Abstract
Background
Immunotherapy has become a powerful treatment option for several solid tumor types. The presence of tumor-infiltrating lymphocytes (TIL) is correlated with better prognosis in ovarian cancer, pointing at the possibility to benefit from harnessing their anti-tumor activity. This preclinical study explores the feasibility of adoptive cell therapy (ACT) with TIL using an improved culture method.
Methods
TIL from high-grade serous ovarian cancer were cultured using a combination of IL-2 with agonistic antibodies targeting 4-1BB and CD3. The cells were phenotyped using flow cytometry in the fresh tissue and after expansion. Tumor reactivity was assessed against HLA-matched ovarian cancer cell lines via IFN-γ ELISPOT.
Results
Ovarian cancer is highly infiltrated with CD8+ TIL that are preferentially and robustly expanded with the addition of the agonistic antibodies. With a 95% success rate, the TIL are grown to ≥ 100 × 106 cells in 2–3 weeks without over differentiation. In addition, the CD8+ TIL grown with this method showed HLA-restricted tumor recognition.
Conclusions
These results indicate the viability of TIL ACT for refractory ovarian cancer by allowing for the large expansion of anti-tumor TIL in a short time and consistent manner.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02402-z) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, Tumor-infiltrating lymphocytes, TIL therapy, Adoptive cell therapy
Introduction
Epithelial ovarian cancer (OvCa) is the deadliest gynecological cancer and is estimated to account for almost 14,000 deaths in 2018 [1]. Although prognosis of early-stage OvCa is favorable, 70% of patients are diagnosed with advanced and metastatic disease [2]. Traditional management of advanced stage OvCa includes tumor reductive surgery and adjuvant platinum–taxane chemotherapy, which results in high rates of initial complete response. However, nearly 90% of patients recur and the 5-year survival rate for late-stage disease is only 28% [3, 4].
Nevertheless, OvCa possesses a strong immune infiltrate that could provide an avenue to greater treatment efficacy and better long-term survival in the context of immunotherapy. Several groups have shown that the presence of CD8+ TIL is associated with a greater 5-year survival in OvCa, suggesting that CD8+ TIL exert some degree of tumor control [5–11]. This provides a rationale for the use of immunotherapy to harness the anti-tumor potential of this immune infiltrate. Checkpoint blockade immunotherapy has already made a tremendous mark in the treatment of cancer. Its success was first observed in the treatment of metastatic melanoma with agents that block the CTLA-4 and PD-1 axes [12–16]. This approach was later transposed to non-small cell lung cancer and renal cell carcinoma [17–19]. Unfortunately, the success of checkpoint blockade has not been reproduced in OvCa to date [20–22].
Since in vivo manipulation of the TIL through checkpoint blockade does not seem to be sufficient to generate a strong clinical response, approaches involving ex vivo manipulation of immune cells, such as adoptive cell therapy (ACT) using autologous TIL, might be able to provide a large quantity of anti-tumor T cells needed for tumor control. Our group and others have demonstrated the effectiveness of TIL ACT in metastatic melanoma [23–26]. With objective response rates (ORR) of 40–50% in metastatic melanoma, TIL ACT is among the best treatment options for this patient population. In the 1990s, several groups attempted to transpose TIL ACT to OvCa either alone or in combination with chemotherapy, or in the adjuvant setting after debulking surgery [27–31]. These early trials had some promising results but suffered from a few limitations. Later studies demonstrated the need for lymphodepleting pre-conditioning regimens for long-term TIL engraftment and improved cell generation methodologies that resulted in the infusion of patients with greater cell numbers [32]. Furthermore, improvements in techniques for TIL enrichment and activation, mainly for CD8+ TIL as they have been correlated with clinical response in melanoma, may increase favorable clinical outcomes in OvCa TIL trials [25, 26].
