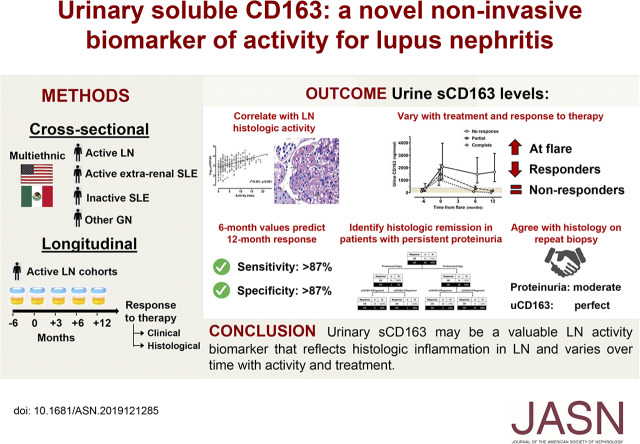
Significance Statement
Although many biomarkers have been tested in lupus nephritis, none have advanced to clinical practice. Treatment is often guided by the level of proteinuria, which lacks the necessary sensitivity to differentiate disease activity from chronic damage to the kidneys. The authors evaluated urinary CD163 as an activity biomarker of lupus nephritis in two large multiethnic populations, with longitudinal follow-up of biomarker expression and testing against clinical and histologic disease activity. They demonstrated that urinary CD163 correlated with disease severity, lupus nephritis class, and histologic activity. Furthermore, the course of urinary CD163 in response to treatment discriminated among response groups and faithfully reflected the disease’s histologic activity in repeated kidney biopsies. These findings suggest that urinary CD163 shows promise as a biomarker reflecting histologic inflammation in lupus nephritis.
Keywords: soluble CD163, CD163 receptor, lupus nephritis, biomarkers, systemic lupus erythematosus, macrophages
Visual Abstract
Abstract
Background
Clinical distinction between patients with lupus nephritis who have active inflammation or chronic kidney damage is challenging. Studies have shown soluble CD163, which derives from cleavage of the CD163 M2c macrophage receptor and can be quantified in urine, correlates with active lupus nephritis.
Methods
We measured urine CD163 at lupus nephritis flares in patients from a Mexican cohort and cross-sectional and longitudinal United States cohorts. We also performed serial urine CD163 measurements during the treatment of flares in a subset of patients from the Mexican and longitudinal United States cohorts, and assessed response to therapy at 12 months. In addition, we evaluated urinary CD163 agreement with histologic activity in 19 patients from the Mexican cohort who had repeated kidney biopsies on follow-up.
Results
Urinary CD163 levels were significantly higher in patients with active lupus nephritis than in patients with active extrarenal SLE, inactive SLE, and other glomerular diseases, and correlated with disease clinical severity, histologic class, and the histologic activity index. Urinary CD163 increased from 6 months preflare to flare, diminishing progressively in complete and partial responders, whereas it remained elevated in nonresponders. Urinary CD163 <370 ng/mmol at 6 months predicted complete renal response at 12 months with >87% sensitivity and >87% specificity. Urinary CD163 <370 ng/mmol or >370 ng/mmol perfectly agreed (κ=1.0) with a histologic activity index ≤1 or >1 in repeated biopsies, respectively. Evaluation of urinary CD163 in patients with persistent proteinuria at 6 months improved the prediction of who would achieve complete renal response at 12 months.
Conclusions
Urinary CD163 reflects histologic inflammation in lupus nephritis and is a promising activity biomarker that varies over time with lupus nephritis activity and treatment.
Lupus nephritis (LN) is a serious and common manifestation of SLE that is associated with high morbidity and mortality.1 The progression rates to ESKD have not been substantially modified in the last several years and 10%–20% of patients still develop irreversible kidney damage.2 Management of LN remains challenging and it is important to be able to distinguish active nephritis from chronic kidney damage, because both often manifest as proteinuria and impaired kidney function. Furthermore, clinical and histologic findings are often discordant3−5 and currently used noninvasive biomarkers lack sufficient sensitivity and specificity to detect active renal inflammation.6
CD163 is a 130-kDa transmembrane protein, a member of the cysteine-rich scavenger receptor superfamily type B originally described as a scavenger receptor for hemoglobin-haptoglobin complexes.7 This receptor is mainly expressed by M2c macrophages that infiltrate tissues during the “healing phase” of inflammation.8,9 The infiltrating macrophages polarize to an alternative activated or M2c phenotype in response to the local microenvironment.8,10,11 CD163+ cells have been found in cellular crescents, proliferative glomerular lesions, and acute tubulointerstitial lesions in patients with LN.12−14 In a previous study from our group, we observed that CD163 gene expression is increased in glomeruli from patients with active LN compared with healthy kidney donors (Supplemental Figure 1).15 This has been corroborated by a single-cell transcriptomic study showing that M2c macrophages expressing the CD163 receptor infiltrate kidney tissue and represent the most numerous cells detected in urine from patients with LN.16
Soluble CD163 (sCD163) is derived from the cleavage of the CD163 macrophage receptor by metalloproteinases.17,18 After cleavage, the sCD163 is shed into the urine where it can be detected in active kidney diseases such as ANCA-associated vasculitis (AAV) and LN.19–23 A previous study in a Japanese lupus population reported elevated urinary sCD163 (uCD163) levels in patients with active LN and showed a correlation between uCD163 levels and the number of infiltrating glomerular macrophages, suggesting uCD163 may be a biomarker of kidney inflammation in LN.20
This study was undertaken to determine whether the reported correlation of uCD163 with active LN was reproducible in independent, ethnically distinct LN cohorts, and to evaluate its ability to predict response to treatment compared with that of currently used clinical and serologic biomarkers.
Methods
Patients
This study consisted of a cross-sectional and a longitudinal evaluation. The cross-sectional evaluation was done to validate a previous Japanese study that suggested uCD163 is a potential disease activity biomarker of LN.20 Biologic samples from patients with biopsy sample-proven active LN were obtained from two biorepositories: a Mexican cohort (n=120) and an Ohio State University (OSU) cohort (n=129). For both biorepositories, plasma and urine samples were obtained preprocedure from all patients who underwent a kidney biopsy, and samples were immediately processed and stored at −70°C. For the cross-sectional study, samples were collected between August 2005 and May 2018 for the OSU cohort at The OSU Wexner Medical Center and between March 2016 and September 2018 for the Mexican cohort at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran. All clinical data from the time of the LN flare/kidney biopsy were obtained from OSU medical records or a prospective LN database established for the Mexican cohort.
For the longitudinal evaluation, 82 patients with active LN from the Mexican cohort were included as a training cohort, and findings were validated in 34 patients from the Ohio SLE Study (OSS) cohort who experienced 49 independent LN flares. The OSS has been previously described.24 Briefly, the OSS prospectively followed patients with LN bimonthly over several years, recording flare activity and response to treatment. In patients with multiple flares, each flare was considered an independent event if the minimum time between flares was ≥12 months. Patients from the Mexican cohort were prospectively followed with samples collected at flare and at 3, 6, and 12 months after treatment at the same center. None of the patients included were lost before 12 months. The agreement between uCD163 at follow-up and histologic findings was assessed in 19 patients from the Mexican cohort who had repeated kidney biopsies performed either for cause (no response [n=8] or relapse [n=2]) or per protocol at 12 months (n=9).
