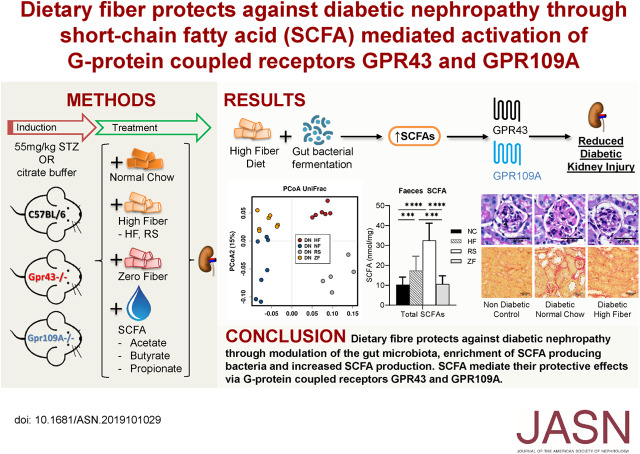
Significance Statement
The gut microbiota and its metabolites, in particular short-chain fatty acids derived from gut microbes’ fermentation of fiber, are emerging therapeutic targets for systemic inflammatory and metabolic diseases, including diabetic nephropathy. The authors report that high-fiber diets or supplementation with short-chain fatty acids (acetate, butyrate, or propionate) afforded protection against development of kidney disease in diabetic mice. Dietary fiber restored gut microbial ecology, corrected “dysbiotic” changes, and increased production of short-chain fatty acids. Mice deficient in the metabolite-sensing G protein–coupled receptors GPR43 or GPR109A were not protected by short-chain fatty acids, suggesting that protection was mediated by downstream binding to these receptors. Tapping into the metabolic potential of the gut microbiota through diet may offer a novel approach to address diabetic nephropathy.
Keywords: diabetic nephropathy, metabolism, chronic inflammation
Visual Abstract
Abstract
Background
Studies have reported “dysbiotic” changes to gut microbiota, such as depletion of gut bacteria that produce short-chain fatty acids (SCFAs) through gut fermentation of fiber, in CKD and diabetes. Dietary fiber is associated with decreased inflammation and mortality in CKD, and SCFAs have been proposed to mediate this effect.
Methods
To explore dietary fiber’s effect on development of experimental diabetic nephropathy, we used streptozotocin to induce diabetes in wild-type C57BL/6 and knockout mice lacking the genes encoding G protein–coupled receptors GPR43 or GPR109A. Diabetic mice were randomized to high-fiber, normal chow, or zero-fiber diets, or SCFAs in drinking water. We used proton nuclear magnetic resonance spectroscopy for metabolic profiling and 16S ribosomal RNA sequencing to assess the gut microbiome.
Results
Diabetic mice fed a high-fiber diet were significantly less likely to develop diabetic nephropathy, exhibiting less albuminuria, glomerular hypertrophy, podocyte injury, and interstitial fibrosis compared with diabetic controls fed normal chow or a zero-fiber diet. Fiber beneficially reshaped gut microbial ecology and improved dysbiosis, promoting expansion of SCFA-producing bacteria of the genera Prevotella and Bifidobacterium, which increased fecal and systemic SCFA concentrations. Fiber reduced expression of genes encoding inflammatory cytokines, chemokines, and fibrosis-promoting proteins in diabetic kidneys. SCFA-treated diabetic mice were protected from nephropathy, but not in the absence of GPR43 or GPR109A. In vitro, SCFAs modulated inflammation in renal tubular cells and podocytes under hyperglycemic conditions.
Conclusions
Dietary fiber protects against diabetic nephropathy through modulation of the gut microbiota, enrichment of SCFA-producing bacteria, and increased SCFA production. GPR43 and GPR109A are critical to SCFA-mediated protection against this condition. Interventions targeting the gut microbiota warrant further investigation as a novel renoprotective therapy in diabetic nephropathy.
Diabetic nephropathy (DN) is the leading cause of CKD globally and continues to grow in incidence and prevalence. Although hyperglycemia has historically been considered the driving force behind diabetic complications, increasing evidence points to chronic inflammation as having a central role.1–4 Intensive glycemic control, blockade of the renin-angiotensin-aldosterone system, and use of SGLT2 inhibitors can delay disease progression in diabetic nephropathy.5,6 However, these regimes provide only partial therapeutic effect, highlighting the need for further insights into the pathogenesis of diabetic nephropathy to mitigate the residual risk of progression to ESKD.
The gut microbiota evolves and exists in a symbiotic relationship with host, contributing to a plethora of physiologic functions including regulation of the gut barrier, development and maintenance of immune responses and energy metabolism.7 Disturbance of the normal gut microbiota (dysbiosis) has been implicated in the pathogenesis of obesity, diabetes, autoimmunity, inflammatory bowel disease, and more recently, kidney disease.8 Multiple studies have consistently demonstrated quantitative and qualitative “dysbiotic” changes to the gut microbiota in CKD and diabetes, which disturb the symbiotic relationship. The hallmark of this dysbiosis is depletion of short-chain fatty acid (SCFA)–producing bacteria, and in the case of CKD, an increase in pathobionts contributing to uremic toxicity.9 Production of uremic toxins, translocation across an impaired intestinal barrier and subsequent activation of the innate immune system with systemic inflammation, are some of the proposed mechanisms by which the gut microbiota may potentiate kidney disease.10 We have previously demonstrated the integral role innate immunity and inflammation play in progression of diabetic nephropathy through TLR2/4, and the potential for blockade of ligand-TLR interactions to prevent diabetic kidney injury and inflammation.3,4,11
Diet remains the largest exogenous determinant of gut microbiota composition12 and has garnered interest as a therapeutic avenue to re-establish symbiosis. Epidemiologic studies have demonstrated a link between higher dietary fiber intake and lower risk of inflammation and mortality, particularly in those with CKD.13 In clinical and experimental studies, the gut microbiome has been shown to determine clinical responses to immune-based anticancer therapies.14–16 Susceptibility to develop experimental type 1 diabetes17 was also shown to be microbiome dependent and manipulation of the microbiome was shown to alter susceptibility. SCFAs, the predominant metabolites produced by bacterial fermentation of dietary fiber, are proposed to play a key role in microbiota–host crosstalk. SCFAs have been demonstrated to reduce inflammatory responses in kidney ischemia reperfusion injury18 and modulate inflammation in vitro.19,20 Pathways regulated by the microbiome in these studies are known to be critically important in the pathogenesis of diabetic nephropathy and CKD; however, the role of the microbiome in diabetic nephropathy has not been elucidated, and evidence that manipulation of the microbiome may retard nephropathy is currently lacking.
In this study, we tested the hypothesis that gut dysbiosis contributes to the pathogenesis of diabetic nephropathy. Using a murine model, we sought to determine whether dietary modification or supplementation, both designed to increase SCFAs, would attenuate renal inflammation and retard the development of diabetic nephropathy. Here, we show for the first time that beneficial reshaping of the gut microbiota prevents nephropathy through the activation of SCFA-sensing receptors.
