natriuresis as well as renal potassium wasting and hypokalemia. However, NCC expression/activity was higher in the double-knockout mice than in Kir4.1 knockout mice.
Conclusions
Nedd4-2 regulates Kir4.1/Kir5.1 expression/activity in the DCT and modulates NCC expression by Kir4.1-dependent and Kir4.1-independent mechanisms. Basolateral Kir4.1/Kir5.1 activity in the DCT partially accounts for the stimulation of NCC activity/expression induced by deletion of Nedd4-2.
It is well established that inwardly rectifying K+ channel 4.1 (Kir4.1) interacts with Kir5.1 to form the Kir4.1/Kir5.1 heterotetramer in the basolateral membrane of the distal convoluted tubule (DCT).1–4 A large of body of evidence indicates that the basolateral Kir4.1/Kir5.1 in the DCT plays an important role in the regulation of NaCl cotransporter (NCC) expression.2,5–8 The mechanism by which the basolateral Kir4.1/Kir5.1 channel regulates NCC expression depends on Cl−-sensitive with-no-lysine kinase (WNK) activity, which stimulates ste20 proline-alanine–rich kinase (SPAK).9–17 Because Kir4.1/Kir5.1 is the only type of K+ channel expressed in the basolateral membrane of DCT,2,6,7,18 the changes in the basolateral K+ channel activity are expected to alter the cell membrane potential, thereby affecting the Cl− movement across the basolateral membrane.5 We have previously demonstrated that a hyperpolarization increased, whereas a depolarization decreased NCC expression and activity.2,6,7,19 Presumably, hyperpolarization should decrease the intracellular Cl− concentration, whereas a depolarization should increase the intracellular Cl− concentrations, thereby either increasing or decreasing WNK activity. Moreover, our in vitro study has shown that Nedd4-2 was able to facilitate the Kir4.1 ubiquitination in the presence of Kir5.1, which serves as a binding/associated protein for Nedd4-2.20 Nedd4-2 has been shown to play a key role in the regulation of epithelial Na+ channel (ENaC).21–31 Moreover, previous study has demonstrated that conditional deletion of Nedd4-2 in the kidney increased the expression of thiazide-sensitive NCC,23 suggesting the role of Nedd4-2 in the degradation of NCC. Because Nedd4-2 is also involved in facilitating the ubiquitination of Kir4.1,20 it is conceivable that the changes in the basolateral Kir4.1/Kir5.1 activity in the DCT may be, at least in part, responsible for the deletion of Nedd4-2–induced increase in NCC expression. Thus, the aim of this study is to test the hypothesis that Nedd4-2 regulates NCC expression that is achieved, at least in part, by regulating the basolateral Kir4.1/Kir5.1 channel activity in the DCT. We have taken advantage of both kidney-specific Nedd4-2 (Ks-Nedd4-2) knockout (KO) mice and Ks-Nedd4-2/Kir4.1 double-KO mice to test the hypothesis.
Methods
All methods used in this study are described in the text and Supplemental Material.
Generating Transgenic Mice
We used Ks-Kir4.1 KO, Ks-Nedd4-2 KO, and Ks-Nedd4-2/Kir4.1 KO mice in this study. Both Ks-Kir4.1 and Ks-Nedd4-2 KO mice have been used and characterized previously.2,23 Briefly, mice expressing Pax8-rtTA and tet-on LC-1, which tightly drove Pax8 and Cre expression under tetracycline-dependent induction, were crossed with Kcnj10-floxed mice to generate inducible Ks-Kir4.1 KO mice or crossed with Nedd4l-floxed mice to generate inducible Ks-Nedd4-2 KO mice. For generating Ks-Nedd4-2/Kir4.1 double-KO mice, male/female pax8(+)-cre(−)-Kcnj10flox/flox-Nedd4lflox/flox were crossed with female/male pax8(+)-Cre(+)-Kcnj10flox/wt-Nedd4lflox/wt mice. This inbreeding strategy should reduce the risk of large chromosome deletion or translocation because Kcnj10, nedd4l, and cre are located in the deferent chromosomes. For starting corresponding gene deletion, we have fed 8- to 10-week-old male/female mice carrying homogenous Nedd4lf/f/Pax8/LC1 (Nedd4-2Pax8/LC1), homogenous Kcnj10f/f/pax8/LC1, or homogenous Kcnj10f/f/Nedd4lf/f/Pax8/LC1 with doxycycline (5 mg/ml, 2% sucrose) in the drinking water for 2 weeks. This was followed by at least 2 additional weeks without doxycycline treatment. However, under some conditions, we have also used the gene KO mice immediately after doxycycline treatment without a 14-day transition period (in this case, it is indicated in the corresponding text). Littermate mice of the same age and genetic background drinking 2% sucrose were used as “wild-type” (WT) mice. The extent and efficacy of Kir4.1 and Nedd4-2 deletion were determined with electrophysiology for Kir4.1 and immunoblotting for Nedd4-2/Kir4.1. The mice are allowed to be maintained in a normal rodent chow with free access to water. We used both male and female mice for experiments, and the animals for the experiments were age and sex matched and have been treated identically. Mice were housed in the New York Medical College animal facility with lights on at 7:00 am and off at 7:00 pm. All of the procedures were reviewed and approved by the institutional animal care and use committee.
Single-Channel Recording
Single-channel patch-clamp experiments were performed in the basolateral membrane of both early DCT and late DCT. Single-K+ channel currents were recorded with an Axon200B amplifier (Axon), low-pass filtered at 1 kHz, and digitized by an Axon interface (Digidata 1332) with sampling rate of 4 kHz. The pipette solution for the single-channel recording contains 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 10 mM HEPES (titrated with KCl to pH 7.4), and the bath solution contains 135 mM NaCl, 5 mM KCl, 2 mM MgCl2, 1.8 mM CaCl2, 5 mM glucose, and 5 mM HEPES (titrated with NaOH to pH 7.4). For the calculation of channel numbers, we selected a channel recording at least 10 minutes long. We determine the channel open probability (Po) from the channel number (N) and NPo (a product of channel number and open probability), which was calculated from data samples of 60-second duration in the steady state. NPo was determined using the following equation:
where ti is the fractional open time spent at each of the observed current levels. The channel conductance was determined by measuring the current amplitudes over several voltages.
Whole-Cell Recording
Whole-cell patch-clamp experiments were performed in the DCT1. An Axon 200A amplifier was used for the measurement of K+ reversal potential and Ba2+-sensitive K+ currents. For measuring K+ reversal potential, the tip of the pipette was filled with pipette solution containing 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 10 mM HEPES (pH 7.4). The pipette was then back filled, with the pipette solution containing amphotericin B (20 μg/0.1 ml). The bath solution is the same as the one that we used to perform the single-channel recordings. For the measurement of whole-cell Ba2+-sensitive K+ current, the bath solution contains 140 mM KCl, 2 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES (pH 7.4). After forming a high resistance seal,32 the membrane capacitance was monitored until the whole-cell patch configuration was formed. The currents were low-pass filtered at 1 kHz and digitized by an Axon interface with 4-kHz sampling rate (Digidata 1440A). Data were analyzed using the pClamp software system 9.0 (Axon).
Immunoblotting
Renal tissue harvested from the top renal cortex was homogenized in a buffer containing 250 mM sucrose, 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, and 1 mM dithiothreitol supplemented with phosphatase and protease inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). For the western blot, the protein sample (40–60 μg; which was treated with <65°C heating) was separated on 4%–12% (wt/vol) Tris-Glycine gel (NovexTM; ThermoFisher Scientific) and transferred to nitrocellulose membrane. The membranes were incubated for 1 hour with LI-COR blocking buffer (PBS) and then, incubated overnight at 4°C with corresponding antibodies. An Odyssey infrared imaging system (LI-COR) was used to capture the images at a wavelength of 680 or 800 nm. The experiments were performed with both male and female mice. However, western blots shown in Results are conducted with the renal tissue of male mice.