As study controls, we included specimens from 31 healthy living kidney donors and 70 patients with a diagnosis of SLE but without active LN (inactive LN [iLN]); these subjects were recruited from both centers. The iLN group (n=70) included 30 patients with active extrarenal systemic SLE (sSLE) and 40 patients with previous LN who had been in complete remission for >12 months and had minimal or no SLE activity (inactive SLE [iSLE]).
To evaluate the disease specificity of the biomarker, we included prebiopsy samples, recruited from both centers, from 13 patients with phospholipase A2 receptor antibody-positive membranous nephropathy, 13 with FSGS, 22 with AAV and kidney involvement, and 27 with IgA nephropathy. All samples were immediately processed and stored at −70°C. All patients were recruited after institutional review board approval and signed informed consent at both centers.
Concurrent corticosteroid treatment was determined for all patients with lupus. The patients were classified as receiving no corticosteroids, low-dose corticosteroids (prednisone equivalent of ≤7.5 mg/d), or moderate/high-dose corticosteroids (prednisone equivalent >7.5 mg/d). In both cohorts, patients with lupus were followed monthly during the first 6 months of treatment and then at least quarterly for the first year. Data for the longitudinal cohorts were obtained from the OSS study database and the prospective LN database established for the Mexican cohort.
Kidney biopsies were classified according to the International Society of Nephrology/Renal Pathology Society criteria25 and scored by the National Institutes of Health (NIH) activity and chronicity indices.26,27 Each item comprising these scores was evaluated as: 0=not present or present in ≤5% of glomeruli, 1+=present in 6%–25% of glomeruli, 2+=present in 26%–50% of glomeruli, or 3+=present in >50% of glomeruli as recently revised.27 Biopsy interpretation was done before the availability of the biomarker results in all cases.
Definitions
Patients with active LN were defined as individuals who had at least four American College of Rheumatology (ACR) lupus criteria,28 who experienced a deterioration of kidney function attributable to LN, and/or proteinuria >500 mg/mg, with biopsy sample-proven immune complex GN.
Patients with iLN was defined as those with an SLE diagnosis according to ACR criteria28 with normal renal function, inactive urine sediment, and 24-hour proteinuria <250 mg maintained for at least 12 months before the time of evaluation (n=70). As defined above, the iLN group comprised patients with active extrarenal systemic manifestations of SLE (sSLE), defined by an SLE Disease Activity Index 2000 (SLEDAI-2K) score of more than six points including four points adjudicated to systemic manifestations, and patients with remitted LN for >12 months, with mild or no SLE activity defined by a SLEDAI-2K score of less than six points (iSLE).
Living kidney donor biopsies routinely done at implantation served as a source of normal tissue after clinical analysis of the tissue was complete. Urine samples were obtained predonation. The diagnosis of other glomerular diseases was based on histologic features of the renal biopsy.
Response to treatment was evaluated at the 12th month of treatment in the patients followed longitudinally.29,30 Complete renal response was defined as stable kidney function (within 15% of baseline) plus a 24-hour urine protein-creatinine ratio (uPCR) <0.5 g/g. Partial response was defined as stable kidney function plus 50% reduction of baseline proteinuria to subnephrotic range if originally nephrotic. Nonresponders did not meet criteria for complete or partial response. Histologic remission was defined as an activity index ≤1 at the repeated biopsy. LN flare severity was classified as we have done previously and outlined in Supplemental Table 1.30
Plasma sCD163 and uCD163 Assessment
Plasma sCD163 and uCD163 were measured using a commercial ELISA kit following manufacturer’s instructions (DuoSet DY1607; R&D systems, Minneapolis, MN). Active LN plasma and urine samples were diluted 100-fold and zero- to 100-fold, respectively, and processed in duplicate with intra- and interassay coefficient of variation <10%. Urine CD163 values were normalized to urine creatinine and expressed as ng per mmol of urine creatinine.
Statistical Analyses
Data distribution was evaluated by the Kolmogorov–Smirnov test. Descriptive statistics were expressed by absolute and relative frequencies or median and interquartile range (IQR). Group comparisons were made by the Kruskal–Wallis, chi-squared or Fisher exact tests as appropriate. Spearman correlations between uCD163 and histologic variables were computed and, in some cases, Pearson correlations between log10-transformed data were used to describe associations. All Spearman correlations are labeled Spearman. If not labeled, the correlation is a Pearson correlation. To evaluate the factors associated with the histologic activity index, we fitted linear regression models with log10-transformed values including age, eGFR, proteinuria, complement protein (C3), C4, plasma sCD163, and uCD163. For the longitudinal analysis, linear mixed models were fitted to evaluate the association between repeated uCD163 measurements and response to therapy in each cohort. The model included log10 uCD163 as the dependent variable; treatment, response group, months of treatment and their interaction, age, sex, eGFR, proteinuria, anti-double stranded DNA (anti-dsDNA) antibodies and C3 course over repeated measurements, and diagnostic biopsy histologic activity index as fixed effects; and subjects as random effects. The estimated means and their 95% confidence intervals were obtained and pairwise comparisons for each time point performed with Bonferroni correction. Diagnostics of the final model included evaluation of the distribution of the standardized residuals and the homogeneity of the residual variance among response groups. The 12-month, response-predictive yields of uCD163, proteinuria, C3, C4, and anti-dsDNA antibodies were determined by receiver operator characteristic (ROC) curves for each time point and the best cutoff was selected at the 6-month time point in the training cohort (Mexican cohort), and then validated in the validation cohort (OSS cohort). Recursive partitioning trees and net reclassification indices were calculated to assess the added value of uCD163 measurement over proteinuria. Agreement between uCD163, uPCR, anti-dsDNA antibodies, C3, C4, and histologic activity in the repeated biopsies was evaluated by Cohen κ. Factors associated with time to doubling of serum creatinine were assessed by Cox regression analysis. Analyses were performed with SPSS version 24.0 (IBM, Armonk, NY) and GraphPad Prism 6 (GraphPad Software, San Diego, CA).
Results
Cross-Sectional Analysis
Urine and serum samples were collected at the time of kidney biopsy in two cohorts of patients with lupus to examine the association of uCD163 levels with active LN, and validate uCD163 as a potential biomarker of LN activity. One cohort, comprised mainly of white and black patients, was established at OSU; the second cohort was established in Mexico City and patients were almost all Mexican Mestizo. The clinical and renal histologic data of these patients are presented in Table 1 and Supplemental Tables 2 and 3. To determine the specificity of uCD163 for LN, CD163 was also measured in the urine of patients with primary membranous nephropathy, FSGS, ANCA-associated nephritis, and IgA nephropathy collected at the time of diagnostic kidney biopsy (Supplemental Table 2).
Table 1.