Methods
Animals
Wild-type (WT) C57BL/6 mice were obtained from the Animal Resource Centre (Perth, Western Australia). Gpr43−/− and Gpr109A−/− mice on a C57BL/6 background were bred and maintained in our facility. Mice were housed in a specific pathogen-free facility at the University of Sydney. Male mice aged 7–9 weeks were used in experiments. Animal care and experimental protocols were approved by the University of Sydney Animal Ethics Committee.
Induction of Diabetes
Diabetes was induced with intraperitoneal injections of streptozotocin (STZ), 55 mg/kg daily for five consecutive days. Age-matched littermates serving as nondiabetic controls received volume- and pH-matched citrate buffer. Mice with a blood glucose level >20 mmol/L were used to assess diabetic kidney injury. Blood glucose level was measured with an Accu-Check glucometer (Roche Diagnostics) using tail vein blood. All mice were euthanized 12 weeks after injection.
Diets and SCFA Treatment
All custom fiber-adjusted diets were purchased from Specialty Feeds, Australia, and on the basis of modifications to normal chow (NC) diet (AIN93G). Resistant starch (RS) diet (SF11–025) is enriched with 63.6% RS, High-fiber (HF) diet (SF11–029) is enriched in guar gum and cellulose (35% crude fiber), and zero-fiber (ZF) diet (SF09–128) is completely devoid of fiber (Supplemental Table 1). Mice received NC upon arrival at our facility, followed by randomization to specific diets. Sodium acetate (SA) 100 mM, sodium butyrate (SB) 50 mM, and sodium propionate (SP) 100 mM (Sigma-Aldrich) were dissolved and administered ad libitum in drinking water. Control mice received pH- and sodium-matched water. Diets and drinking solutions were refreshed three times per week.
Study Design
Diet Experiment
To assess the influence of dietary fiber supplementation, 3 weeks after STZ injection diabetic WT mice were randomized to four specific diet groups (with five mice per nondiabetic control group): (1) DN+RS, n=5; (2) DN+HF, n=10; (3) DN+NC, n=9; and (4) DN+ZF, n=9.
SCFA Experiment
WT diabetic mice were maintained on the control diet and randomized to receive acetate or butyrate in drinking water from 3 weeks after STZ injection, in the following groups (with five mice per nondiabetic control group): (1) DN+SA, n=10; (2) DN+SB, n=10; (3) DN+SP, n=10; and (4) DN+ water n=10.
Mechanistic Experiments
Diabetic mice deficient in G protein–coupled receptors (GPRs) GPR43 and GPR109A were randomized to RS diet or SCFA treatment at 3 weeks after STZ injection in the following groups: (1) Gpr43−/−+SA, n=10; (2) Gpr43−/−+ water, n=10; (3) Gpr109A−/−+SB, n=11; (4) Gpr109A−/−+ water, n=10; (5) WT B6+ water, n=10; (6) Gpr43−/−+RS, n=10; (7) Gpr43−/−+NC, n=10; (8) WT B6+RS, n=10; and (9) WT B6+NC, n=10.
Sample Collection
Blood, urine, and kidney tissue were harvested at 12 weeks as previously described.11 Feces were collected under sterile conditions, frozen on dry ice immediately after collection, and stored at −80°C.
Quantification of Urinary Albumin Excretion
Urinary albumin excretion was quantified using the Murine Albumin ELISA Quantitation Set (Bethyl Laboratories) as described previously.11 Urine creatinine was measured enzymatically by the Biochemistry Department of Royal Prince Alfred Hospital, Australia.
Histology
Periodic acid–Schiff (PAS) and picrosirius red staining were performed on 3 and 5 μm formalin-fixed kidney sections, respectively. Glomerular tuft area (AG) was measured in 20 glomerular profiles per mouse, using DP2-BSW software v2.2 (OLYMPUS). Glomerular volume (VG) was calculated using the following formula: VG=(β/κ) × (AG)3/2, where β=1.38 (shape coefficient for spheres) and κ=1.1 (size distribution coefficient).21 Glomerular extracellular matrix was defined as the PAS-positive area quantified by image analysis software (ImagePro Premier 9). Interstitial collagen was assessed in a blinded fashion by point counting using an ocular grid at ×400 magnification, in 20 consecutive fields.22
Immunohistochemistry
Staining for CD68+ and Wilms Tumor 1 (WT-1) was performed on 7 μm acetone-fixed frozen sections after application of a biotin blocker system (DAKO). Staining for type 1 collagen (Col-1) was performed on formalin-fixed paraffin sections (5 μm) after deparaffinization and antigen retrieval by boiling in 10 mM sodium citrate buffer (pH 6). After blocking with 20% normal horse serum, primary antibodies rat anti-mouse CD68 (ABD Serotec Inc), rabbit anti-WT1 (Abcam), or rabbit anti–Col-1 (Abcam) were applied and incubated for 60 minutes. For visualization of bound primary antibodies, sections were incubated with secondary antibodies biotinylated anti-rat IgG or anti-rabbit IgG (BD Pharmingen). Vector stain ABC kit (Vector Laboratories Inc) was applied, followed by 3,3′-diaminobenzidine solution (DAKO), and counterstaining with Harris hematoxylin.
Quantification of Immunostaining
Macrophage (CD68+) staining was assessed in a blinded manner by analysis of 20 consecutive high-power fields (×400 magnification) of renal cortex in each section. CD68-positive cells were counted using an ocular grid and expressed as cells per field. WT-1–positive cells were counted in 20 glomerular cross-sections (high-power fields, ×400 magnification). Results were expressed as podocytes per glomerulus using the equation Npodglom=NVpodglom×Vglom.23
Real-Time PCR
RNA was extracted from kidney tissue using TRIzol (Invitrogen). Complementary DNA was synthesized using oligo d(T) primers (Applied Biosystems) and the SuperScript III reverse transcription kit (Invitrogen). Complementary DNA was amplified in Universal Master Mix (Applied Biosystems) with gene-specific primers and probes, using the Roche Lightcycler 480 (Roche Applied Science). Specific TaqMan primers and probes used for TLR2, TLR4, IL6, IFNγ, TNFα, CCL2, CXCL10, TGFβ1, fibronectin, and GAPDH were obtained from Applied Biosystems. Results were normalized to GAPDH expression.
Bacterial DNA Sequencing
Bacterial genomic DNA was extracted from feces and purified using QIAamp DNA stool mini kit (QIAGEN), according to the manufacturer’s protocol. DNA was amplified using tagged amplicons spanning the V4 region of bacterial 16S ribosomal RNA gene (515f/806r) on the Illumina MiSeq Platform (2×250 bp), at the Ramaciotti Centre for Genomics (University of New South Wales, Sydney, Australia). Data were deposited in the European Nucleotide Archive under accession number PRJEB36826.
Bioinformatics Analysis
Bioinformatics analysis was performed using the QIIME v1.9.1 pipeline. Paired-end reads were assembled, chimeric sequences were detected and removed using Chimeraslayer, and operational taxonomic units were picked at 97% sequence identity using the uclust algorithm. Taxonomies were assigned with BLAST against the Greengenes database. Taxa present at <0.01% were filtered. Rarefaction analysis was used to compare the adequacy of sequencing depth (Supplemental Figure 1). Data were log2-transformed to account for non-normal distribution of taxonomic count data. α Diversity was measured using Shannon diversity and evenness. Bacterial community profiles were compared using weighted UniFrac clustering of OTU abundances. A Pearson correlation-based network showing relationships between albuminuria and bacterial taxa was visualized in Calypso (http://cgenome.net/calypso/).