Procedures for Renal Clearance
Animal were anesthetized by 2%–4% isoflurane through an inhaling mask. The mice were placed on a small heated blanket to maintain body temperature at 37°C. The trachea was cannulated to clear any mucus that may be produced during the experiment. A carotid artery was catheterized with PE10 tubing for blood collections, and the jugular vein was also cannulated for intravenous infusion. The bladder was exposed and catheterized via a suprapubic incision with a 10-cm piece of PE10 tubing for urine collections. After completion of surgery, isotonic saline was given intravenously for 4 hours (0.25–0.3 ml/h and total 1–1.2 ml 0.9% saline) to replace surgical fluid losses and to maintain hemodynamics. Urine collections were started 1 hour after infusion of 0.3 ml saline, and a total of six collections (every 30 minutes) were performed. Two collections before hydrochlorothiazide (HCTZ) were pooled as the control group, and four collections after HCTZ application (30 mg/kg body wt) were also pooled (total of 120 minutes) as the experimental group. For measuring the basal level of urinary K+ excretion, urine samples were collected every 30 minutes for a total of six collections, and samples were pooled. After renal clearance experiment, the mice were euthanized by intravenous somnasol.
Immunofluorescence
Mice were anesthetized with intraperitoneal injection of ketamine (80 mg/kg), xylazine (10 mg/kg), and acepromazine (2 mg/kg). Kidneys were fixed by retrograde abdominal aortic perfusion of 3% paraformaldehyde in PBS (pH 7.4) and frozen in ornithine carbamyl transferase; 5-mm sections were washed in 1× PBS and permeabilized with 0.5% Triton X-100 for 30 minutes followed by 30 minutes of blockade in 5% BSA/PBS. Primary antibody rabbit antitotal NaCl cotransporter (anti-tNCC) in 0.3% BSA/PBS was incubated overnight at 4°C. Sections were incubated for 1 hour with 1:1000 Alexa 488-conjugated antibody (Invitrogen, Carlsbad, CA).
Material and Statistical Analyses
HCTZ was purchased from Sigma-Aldrich. We have obtained antibodies for phosphorylated NaCl cotransporter (pNCC) at Thr53 (Phospho Solution), NCC (Millipore), ENaCα (Stressmarq), Kir4.1 (Alomone), Nedd4-2 (Cell Signaling), glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling), and β-actin (Abcam). Detailed information of the antibodies is presented in Supplemental Material. The specificity of pNCC, NCC, Kir4.1, and Nedd4-2 antibodies was verified using the tissues from corresponding gene KO mice. Data were analyzed using t test for comparisons between two groups or using one-way ANOVA for more than two groups, and the Holm–Sidak test was used as post hoc analysis. P values <0.05 were considered statistically significant. Data are presented as the mean ± SEM.
Results
Nedd4-2 Regulates Kir4.1/Kir5.1 in the DCT
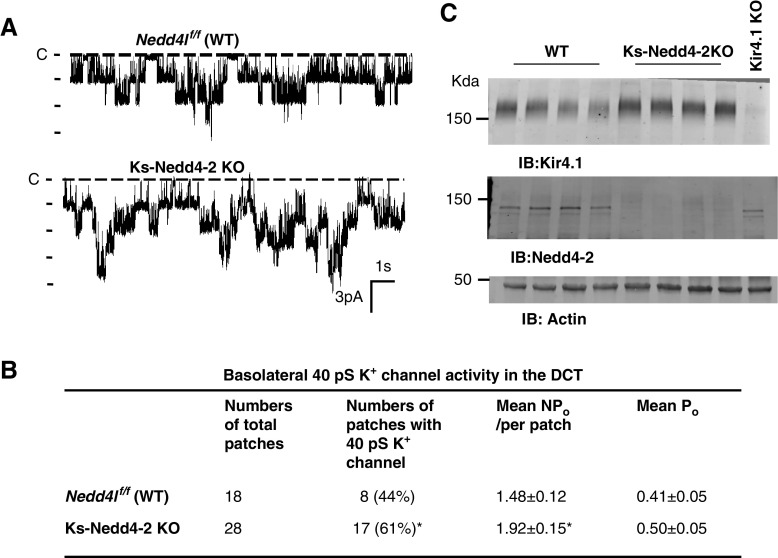
Our previous study has demonstrated that Nedd4-2 was able to inhibit Kir4.1/Kir5.1 in vitro by stimulating the ubiquitination of Kir4.1.20 To test the role of Nedd4-2 in the regulation of the Kir4.1/Kir5.1 in vivo animal model, we used the single-channel recording technique to examine the basolateral K+ channel activity in the isolated DCT of the WT and Ks-Nedd4-2 KO mice. Figure 1A is a recording showing the basolateral 40-pS K+ channel activity (a Kir4.1/Kir5.1 heterotetramer) in the WT and Ks-Nedd4-2 KO mice, respectively. From the inspection of Figure 1A, it is apparent that the basolateral K+ channel activity in the DCT was higher in Ks-Nedd4-2 KO mice than in the WT mice. Figure 1B is a table summarizing the results of experiments in which we measured the channel open probability (Po), the probability of finding the basolateral 40-pS K+ channel in the DCT, and NPo, a product of channel number (N) and Po. We found the 40-pS K+ channel in the DCT in 8 of a total of 18 patches (44%) in the WT mice but in 17 of a total of 28 patches in Ks-Nedd4-2 KO mice (61%). Although the mean Po was not significantly different between WT and Ks-Nedd4-2 KO mice (0.41±0.05 versus 0.50±0.05), the mean NPo of the 40-pS K+ channel was significantly higher in Ks-Nedd4-2 KO mice (1.92±0.15) than in the WT mice (1.48±0.12). The notion that the deletion of Nedd4-2 increased the basolateral Kir4.1/Kir5.1 activity was also suggested by the western blot analysis. Figure 1C is a western blot showing the expression of Kir4.1 in the WT and in the Ks-Nedd4-2 KO mice (full-size gel is shown in Supplemental Figure 1A). We confirmed our previous finding that the deletion of Nedd4-2 significantly increased the expression of Kir4.1 to 146%±9% (n=5) of the WT control.20
Figure 1.
Deletion of Nedd4-2 stimulates Kir4.1/Kir5.1 in the DCT. (A) Representative single-channel recordings showing the basolateral K+ channel activity in the DCT of WT and Ks-Nedd4-2 KO mice. The experiments were performed in cell-attached patches with bath solution containing 140 mM NaCl and 5 mM KCl and pipette solution containing 140 mM KCl. The channel closed level is indicated by “C,” and the holding potential was 0 mV. (B) Probability of finding basolateral K+ channel activity, mean NPo, and Po in the DCT of WT and Ks-Nedd4-2 KO mice. *Significant difference between WT and Ks-Nedd4-2 KO mice. (C) An immunoblot showing the expression of Kir4.1 and Nedd4-2 in WT and Ks-Nedd4-2 KO mice. The sample from Ks-Kir4.1 KO mice serves as a negative control for Kir4.1. IB, immunoblot.
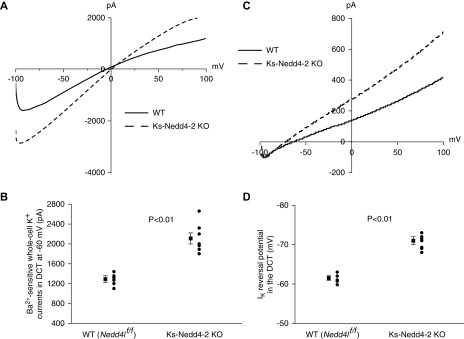
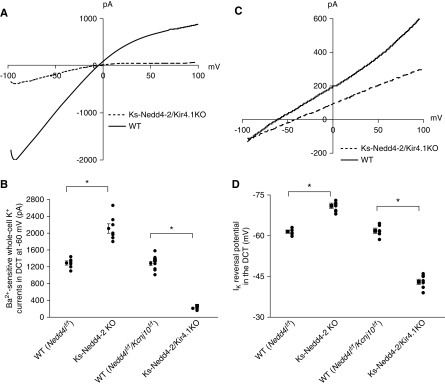
Because the basolateral Kir4.1 in the DCT is the only type of K+ channel providing K+ conductance in the early part of the DCT,1–3,7 it is conceivable that increased K+ channel activity should augment the whole-cell K+ conductance in the DCT1. Thus, we used whole-cell recording to measure the Ba2+-sensitive whole-cell K+ current in the DCT. Figure 2A is a representative trace of the whole-cell K+ currents in the DCT1 measured with ramp protocol (from −100 to 100 mV) in the WT (Figure 2A, solid line) and in the Ks-Nedd4-2 KO mice (Figure 2A, dotted line). It is apparent that deletion of Nedd4-2 increased Ba2+-sensitive whole-cell K+ current in the DCT1. Figure 2B is a scatter plot summarizing each individual data point and mean value of the experiments in which the Ba2+-sensitive whole-cell K+ currents were measured at −60 mV. The deletion of Nedd4-2 increased Ba2+-sensitive whole-cell K+ current from 1290±70 (WT) to 2110±12 pA (n=7). Because the basolateral membrane potential is determined by Kir4.1/Kir5.1 in DCT, we speculate that the deletion of Nedd4-2 should hyperpolarize the DCT membrane. Thus, we next measured the K+ current (IK) reversal potential of the DCT with whole-cell recording in WT and Ks-Nedd4-2 KO mice. Figure 2C is a recording of the current/voltage (I/V) curve in the WT (Figure 2C, solid line) and in the Ks-Nedd4-2 KO mice (Figure 2C, dotted line). As expected, the IK reversal potential of the DCT (an index of the membrane potential) was shifted to the left in the Ks-Nedd4-2 KO mice in comparison with WT mice, suggesting that the deletion of Nedd4-2 hyperpolarizes the DCT membrane. Figure 2D is a scatter plot summarizing each individual data point and the mean value of the experiments in which IK reversal potential was measured. The deletion of Nedd4-2 hyperpolarized the DCT membrane from 61.5±0.6 to 71.0±1.0 mV (n=6). Thus, data support the notion that Nedd4-2 plays a role in the regulation of the basolateral Kir4.1/Kir5.1 in the DCT.