Characteristics of the patients included in the cross-sectional evaluation
| Characteristics | Active LN Mexican Cohort (n=120) | Active LN OSU Cohort (n=129) | iLN (n=70) | Healthy Kidney Donors (n=31) | |
|---|---|---|---|---|---|
| iSLE (n=40) | Active Extrarenal SLE (n=30) | ||||
| Age, yr | 32 (25–39) | 31 (26–40) | 44 (30–57) | 42 (27–50) | 34 (29–41) |
| Female, n (%) | 108 (90) | 106 (82) | 37 (93) | 27 (90) | 23 (74) |
| Race, n (%) | |||||
| White | 1 (1) | 61 (47) | 5 (13) | 16 (53) | 12 (39) |
| Black | 0 (0) | 56 (44) | 1 (2) | 10 (33) | 5 (16) |
| Asian | 0 (0) | 4 (3) | 0 (0) | 0 (0) | 0 (0) |
| Mexican Mestizo | 119 (99) | 0 (0) | 34 (85) | 4 (13) | 10 (32) |
| Other | 0 (0) | 8 (6) | 0 (0) | 0 (0) | 4 (13) |
| Creatinine, mg/dl | 1.1 (0.7–2.0) | 0.9 (0.7–1.6) | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| eGFR, ml/min per 1.73 m2 | 68 (35–110) | 90 (48–123) | 105 (90–119) | 98 (85–116) | 101 (96–109) |
| Proteinuria, 24 h g/g | 3.3 (2.2–5.2) | 2.6 (0.9–4.8) | 0.1 (0.0–0.25) | 0.1 (0.0–0.25) | 0.1 (0.0–0.1) |
| Positive dsDNA antibodies | 101 (84) | 89 (69) | 23 (58) | 26 (87) | — |
| C3, mg/dl | 68 (44–88) | 62 (43–86) | 104 (88–122) | 94 (65–109) | — |
| C4, mg/dl | 8 (8–13) | 11 (8–18) | 21 (10–27) | 15 (11–35) | — |
| SLEDAI-2K, points | 16 (14–22) | 17 (9–24) | 0 (0–4) | 17 (10–24) | — |
All values are expressed as median (IQR) or number (relative frequency). eGFR calculated by the CKD-Epidemiology Collaboration formula.
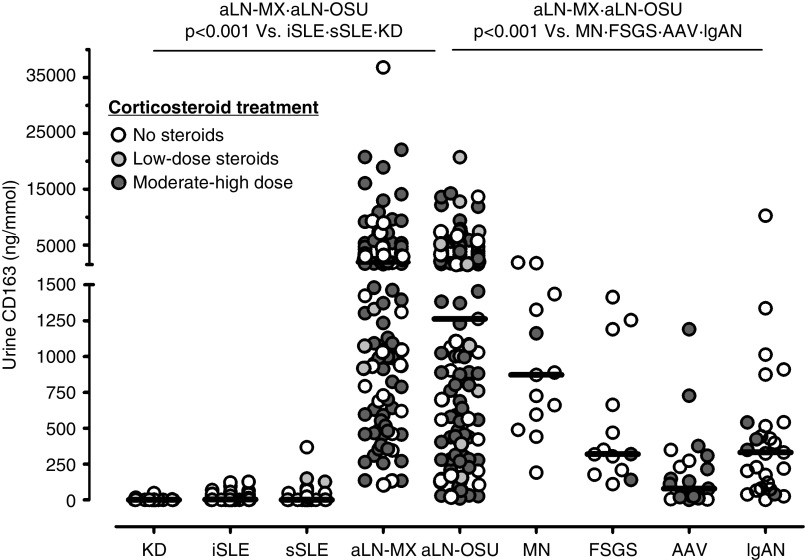
Urine CD163 levels were significantly higher (P<0.001) in patients with active LN (1596 ng/mmol; IQR, 588–3899 ng/mmol) than healthy controls (0 ng/mmol; IQR, 0–1 ng/mmol), patients with iSLE (2.8 ng/mmol; IQR, 0–35 ng/mmol), and patients with active extrarenal SLE (0 ng/mmol; IQR, 0–31 ng/mmol) (Figure 1). Urine CD163 levels did not correlate with corticosteroid dose (Spearman r=0.12, P=0.07). There were no differences in baseline characteristics between those with undetectable or detectable uCD163 values in any of the control groups.
Figure 1.
Urine CD163 levels are higher in active lupus nephritis than in patients with lupus without nephritis and other glomerular diseases. There was no correlation between corticosteroid dose and uCD163 in all patients or in individual groups. AAV, n=23; aLN-MX, active LN Mexican cohort, n=120; aLN-OSU, active LN OSU cohort, n=129; FSGS, n=13; IgAN, IgA nephropathy, n=27; iSLE, n=40; KD, kidney donors, n=30; MN, membranous nephropathy, n=13; sSLE, n=30.
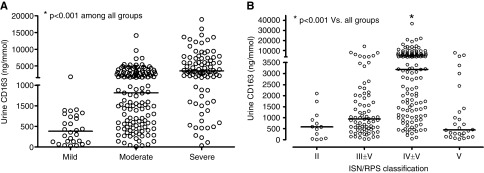
Among glomerular diseases, uCD163 levels were highest in LN (Figure 1). The level of uCD163 varied by pathologic class of LN, increased with the clinical severity of the flare (Figure 2), and was higher in patients presenting with nephritic syndrome (2376 ng/mmol; IQR, 1011–5208 ng/mmol) or nephrotic syndrome (2678 ng/mmol; IQR, 982–6019 ng/mmol) compared with those presenting with non-nephrotic proteinuria, hematuria, and normal renal function (760 ng/mmol; IQR 307–1778 ng/mmol; P<0.001).
Figure 2.
Urine CD163 levels increase with the clinical and histological severity of lupus nephritis. Urinary CD163 as (A) a function of LN severity and (B) International Society of Nephrology/Renal Pathology Society (ISN/RPS) class.
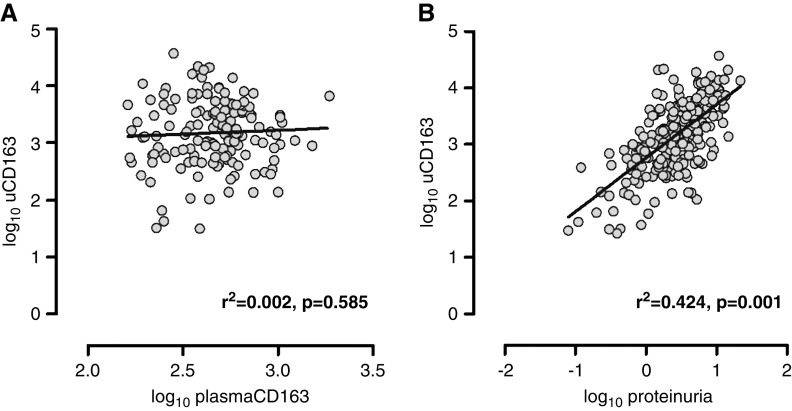
There was no correlation between log10-transformed plasma and log10-uCD163 (Pearson r=0.04; Figure 3), and plasma levels did not vary with flare severity, histologic class, or disease presentation. Proteinuria accounted for about 40% of the variability in log10-uCD163 levels (r2=0.42; Figure 3). The levels of uCD163 remained elevated after correction by total proteinuria (Supplemental Figure 2). Immunoblotting done with the CD163 ELISA capture antibody detected the same CD163 mol wt species in plasma, serum, and urine (data not shown).
Figure 3.
Urine CD163 correlates modestly with proteinuria but not with plasma CD163 in lupus nephritis patients. Correlation between (A) plasma and urinary CD163, and (B) between uCD163 and proteinuria.
Spearman correlations were calculated between uCD163, the histologic activity index, and each of its components (Table 2). Urinary CD163 levels positively correlated with the histologic activity index in the OSU (r=0.59, P<0.001) and Mexican (r=0.48, P<0.001) cohorts. Urinary CD163 correlated best with activity in the glomerular compartment compared with the tubulointerstitium. There was no correlation between uCD163 and the histologic chronicity index or its components (Table 2). The correlation between uCD163 and the activity index was maintained across all levels of histologic chronicity (Supplemental Figure 3).
Table 2.