SCFA Measurements
Proton nuclear magnetic resonance (1H NMR) spectroscopy was used for metabolic profiling of feces and serum. Fecal pellets were homogenized in cold deuterated water (D2O) by bead beating at 5 m/s for 40 seconds. The fecal slurry was centrifuged at 16,000×g for 5 minutes at 4°C. Aliquots of serum or fecal supernatant were passed through Amicon ultra cellulose filters with a cut-off molecular mass of 3 kDa (Millipore), mixed with buffers (200 mM Na3PO4/D2O and 5.0 mM DSS/D2O, pH 7.0), before being transferred into 3-mm NMR tubes for analysis. Spectra were recorded at 298K on a Bruker 600 MHz AVANCE III spectrometer. The spectra were processed and analyzed using Chenomx NMR Suite v8.4 (Chenomx Inc). Phase and baseline corrections were carried out, with the known concentration of DSS used to determine the concentrations of metabolites.
Primary Culture and Stimulation of Mouse Tubular Epithelial Cells and Podocytes
Mouse kidney tubular epithelial cells (TECs) and podocytes were isolated from C57BL/6 mice, then cultured as described previously.4 Cultured podocytes and TECs at 80% confluence were rinsed and incubated with serum-free DMEM/F12 medium for 48 hours. The cells were then exposed to 30 mM D-glucose (Invitrogen) or mannitol (5.5 mM glucose plus 24.5 mM mannitol) in the presence and/or absence of acetate (25 mM), propionate (12 mM), or butyrate (3.2 mM) for 12 hours.18 After stimulation, cells were harvested for RNA extraction and assessment of mRNA expression by RT-PCR.
Statistical Analyses
All results are expressed as mean±SD or mean±SEM. Data were analyzed using two-tailed t tests, or one- or two-way ANOVA with post hoc Bonferroni correction (GraphPad, San Diego, CA) where appropriate. A P value of <0.05 was considered statistically significant.
Results
Dietary Fiber Provides Protection against Albuminuria in Diabetic Nephropathy
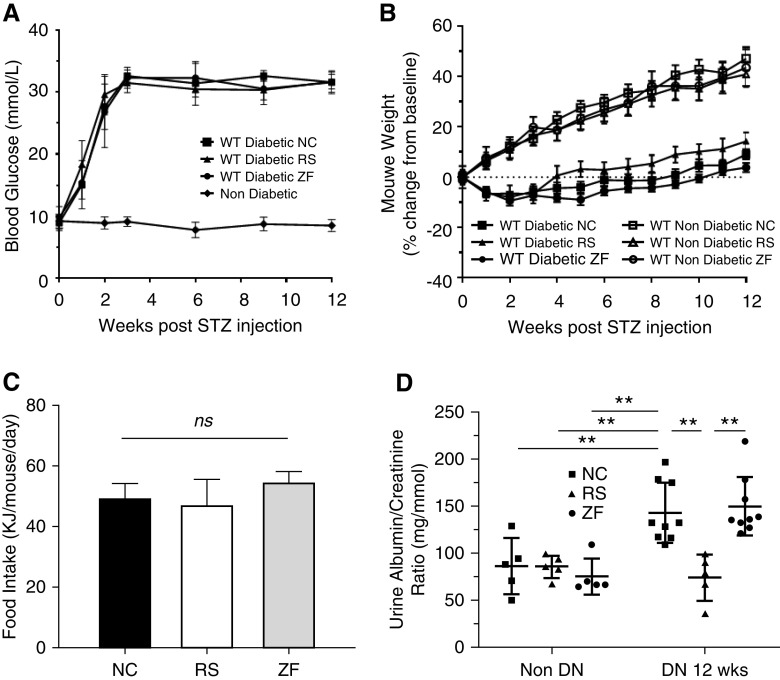
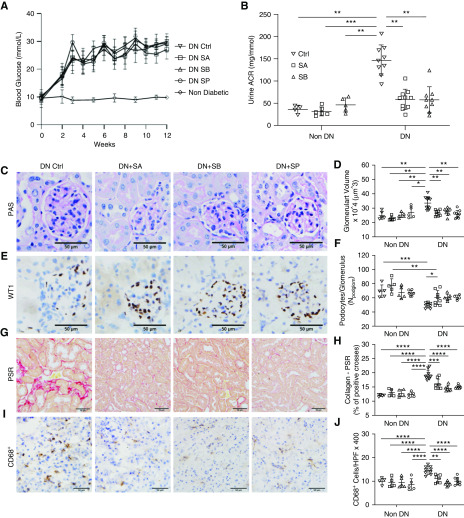
To assess the effect of dietary fiber on diabetic nephropathy, we fed WT diabetic mice diets containing standard (NC), deprived (ZF), or enriched amounts of fiber (RS), which are known to alter SCFA levels.24 After 12 weeks, all mice displayed a similar profile in the progression of hyperglycemia (Figure 1A), weight change (Figure 1B), and food intake (Figure 1C) over a 12-week period after STZ injection. Importantly, feeding with RS or ZF did not significantly alter the glucose profile relative to diabetic controls on NC.
Figure 1.
RS feeding provided protection against albuminuria in DN. STZ treatment induced diabetes in WT mice with similar severity, as indicated by (A) blood glucose, (B) body weight, and (C) food intake profiles. Progressive albuminuria is seen in diabetic ZF- and NC-fed mice compared with controls. (D) However, diabetic mice fed RS developed significantly less albuminuria, with no difference compared with nondiabetic controls at week 12. Data are shown as means±SD; **P<0.01.
Albuminuria is the earliest sign of diabetic nephropathy, reflecting damage to the glomerular filtration barrier, perpetuating disease progression through downstream inflammatory and fibrogenic pathways. NC- and ZF-fed diabetic mice developed progressive albuminuria, with significant elevation at 12 weeks after STZ injection compared with nondiabetic controls (U/ACR 138.7±28 and 149.88±44 versus 86.56±29 mg/mmol; P<0.01). Despite equivalent degrees of hyperglycemia, RS-fed diabetic mice were protected from the development of albuminuria with a significantly lower U/ACR at 12 weeks compared with NC- or ZF-fed diabetic mice (74.4±24 versus 138.7±28 and 149.88±4 mg/mmol; P<0.01) with no difference compared with nondiabetic controls (Figure 1D).