Our group showed that manipulating 4-1BB/CD137 through agonistic stimulation (Urelumab, BMS) increased CD8+ TIL proliferation in melanoma, triple-negative breast cancer and pancreatic cancer [33–35]. Likewise, our group further showed that the addition of an anti-CD3 antibody (OKT3) to the early TIL culture resulted in faster and more consistent expansion of CD8+ melanoma TIL [36]. Based on this previous work, we posited that the use of this novel 3-signal approach in OvCa TIL culture would provide similar benefits of increased CD8+ TIL yield.
Here, we demonstrate that the addition of an agonistic 4-1BB mAb and OKT3 increases the ability to grow TIL from OvCa, improves the total yield, and stimulates the proliferation of activated CD8+ T cells. In addition, these CD8+ TIL displayed HLA-restricted tumor recognition. These results support the use of TIL expanded with agonistic 4-1BB and CD3 mAbs in ACT strategies for patients with OvCa.
Materials and methods
Patient selection
Patients with primary or metastatic high-grade serous ovarian carcinoma underwent surgical resection (n = 98, 84 evaluable for flow cytometry assays). In 47 patients, platinum-based chemotherapy and/or chemoradiation was administered. Patients are referred to by their de-identified number.
Reagents and cell lines
A fully human and purified IgG4 monoclonal antibody (mAb) against human CD137/4-1BB, Urelumab (BMS-663513), was kindly provided by Bristol-Myers Squibb (BMS, New York, NY, USA). Human recombinant interleukin-2 (IL-2) (Proleukin™) was generously provided by Prometheus Therapeutics & Diagnostics (San Diego, CA, USA). The GMP-grade soluble anti-CD3 mAb (Mouse IgG2a, clone OKT3) was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). The HLA-ABC monoclonal antibody (clone W6/32) and its isotype control (Mouse IgG2a κ, clone eBM2a) were purchased from ThermoFisher (Waltham, MA). The human epithelial ovarian cancer cell lines COV318, COV362, and SKOV3 were used for the recognition ELISPOT assays.
Isolation and expansion of TIL from human OvCa tumors
The tumor samples were cut into six 1–3 mm3 fragments and placed in TIL complete medium [TIL-CM: RPMI-1640 with GlutaMax (Gibco/Invitrogen), 1 × Pen–Strep (Gibco/Invitrogen), 50 μmol/L 2-mercaptoethanol (Gibco/Invitrogen), 20 μg/mL Gentamicin (Gibco/Invitrogen), and 1 mmol/L pyruvate (Gibco/Invitrogen)] with 6000 IU/mL IL-2 in 24-well plates for a period of 4–5 weeks, as previously described [37]. For the 4-1BB mAb (a41BB) condition, both 6000 IU/mL IL-2 and 10 ug/mL 4-1BB mAb were added in the culture plates on day 0 and day 4 or 5. For the a41BB + OKT3 condition, five tumor fragments were put in a G-Rex 10 flask (Wilson Wolf Manufacturing, New Brighton, MN, USA) in 20 mL TIL-CM with 6000 IU/mL IL-2, 10 μg/mL 4-1BB mAb, and 30 ng/mL anti-CD3 (OKT3) as previously described [36]. Four to five days after culture initiation, 20 mL of additional TIL-CM with 6000 IU/mL IL-2 was added. Half-media changes were done every 3–4 days with fresh TIL-CM containing 6000 IU/mL IL-2 for up to 35 days or until the cells formed a thick layer completely covering the bottom of the flask. The cell suspensions were collected and cryopreserved for later testing.