Correlation between uCD163 levels and histologic activity and chronicity components
| Histologic Component | All Patients (n=249) | Mexican Cohort (n=120) | OSU Biopsy Cohort (n=129) | |||
|---|---|---|---|---|---|---|
| Spearman r | P Value | Spearman r | P Value | Spearman r | P Value | |
| Activity index | 0.53 | <0.001 | 0.48 | <0.001 | 0.59 | <0.001 |
| Endocapillary hypercellularity | 0.37 | <0.001 | 0.43 | <0.001 | 0.45 | <0.001 |
| Leukostasis | 0.43 | <0.001 | 0.44 | <0.001 | 0.41 | <0.001 |
| Fibrinoid necrosis | 0.33 | <0.001 | 0.27 | 0.003 | 0.42 | <0.001 |
| Hyaline thrombi | 0.38 | <0.001 | 0.37 | <0.001 | 0.38 | <0.001 |
| Wire loops | 0.42 | <0.001 | 0.35 | <0.001 | 0.51 | <0.001 |
| Karyorrhexis | 0.34 | <0.001 | 0.30 | 0.001 | 0.42 | <0.001 |
| Cellular crescents | 0.39 | <0.001 | 0.33 | <0.001 | 0.46 | <0.001 |
| Interstitial inflammation | 0.28 | <0.001 | 0.31 | <0.001 | 0.25 | 0.004 |
| Chronicity index | 0.11 | 0.10 | 0.25 | 0.10 | −0.05 | 0.58 |
| Glomerular sclerosis | −0.02 | 0.76 | −0.16 | 0.08 | −0.10 | 0.24 |
| Fibrous crescents | 0.03 | 0.62 | 0.19 | 0.07 | −0.12 | 0.19 |
| Interstitial fibrosis | 0.07 | 0.30 | 0.14 | 0.14 | −0.01 | 0.90 |
| Tubular atrophy | 0.07 | 0.24 | 0.16 | 0.08 | −0.01 | 0.88 |
Histologic LN Activity Predictors
Factors associated with the histologic activity index were explored by linear regression analyses with log10-transformed variables. As shown in Supplemental Table 4, a model including age, eGFR, proteinuria, and C3 was significant with r2=0.30. This model improved by substituting uCD163 for proteinuria (r2=0.36), but did not improve further by including proteinuria and uCD163 (r2=0.36).
Urinary CD163 Differentiates Active from iLN
As a last step of the cross-sectional evaluation, uCD163 levels were evaluated by ROC curves in the predefined groups of active and iLN. The area under the curve was 0.998 (95% CI, 0.995 to 1.00) and 0.980 (95% CI, 0.96 to 0.99) in the Mexican and OSU cohorts, respectively. A cutoff >130 ng/mmol had 97% (95% CI, 90% to 99%) sensitivity and 94% (95% CI, 91% to 97%) specificity to differentiate active LN from iLN in the combined sample (Supplemental Figure 4).
Longitudinal Analysis
The course of uCD163 expression over time was studied. Serial urine samples were available from 49 OSS LN flare cycles and 82 Mexican LN flare cycles (Supplemental Table 5). From the OSS cohort, 34 patients experienced 49 independent LN flares. These patients were distinct from the cross-sectional (OSU) cohort. In the OSS, samples were collected before 25 flares, at flare, and at 6 and 12 months postflare for all flares. After 12 months of treatment, 22 (45%), nine (18%), and 18 (37%) OSS flares culminated in complete, partial, or nonresponse to treatment, respectively. In the Mexican cohort, 29 (35%), 22 (27%), and 31 (38%) flares culminated in complete, partial, or nonresponse to treatment, respectively. There was no significant difference in these proportions for the two cohorts (P=0.45, Fisher test).
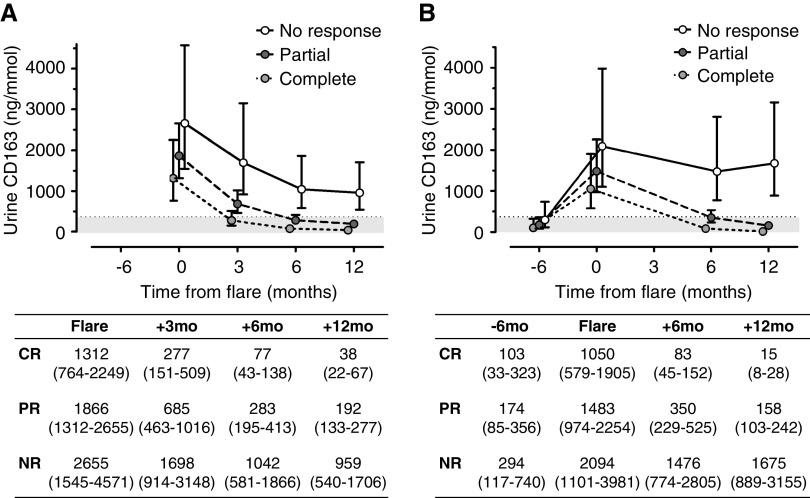
As shown in Figure 4, profiles of average uCD163 values over the course of an LN flare were different for flares culminating in clinical complete, partial, or no response at 12 months (interaction effect P<0.001). The mean uCD163 increased from preflare to flare and then fell during treatment in patients who had a clinical complete or partial response at 12 months in both cohorts (Figure 4, Supplemental Figure 5). Although uCD163 at flare was numerically higher in nonresponders, there were no statistically significant differences of uCD163 at flare between response groups. In nonresponders, uCD163 did not drop consistently or as profoundly as in responders during treatment (Figure 4). The course of uCD163 was conditionally associated with the group of response to therapy after controlling for age, sex, course of eGFR, proteinuria and C3, histologic class, biopsy activity index, and treatment received. The estimated means from this model and their 95% confidence intervals are shown in Figure 4.
Figure 4.
Urine CD163 levels during treatment of lupus nephritis are associated with response to therapy. Course of uCD163 in response to therapy in complete (CR), partial (PR), and nonresponders (NR) in (A) the Mexican cohort and (B) the OSS cohort. The graph shows the estimated means and their 95% confidence intervals obtained from the linear mixed model analysis. The gray area identifies the cutoff of uCD163=370 ng/mmol.
ROC curves were constructed from the training (Mexican) cohort to determine the best 6-month biomarker cutoffs for predicting a 12-month complete renal response (Supplemental Table 6). Urine CD163, proteinuria, anti-dsDNA antibodies, C3, and C4 were individually examined as a biomarker. Urine CD163 <370 ng/mmol at 6 months had 90% sensitivity and 87% specificity to predict a complete renal response by month 12, compared with a uPCR <1.5 g/g at 6 months which had 86% sensitivity and 81% specificity for complete renal response.
In the validation (OSS) cohort, uCD163 <370 ng/mmol at 6 months had 87% sensitivity and 89% specificity for predicting complete renal response by 12 months, verifying this observation from the training cohort (Table 3). Although proteinuria is the most important component of clinical renal response in LN, it is also often viewed as an LN activity biomarker. The diagnostic performance of uCD163 was therefore compared with a 6-month proteinuria level of <1.5 g/g, determined to be the best cutoff by ROC analysis, and a 6-month proteinuria level of <0.5 g/g, generally used to represent complete renal remission (Table 3). Urine CD163 was similar to proteinuria at 1.5 g/g for predicting complete remission at 12 months, and far more sensitive albeit less specific than proteinuria at 0.5 g/g. Finally, uCD163 was compared with several serologic biomarkers that are often examined early in the course of LN treatment and are considered to be useful in gauging how successful therapy will be. As shown in Table 3, the diagnostic performance of uCD163 was superior to that of conventional serologic biomarkers.
Table 3.