Dietary Fiber Attenuated Kidney Hypertrophy and Glomerular Injury
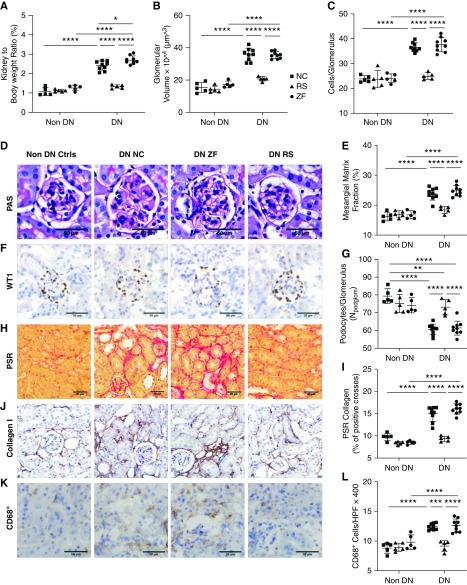
Diabetes-induced kidney hypertrophy was evident in NC- and ZF-fed mice, with an increase in kidney-to-body weight ratio compared with nondiabetic WT controls. RS feeding prevented kidney hypertrophy, with no significant increase in kidney-to-body weight ratio compared with nondiabetic controls at 12 weeks (Figure 2A). Histologic examination by light microscopy revealed attenuation of diabetic changes in the kidneys of RS-fed mice. Computerized morphometric analysis of PAS-stained sections demonstrated significant glomerular hypertrophy, hypercellularity, and increased mesangial matrix in NC- and ZF-fed diabetic mice, whereas these parameters were significantly reduced in RS-fed diabetic mice (Figure 2, B–E).
Figure 2.
RS feeding reduced glomerular and interstitial injury in DN. Diabetic mice fed RS are relatively protected from diabetes-induced glomerular and interstitial injury compared with NC- and ZF-fed mice, as indicated by (A) decreased kidney-to-body weight ratio, (B and D) VG, (C) glomerular hypercellularity, and (E) mesangial expansion. (D) Representative sections of glomeruli from NC-, ZF-, and RS-fed diabetic and NC-fed nondiabetic mice at 12 weeks (PAS-stained, ×400 magnification). (F and G) Representative sections of glomeruli stained for WT-1 at 12 weeks (×400 magnification), demonstrating podocyte density in nondiabetic mice, which is reduced in NC- and ZF-fed mice, with relative preservation in with RS feeding. Representative sections of kidney from diabetic and nondiabetic mice at 12 weeks demonstrating the increased interstitial collagen deposition being attenuated by RS feeding using (H and I) picrosirius red staining and immunostaining for (J) Col-I. (K) Representative sections of kidney from diabetic and nondiabetic mice stained for CD68. (L) Increased interstitial CD68+ macrophage accumulation is evident in diabetic NC- and ZF-fed, but not RS-fed mice. Data are shown as means±SD; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Progression of diabetic nephropathy is also characterized by loss of podocyte density, which typically parallels albuminuria. This was quantified by WT-1 expressed as a ratio to mean VG. Mice fed NC showed a marked reduction in the number of podocytes per glomerulus compared with nondiabetic WT controls at 12 weeks. Podocyte number was preserved in diabetic mice fed RS and was similar to nondiabetic WT mice of similar age (Figure 2, F and G).
Dietary Fiber Decreased Renal Fibrosis and Interstitial Macrophage Recruitment
Accumulation of extracellular matrix components, particularly collagen, is a prognostic marker for progressive diabetic nephropathy. Col-1 is minimally expressed in healthy kidneys but increases with disease processes causing fibrosis. To quantify collagen deposition, sections were stained using picrosirius red and immunostained for Col-1. Collagen deposition was significantly increased in NC-fed diabetic mice, predominantly localized to the tubulointerstitial space, whereas these changes were attenuated with RS feeding (Figure 2, H–J).
Macrophages are the predominant immune cell infiltrating diabetic kidneys and contribute to progression of renal injury. Immunostaining for the pan-macrophage marker CD68 revealed a dramatically increased number of interstitial macrophages at week 12 in the kidneys of diabetic NC- or ZF-fed mice. This accumulation of CD68-positive cells was significantly reduced in mice fed RS (Figure 2, K and L).
Collectively, our results support a protective role for dietary fiber against progression of diabetic nephropathy. This is reinforced by the finding that diabetic mice fed a second type of HF diet enriched in guar gum were also protected against nephropathy (Supplemental Figure 2).
Dietary Fiber Reduced the Expression of Inflammatory and Fibrotic Genes in Diabetic Kidneys
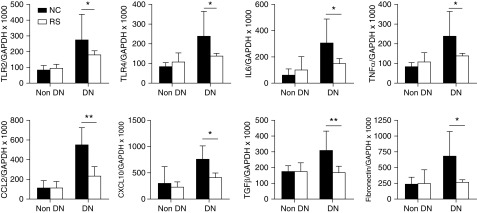
We next examined the expression of inflammatory molecules in diabetic kidneys. mRNA expression of innate immune receptors TLR2 and TLR4 was significantly increased in diabetic mice at 12 weeks; however, this upregulation was not observed in the kidneys of RS-fed mice. Downstream inflammatory cytokines and chemokines (IL6, TNFα, CCL2 and CXCL10) and fibrosis-related genes (TGFβ and fibronectin) were also upregulated in diabetic kidneys, and abrogated with RS feeding (Figure 3).
Figure 3.
RS feeding reduced inflammatory cytokine and chemokine mRNA expression in diabetic kidneys. mRNA expression of innate immune receptors (TLR2, TLR4), proinflammatory cytokines (IL6, TNFα), chemokines (CCL2, CXCL10), and fibrosis related genes (TGFβ, fibronectin) were significantly reduced in RS-fed mice compared with NC-fed diabetic controls. Data are shown as means±SD; *P<0.05; **P<0.01.
Dietary Fiber Altered Gut Microbiota Composition and Improved Dysbiosis in Diabetic Mice
With the observation that dietary fiber could attenuate the development of diabetic nephropathy, we next investigated whether the observed renoprotective effects were attributable to diet-induced alterations in gut microbial ecology. Feces were collected before and after STZ injection, and after maintenance on treatment diet. Microbiota composition was assessed by analyses of amplicons generated across the V4 region of the bacterial 16S ribosomal RNA genes.
We first assessed gut microbiota α diversity and composition before and after STZ injection with mice on NC. Although most commonly used in research as a diabetogenic drug for its cytotoxic effect on pancreatic islet β cells, STZ, derived from Streptomyces achromogenes, also has antibiotic activity.25 Expectedly, STZ caused a reduction in α diversity and disruption of the fecal bacterial community shortly after injection (Supplemental Figure 3). However, this change was relatively short-lived, with mice that remained on NC demonstrating spontaneous recovery of α diversity by 3 weeks after injection and before diet randomization. After randomization, the microbial composition in NC-fed, fiber-supplemented, and depleted groups all polarized according to diet type, highlighting the profound ability of diet to influence the gut microbiota.