Flow cytometric analysis of TIL
Fresh tumor samples were manually disaggregated between frosted-glass slides to obtain a single-cell suspension for analysis. Both the disaggregated tissue samples and expanded TIL were stained on ice in FACS Wash Buffer (Dulbecco’s Phosphate Buffered Saline 1× with 1% Bovine Serum Albumin) for 30 min with fluorochrome-conjugated monoclonal antibodies for CD3 FITC (SK7), CD4 PerCP-Cy5.5 (RPA-T4), CD8 PB (RPA-T8), CD16 PE (B159), CD28 PE-Cy7 (CD28.2), CD56 PE-Cy7 (B159), TCR γδ APC (B1), BTLA PE (J168 & J168-540), PD-1 BV650 (EH12), HLA-ABC PE (G46-2.6) (all BD Bioscience, San Jose, CA, USA), and PD-1 PerCP-Cy5.5 (EH12.2H7) (Biolegend, San Diego, CA, USA). Dead cells were excluded using the Aqua or Yellow LIVE/DEAD viability stain (ThermoFisher). Stained cells were fixed in 1% paraformaldehyde solution for 20 min at room temperature. Samples were acquired using the BD FACSCanto™ II or BD LSRFortessa X-20 and analyzed using FlowJo Software v10.5 (Tree Star). Subpopulations were excluded from analysis if comprised of less than 100 events.
Cell sorting and rapid expansion of CD8+ OvCa TIL
To control for reactivity of CD8+ TIL, bulk TIL products from eight different patients were stained with CD3 FITC, CD8 APC-H7, and SYTOX Blue Dead Cell Stain to isolate the CD8+ T cells using a BD FACSAria IIIu in the MD Anderson Cancer Center (MDACC) Flow Cytometry and Cellular Imaging Core Facility. Then, to provide greater cell numbers for functional assays, the sorted CD8+ TIL underwent the rapid expansion protocol in G-Rex10 flasks, which was previously described by Forget et al, and then viably frozen [37].
Recognition assay via IFN-γ ELISPOT
One day prior to the assay, the TIL were thawed and rested overnight in TIL-CM with 6000 IU/mL IL-2. Six hours prior to the assay, TIL were washed and rested in TIL-CM without IL-2. The tumor lines were put at 1 × 106 cells/mL and incubated with 80 μg/mL of the HLA-ABC blocking antibody or 80 μg/mL of its isotype control for 3 h in 15 mL conical tubes at 37 °C. The tumor cells were then added directly to the ELISPOT plate. TIL were then put at a 2:1 or 4:1 ratio with an HLA-matched OvCa cell line (either SKOV3, COV318, or COV362) in the presence of an HLA-ABC blocking antibody, its isotype control, PMA/Ionomycin, or media (TIL alone). The conditions were performed in triplicate and the cells were co-cultured for 15 h before developing as previously described [34]. Spots on ELISPOT plates were counted with the ImmunoSpot machine (Cellular Technology Ltd., Shaker Heights, OH, USA).
Statistical analysis
GraphPad Prism v7.01 (GraphPad Software) was used for graphing and statistical analysis. Differences between groups or experimental conditions were determined using non-parametric, two-tailed t tests (paired or unpaired as appropriate).
Results
Primary and metastatic OvCa TIL infiltrate is predominantly CD8+ T cell rich
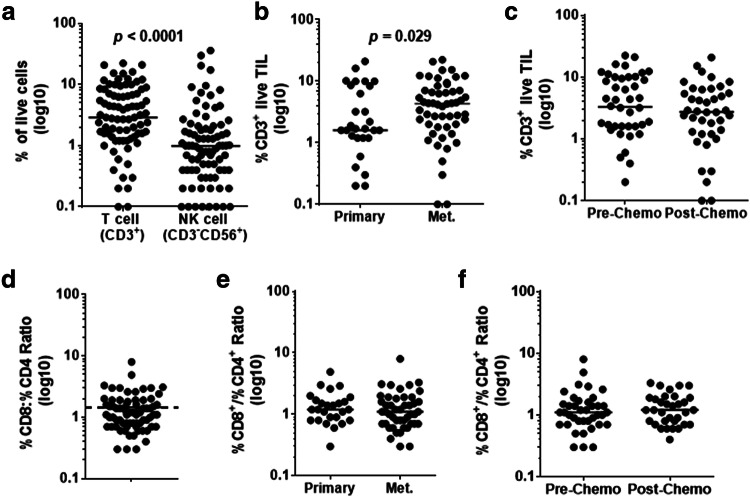
We assessed the lymphoid immune infiltrate at the onset of the culture by performing flow cytometry on manually disaggregated primary and metastatic tumor samples (n = 84). The proportion of CD3+ TIL among live cells recovered varied widely among samples (median 3%, range 0.1–20%) (Fig. 1a). Infiltration by NK cells was overall less (median 1% range 0.1–35%) (Fig. 1a). However, CD3+ TIL were a significantly greater portion of the infiltrate than NK cells in most cases (p < 0.0001).