Diagnostic performance of 6-month biomarkers to discriminate 12-month complete renal response from no response in the training and validation cohorts
| Biomarker | Cohort | Sensitivity | Specificity | PPV | NPV | +LR | −LR |
|---|---|---|---|---|---|---|---|
| uCD163<370 ng/mmol | Training cohort | 0.90 | 0.87 | 0.87 | 0.90 | 6.95 | 0.12 |
| Validation cohort | 0.87 | 0.89 | 0.91 | 0.84 | 7.82 | 0.15 | |
| uPCR<0.5 g/g | Training cohort | 0.52 | 0.97 | 0.94 | 0.68 | 16.0 | 0.22 |
| Validation cohort | 0.48 | 1.00 | 1.00 | 0.60 | a | 0.52 | |
| uPCR<1.5 g/g | Training cohort | 0.86 | 0.81 | 0.81 | 0.86 | 4.45 | 0.17 |
| Validation cohort | 0.87 | 0.89 | 0.91 | 0.84 | 7.82 | 0.15 | |
| 25% uPCR reduction | Training cohort | 0.86 | 0.65 | 0.69 | 0.83 | 2.43 | 0.21 |
| Validation cohort | 0.87 | 0.56 | 0.71 | 0.77 | 1.96 | 0.23 | |
| Anti-dsDNA antibody disappearance | Training cohort | 0.66 | 0.48 | 0.54 | 0.60 | 1.27 | 0.71 |
| Validation cohort | 0.70 | 0.56 | 0.67 | 0.59 | 1.57 | 0.55 | |
| 25% anti-dsDNA antibody reductionb | Training cohort | 0.83 | 0.45 | 0.59 | 0.74 | 1.51 | 0.38 |
| Validation cohort | — | — | — | — | — | — | |
| C3 normalization | Training cohort | 0.76 | 0.55 | 0.61 | 0.71 | 1.68 | 0.44 |
| Validation cohort | 0.70 | 0.67 | 0.73 | 0.63 | 2.09 | 0.46 | |
| 25% C3 increase | Training cohort | 0.76 | 0.68 | 0.69 | 0.75 | 2.35 | 0.36 |
| Validation cohort | 0.65 | 0.72 | 0.75 | 0.62 | 2.35 | 0.48 |
PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
The denominator is zero.
Anti-dsDNA antibody values were only available as positive/negative from the validation cohort.
Early Prediction of Renal Response
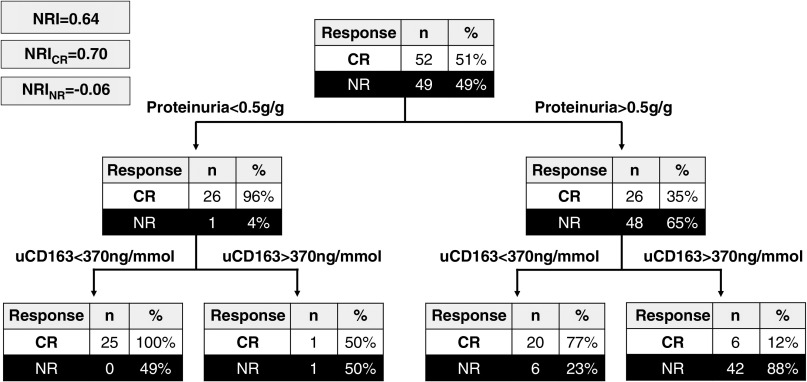
Survival analysis demonstrated that the median time to reach a level of uCD163 <370 ng/mmol was 3 months (IQR, 2–4 months), compared with a median of 8 months (IQR, 5–11 months) for proteinuria to reach 0.5 g/g, the level of complete renal response (Supplemental Figure 6). This suggested that evaluating uCD163 plus proteinuria may provide added value to the early determination of 12-month renal response. This was tested using recursive partitioning trees to determine the net reclassification index (NRI). As shown in Figure 5, uCD163 measurement after proteinuria evaluation increased the identification of complete responders from 35% to 77% in the group with persisting proteinuria >0.5 g/g with an NRI of 0.64. Similarly, in patients with proteinuria >1.0 g/g at 6 months, uCD163 increased the identification of complete responders from 24% to 69% with an NRI of 0.13.
Figure 5.
Urine CD163 measurement allows to better identify responders in patients with persisting proteinuria. At 6 months, 27 patients had proteinuria <0.5g/g (left arm); 26 (96%) achieved complete remission (CR) by 12 months. There was little added value of measuring uCD163 in these patients. At 6 months, there were 74 patients with proteinuria >0.5g/g (right arm); 26 (35%) of these patients achieved CR by 12 months. Measuring uCD163 helped correctly identify the 20/26 patients and 42/48 patients who achieved CR and nonresponse (NR), respectively by 12 months. NRI, net reclassification index; NRICR, NRI component for complete remission; NRINR, NRI component for nonresponders.
Relationship Between uCD163 Levels and Histologic Response
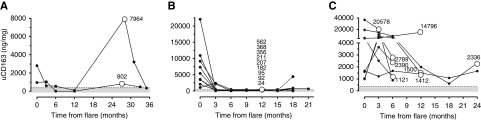
Due to the reported discordance between clinical and histologic response,3−5 and to demonstrate that a reduction or increase in uCD163 reflects a decline or increase, respectively, in the histologic activity of LN, uCD163 was measured in 19 patients from the Mexican cohort who had a repeat biopsy during follow-up (Table 4). Nine patients with complete and partial response were biopsied per protocol at 12 months (patients 45, 46, 49, 50, 52, 99, 118, 119, 120). Of these, the uCD163 level was <370 ng/mmol in eight patients, and all eight had a histologic activity index ≤1. One partial responder (patient 49) had a uCD163 level of 562 ng/mmol and an activity index of 4. Two complete responders (patients 11 and 12) at 12 months, who had uCD163 levels <370 ng/mmol, relapsed during follow-up and were rebiopsied. At relapse, uCD163 increased to well over 370 ng/mmol. Finally, eight patients (patients 14, 16, 35, 37, 56, 65, 105, 117) who were rebiopsied at variable time points for no response to therapy had persistently elevated (>1000 ng/mmol) uCD163 and persistent histologic activity (Figure 6).
Table 4.