Figure 2.
Deletion of Nedd4-2 hyperpolarizes DCT membrane. (A) Representative whole-cell recordings showing the Ba2+-sensitive K+ currents measured with ramp protocol from −100 to 100 mV in the DCT1 of WT and Ks-Nedd4-2 KO mice. (B) A scatter graph summarizes the values measured at–60 mV with whole-cell recording. The mean value and SEM are shown on the left of each row. For the whole-cell recording, a symmetric 140 mM K+ solution was used for the bath and the pipette. (C) A perforated whole-cell recording showing the K+ current (IK) reversal potential in the DCT of WT and Ks-Nedd4-2 KO mice. (D) A scatter graph summarizes the results of experiments in which the IK reversal potentials were measured in WT and Ks-Nedd4-2 KO mice. The mean value and SEM are shown on the left of each row. For the measurement of IK reversal potential, the bath solution contains 140 mM NaCl and 5 mM KCl, whereas the pipette solution has 140 mM KCl.
Deletion of Nedd4-2 Stimulates NCC
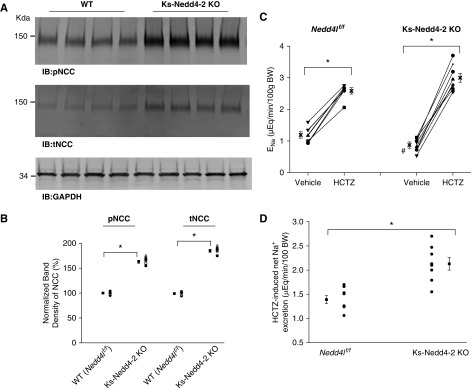
Because the basolateral Kir4.1/Kir5.1 activity in DCT has been shown to determine the NCC expression/activity,2,6,7,18 increased Kir4.1/Kir5.1 activity is expected to stimulate NCC expression/activity. To test this hypothesis, we used western blot to examine the expression of NCC in the WT mice and in the Ks-Nedd4-2 KO mice. Figure 3A is a western blot from seven experiments in which tNCC and pNCC expressions were examined (Supplemental Figure 1B shows the full-size gel). We confirmed the previous report that the deletion of Nedd4-2 increased the expression of tNCC and pNCC.23 Figure 3B is a scatter plot summarizing the mean value and each data point of the normalized band density of pNCC and tNCC. The deletion of Nedd4-2 increased the expression of pNCC (163%±2% of the WT) and tNCC (185%±3% of the WT). The notion that the deletion of Nedd4-2 stimulated NCC was also suggested by the finding that HCTZ-induced natriuresis was augmented in Ks-Nedd4-2 KO mice. Figure 3, C and D summarizes the results of experiments in which we have used the renal clearance technique to examine thiazide-induced renal Na+ excretion (ENa) in the WT and Ks-Nedd4-2 KO mice (four males and four females for each group). From the inspection of Figure 3C, it is apparent that the basal level of ENa (0.87±0.10 μeq/min per 100 g body wt) in Ks-Nedd4-2 KO mice was significantly lower than in the WT mice (1.19±0.10 μeq/min per 100 g body wt), suggesting enhanced renal Na+ absorption in Ks-Nedd4-2 KO mice. Moreover, HCTZ at 30 mg/kg body wt had a larger net natriuretic effect in Ks-Nedd4-2 KO mice (Δ value, 2.13±0.13 from 0.87±0.10 to 3.00±0.14 μeq/min per 100 g body wt) than in WT mice (Δ value, 1.39±0.08 from 1.19±0.10 to 2.58±0.10 μeq/min per 100 g body wt). This suggests that the deletion of Nedd4-2 stimulates NCC expression/activity.
Figure 3.
Deletion of Nedd4-2 stimulates NCC. (A) An immunoblot showing the expression of pNCC and tNCC in WT and Ks-Nedd4-2 KO mice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblot. (B) A scatter graph summarizes the normalized band intensity of the above experiments for pNCC and tNCC, respectively. The mean value and SEM are shown on the left of each row. (C) A scatter line graph showing the results of each experiment in which the effect of single-dose HCTZ (30 mg/kg body wt [BW]) on urinary Na+ excretion (ENa) within 120 minutes was measured with the renal clearance method in WT (Nedd4lflox/flox) and Ks-Nedd4-2 KO mice. (D) A scatter plot showing Δ values of HCTZ-induced net changes in ENa. *Significant difference (P=0.05) determined by t test; #significant difference of the basal ENa between WT (Nedd4lf/f) and Ks-Nedd4-2 KO mice.
Kir4.1 Activity Was Inhibited in Ks-Nedd4-2/Kir4.1 KO Mice
We then explored the possibility that the deletion of Nedd4-2–induced stimulation of NCC may be partially due to the activation of the basolateral K+ channel in the DCT. To test this hypothesis, we generated Ks-Nedd4-2/Kir4.1 KO mice and corresponding WT mice. Western blot analysis has confirmed that the expression of Kir4.1 and Nedd4-2 was almost absent in Ks-Nedd4-2/Kir4.1 KO mice (Supplemental Figure 2A). The single-channel recording detected a 40-pS K+ channel in the basolateral membrane of the DCT of the corresponding WT mice (Supplemental Figure 2B).
We then used the whole-cell recording to measure the Ba2+-sensitive K+ currents in the WT mice (Nedd4f/f and Nedd4lf/f/Kcnj10f/f) and in Ks-Nedd4-2 KO and Ks-Nedd4-2/Kir4.1 KO mice. Figure 4A is a recording showing Ba2+-sensitive K+ currents of the DCT measured with ramp protocol from −100 to 100 mV in Nedd4lf/f/Kcnj10f/f mice (WT) (Figure 4A, solid line) and in Ks-Nedd4-2/Kir4.1 KO mice (Figure 4A, dotted line). Like in Ks-Kir4.1 KO mice,2,6,18 the whole-cell K+ currents were largely inhibited in Ks-Nedd4-2/Kir4.1 KO mice. Figure 4B is a scatter plot summarizing each data point and the mean value of the experiments (n=7–9 for each group) in which the Ba2+-sensitive K+ currents in the DCT were measured at −60 mV. The Ba2+-sensitive K+ currents in Nedd4lf/f/Kcnj10f/f mice (1280±50 pA) were the same as those in Nedd4f/f mice (1290±50 pA), whereas the whole-cell K+ currents in Ks-Nedd4-2/Kir4.1 KO mice were largely abolished (210±15 pA). Consequently, the DCT membrane was depolarized in Ks-Nedd4-2/Kir4.1 KO mice. Figure 4C is a recording showing the I/V curve measured with the whole-cell recording in WT and Ks-Nedd4-2/Kir4.1 KO mice. It is apparent that DCT membrane was depolarized because the I/V curve in the Ks-Nedd4-2/Kir4.1 KO mice (Figure 4C, dotted line) shifted to the right in comparison with the WT (Figure 4C, solid line). Figure 4D is a scatter plot showing each data point and mean value of IK reversal potentials of the DCT in the WT, Ks-Nedd4-2 KO, and Ks-Nedd4-2/kir4.1 KO mice (n=7–9). The IK reversal potential of the DCT in the Ks-Nedd4-2/Kir4.1 KO mice was 43.0±1.0 mV, a value that was significantly lower than in WT (Nedd4lf/f, 61.5±0.6 mV and Nedd4lf/f/Kcnj10f/f, 62±1 mV) and in Ks-Nedd4-2 KO mice (71.0±1.0 mV). Thus, we confirmed that Kir4.1 activity was largely absent in the Ks-Nedd4-2/Kir4.1 KO mice.