Fig. 1.
Characterization of lymphocyte infiltrate in primary/metastatic and pre/post-chemo OvCa samples. a T cell (CD3+) and NK cell (CD3−CD56+) infiltration is compared within the live lymphocyte population using flow cytometry, with the horizontal bar representing the median value of each population (n = 84). T cell (CD3+) infiltration is compared between b primary and metastatic sites, and c pre- and post-chemo samples, with the horizontal bars indicating the median value (n = 84). d The ratio of CD8+ to CD4+ % T cells within the CD3+ compartment is displayed. The dotted line indicates where a ratio of 1 is in relation to the average ratio which is represented by the solid horizontal line (n = 72). The CD8/CD4 ratio is compared between e primary and metastatic sites and f pre- and post-chemo samples, with the horizontal bars indicating the median value (n = 72). Samples that had less than 100 cells within the CD8 and CD4 gates were excluded from analysis
Because TIL ACT for OvCa would most likely target patients who progressed on standard of care and thus would be administered to chemo-refractory patients likely to be metastatic, we investigated the contribution of those parameters on the T cell infiltration. When patients were stratified by chemotherapy exposure and surgery site, metastatic tumors were found to have more CD3+ TIL than primary tumors (median 4% vs. 1.5%, p = 0.029) as a component of live cells (Fig. 1b). No significant difference in the proportion of CD3+ TIL was found between pre-chemotherapy and post-chemotherapy samples (Fig. 1c).
Within the CD3+ TIL compartment, the mean CD8:CD4 ratio was 1.5 demonstrating that OvCa is predominately infiltrated by CD8+ TIL (Fig. 1d). No difference in the CD8:CD4 ratio was found in the context of primary/metastasis and pre/post-chemotherapy (Fig. 1e, f). As a point of comparison, both the CD3+ TIL infiltration and CD8:CD4 ratio were found to be similar to what our group has previously reported in metastatic melanoma [35], suggesting that OvCa tumors are relatively well infiltrated by T cells.
Use of agonistic 4-1BB mAb and anti-CD3 increases TIL growth and success rate
Prior work by our group detailed how infusion of melanoma patients with a higher proportion of CD8+ T cells and larger amount of TIL in general correlates with clinical response [25, 26]. We reasoned that CD8+ TIL could also be important in other solid tumor types as their presence correlates with improved survival. Thus, we next tested the two new methodologies developed by our group which aimed at facilitating the expansion of CD8+ TIL from ovarian cancer tissue.