uCD163 in patients with repeated biopsies on follow-up
| Patient | First Biopsy (diagnostic biopsy at flare) | Follow-up | Second Biopsy (per protocol or per-cause) | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Histopathology | uCD163 (ng/mmol) | 12-mo Response | uCD163 (ng/mmol) | Indication | Histopathology | uCD163 (ng/mmol) | Last Follow-up (mo) | |
| 11 | Class IV+V | 2807 | Complete response | 3 mo: 595 | Relapse at 28th mo | Class IV+V | 7964 | No response to reinduction (36 mo) |
| AI=12 | 6 mo: 248 | AI=6 | ||||||
| CI=3 | 12 mo: 87 | CI=7 | ||||||
| 12 | Class III+V | 950 | Complete response | 3 mo: 1032 | Relapse at 27th mo | Class V | 802 | Complete response to reinduction (35 mo) |
| AI=2 | 6 mo: 0 | AI=0 | ||||||
| CI=4 | 12 mo: 0 | CI=3 | ||||||
| 45 | Class IV+V | 1075 | Partial response | 3 mo: 2397 | Protocol at 12th mo | Class III | 368 | Complete response (20 mo) |
| AI=8 | 6 mo: 457 | AI=1 | ||||||
| CI=3 | 12 mo: 368 | CI=2 | ||||||
| 46 | Class IV+V | 22,072 | Partial response | 3 mo: 330 | Protocol at 12th mo | Class III | 211 | Partial response (20 mo) |
| AI=11 | 6 mo: 169 | AI=1 | ||||||
| CI=3 | 12 mo: 211 | CI=3 | ||||||
| 49 | Class IV+V | 3581 | Partial response | 3 mo: 428 | Protocol at 12th mo | Class III+V | 562 | Renal relapse (19 mo) |
| AI=10 | 6 mo: 308 | AI=4 | ||||||
| CI=5 | 12 mo: 562 | CI=3 | ||||||
| 50 | Class IV+V | 5401 | Complete response | 3 mo: 428 | Protocol at 12th mo | Class IV | 182 | Complete response (18 mo) |
| AI=14 | 6 mo: 232 | AI=1 | ||||||
| CI=2 | 12 mo: 182 | CI=4 | ||||||
| 52 | Class IV+V | 10,889 | Complete response | 3 mo: 125 | Protocol at 12th mo | Class III | 92 | Complete response (18 mo) |
| AI=16 | 6 mo: 114 | AI=1 | ||||||
| CI=4 | 12 mo: 92 | CI=4 | ||||||
| 99 | Class III+V | 3492 | Complete response | 3 mo: 50 | Protocol at 12th mo | Class III | 24 | Complete response (15 mo) |
| AI=3 | 6 mo: 87 | AI=0 | ||||||
| CI=3 | 12 mo: 24 | CI=4 | ||||||
| 118 | Class III+V | 2082 | Partial response | 3 mo: 238 | Protocol at 12th mo | Class III | 356 | Partial response (12 mo) |
| AI=10 | 6 mo: 137 | AI= 1 | ||||||
| CI=3 | 12 mo: 356 | CI= 2 | ||||||
| 119 | Class III+V | 7338 | Complete response | 3 mo: 352 | Protocol at 12th mo | Class III | 95 | Complete response (12 mo) |
| AI=4 | 6 mo: 0 | AI= 1 | ||||||
| CI=4 | 12 mo: 95 | CI = 7 | ||||||
| 120 | Class IV+V | 9169 | Partial response | 3 mo: 2138 | Protocol at 12th mo | Class III | 207 | Partial response (12mo) |
| AI=4 | 6 mo: 0 | AI= 1 | ||||||
| CI=4 | 12 mo: 207 | CI= 3 | ||||||
| 14 | Class IV+V | 1048 | No response | 3 mo: 7189 | No response at 12th mo | Class IV+V | 1500 | No response (32 mo) |
| AI=12 | 6 mo: 5738 | AI=12 | ||||||
| CI=6 | 12 mo: 1500 | CI=6 | ||||||
| 16 | Class III | 4267 | No response | 3 mo: 5098 | No response at 12th mo | Class IV+V | 14,796 | Progression to ESKD (16 mo) |
| AI=7 | 6 mo: 8318 | AI=17 | ||||||
| CI=1 | 12 mo: 14,796 | CI=5 | ||||||
| 35 | Class IV | 1675 | No response | 3 mo: 1220 | No response at 12th mo | Class IV+V | 1412 | DSCr (21 mo) |
| AI=3 | 6 mo: 1676 | AI=6 | ||||||
| CI=6 | 12 mo: 1412 | CI=7 | ||||||
| 37 | Class IV+V | 36,803 | No response | 3 mo: 20,578 | No response at third mo | Class IV+V | 20,578 | Progression to ESKD (3 mo) |
| AI=12 | AI=13 | |||||||
| CI=6 | CI=10 | |||||||
| 56 | Class IV+V | 18,922 | No response | 3 mo: 14,690 | No response at 24th mo | Class IV | 2336 | Response to change of IS (31 mo) |
| AI=11 | 6 mo: 8281 | AI=4 | ||||||
| CI=4 | 12 mo: 1998 | CI=7 | ||||||
| 65 | Class IV+V | 991 | No response | 3 mo: 10,462 | No response at sixth mo | Class IV+V | 2789 | Response to change of IS (14 mo) |
| AI=8 | 6 mo: 2789 | AI=10 | ||||||
| CI=6 | CI=5 | |||||||
| 105 | Class IV+V | 4718 | No response | 3 mo: 2526 | No response at sixth mo | Class IV+V | 1121 | Progression to ESKD (10 mo) |
| AI=5 | 6 mo: 1121 | AI=12 | ||||||
| CI=3 | CI=5 | |||||||
| 117 | Class IV+V | 1600 | No response | 3 mo: 3646 | No response at sixth mo | Class IV | 2390 | DSCr (12 mo) |
| AI=9 | 6 mo: 2390 | AI=8 | ||||||
| CI=7 | CI=10 | |||||||
AI, activity index; CI, chronicity index; IS, immunosuppression.
Figure 6.
Urine CD163 levels reflect histologic activity in lupus nephritis patients with repeated biopsies. Course of uCD163 in patients with repeated biopsies performed (A) for renal relapse, (B) per protocol at 12 months, or (C) for nonresponse to therapy. The white circle shows the time point where a kidney biopsy was performed and the numbers correspond to uCD163 levels (ng/mmol). The gray area identifies the cutoff of uCD163=370 ng/mmol.
There was a positive correlation (Spearman r=0.83, P<0.001) between the activity index and uCD163 in repeated biopsies and there was a perfect agreement (κ=1.0) between patients with activity index ≤1 and uCD163 <370 ng/mmol, and patients with activity index >1 and uCD163 >370 ng/mmol. In contrast, agreement between proteinuria <0.5 g/g and histologic activity index was only moderate (κ<0.6) because three patients with persistent proteinuria >0.5 g/g were demonstrated to have no histologic activity (Table 5, Supplemental Table 7).
Table 5.
Concordance analysis between clinical parameters at the time of a repeated biopsy and histologic activity index ≤1
| Parameter | Cohen κ | Interpretation |
|---|---|---|
| uCD163<370 ng/mmol | 1.000 | Perfect agreement |
| uPCR<0.5 g/g | 0.526 | Moderate agreement |
| uPCR<1.0 g/g | 0.553 | Moderate agreement |
| uPCR<1.5 g/g | 0.553 | Moderate agreement |
| Negative dsDNA-Ab | 0.526 | Moderate agreement |
| C3 normalization | 0.561 | Moderate agreement |
| C4 normalization | 0.658 | Moderate agreement |
The course of uCD163 and proteinuria was analyzed according to histologic response in patients with repeated biopsies. As shown in Supplemental Figure 7, both uCD163 and proteinuria remained elevated in patients without histologic response, whereas uCD163 and proteinuria values decreased in histologic responders. Urinary CD163 levels decreased to <370 ng/mmol by 3 and 6 months in five of eight (63%) and eight of eight (100%) of histologic responders, respectively; whereas proteinuria remained >0.5 g/g by 3, 6, and 12 months in two of eight (25%), two of eight (25%), and four of eight (50%) of histologic responders, respectively.
uCD163 and Kidney Outcomes
Over a median follow-up of 28 months (IQR, 19–41 months), 23/116 (20%) of patients from the combined cohorts developed a doubling of serum creatinine (DSCr). On univariate Cox regression analysis, baseline eGFR, histologic chronicity index, the 6- and 12-month treatment response, eGFR, proteinuria, and uCD163 levels were associated with DSCr (Supplemental Table 8). A persistence of uCD163 >370 ng/mmol at 6 and 12 months increased the risk for DSCr with a hazard ratio of 2.82 (95% CI, 1.11 to 7.16) and 3.62 (95% CI, 1.43 to 9.20), respectively.