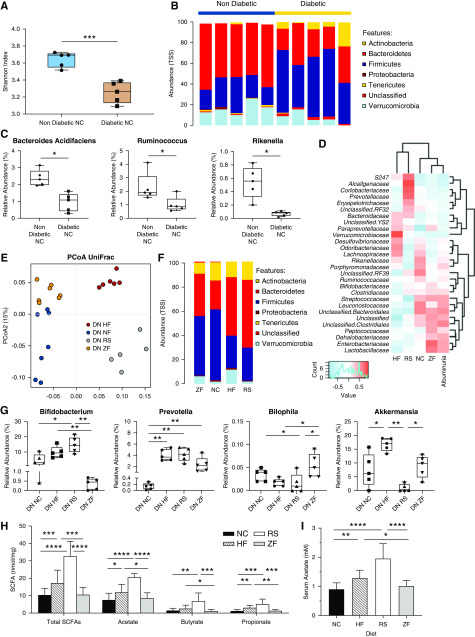
Diabetes induced a disease-related dysbiosis, evidenced by reduced α diversity as expressed by Shannon index (P=0.007) at 12 weeks compared with nondiabetic mice on an equivalent diet (Figure 4A). Relative abundance of bacteria at a phylum level was altered in diabetic mice, exhibiting increased Firmicutes and Actinobacteria but reduced Bacteroidetes compared with nondiabetic mice (Figure 4B). At the genus level, this correlated with relative loss of SCFA producers Bacteroides (species Bacteroides acidifaciens), Ruminococcus, and Rikenella (Figure 4C). Diabetes was also associated with a reduction in the genus Akkermansia (species Akkermansia muciniphila), a mucin-degrading bacteria integral to maintenance of gut integrity.
Figure 4.
Diet-induced alterations in microbial ecology contribute to protection against DN through enrichment of SCFA-producing species. (A) Diabetes influences microbiota composition, with OTU-based α diversity metrics showing loss of diversity in diabetic versus nondiabetic mice (Shannon index). (B) Relative abundance of bacteria presented at the phylum level, depicting changes in microbiota composition in diabetic versus nondiabetic mice. (C) At the genus and species levels, diabetic mice had lower levels of known SCFA producing Ruminococcus, Rikenella genera, and bacteria from the Bacteroides acidifaciens species than nondiabetic controls. (E) Fecal microbiota composition was analyzed by 16S ribosomal RNA sequencing from feces of diabetic mice at 12 weeks. The composition of gut microbiota in diabetes is influenced by diet. Principal coordinates analysis using weighted UniFrac distances depicts significant differences in the composition of the gut microbiota between HF-, RS-, NC-, and ZF-fed diabetic mice (analysis of similarities P<0.001). (F) Distribution of OTUs (key) in feces from diabetic mice fed different diets (horizontal axis); each OTU is presented as relative abundance at the phylum level. (G) RS and HF feeding ameliorates disease-related dysbiosis, resulting in expansion of the SCFA-producing Prevotella and Bifidobacterium genera, with relative reduction in pathobiont Bilophila. RS and HF diets had contrasting effects on relative abundance of Akkermansia. (D) Pearson correlation-based heatmap at a family level, identifying the bacteria associated with severity of albuminuria, with cool colors representing negative correlation and hot colors representing positive correlations. (H and I) Quantification of SCFA levels in feces and serum from diabetic mice fed fiber supplemented (HF, RS), standard (NC), or deplete (ZF) diets by 1H NMR spectroscopy. Data are shown as means±SD with a minimum of five mice per group; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
To assess the effect of dietary fiber on dysbiosis, we analyzed the gut microbial composition associated with our four diets in diabetic mice: NC, which has an equivalent amount of fiber to that recommended for humans; ZF; and the two different HF diets (RS and guar gum amylose). Principal coordinate analysis of UniFrac distances showed clear cluster separation of diabetic mice according to diet type (analysis of similarities P<0.001; R=0.93) (Figure 4E). The relative abundance of bacterial phyla in each group of mice is shown in Figure 4F. RS and HF feeding ameliorated diabetes-related dysbiosis, with increased relative abundance of phylum Bacteroidetes at the expense of Firmicutes. Expansion of the SCFA producing genera Prevotella and Bifidobacterium with relative reduction of Bilophila (containing the known pathobiont Bilophila wadsworthia) was also seen in fiber-fed diabetic mice compared with NC-fed controls. HF diet, but not RS diet, increased the relative abundance of A. muciniphila in diabetic mice (Figure 4G).
To identify bacteria associated with severity of albuminuria, an early hallmark of diabetic nephropathy, we used Pearson correlation analyses shown as a heatmap in Figure 4D. At a family level, Lactobacillaceae, Enterobacteriaceae, unclassified Clostridiales, and Streptococcaceae were most strongly associated with increased albuminuria, all of which were most abundant in NC- and ZF-fed diabetic mice. In contrast, “protective bacteria” associated with lower levels of albuminuria were found predominantly in diabetic mice fed RS or HF diets.
Dietary Fiber Promotes Increased SCFA Production
As dietary fiber is known to be fermented by the gut microbiota to release SCFA, we next measured SCFA levels in feces and serum of diabetic mice using 1H NMR spectroscopy. In accordance with the changes in gut microbial ecology, diabetic mice fed RS and HF diets had significantly greater levels of serum acetate (Figure 4I) and fecal SCFAs (Figure 4H) compared with those on NC. However, SCFA levels in ZF-fed mice remained similar to NC-fed controls.
SCFA Are a Key Mediator of the Renoprotective Effects of Dietary Fiber
To determine whether changes in SCFA levels could directly mediate the renoprotective effects seen with dietary fiber, we treated diabetic mice with drinking water containing 100 mM acetate, 50 mM butyrate, or 100 mM propionate from week 3 postinjection while maintained on control diet. Mice treated with acetate in drinking water achieved similar peripheral concentrations of acetate to fiber-fed mice, with no changes to systemic acid-base balance (data not shown). Protection against progression of diabetic nephropathy was found in SCFA-treated mice, with reductions in albuminuria, glomerular hypertrophy, mesangial expansion, and interstitial fibrosis compared with controls (Figure 5). Similar to the effect seen with HF feeding, protection against diabetic nephropathy was accompanied by preservation of podocyte number (Figure 5, E and F) and a reduction in interstitial macrophage infiltration (Figure 5, I and J). We have previously demonstrated that SCFA supplementation in drinking water does not alter gut microbiota composition,26 supporting a direct effect of SCFA in mediating protection against diabetic nephropathy.
Figure 5.
Treatment with SCFA acetate, butyrate, and propionate protects against DN. (A) Diabetic mice remained similarly hyperglycemic regardless of whether they received acetate, butyrate, propionate, or pH- and sodium-matched water. (B) Acetate and butyrate treatment reduced albuminuria at 12 weeks compared with control diabetic mice. Diabetic glomerular and interstitial changes including (C and D) VG, (E and F) podocyte injury, and (G and H) interstitial macrophage accumulation (I and J) were diminished with SCFA treatment versus control diabetic mice. Data are shown as means±SD; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
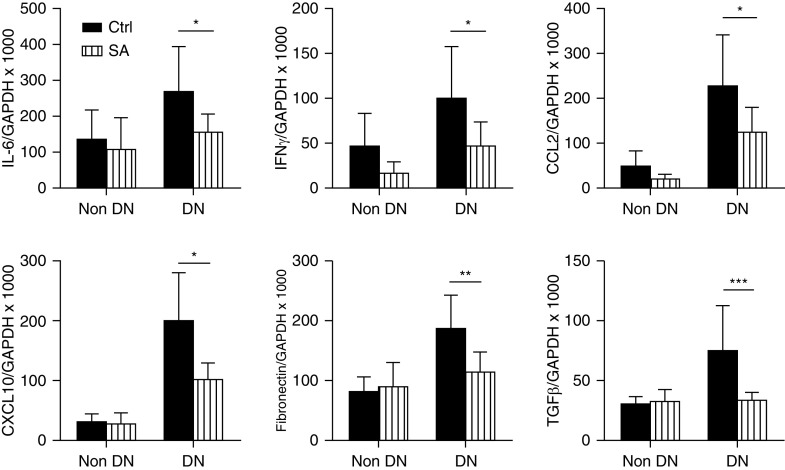
Acetate Treatment Suppressed Expression of Inflammatory and Fibrotic Genes in Diabetic Kidneys
To further explore the molecular mechanisms underlying the effect of SCFA, real-time PCR was used to evaluate gene expression of inflammatory cytokines, chemokines, and fibrosis-related genes in diabetic kidneys. Expression of IL6 and IFNγ mRNAs were upregulated in WT diabetic kidneys compared with nondiabetic WT controls; however, this upregulation was not observed in the kidneys of acetate supplemented mice. Gene expression of chemokines CCL2 and CXCL10 were also substantially increased in WT diabetic kidneys, but not in acetate supplemented mice. Expression of the fibrosis-related genes TGFβ and fibronectin were also suppressed with acetate supplementation (Figure 6).