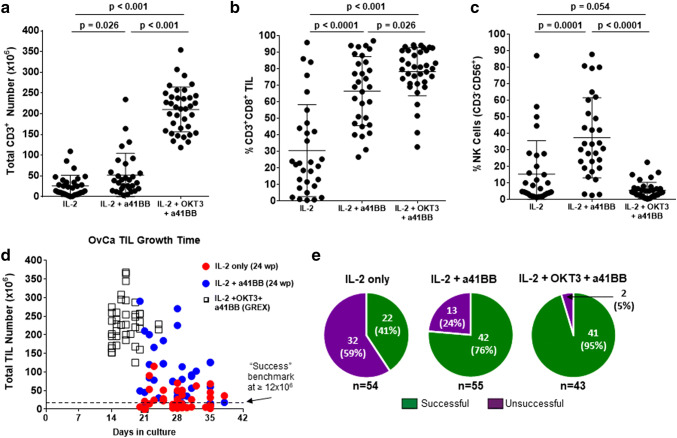
Fresh tumor samples were set up for TIL culture using the different expansion methods. The first method compared IL-2 only versus IL-2 plus an agonistic 4-1BB mAb (a41BB). The use of a41BB significantly increased the average total CD3+ TIL growth from 30x106 cells for IL-2 alone to 50 × 106 for IL-2 + a41BB (Fig. 2a). Additionally, a41BB greatly increased the average percentage of CD8+ TIL in culture from 30% for IL-2 alone to 65% for IL-2 + a41BB (Fig. 2b). A corresponding decrease in the average percentage of CD4+ TIL from the IL-2 only condition to the IL-2 + a41BB condition was also observed (Supplementary Fig. 1). The mean CD4+ percentage dropped from 50% to 5% (Supplementary Fig. 1a) and the median CD8-to-CD4 ratio increased from 0.4 to 60, respectively (Supplementary Fig. 1b).
Fig. 2.
Characterization of TIL growth across culture conditions. Comparison of the a total CD3+ TIL number, b percentage of CD3+CD8+ TIL, and c percentage of NK cells generated between the different culture methods. Samples in IL-2 and IL-2 + a41BB conditions are paired (n = 30) while samples in IL-2 + OKT3 + a41BB are unpaired (n = 36). For a–c, only samples that grew are shown. Some samples are not shown due to dropout after QC. The horizontal bars indicate the mean value and SD are shown for each population. Comparison between culture methods of the d time of culture and e success rate of growth for all attempted cultures. In e, IL-2 + a41BB has 55 samples because one sample was set up without an IL-2 only counterpart culture
However, since NK cells can also express 4-1BB, the addition of the a41BB mAb increased their growth as well from 15% to 40% of the expanded culture on average (Fig. 2c). To avoid expansion of NK cells, a 3rd culture strategy using an agonistic stimulation of CD3 with an anti-CD3 mAb (OKT3) was implemented. Similar to previous work for metastatic melanoma TIL expansion, this strategy (named TIL 3.0) led to a high percentage of CD8+ TIL (80% average, Fig. 2b) with a low percentage of CD4+ TIL (10% average, Supplementary Fig. 1), and NK cells (5% average, Fig. 2c) [36]. Protocol-induced changes in CD4+ Tregs were not assessed due to prior observations that high-dose IL-2 does not allow Treg growth and abrogates their function [33, 38]. To show the benefit of a41BB, a subset of ten samples was cultured with IL-2 + OKT3 (no a41BB) and compared with TIL 3.0 (Supplementary Fig. 2). Without a41BB, the total number of CD3+ TIL and the percentage of that which were CD8+ TIL were both diminished (Supplementary Fig. 2a). NK cells remained low.
TIL 3.0 also produced a greater number of OvCa TIL (mean of 200 × 106 cells) after a period of 2–3 weeks, as opposed to the 3–5 weeks required for the other two methods (Fig. 2a, d). The consistent reduction in culture time was lost when IL-2 + OKT3 without a41BB was used and the yield of TIL was inferior (Supplementary Fig. 2b). The time of expansion did not appear to depend on chemotherapy exposure (Supplementary Fig. 3a), although CD3+ TIL from post-chemotherapy samples grew slightly, but significantly, less (225 × 106 vs. 200 × 106, p = 0.028) using TIL 3.0 (Supplementary Fig. 3b). Regardless, all TIL 3.0 cultures produced markedly more TIL than IL-2 only or IL-2 + a41BB.