Discussion
Studies of biomarkers in LN have been limited by their cross-sectional approach, small size, and evaluation in a single cohort without a validation cohort.6 In this study, we evaluated large, ethnically and clinically heterogeneous lupus populations using cross-sectional and longitudinal approaches. Urinary CD163 proved to be associated with the clinical severity and histologic activity of the LN, differentiated between active and inactive disease in predefined LN groups, and was modified by treatment with the response of the biomarker paralleling the clinical response. In patients responding to treatment, uCD163 declined faster than proteinuria and the improvement in serologic biomarkers and a uCD163 value <370 ng/mmol after induction treatment predicted complete renal response 6 months later. For patients with persistent proteinuria at 6 months, uCD163 accurately identified those patients who would go on to a complete clinical response. Importantly, in contrast to proteinuria, uCD163 perfectly agreed with the histologic activity findings, the gold standard for renal activity in patients with repeated biopsies on follow-up.
sCD163 derives from the cleavage of the CD163 M2c macrophage receptor.31,32 A role for macrophages and innate immunity in the pathogenesis of LN is well known,33 with previous studies demonstrating an association between the degree of macrophage kidney infiltration and renal prognosis.34,35 More recent studies have shown that the macrophage infiltrate in LN kidneys is predominantly composed of CD163+ cells.12−16 These “polarized” macrophages infiltrate inflamed tissues presumably during the healing phase of acute inflammation,8 although our data suggest more of an association with inflammatory activity in LN. Also, transcriptomic analysis of human glomeruli demonstrated an increased expression of myeloid lineage transcripts in LN, and specifically the CD163 gene.15,16,36
Urine CD163 has been previously shown to increase in several active glomerular diseases. In AAV, a cutoff of 300–350 ng/mmol differentiated active from inactive renal vasculitis with >70% sensitivity and >94% specificity.19,22 Similarly, we found an increase of uCD163 in several active glomerular diseases, but the highest uCD163 levels were found in active LN.
In patients with lupus, a previous study demonstrated that serum CD163 plasma levels increase in active nonrenal lupus.37 We found that CD163 increases both in plasma and urine during active LN, however, only uCD163 is associated with the clinical severity and the histologic activity of the disease. Moreover, uCD163 is not significantly increased in patients with extrarenal sSLE activity and does not correlate with plasma CD163, suggesting uCD163 is a specific biomarker of kidney inflammation.
Urinary CD163 behaves as a histologic biomarker that correlates with the number of CD163+ cells infiltrating the glomeruli. A previous study20 showed that uCD163 correlates with the histologic activity index, which we corroborated and extended by demonstrating individual correlations with each component of the activity index. We found that these associations are independent of renal function and proteinuria. Because uCD163 correlates with the degree of inflammatory glomerular infiltrates, it likely also differentiates between LN histologic classes with a high degree of macrophage infiltration (III/IV) and less inflammatory classes (II, V).
Urinary CD163 increases from preflare levels to LN flare, and then decreases during treatment, especially in patients who respond clinically. Urinary CD163 levels improved by the end of the induction phase of treatment, and a uCD163 level <370 ng/mmol predicted complete response to therapy by 12 months with high sensitivity and specificity. In contrast, proteinuria is well known to improve slowly, reaching its nadir well into the maintenance phase of treatment.38 Thus, uCD163 levels at the end of the intensive immunosuppressive phase of LN therapy may facilitate treatment decisions by discriminating patients who will undergo complete remission from nonresponders.
Moreover, uCD163 showed higher sensitivity and specificity to predict a clinical response to therapy compared with other serologic biomarkers. The few nonresponders with uCD163 <370 ng/mmol were patients with advanced chronic damage who progressed to ESKD, probably reflecting individuals with persistent proteinuria due to chronic damage as opposed to significant intrarenal inflammation. This observation remains to be validated in future studies with serial biopsies in patients with this specific clinical scenario. Furthermore, a persistently elevated uCD163 at 6 and 12 months was associated with worse kidney outcomes.
Proteinuria is used clinically as a biomarker of LN response and as a surrogate marker of LN remission. However, it has been demonstrated that the clinical and histologic responses in LN may be discordant, that is, the presence of proteinuria does not always reflect ongoing renal histologic activity and its absence does not always reflect resolution of kidney inflammation.3,4,5 We therefore measured uCD163 in patients who had serial biopsies, and showed that uCD163 levels accurately reflected histologic activity. In contrast, proteinuria has only moderate agreement with histologic findings in repeated biopsies. Urinary CD163 levels were persistently elevated (>370 ng/mmol) in cases with ongoing histologic activity and increased during renal relapse, whereas uCD163 decreased <370 ng/mmol in patients with histologic response. Interestingly, although patient 49 had a partial clinical response by 12 months, uCD163 remained >370 ng/mmol. Repeat kidney biopsy samples from this patient showed persistent histologic activity (activity index=4). Subsequently, the patient relapsed (uPCR>7 g/g) and uCD163 increased to 4410 ng/mmol. This case illustrates how uCD163 levels reflect kidney inflammation and may support therapeutic decisions or immunosuppression adjustments.
The optimal cutoff for uCD163 in LN remains to be defined with further studies. The observation that uCD163 decreased to zero in 23% of responders along with the median uCD163 of 2.8 ng/mmol in patients with iSLE suggests that uCD163 levels progressively decrease to barely detectable levels in patients who respond to therapy, possibly reflecting the complete resolution of intrarenal inflammation. An optimal early and late cutoff level in response to therapy should be further defined based on associations with histologic response and long-term outcomes (e.g., progression to CKD/ESKD). Urinary CD163 may be useful in additional clinical scenarios. For example, when attempting to taper or suspend maintenance immunosuppression, a high uCD163 may noninvasively identify ongoing intrarenal inflammation and a higher risk of relapse if treatment were to be stopped. This would be an important step forward because recent work demonstrated a higher risk of relapse after tapering maintenance therapy for patients with subclinical glomerular inflammation that was identified by repeat kidney biopsy.5
As we and others have shown, uCD163 is not specific for LN and its levels are elevated in several other glomerular diseases.12,13,20 For example, uCD163 has been recently proposed as an activity biomarker in AAV.22,23 Therefore, although uCD163 is useful to identify the inflammatory activity of LN, it does not differentiate between active LN and other inflammatory glomerular diseases.
A number of serum and urine biomarker candidates have been studied in LN in the last several years.6,39,40 Most of them assess the biomarker of interest in active LN groups based on clinical definitions or compare the biomarker to clinically defined response groups.41,42 Because proteinuria is an imperfect biomarker and a component of the clinical definition of remission, novel biomarkers should be tested against the gold standard of kidney histology as was done in this study.
The major limitation of this study is that we only had repeat kidney biopsies from a handful of patients to demonstrate that uCD163 reflects histologic as well as clinical renal response. To qualify uCD163 as a response biomarker and to demonstrate superiority over proteinuria and eGFR, a prospective follow-up study comparing renal histologic response and uCD163 will be needed. Nonetheless, correlation and agreement between uCD163 and histologic activity was very high in the subset of patients with repeated biopsies. Additionally, the data seem to suggest that persistently elevated uCD163 may be associated with a dismal renal prognosis but, to verify, longer follow-up is necessary.
In conclusion, our data coupled with previously published data demonstrate that uCD163 reflects LN activity in three independent cohorts that together present a significant sample size compared with most biomarker studies in LN. Urine CD163 also varies over time with LN histologic activity and treatment. These observations suggest that uCD163 may be a valuable LN activity biomarker.