Figure 6.
Acetate treatment reduced inflammatory and fibrotic mRNA gene expression in diabetic kidneys at 12 weeks. RT-PCR demonstrated substantial upregulation of mRNA expression of IL-6, IFNγ, CCL2, CXCL10, fibronectin, and TGFβ in WT diabetic kidneys, all of which were markedly diminished by acetate treatment. Data are shown as means±SD; *P<0.05; **P<0.01; ***P<0.001.
GPR43 and GPR109A, GPRs for Acetate and Butyrate, Are Necessary for Fiber and SCFA Mediated Protection against Diabetic Nephropathy
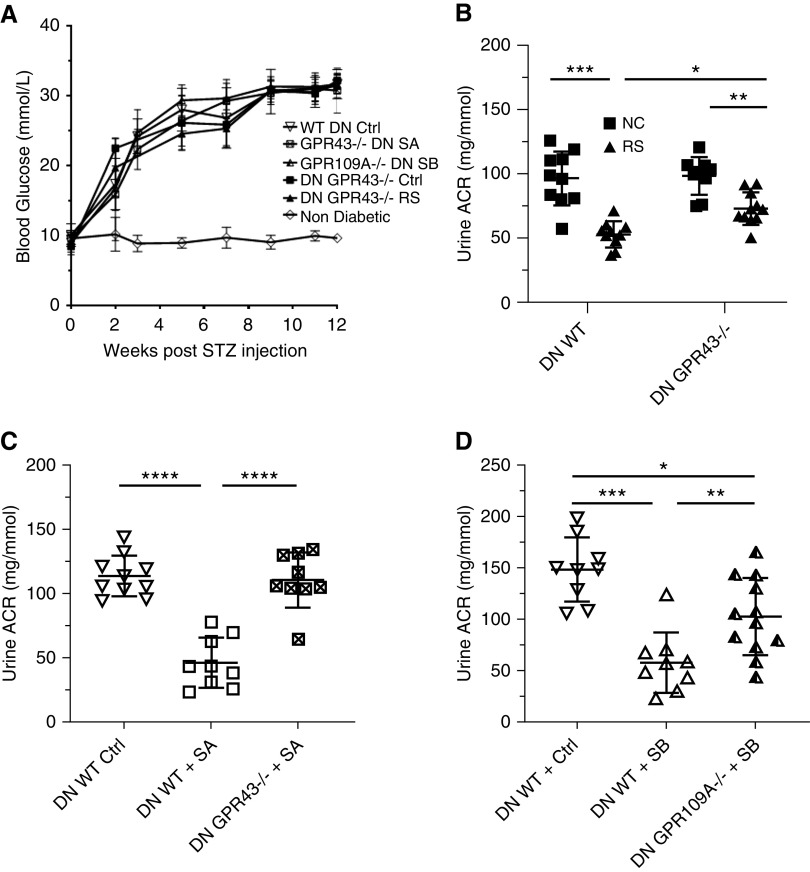
The molecular actions of SCFA on cell function are mediated through diverse pathways, including signaling via “metabolite-sensing” GPRs.27 To determine whether the beneficial effects of SCFA on diabetic nephropathy were mediated via GPRs, we treated groups of diabetic Gpr43−/− and Gpr109A−/− mice on a C57BL/6 background with acetate- and butyrate-supplemented drinking water, respectively. Gpr43−/− and Gpr109A−/− mice were equally susceptible to STZ-induced diabetes, with similar progression of hyperglycemia to WT mice (Figure 7A).
Figure 7.
GPR43 and GPR109A are required for SCFA- and fiber-mediated protection against DN. (A) Gpr43−/− and Gpr109A−/− mice displayed a similar progression of hyperglycemia to WT counterparts. Blood glucose profiles were not significantly altered with SCFA treatment or RS diet. (B) Albuminuria in Gpr43−/− mice fed a HF diet, demonstrating partial reduction at 12 weeks. (C and D) Gpr43−/− and Gpr109A−/− mice were treated with oral acetate and butyrate, respectively, after induction of diabetes. Albuminuria measured at 12 weeks in (C) Gpr43−/− mice and (D) Gpr109A−/− mice. Data are shown as means±SD of at least n=5 mice per group; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Acetate is known to be a selective and strong agonist for GPR43. WT B6 Gpr43+/+ mice supplemented with acetate were largely protected from the development of diabetic nephropathy, whereas Gpr43−/− mice were not protected and albuminuria was exacerbated (Figure 7C). We next tested GPR109A, the primary receptor for butyrate. Butyrate supplementation in Gpr109A−/− mice provided partial protection from diabetic nephropathy, with a small reduction in albuminuria compared with diabetic WT controls (Figure 7D). Thus although acetate-mediated protection appears dependent upon GPR43, butyrate facilitates its beneficial effects via both GPR109A and additional pathways.
To determine whether the beneficial effects of dietary fiber on DN were mediated via these same GPRs, we fed diabetic WT C57BL/6 Gpr43+/+ and Gpr43−/− mice RS, the diet that produced our highest SCFA levels. RS-fed Gpr43−/− mice showed some improvement in albuminuria (P<0.01) compared with WT controls, but absence of GPR43 did not account for the full protective effects of a HF diet (Figure 7B).