Figure 4.
Double deletion of Nedd4-2 and Kir4.1 depolarizes DCT membrane. (A) Representative whole-cell recordings showing the Ba2+-sensitive K+ currents measured with ramp protocol from −100 to 100 mV in the DCT1 of WT mice (Kcnj10flox/flox/Nedd4lflox/flox) and Ks-Nedd4-2/Kir4.1 KO mice (dotted line). (B) A scatter graph summarizes the values measured at–60 mV with whole-cell recording. The mean value and SEM are shown on the left of each row. For the whole-cell recording, a symmetric 140 mM K+ solution was used for the bath and the pipette. (C) A perforated whole-cell recording showing the K+ current (IK) reversal potential in the DCT of WT and Ks-Nedd4-2/Kir4.1 KO mice. (D) A scatter graph summarizes the results of experiments in which the IK reversal potentials were measured in WT and Ks-Nedd4-2/Kir4.1 KO mice. The mean value and SEM are shown on the left of each row. For the measurement of IK reversal potential, the bath solution contains 140 mM NaCl and 5 mM KCl, whereas the pipette solution has 140 mM KCl. Data for WT (Nedd4lf/f) and Ks-Nedd4-2 KO mice in (B) and (D) are duplicated from Figure 2, B and D, respectively, for the comparison. *Significant difference (P=0.05) determined by t test.
Deletion of Nedd4-2 Increases ENaC Expression
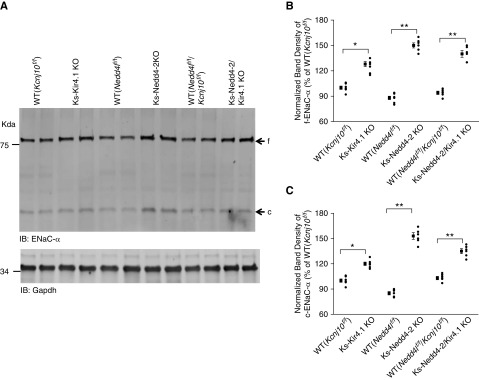
Previous study has demonstrated that the deletion of Nedd4-2 in the kidney increased the expression of ENaC.21,33 To determine whether the Nedd4-2 deletion-induced stimulation of ENaC was preserved in the Ks-Nedd4-2/Kir4.1 KO mice, we next examined the expression of ENaCα in Ks-Kir4.1 KO, Ks-Nedd4-2 KO, Ks-Nedd4-2/Kir4.1 KO, and the corresponding WT mice. Figure 5A is a western blot showing that the expression of full-length ENaCα increased in Ks-Nedd4-2/Kir4.1 KO and in Ks-Nedd4-2 KO mice, whereas it increased modestly in Ks-Kir4.1 KO mice, possibly due to high aldosterone level as a compensative response to Na+ wasting.2 Figure 5B is a scatter plot summarizing each data point and mean value of normalized band density of full-length ENaCα (n=6 from each group). The expression of full-length ENaCα was 128%±3% (Kir4.1 KO mice), 150%±3% (Ks-Nedd4-2 KO mice), and 140%±4% (Ks-Nedd4-2/Kir4.1 KO mice) of the control value (WT, Kcnj10f/f). From the inspection of Figure 5A, it is also clear that the deletion of Nedd4-2 increased cleaved ENaCα. Figure 5C is a scatter plot summarizing each data point and mean value of normalized band density of cleaved ENaCα. The expression of the cleaved ENaCα was 120%±2% (Kir4.1 KO mice), 155%±3% (Ks-Nedd4-2 KO mice), and 135%±4% (Ks-Nedd4-2/Kir4.1 double-KO mice) of the control value (WT, Kcnj10f/f). Thus, like in Ks-Nedd4-2 KO mice, ENaCα expression was increased in Ks-Nedd4-2/Kir4.1 KO mice.
Figure 5.
Deletion of Nedd4-2 increases the expression of ENaCα. (A) An immunoblot showing the expression of full-length (f) ENaCα and cleaved (c) ENaCα in WT (Kcnj10flox/flox, Nedd4lflox/flox, and Nedd4lflox/flox-Kcnj10flox/flox), Ks-Kir4.1 KO, Ks-Nedd4-2 KO, and Ks-Nedd4-2/Kir4.1 KO mice. Gapdh, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblot. A scatter graph summarizes the normalized band intensity of the above experiments for (B) f-ENaCα and (C) c-ENaCα. The mean value and SEM are shown on the left of each row. *Difference is significant at P=0.05; **difference is significant at P=0.01.
Kir4.1 Activity Is Involved in Nedd4-2 Deletion-Induced Activation of NCC
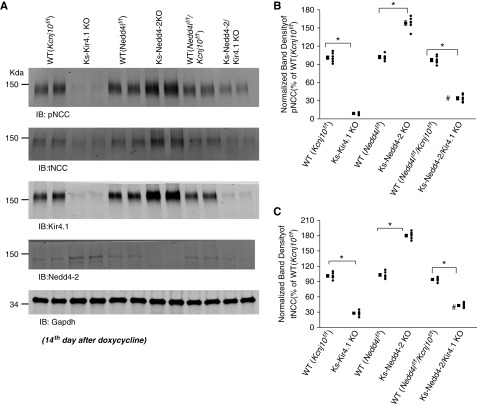
We next examined the expression of pNCC and tNCC in Ks-Kir4.1 KO mice, Ks-Nedd4-2 KO mice, Ks-Nedd4-2/Kir4.1 KO mice, and corresponding WT mice. Figure 6A is a western blot showing the expression of pNCC, tNCC, Kir4.1, and Nedd4-2 in different mice (full-size gel is shown in Supplemental Figure 3). We confirmed that the expression of pNCC and tNCC was inhibited in Ks-Kir4.1 KO mice2,6 but that it increased in Ks-Nedd4-2 KO mice.23 However, in sharp contrast to Ks-Nedd4-2 KO mice, the expression of pNCC and tNCC was inhibited in the Ks-Nedd4-2/Kir4.1 KO mice in comparison with the corresponding WT mice. Figure 6, B and C is scatter plots summarizing each data point and the mean value of the normalized band density for pNCC and tNCC, respectively. The expression of pNCC was 9%±1% in Ks-Kir4.1 KO mice (n=6), 160%±4% in Ks-Nedd4-2 KO mice (n=6), and 34%±3% in Ks-Nedd4-2/Kir4.1 KO mice (n=6) in comparison with their corresponding WT control. The expression of tNCC was 28%±2% in Ks-Kir4.1 KO mice (n=6), 180%±3% in Ks-Nedd4-2 KO mice (n=6), and 43%±3% in Ks-Nedd4-2/Kir4.1 KO mice (n=6) in comparison with their corresponding WT control. Moreover, the expression of pNCC and tNCC was slightly but significantly higher in Ks-Nedd4-2/Kir4.1 KO mice than in Ks-Kir4.1 KO mice (Figure 6, B and C). This finding supports the role of Nedd4-2 in mediating NCC membrane retrieval from the plasma membrane by a Kir4.1-independent mechanism.
Figure 6.