Disclosures
Dr. Rovin reports personal fees from Astra Zenica, Aurinia, Bristol Myers Squibb, Callidatis, Chemocentryx, EMD Serono, Janssen, Morphosys, Novartis, Omeros, and Retrophin; nonfinancial support from the Lupus Foundation of America; and grants from NIH; outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT; Mexican National Council of Science and Technology) grant FOSSIS 2017-2-289663 (to Dr. Mejia-Vilet) and National Institute of Arthritis and Musculoskeletal and Skin Disorders grant RO1 AR071947 (to Dr. Rovin).
Supplementary Material
Acknowledgments
Dr. Mejia-Vilet and Dr. Rovin designed the study; Dr. Cano-Verduzco, Dr. Cruz, Dr. Mejia-Vilet, Dr. Morales-Buenrostro, Dr. Shapiro, and Dr. Zhang collaborated in patient recruitment, data acquisition, and organization; Dr. Cano-Verduzco, Dr. Cruz, and Dr. Zhang performed the laboratory analyses; Dr. Mejia-Vilet, Dr. Morales-Buenrostro, and Dr. Nagaraja analyzed the data; Dr. Cano-Verduzco and Dr. Mejia-Vilet made the figures; Dr. Mejia-Vilet, Dr. Nagaraja, and Dr. Rovin drafted and revised the manuscript; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019121285/-/DCSupplemental.
Supplemental Figure 1. Glomerular CD163 transcript expression.
Supplemental Figure 2. Urinary CD163 levels corrected by total proteinuria in different glomerular diseases.
Supplemental Figure 3. Heatmap showing correlation (Spearman r) of uCD163 with histological activity and chronicity indices.
Supplemental Figure 4. Diagnostic yield of uCD163 to differentiate between active and inactive lupus nephritis.
Supplemental Figure 5. Urinary CD163 levels over time in response to treatment.
Supplemental Figure 6. Time course of proteinuria and uCD163 by survival analysis.
Supplemental Figure 7. Urinary CD163 (A) and proteinuria (B) over time according to histological response.
Supplemental Table 1. Criteria for the clinical classification of severity of lupus nephritis flares.
Supplemental Table 2. Characteristics of the patients included in the cross-sectional evaluation.
Supplemental Table 3. Histological characteristics of the patients included in the study.
Supplemental Table 4. Factors associated with the histological activity index determined by linear regression analysis.
Supplemental Table 5. Characteristics of the lupus nephritis flares included in the longitudinal evaluation.
Supplemental Table 6. Predictors of a 12-month complete clinical renal response.
Supplemental Table 7. Agreement tables between histological activity and clinical parameters in patients with repeated biopsies.
Supplemental Table 8. Univariate Cox-regression analysis for doubling of serum creatinine over follow-up.
References
- 1.Mok CC, Kwok RC, Yip PS: Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 65: 2154–2160, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tektonidou MG, Dasgupta A, Ward MM: Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: A systematic review and Bayesian meta-analysis. Arthritis Rheumatol 68: 1432–1441, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarado AS, Malvar A, Lococo B, Alberton V, Toniolo F, Nagaraja HN, et al.: The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus 23: 840–847, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, et al.: Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant 32: 1338–1344, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Rosa M, Azzato F, Toblli JE, De Rosa G, Fuentes F, Nagaraja HN, et al.: A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int 94: 788–794, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Birmingham DJ, Merchant M, Waikar SS, Nagaraja H, Klein JB, Rovin BH: Biomarkers of lupus nephritis histology and flare: Deciphering the relevant amidst the noise. Nephrol Dial Transplant 32[Suppl 1]: i71–i79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al.: Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Anders HJ, Ryu M: Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Murray PJ: Macrophage polarization. Annu Rev Physiol 79: 541–566, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Amit I, Winter DR, Jung S: The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17: 18–25, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Glass CK, Natoli G: Molecular control of activation and priming in macrophages. Nat Immunol 17: 26–33, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Liu CH, Xu DL, Gao B: Significance of CD163-positive macrophages in proliferative glomerulonephritis. Am J Med Sci 350: 387–392, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Li J, Yu YF, Liu CH, Wang CM: Significance of M2 macrophages in glomerulonephritis with crescents. Pathol Res Pract 213: 1215–1220, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Olmes G, Büttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C: CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther 18: 90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mejia-Vilet JM, Parikh SV, Song H, Fadda P, Shapiro JP, Ayoub I, et al.: Immune gene expression in kidney biopsies of lupus nephritis patients at diagnosis and at renal flare. Nephrol Dial Transplant 34: 1197–1206, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, et al.; Accelerating Medicines Partnership in SLE network: The immune cell landscape in kidneys of patients with lupus nephritis [published correction appears in Nat Immunol 20: 1404, 2019]. Nat Immunol 20: 902–914, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Møller HJ, Nielsen MJ, Maniecki MB, Madsen M, Moestrup SK: Soluble macrophage-derived CD163: A homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 215: 406–412, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Etzerodt A, Maniecki MB, Møller K, Møller HJ, Moestrup SK: Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol 88: 1201–1205, 2010 [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly VP, Wong L, Kennedy C, Elliot LA, O’Meachair S, Coughlan AM, et al.: Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 27: 2906–2916, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo N, Tsuboi N, Furuhashi K, Shi Y, Du Q, Abe T, et al.: Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 31: 2023–2033, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa A, Tsuboi N, Yokoe Y, Katsuno T, Ikeuchi H, Kajiyama H, et al.: Urinary levels of the leukocyte surface molecule CD11b associate with glomerular inflammation in lupus nephritis. Kidney Int 95: 680–692, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Dekkema GJ, Abdulahad WH, Bijma T, Moran SM, Ryan L, Little MA, et al.: Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: A cohort study. Nephrol Dial Transplant 34: 234–242, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Moran SM, Monach PA, Zgaga L, Cuthbertson D, Carette S, Khalidi NA, et al.: Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 35: 283–291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN: Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol 16: 467–473, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al.: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, et al.: Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 75: 382–391, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al.: Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93: 789–796, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al.; American College of Rheumatology: American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64: 797–808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidney Disease Improving Global Outcomes: KDIGO Clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 1–274, 2012 [Google Scholar]
- 31.Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bächli E, Fehr J, et al.: Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol 74: 6–10, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Martinez FO, Sica A, Mantovani A, Locati M: Macrophage activation and polarization. Front Biosci 13: 453–461, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Maria NI, Davidson A: Renal macrophages and dendritic cells in SLE nephritis. Curr Rheumatol Rep 19: 81, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Hill GS, Delahousse M, Nochy D, Tomkiewicz E, Rémy P, Mignon F, et al.: A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int 58: 1160–1173, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, et al.: Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int 59: 304–316, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Peterson KS, Huang JF, Zhu J, D’Agati V, Liu X, Miller N, et al.: Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest 113: 1722–1733, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zizzo G, Guerrieri J, Dittman LM, Merrill JT, Cohen PL: Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Res Ther 15: R212, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korbet SM, Lewis EJ; Collaborative Study Group: Complete remission in severe lupus nephritis: assessing the rate of loss in proteinuria. Nephrol Dial Transplant 27: 2813–2819, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Caster DJ, Powell DW: Utilization of biomarkers in lupus nephritis. Adv Chronic Kidney Dis 26: 351–359, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monroy Trujillo JM, Fine DM: Lupus nephritis in the era of biomarkers. Clin J Am Soc Nephrol 11: 4–5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunner HI, Bennett MR, Gulati G, Abulaban K, Klein-Gitelman MS, Ardoin SP, et al.: Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J Rheumatol 44: 1239–1248, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R, Yadav A, Aggarwal A: Longitudinal assessment of monocyte chemoattractant protein-1 in lupus nephritis as a biomarker of disease activity. Clin Rheumatol 35: 2707–2714, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.