SCFA Treatment Inhibits Proinflammatory Responses in TECs and Podocytes Exposed to High Glucose
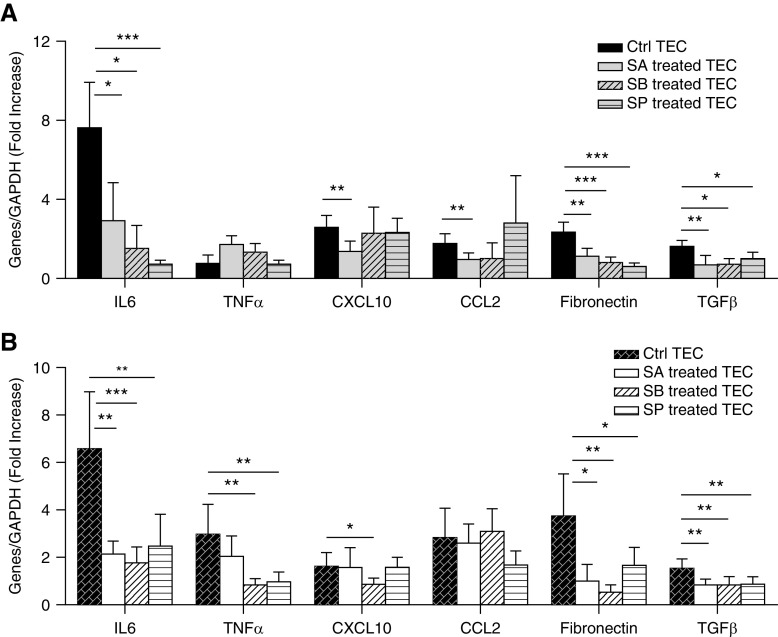
To determine the effects of SCFA on kidney cells under hyperglycemic conditions, primary TEC and podocyte cultures were treated with acetate, butyrate, or propionate under high and normal glucose conditions. mRNA expression of proinflammatory cytokine (IL6), chemokines (CCL2, CXCL10), and profibrotic genes (TGFβ, fibronectin) were increased two- to seven-fold in TECs exposed to high glucose relative to osmotic controls. Upregulation of IL6, fibronectin, and TGFβ was significantly attenuated by all three SCFAs. CCL2 and CXCL10 expression was diminished by only acetate (Figure 8A).
Figure 8.
SCFA treatment reduced proinflammatory gene expression in glucose stimulated TECs and podocytes. Primary TECs and podocytes from WT mice were exposed to high glucose or an osmotic control in the presence and/or absence of SCFA for 12 hours. (A and B) High-glucose conditions induced upregulation of proinflammatory- and fibrosis-related genes two- to seven-fold in TECs and podocytes. Expression of IL6, fibronectin, and TGFβ were diminished after SCFA treatment in both TECs and podocytes. (A) Acetate reduced expression of chemokines (CCL2, CXCL10) in TECs. (B) Podocytes expressed higher levels of TNFα after glucose stimulation, which was diminished by butyrate and propionate. Data are shown as means±SD (n=6); *P<0.05; **P<0.01; ***P<0.001.
In podocytes, upregulation of IL6, TGFβ, and fibronectin was similarly attenuated by all three SCFAs. In contrast to TECs, mRNA expression of TNFα in podocytes was exacerbated by exposure to glucose, and diminished by butyrate and propionate. No significant difference was seen in CCL2 expression (Figure 8B).
Discussion
The gut microbiota and its metabolites play an integral role in the pathogenesis and development of CKD and other types of inflammatory and metabolic diseases.28,29 Higher fiber intake has been associated with decreased inflammation and mortality in CKD,13 yet the mechanisms of the gut–kidney axis are yet to be fully explored. Using a murine model of STZ induced diabetes, our study provides the first insights into the interplay between diet, the gut microbiome and development of pathologic inflammatory responses in diabetic nephropathy. In this study, we observed that dietary fiber is integral to a healthy gut microbiota, the maintenance of which protected mice against the clinical and histologic manifestations of diabetic nephropathy without altering glycemia. Supplementation with microbial-derived metabolites acetate and butyrate afforded similar protection, achieved principally through binding to metabolite-sensing receptors GPR43 and GPR109A. Our results reveal a role for dietary manipulation of the gut microbiome in attempting to delay progression of diabetic nephropathy, with SCFA being the key mediators of the protection afforded by fiber consumption.
Diet is the dominant exogenous factor known to influence the gut microbiome, and a key determinant of its composition and diversity.12 In our study, changes in fiber consumption led to clear changes in the microbiota, evidenced by distinct cluster separation of zero-, normal-, and high fiber-fed diabetic mice using principal coordinate analysis. A high fiber diet ameliorated diabetes-related dysbiosis, and supported expansion of SCFA producers, in contrast to reduction of these groups in NC-fed diabetic controls. We also detected increased numbers of Bilophila wadsworthia in diabetic mice, a known urease producing pathobiont linked to increased gut permeability and systemic inflammation in mice.30,31 These results parallel findings in human studies of a “dysbiotic” microbiota in CKD, with expansion of urease producing and contraction of SCFA-producing bacteria being predominant features.9,31 The expansion of SCFA-producing bacteria seen in our diabetic fiber-fed mice highlights the capacity of diet to influence gut microbial composition. Despite this, treatment with pre-, pro-, or synbiotics in humans with CKD have produced conflicting results,32 emphasizing the need for greater mechanistic insights to guide future translational studies.
Our two high fiber diets both resulted in expansion of the SCFA-producing Bifidobacterium and Prevotella genera, but had contrasting effects on Akkermansia. A. muciniphila has attracted interest as a probiotic for its beneficial effects in murine studies on metabolic syndrome, inflammation, and gut permeability, with a growing body of human translational work targeting obesity.33,34 The HF diet promoted a three-fold expansion in contrast to relatively low abundance in RS-fed mice, yet both yielded similar degrees of protection against diabetic nephropathy. This finding highlights the complexity of the microbial ecosystem and provides support for the concept that no single species is responsible, rather that diet can induce net microbial changes, which tip the balance away from dysbiosis.
Dietary fibers are fermented by commensal colonic bacteria, resulting in the release of SCFAs locally and via the portal circulation to mediate extraintestinal effects.35,36 Our HF diets induced changes in microbial composition that correlated with increased fecal and circulating SCFA levels. Systemic SCFA levels have been shown to respond in a dose-dependent manner to dietary fiber, confirming gut microbial fermentation of fiber as the primary factor influencing circulating levels.37 In keeping with this, our RS diet, which contained the highest proportion of nondigestible fiber suitable for bacterial fermentation, produced the highest SCFA levels, followed by HF and then NC.
SCFA supplementation reproduced the beneficial effects of HF diets on diabetic nephropathy, pointing to these as key mediators of gut microbiota–host crosstalk. Although previous studies have demonstrated a role for SCFA in improving glucose tolerance through AMPK-, PPY-, and GLP-1–dependent mechanisms,38–40 we found no difference in glucose profiles between SCFA-treated diabetic WT, Gpr43−/−, or Gpr109A−/− mice. This likely reflects these studies being performed in high-fat feeding models of type 2 diabetes. The improvements found highlight the effect of SCFA on obesity and insulin resistance,41,42 with these effects yielding no glucose difference in our lean diabetic mice.