NCC expression is not increased in the double-KO mice. (A) An immunoblot showing the expression of pNCC, tNCC, Kir4.1, and Nedd4-2 in WT (Kcnj10flox/flox, Nedd4lflox/flox, and Kcnj10flox/flox/Nedd4lflox/flox), Ks-Kir4.1 KO, Ks-Nedd4-2 KO, and Ks-Nedd4-2/Kir4.1 KO mice. Gapdh, glyceraldehyde-3-phosphate dehydrogenase; IB, immunoblot. A scatter graph summarizes the normalized band intensity of the (B) pNCC and (C) tNCC in WT (Kcnj10flox/flox, Nedd4lflox/flox, and Kcnj10flox/flox/Nedd4lflox/flox), Ks-Kir4.1 KO, Ks-Nedd4-2 KO, and Ks-Nedd4-2/Kir4.1 KO mice, respectively. The mean value and SEM are shown on the left of each row. Both male and female mice were used for the western blot, and the data obtained from male/female mice were normalized with corresponding male/female WT mice. *Significant difference (P=0.05) determined by t test; #value is significantly different between Ks-Kir4.1 KO and Ks-Nedd4-2/Kir4.1 KO mice.
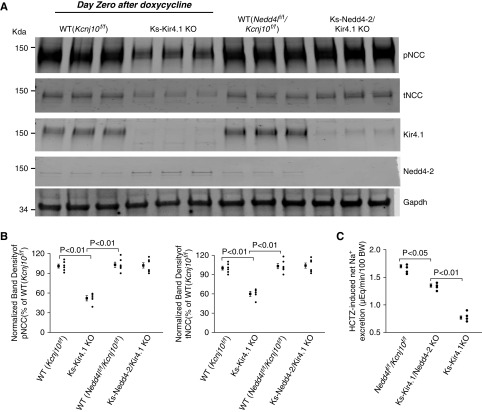
To exclude the possibility that Kir4.1 deletion may have a dominant negative phenotype, we performed experiments in which we used the mice immediately after treatment of doxycycline (without a 2-week transition period). This approach provides an advantage in comparison with the standard protocol with a 2-week transition period because we are able to observe the intermediate phase of NCC response to Kir4.1 deletion between Ks-Kir4.1 KO and Ks-Nedd4-2/Kir4.1 double-KO mice. Figure 7A is a western blot showing the expression of pNCC, tNCC, Kir4.1, and Nedd4-2 in different mice (full-size gel is shown in Supplemental Figure 4). Two scatter plots in Figure 7B summarize the mean value and each data point of the normalized band density for pNCC (Figure 7B, left panel) and tNCC (Figure 7B, right panel), respectively. The expression of pNCC was 52%±4% in Ks-Kir4.1 KO mice (n=5) and 99%±5% in Ks-Nedd4-2/Kir4.1 KO mice (n=5) in comparison with their corresponding WT. The expression of tNCC was 60%±4% in Ks-Kir4.1 KO mice (n=5) and 101%±6% in Ks-Nedd4-2/Kir4.1 KO mice (n=5) in comparison with their corresponding WT. Moreover, the renal clearance study also shows that HCTZ-induced net renal Na+ excretion was significantly decreased (0.77±0.03 μeq/min per 100 g body wt, n=4) in Ks-Kir4.1 KO mice (immediately after doxycycline) in comparison with WT mice (1.70±0.03 μeq/min per 100 g body wt, n=4). In contrast, HCTZ-induced net Na+ excretion was only modestly decreased in the double-KO mice immediately after doxycycline treatment (1.30±0.03 μeq/min per 100 g body wt, n=4). Finally, quantitative RT-PCR also demonstrated that the deletion of Kir4.1 decreased NCC mRNA (27%±2%, n=4) but that it had no effect (110%±8%) in the double-KO mice in comparison with the WT mice (Kcnj10f/f) (Supplemental Figure 5A). This was not due to the failure of inhibiting Kir4.1/Kir5.1 in the DCT of the double-KO mice. Western blot analysis confirmed the downregulation of Kir4.1 in Ks-Kir4.1 KO mice (by 85%±5%) and in Ks-Nedd4-2/Kir4.1 KO mice (by 80%±5%) (Figure 7A). The electrophysiologic experiments have also demonstrated that Kir4.1/Kir5.1 activity in the DCT was similarly inhibited in Ks-Kir4.1 KO mice (290±35 pA, n=8) and in the Nedd4-2/Kir4.1 double-KO mice (350±30 pA, n=8), whereas it was 1200±40 pA (n=8) in WT mice (Supplemental Figure 5B). Thus, our data indicate that deletion of Kir4.1 does not have a dominant phenotype as observed in WNK4 KO mice.34 Also, the results further support the role of Nedd4-2 in mediating NCC retrieval from the apical membrane of the DCT.
Figure 7.
Nedd4-2 deletion delays Kir4.1 deletion-induced inhibition of NCC. (A) An immunoblot showing the expression of pNCC, tNCC, Kir4.1, and Nedd4-2 in WT (Kcnj10flox/flox and Kcnj10flox/flox/Nedd4lflox/flox), Ks-Kir4.1 KO, and Ks-Nedd4-2/Kir4.1 KO mice. The experiments was conducted in the mice immediately after treatment of doxycycline without a 14-day transition period. Gapdh, glyceraldehyde-3-phosphate dehydrogenase. (B) A scatter graph summarizes the normalized band intensity of the pNCC (left panel) and tNCC (right panel) in WT (Kcnj10flox/flox and Kcnj10flox/flox/Nedd4lflox/flox), Ks-Kir4.1 KO, and Ks-Nedd4-2/Kir4.1 KO mice. The mean value and SEM are shown on the left of each row. Male mice were used for the western blot. (C) A scatter plot shows the HCTZ-induced net renal Na+ excretion in WT, Ks-Nedd4-2/Kir4.1 KO, and Ks-Kir4.1 KO mice. The mice were used immediately after either doxycycline or vehicle treatment. BW, body weight.
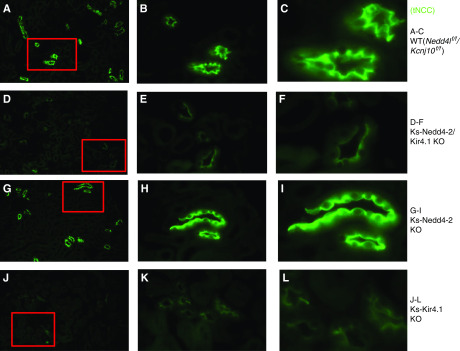
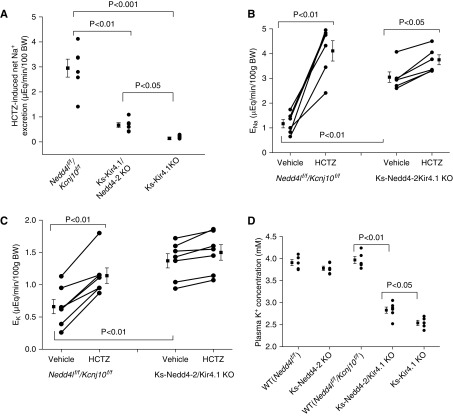
The notion that the stimulation of the basolateral Kir4.1/Kir5.1 in the DCT is partially responsible for the Nedd4-2 deletion-induced stimulation of NCC was also suggested by immunostaining images. Figure 8 shows immunofluorescence staining of NCC from low (×4) (Figure 8, A, D, G, and J) and intermediate (×20) (Figure 8, B, E, H, and K) to high magnification (×60) (Figure 8, C, F, I, and L) in WT (Figure 8, A–C), Ks-Nedd4-2/Kir4.1 KO (Figure 8, D–F), Ks-Nedd4-1 (Figure 8, G–I), and Ks-Kir4.1 KO mice (Figure 8, J–L). It is apparent that the expression of tNCC was decreased in Ks-Nedd4-2/Kir4.1 KO mice in comparison with WT and Ks-Nedd4-2 KO mice. Also, apical membrane NCC staining seems to be decreased in both Ks-Kir4.1 KO mice and Ks-Nedd4-2/Kir4.1 KO mice, but it seems to be increased in Ks-Nedd4-2 KO mice. However, the apical membrane staining of tNCC seems to be slightly higher in the double-KO mice than in the Ks-Kir4.1 KO mice, suggesting possibly higher NCC activity in the double-KO mice than in Kir4.1 KO mice. This notion was also confirmed by examining HCTZ-induced net renal Na+ excretion (an index of NCC activity or pNCC) with renal clearance in WT (Nedd4lf/f/Kcnj10f/f), Ks-Nedd4-2/Kir4.1 KO, and Ks-Kir4.1 KO mice. Figure 9A is a scatter plot summarizing the mean value and that each data point of experiments shows that HCTZ-induced net renal Na+ excretion in Ks-Nedd4-2/Kir4.1 KO mice (0.67±0.10 μeq/min per 100 g body wt, n=6) was significantly higher than in Ks-Kir4.1 KO mice (0.14±0.05 μeq/min per 100 g body wt, n=8). This suggests that Nedd4-2 regulates NCC activity in a Kir4.1/Kir5.1-independent way. However, NCC activity was still significantly inhibited in Ks-Nedd4-2/Kir4.1 KO mice in comparison with WT mice. Figure 9B is a line graph summarizing each individual experiment in which HCTZ-induced natriuresis was examined in WT (Nedd4lf/f/Kcnj10f/f) and Ks-Nedd4-2/Kir4.1 KO mice. From the inspection of Figure 9B, it is apparent that the basal level of renal Na+ excretion (3.15±0.21 μeq/min per 100 g body wt, n=6) in Ks-Nedd4-2/Kir4.1 KO mice was higher than in WT mice (1.17±0.17 μeq/min per 100 g body wt, n=6), indicating renal Na+ wasting even with high ENaC expression. Moreover, the HCTZ application increased renal Na+ excretion from 1.17±0.17 to 4.11±0.41 μeq/min per 100 g body wt in WT mice (Nedd4lf/f/Kcnj10f/f), and the HCTZ-induced net Na+ excretion was 2.94±0.36 μeq/min per 100 g body wt (n=6), which was significantly higher than that in the double-KO mice (Figure 9A). By calculating the difference of HCTZ-induced net natriuresis, the deletion of Kir4.1 inhibited the NCC function by 95%, whereas the double deletion inhibited the NCC by 77%, suggesting that the deletion of Nedd4-2 prevented NCC retrieval.