At the molecular level, SCFA mediate cellular effects through multiple mechanisms, including GPRs, effects on cell proliferation, apoptosis, and histone acetylation.27 GPR43 and GPR109A are expressed on gut epithelial cells and innate immune cells that respond to SCFA produced in the colon, indicating a broad role in inflammatory and immune responses.43,44 We and others have previously demonstrated the SCFA-GPR signaling pathway as critical to regulation of immune and inflammatory responses by commensal bacteria.24,45 The importance of these receptors in mediating SCFA’s effects in diabetic nephropathy was clearly evident, with the protective effects unable to be fully replicated in GPR43- and GPR109A-deficient mice. Although the benefit of acetate treatment was abrogated in Gpr43−/− mice, butyrate provided partial protection in Gpr109A−/− diabetic mice, likely because of its ability to also signal through GPR43. Studies of renal cell lines have supported the importance of GPR’s, confirming their presence in kidney tissue, vessels, and podocytes.46–48 Consistent with our findings in vivo, we demonstrated that under high-glucose conditions, treatment with SCFA was able to reduce inflammation in cultured TECs and podocytes in vitro. Similarly, Huang et al.19 found incubation of glomerular mesangial cells induced under high glucose or LPS, with SCFA or a GPR43 agonist, inhibited proliferation and reduced production of reactive oxygen species and expression of MCP-1 and IL-1β, supporting this pathway as a target in diabetic nephropathy.
SCFAs also modulate the production of inflammatory mediators by macrophages independent of GPRs. Chang et al.49 demonstrated suppression of LPS and cytokine-stimulated production of proinflammatory mediators (TNFα, IL6, and NO) and enhanced release of the anti-inflammatory cytokine IL10 after treatment of macrophages with butyrate. Similarly, we found a significant reduction in macrophage accumulation within kidneys of diabetic mice treated with SCFA or high fiber, and lower expression of pathogenic cytokines and chemokines. Reduced expression of innate immune receptors TLR2 and TLR4 was also seen in our fiber-fed mice, supporting activation of innate immunity and TLRs as pathways through which the microbiota might modulate renal inflammation and injury.
Our studies were performed in a STZ model of type 1 diabetes. As STZ has antibiotic activity, confounding effects on gut microbiota were present. To exclude this, we studied the effects of STZ and found that gut microbiota was transiently altered after injection, as mice showed resilience to these perturbations,50 with α diversity and microbial composition largely restored within 3 weeks. Our model has other potential limitations, including the lack of kidney failure after development of nephropathy. Thus, caution in extrapolating to human disease is advisable and replication in other murine models is warranted.
Our results provide evidence of the importance of the gut microbiota in diabetic kidney disease, and support the hypothesis of a gut–kidney axis linking dietary fiber, the gut microbiota, SCFA, and kidney disease. Our study also provides mechanistic insights, demonstrating that dietary fiber and SCFAs protected against diabetic nephropathy through regulation of key pathways and genes involved in innate immunity, inflammation, and macrophage recruitment. Future studies are required to determine the ability of diet to not only delay progression, but also reverse established disease. Strategies targeting the modifiable capacity of the gut microbiota through dietary manipulation represent a novel and cost-effective therapeutic strategy that warrants further exploration.
Disclosures
None.
Funding
None.
Supplementary Material
Acknowledgments
Prof. Chadban and Prof. Wu conceived and directed the study. Dr. Li, Dr. Loh, Dr. Chen, Dr. Singer, Dr. Kwan, Dr. Ma, and Dr. Liu carried out experiments. Dr. Li and Dr. Chen analyzed and interpreted data. Dr. Li drafted the manuscript. Prof. Chadban, Prof. Wu, Dr. Macia, Dr. Tan, and Prof. Mackay edited the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101029/-/DCSupplemental.
Supplemental Figure 1. Rarefaction curve.
Supplemental Figure 2. HF diet attenuates the clinical and histological manifestations of diabetic nephropathy.
Supplemental Figure 3. Changes to gut microbiota before and after STZ injection.
Supplemental Table 1. Nutritional parameters of experimental diets.
References
- 1.Awad AS, You H, Gao T, Cooper TK, Nedospasov SA, Vacher J, et al.: Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int 88: 722–733, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF: Inflammatory cytokines in diabetic nephropathy. J Diabetes Res 2015: 948417, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, et al.: TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One 9: e97985, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Wu H, Zhao CY, Panchapakesan U, Pollock C, Chadban SJ: Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int J Clin Exp Pathol 7: 481–495, 2014 [PMC free article] [PubMed] [Google Scholar]
- 5.Alicic RZ, Johnson EJ, Tuttle KR: SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: A review. Am J Kidney Dis 72: 267–277, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R: Current understanding of the human microbiome. Nat Med 24: 392–400, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, Richards EM, Pepine CJ, Raizada MK: The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 14: 442–456, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al.: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Ma J, Kwan T, Stribos EGD, Messchendorp AL, Loh YW, et al.: Blockade of HMGB1 attenuates diabetic nephropathy in mice. Sci Rep 8: 8319, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z, Knight R: Dietary effects on human gut microbiome diversity. Br J Nutr 113[Suppl]: S1–S5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, et al.: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al.: Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359: 91–97, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al.: Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359: 97–103, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser J: Gut microbes shape response to cancer immunotherapy. Science 358: 573, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Mariño E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, et al.: Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18: 552–562, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al.: Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26: 1877–1888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, et al. : Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp Clin Endocrinol Diabetes 125: 98–105, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto N, Riley S, Fraser D, Al-Assaf S, Ishimura E, Wolever T, et al.: Butyrate modulates TGF-beta1 generation and function: Potential renal benefit for Acacia(sen) SUPERGUM (gum arabic)? Kidney Int 69: 257–265, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Weibel ER, Gomez DM: A principle for counting tissue structures on random sections. J Appl Physiol 17: 343–348, 1962 [DOI] [PubMed] [Google Scholar]
- 22.McWhinnie DL, Thompson JF, Taylor HM, Chapman JR, Bolton EM, Carter NP, et al.: Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation 42: 352–358, 1986 [DOI] [PubMed] [Google Scholar]
- 23.White KE, Bilous RW: Estimation of podocyte number: A comparison of methods. Kidney Int 66: 663–667, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al.: Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6: 6734, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT: Streptozotocin, a new antibacterial antibiotic. Antibiot Annu 7: 230–235, 1959-1960 [PubMed] [Google Scholar]
- 26.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al.: Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15: 2809–2824, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L: The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F: From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165: 1332–1345, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al.: High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9: e114881, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Z, Long W, Hao B, Ding D, Ma X, Zhao L, et al.: A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog 9: 59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND: Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koppe L, Mafra D, Fouque D: Probiotics and chronic kidney disease. Kidney Int 88: 958–966, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al.: Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110: 9066–9071, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al.: Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med 25: 1096–1103, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT: Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murase M, Kimura Y, Nagata Y: Determination of portal short-chain fatty acids in rats fed various dietary fibers by capillary gas chromatography. J Chromatogr B Biomed Appl 664: 415–420, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al.: Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T: Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun 344: 597–604, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al.: Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 295: E1160–E1166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al.: Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, et al.: Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7: e35240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF,et al. : Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome [published correction appears in Gastroenterology 144: 250, 2013]. Gastroenterology 143: 913-6 e7, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. : The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al.: (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al.: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al.: Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajkumar P, Aisenberg WH, Acres OW, Protzko RJ, Pluznick JL: Identification and characterization of novel renal sensory receptors. PLoS One 9: e111053, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felizardo RJF, de Almeida DC, Pereira RL, Watanabe IKM, Doimo NTS, Ribeiro WR, et al.: Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J 33: 11894–11908, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Chang PV, Hao L, Offermanns S, Medzhitov R: The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111: 2247–2252, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De La Cochetière MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Doré J: Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol 43: 5588–5592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.