Figure 8.
NCC expression is suppressed in Ks-Nedd4-2/Kir4.1 KO mice. Images show tNCC immunostaining in (A–C) WT (Nedd4lflox/flox/Kcnj10flox/flox), (D–F) Ks-Nedd4-2/Kir4.1 KO, (G–I) Ks-Nedd4-2 KO, and (J–L) Ks-Kir4.1 KO mice. The images in red squares in (A, D, G, and J) are magnified with intermediate (B, E, H, and K) and large resolution (C, F, I, and L). Original magnification, ×4 in A, D, G, and J; ×20 in B, E, H, and K; ×60 in C, F, I, and L.
Figure 9.
Double deletion of Kir4.1 and Nedd4-2 impairs NCC function. (A) A scatter plot showing the Δ values of HCTZ-induced net ENa in WT (Nedd4lflox/flox/Kcnj10flox/flox), Ks-Nedd4-2/Kir4.1 KO, and Ks-Kir4.1 KO mice. (B) A scatter line graph showing the results of each experiment in which the effect of single-dose HCTZ (30 mg/kg body wt [BW]) on urinary Na+ excretion (ENa) within 120 minutes was measured with the renal clearance method in WT (Nedd4lflox/flox/Kcnj10flox/flox) and Ks-Nedd4-2/Kir4.1 KO mice. The significance is determined by t test. (C) A scatter line graph showing the results of each experiment in which the effect of single-dose HCTZ (30 mg/kg BW) on urinary K+ excretion (EK) within 120 minutes was measured with the renal clearance method in WT (Nedd4lflox/flox/Kcnj10flox/flox) and Ks-Nedd4-2/Kir4.1 KO mice. (D) A scatter plot shows the plasma K+ concentrations in WT (Nedd4lflox/flox and Nedd4lflox/flox/Kcnj10flox/flox), Ks-Nedd4-2 KO, Ks-Nedd4-2/Kir4.1 KO, and Ks-Kir4.1 KO mice. The experiments were performed in the mice treated with doxycycline followed by a 14-day recovery period.
Ks-Nedd4-2/Kir4.1 Double-KO Mice Were Hypokalemic
Because the inhibition of NCC has been shown to cause renal K+ wasting,35 we speculate that Ks-Nedd4-2/Kir4.1 KO mice have a phenotype of renal K+ wasting. Thus, we used renal clearance to examine the basal level of renal K+ excretion and HCTZ-induced renal K+ excretion. Figure 9C is a line graph summarizing each individual experiment, the mean value, and statistical information. It is apparent that the basal level of renal K+ excretion (1.37±0.11 μeq/min per 100 g body wt) in Ks-Nedd4-2/Kir4.1 KO mice was significantly higher than in WT mice (0.66±0.11 μeq/min per 100 g body wt). Application of HCTZ increased EK to 1.14±0.12 μeq/min per 100 g body wt in WT mice, but it had no significant effect on EK in Ks-Nedd4-2/Kir4.1 KO mice (1.50±0.12 μeq/min per 100 g body wt). As a consequence of renal K+ wasting, Ks-Nedd4-2/Kir4.1 KO mice were hypokalemic. Figure 9D is a scatter plot summarizing the mean value and each data point of plasma K+, and Supplemental Figure 6A shows a table summarizing the mean value of the plasma K+ and Na+ concentration and statistical information in different genotypes. The plasma K+ levels were 2.54±0.06 mM (n=5) in Ks-Kir4.1 KO mice and 2.83±0.07 mM (n=6) in Ks-Nedd4-2/Kir4.1 KO mice. In contrast, they were 3.91±0.07 mM (Nedd4lf/f)), 3.97±0.08 mM (Nedd4lf/f/Kcnj10f/f), and 3.78±0.1 mM (Ks-Nedd4-2 KO mice). However, the plasma Na+ levels were similar in all groups. Although the expression of NCC in the double-KO mice used at day 0 after doxycycline treatment was similar to that in WT mice (Figure 7A), the basal level of urinary EK (0.84±0.07 μeq/min per 100 g body wt, n=7) was significantly higher in the double-KO mice (used immediately after doxycycline) than in WT mice (0.52±0.05 μeq/min per 100 g body wt), but it is was lower than in Ks-Kir4.1 KO mice used immediately after doxycycline (1.13±0.08 μeq/min per 100 g body wt) (Supplemental Figure 6B). Consequently, Ks-Nedd4-2/Kir4.1 mice used immediately after doxycycline were still hypokalemic (3.02±0.12 mM, n=6), but it was significantly higher than in Ks-Kir4.1 KO mice used immediately after doxycycline (2.58±0.11 mM) (Supplemental Figure 6C). It is possible that deletion of Kir4.1 may also affect NKCC2 function in the thick ascending limb (TAL) because Kir4.1/Kir5.1 is expressed in the TAL.36 Thus, Kir4.1/Kir5.1 activity should regulate WNK4 in the TAL, thereby regulating NKCC2 expression/activity.37
Discussion
The basolateral K+ channel in the DCT is composed of Kir4.1 and Kir5.1 and plays an important role in the regulation of NCC expression in response to dietary K+ and Na+ intake.2,6,18 Loss-of-function mutations of Kir4.1 in the kidney have been shown to cause abnormal electrolyte transport, including renal K+ wasting and metabolic alkalosis.38–40 Although Kir4.1 is the subunit providing K+ permeability of the heterotetramer, Kir5.1 is a regulatory subunit for Kir4.1/Kir5.1.3,20,41 Our previous in vitro experiment has demonstrated that Kir5.1 was able to bind E3 ubiquitin ligase, Nedd4-2.20 Three lines of evidence obtained in Ks-Nedd4-2 KO mice have suggested that Nedd4-2 was involved in the regulation of Kir4.1/Kir5.1 in the DCT. First, the deletion of Nedd4-2 increased the basolateral 40-pS K+ channel (a Kir4.1/Kir5.1 heterotetramer) activity and augmented the whole-cell K+ conductance in the DCT. Second, the DCT membrane potential in Ks-Nedd4-2 KO mice was more negative than those in corresponding WT mice. Third, the deletion of Nedd4-2 increased the expression of Kir4.1 in the kidney. Although Kir4.1 is also expressed in the nephron segments other than the DCT and plays a role in regulating NKCC2 by calcium-sensing receptor,36,42–45 Nedd4-2 deletion-induced stimulation of Kir4.1 should be largely due to augmented Kir4.1 expression in the DCT because the DCT is a main site for Kir4.1.32 Thus, Nedd4-2 is involved in the regulation of the basolateral Kir4.1/Kir5.1 channel in the DCT.
Although it has been reported previously that the deletion of Nedd4-2 increases NCC,21,23 the mechanism by which Nedd4-2 regulates NCC is not well explored. Thus, the novelty of this study is to demonstrate that Nedd4-2–induced regulation of Kir4.1/Kir5.1 is, at least in part, a mechanism by which Nedd4-2 regulates NCC because NCC expression/activity was inhibited in the double-KO mice in comparison with corresponding WT mice. However, the finding that the NCC expression/activity in Ks-Nedd4-2/Kir4.1 double-KO mice was slightly but significantly higher than that in Ks-Kir4.1 KO mice suggests that Nedd4-2 also has a role in directly regulating NCC retrieval from the apical membrane by a Kir4.1-independent mechanism. This may be achieved by directly regulating NCC ubiquitination or by regulating WNK1 expression, thereby modulating NCC activity. However, the results have also suggested that Nedd4-2–dependent regulation of NCC expression/activity is partially achieved by stimulating a Kir4.1/Kir5.1-dependent mechanism. However, further study is required to explore how Nedd4-2 and the basolateral Kir4.1/Kir5.1 work in concert to regulating NCC expression. Our previous study demonstrated that Nedd4-2 was able to bind to TPVT motif in the C terminus of Kir5.1.20 Thus, it is possible that Kir5.1 serves as an adapting protein for Nedd4-2, which is responsible for the ubiquitination of Kir4.1. This notion was supported by our in vitro study demonstrating that Nedd4-2 was able to increase the ubiquitination of Kir4.1 in the presence of Kir5.1 but not in the absence of Kir5.1. Moreover, coexpression of Nedd4-2 inhibited K+ currents in HEK cells transfected with Kir4.1/Kir5.1 but not with Kir4.1 alone, suggesting that Nedd4-2 inhibits Kir4.1/Kir5.1 activity. Thus, the deletion of Nedd4-2 should impair the regulation of the basolateral Kir4.1/Kir5.1 in response to a variety of physiologic stimuli. Although further experiments are required to test whether the deletion of Nedd4-2 inhibits the Kir4.1 ubiquitination, the observation that Kir4.1 expression was increased in Ks-Nedd4-2 KO mice has indicated that Nedd4-2 regulates the basolateral Kir4.1/Kir5.1 in the DCT.
Nedd4-2 has been shown to be expressed in the aldosterone-sensitive distal nephron (ASDN), including the DCT, connecting tubule and collecting duct.22,23,27,46 The deletion of Nedd4-2 in adult animals has been shown to increase the NCC expression, and the Nedd4-2–deficient mice developed salt-sensitive hypertension.23 Our experiments have confirmed this finding that the deletion of Nedd4-2 at adult mice increased the expression of NCC. Although the role of Nedd4-2 in regulating NCC is well established, the mechanism by which Nedd4-2 inhibits NCC expression is not completely understood. An in vitro study has demonstrated that Nedd4-2 regulates NCC expression through an aldosterone-SGK1–dependent mechanism.26,47 However, it is still a debatable issue regarding the role of aldosterone on NCC. The recent development in the field has built a consensus that the regulation of NCC may not depend on mineralocorticoids receptor.48 However, aldosterone may regulate NCC by an MR-independent mechanism.49 Thus, another in vivo study is required to test the role of aldosterone-SGK1 in the regulation of NCC. The observation that the deletion of Nedd4-2 increased the negativity of DCT membrane (hyperpolarization) has suggested the possibility that Nedd4-2 deletion-induced stimulation of NCC may be partially achieved by stimulating the basolateral K+ channel activity in the DCT. This notion is strongly suggested by the results obtained in Ks-Nedd4-2/Kir4.1 KO mice. First, renal clearance experiments showed that HCTZ-induced natriuresis was significantly blunted in Ks-Nedd4-2/Kir4.1KO mice, suggesting the inhibition of NCC. Second, the expression of pNCC and tNCC was inhibited in Ks-Nedd4-2/Kir4.1 KO mice, but it was increased in Ks-Nedd4-2 KO mice. We speculate that the deletion of Nedd4-2–induced activation of basolateral K+ channels in the DCT and hyperpolarization should activate Cl−-sensitive WNK, thereby increasing the expression of pNCC. Because pNCC is more stable than non-pNCC,50 the deletion of Nedd4-2 also increases the expression of tNCC proteins. Thus, we believe that Nedd4-2–dependent regulation of basolateral Kir4.1/Kir5.1 in the DCT plays a role in regulating NCC activity/expression.
A large body of in vitro and in vivo evidence has convincingly demonstrated that Nedd4-2 regulates ENaC in the ASDN.21,23–25,27–31 Indeed, the deletion of Nedd4-2 increased the ENaC expression and caused salt-sensitive hypertension.23,33 Our study has confirmed the previous finding that the deletion of Nedd4-2 increases ENaC expression. However, the previous study by Ronzaud et al.23 has demonstrated that the deletion of Nedd4-2 increased the expression of ENaCβ and ENaCγ but not the ENaCα subunit. However, we have observed that the deletion of Nedd4-2 increased the expression of ENaCα. Considering the fact that dietary sodium content in the study by Ronzaud et al.23 was lower (0.18%) than that in our study (0.4%), it is possible that plasma aldosterone level in the mice on 0.18% Na+ should be higher than on 0.4% Na+. Thus, a high aldosterone level increased ENaCα expression, thereby masking the difference between the WT and Nedd4-2–deficient mice. Indeed, a separate study performed by the same laboratory reported that ENaCα expression was higher in Nedd4-2–deficient mice than in the WT mice on a different diet that reduced plasma aldosterone level.21 Thus, an increase in ENaCα in Ks-Nedd4-2/Kir4.1 KO mice should be the result of Nedd4-2 deletion and a possible high aldosterone level in the double-KO mice.
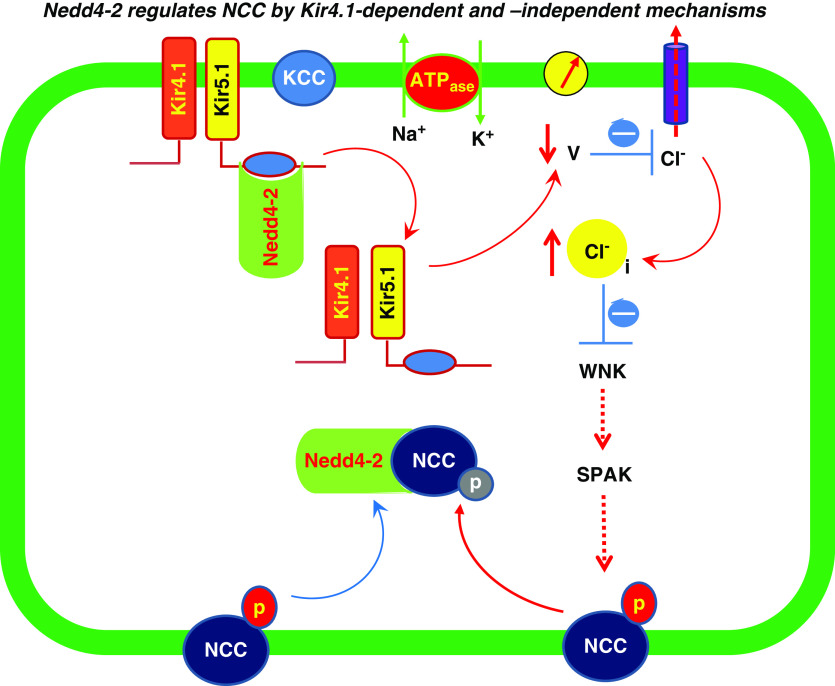
Although ENaC expression was upregulated in Ks-Nedd4-2 KO mice, the mice were normokalemic as reported previously.21 It is possible that a high NCC activity in Ks-Nedd4-2 KO mice reduced Na+ and volume delivery to the late ASDN, thereby offsetting the effect of high ENaC activity on ROMK-dependent K+ secretion. In contrast, Ks-Kir4.1/Nedd4-2 double-KO mice were hypokalemic, presumably due to high Na+ and volume delivery to the late ASDN and high ENaC activity. As a consequence of high ENaC expression and high Na+ delivery, renal K+ excretion was increased in Ks-Nedd4-2/Kir4.1 KO mice. The physiologic significance of this study is the illustration of the mechanism by which Nedd4-2 regulates NCC activity. Figure 10 is a cell model illustrating the role of Kir4.1/Kir5.1 in mediating the effect of Nedd4-2 on thiazide-sensitive NCC. The inhibition of Kir4.1/Kir5.1 activity by Nedd4-2 should depolarize DCT membrane. A decrease in DCT membrane negativity increases the intracellular Cl− concentrations and inhibits WNK/SPAK activity, thereby inhibiting NCC expression/activity. In addition, Nedd4-2 is critically involved in mediating the degradation of NCC protein and might also play a role directly/indirectly in regulating WNK/SPAK pathways. In this regard, it has been reported that the deletion of Nedd4-2 increases the expression of WNK1.21 Because tenfold higher Cl− concentrations are required to inhibit WNK1,16 it is possible that high WNK1 expression may be able to transiently compensate the WNK4 activity inhibited by the deletion of Kir4.1. Thus, the possible interpretation for the observation that the expression of NCC in the double-KO mice (0 day after doxycycline treatment) was not decreased immediately, although Kir4.1 activity was inhibited, may be an Nedd4-2 deletion-induced increase in WNK1 expression, which phosphorylates SPAK and NCC. Thus, more studies are necessary to fully understand the mechanism by which the interaction between Kir4.1/Kir5.1 and Nedd4-2 regulates NCC. However, it is safe to conclude that the basolateral Kir4.1/Kir5.1 activity is, at least partially, involved in mediating the Nedd4-2 deletion-induced stimulation of NCC.
Figure 10.
A cell scheme illustrates the role of Kir4.1/Kir5.1 in mediating the effect of Nedd4-2 on thiazide-sensitive NCC. The inhibition of Kir4.1/Kir5.1 activity by Nedd4-2 should depolarize the DCT membrane voltage (V). A decrease in the cell negativity reduces the driving force for Cl− exit by basolateral Cl− channels, thereby increasing intracellular Cl− (Cl−i), which inhibits WNK and SPAK. This leads to inhibition of NCC phosphorylation and activity. Nedd4-2 is expected to facilitate the degradation of non-pNCC. In addition, Nedd4-2 also regulates NCC retrieval from the apical membrane of the DCT by Kir4.1-indipendent mechanism (blue arrow). The dotted line indicates the inhibition.
Disclosures
None.
Funding
The work is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease grants DK54983 (to Dr. Wang) and DK115366 (to Dr. Lin).
Supplementary Material
Acknowledgments
The authors thank Ms. Gail Anderson for her assistance in editing the manuscript.
Dr. Su and Dr. Wu participated in designing and conducting experiments and in analyzing data and preparing figures. Dr. Gao performed renal clearance and immunostaining. Dr. Duan, Dr. Xiao, and Dr. Zhang performed the patch clamp, renal clearance, and measurement of plasma electrolytes. Dr. Staub and Dr. Wang are involved in interpretation of the data and in preparation of the draft. Dr. Lin is responsible for designing and executing the experiments and for drafting and final approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019090923/-/DCSupplemental.
Supplemental Figure 1. Deletion of Nedd4-2 increases the expression of inwardly rectifying K+ channel 4.1 and NaCl cotransporter.
Supplemental Figure 2. Basolateral 40-pS K+ channel in the distal convoluted tubule of floxed Nedd4l/Kcnaj10 (wild type).
Supplemental Figure 3. Expression of phosphorylated NaCl cotransporter and total NaCl cotransporter in kidney-specific Nedd4-2/inwardly rectifying K+ channel 4.1 knockout mice.
Supplemental Figure 4. Expression of NaCl cotransporter in the mice at 0 day after doxycycline treatment.
Supplemental Figure 5. NaCl cotransporter mRNA expression and inwardly rectifying K+ channel 4.1/inwardly rectifying K+ channel 5.1 activity.
Supplemental Figure 6. Kidney-specific Nedd4-2/inwardly rectifying K+ channel 4.1 knockout mice were hypokalemic.
Supplemental Material. Supplemental methods include genotyping, preparation of the distal convoluted tubule, quantitative RT-PCR, and antibodies.
References
- 1.Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, et al.: Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci U S A 108: 10361–10366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, et al.: Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, et al.: Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, et al.: An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Gao ZX, Su XT, Wang MX, Wang WH, Lin DH: Kir4.1/Kir5.1 activity is essential for dietary sodium intake-induced modulation of Na-Cl cotransporter. J Am Soc Nephrol 30: 216–227, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, et al.: KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111: 11864–11869, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik S, Lambert E, Zhang J, Wang T, Clark HL, Cypress M, et al.: Potassium conservation is impaired in mice with reduced renal expression of Kir4.1. Am J Physiol Renal Physiol 315: F1271–F1282, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, et al.: The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm PR, Coleman R, Delpire E, Welling PA: Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH: The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahle KT, Ring AM, Lifton RP: Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, et al.: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 14.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, et al.: A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazúa-Valenti S, Gamba G: Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am J Physiol Cell Physiol 308: C779–C791, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang MX, Cuevas CA, Su XT, Wu P, Gao ZX, Lin DH, et al.: Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu P, Gao ZX, Zhang DD, Su XT, Wang WH, Lin DH: Deletion of Kir5.1 impairs renal ability to excrete potassium during increased dietary potassium intake. J Am Soc Nephrol 30: 1425–1438, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang MX, Su XT, Wu P, Gao ZX, Wang WH, Staub O, et al.: Kir5.1 regulates Nedd4-2-mediated ubiquitination of Kir4.1 in distal nephron. Am J Physiol Renal Physiol 315: F986–F996, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Qusairi L, Basquin D, Roy A, Rajaram RD, Maillard MP, Subramanya AR, et al.: Renal tubular ubiquitin-protein ligase NEDD4-2 is required for renal adaptation during long-term potassium depletion. J Am Soc Nephrol 28: 2431–2442, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo F, Staub O: NEDD4-2 and salt-sensitive hypertension. Curr Opin Nephrol Hypertens 24: 111–116, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, et al.: Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, et al.: AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bhalla V, Hallows KR: Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Flores SY, Loffing-Cueni D, Kamynina E, Daidié D, Gerbex C, Chabanel S, et al.: Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4-2 phosphorylation and increased Na+ transport in cortical collecting duct cells. J Am Soc Nephrol 16: 2279–2287, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, et al.: Dietary sodium intake regulates the ubiquitin-protein ligase nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pearce D, Kleyman TR: Salt, sodium channels, and SGK1. J Clin Invest 117: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder PM, Olson DR, Thomas BC: Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem 277: 5–8, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Snyder PM: The epithelial Na+ channel: Cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Zhou R, Patel SV, Snyder PM: Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, et al.: Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett 388: 11–15, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, et al.: Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 295: F462–F470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, et al. : Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al.: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Wang L, Su XT, Lin DH, Wang WH: KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol 308: F1288–F1296, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terker AS, Castañeda-Bueno M, Ferdaus MZ, Cornelius RJ, Erspamer KJ, Su XT, et al.: With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am J Physiol Renal Physiol 315: F781–F790, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, et al.: Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, et al.: KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A 107: 14490–14495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, et al.: Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker SJ, Imbrici P, Salvatore L, D’Adamo MC, Pessia M: pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem 275: 16404–16407, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Su XT, Wang WH: The expression, regulation, and function of Kir4.1 (Kcnj10) in the mammalian kidney. Am J Physiol Renal Physiol 311: F12–F15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha SK, Huang C, Ding Y, Qi X, Huang CL, Miller RT: Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem 286: 1828–1835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, et al.: Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH: Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol 310: F985–F993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staub O, Yeger H, Plant PJ, Kim H, Ernst SA, Rotin D: Immunolocalization of the ubiquitin-protein ligase Nedd4 in tissues expressing the epithelial Na+ channel (ENaC). Am J Physiol 272: C1871–C1880, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, et al.: Nedd4-2 modulates renal Na+-Cl- cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng L, Poulsen SB, Wu Q, Esteva-Font C, Olesen ETB, Peng L, et al.: Rapid aldosterone-mediated signaling in the DCT increases activity of the thiazide-sensitive NaCl cotransporter. J Am Soc Nephrol 30: 1454–1470, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenbaek LL, Kortenoeven MLA, Aroankins TS, Fenton RA: Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 289: 13347–13361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.