The overall success rate of establishing an OvCa TIL culture was greatly increased from 41% (22/54) for IL-2 only and 76% (42/55) for IL-2 + a41BB to 95% (41/43) for the IL-2 + OKT3 + a41BB method (Fig. 2e). The benchmark for a successful TIL culture, 12 × 106 total cells, was established from scaling down the MDACC Clinical Melanoma TIL Lab’s criterion for success where 20 fragments are set up for TIL expansion and 40 × 106 cells is considered the minimum to treat a patient [35]. Chemotherapy exposure only affected the success rate of expansion for the IL-2 only condition, with the success being 45% (13/29) pre-chemotherapy and 36% (9/25) post-chemotherapy (Supplementary Fig. 3c). This discrepancy was negated for the IL-2 + a41BB and TIL 3.0 methods.
We assessed if the presence of TIL in the fresh sample affected the success of growth (Supplementary Fig. 4). When comparing the percentage of CD3+ TIL in the fresh sample vs the number grown, there was a strong correlation (rS > 0.5) for the IL-2 only culture method where more CD3+ TIL grew if there were a higher percentage of CD3+ TIL present in the initial tumor tissue (Supplementary Fig. 4a). Moreover, it was apparent that cultures with less than 2% CD3+ TIL in the initial tumor tissue cultured with IL-2 alone did not grow, but cultures initiated with as little as 0.2% CD3+ T cell infiltrate could yield appreciable number of TIL when cultured with IL-2 + OKT3 + a41BB. However, while the IL-2 + a41BB and TIL 3.0 culture conditions did not show as strong of a correlation with T cell infiltration (rS < 0.5), the overall trend was the same as indicated by the positive slopes of the linear regression lines (Supplementary Fig. 4a). There was no strong correlation with respect to the CD8+/CD4+ ratio of fresh TIL and the amount of CD8+ TIL generated, particularly for the IL-2 only condition due to the lack of CD8+ TIL generated by this method (Supplementary Fig. 4b). Overall, there was a positive trend toward growing more CD8+ TIL if there was a higher CD8+/CD4+ ratio initially as indicated by the slopes of the regression line for IL-2 + a41BB and IL-2 + OKT3 + a41BB.
Concurrent engagement of 4-1BB and CD3 prevents CD8+ TIL over differentiation
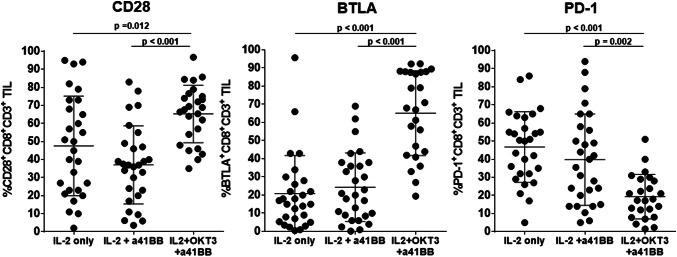
To understand how the different culture methods affected the CD8+ TIL, the expression of CD28, BTLA, and PD-1 was explored as surrogates for T cell activation and differentiation (Fig. 3). These three markers were strategically selected for the power of assessment they provide individually as well as together. BTLA+CD8+ TIL have been reported by our group to be less differentiated cells, capable of prolonged persistence and serial killer capacities [39, 40]. Expression of CD28 is also a trait of lesser differentiation, while PD1 is often associated with exhaustion when highly expressed [41, 42]. When looking at expression of the three markers on OvCa TIL expanded with IL-2 only and IL-2 + a41BB, no significant difference was observed between both groups. Nonetheless, both showed a spread in CD28 and PD1 expression (Fig. 3, left and right graph) with a low expression of BTLA (Fig. 3, middle graph). However, TIL products grown with the TIL 3.0 method had significantly greater percentage of TIL expressing BTLA and CD28, and a significantly smaller percentage expressing PD-1 altogether suggesting a less differentiated profile (Fig. 3).
Fig. 3.
Phenotyping of activation and differentiation of grown TIL. Comparison between the different culture methods of the percentage of CD28, BTLA, and PD-1 expressing CD8+ TIL generated. Samples in IL-2 and IL-2 + a41BB conditions are paired (n = 28) while samples in IL-2 + OKT3 + a41BB are unpaired (n = 25). Samples not passing QC were excluded from the analysis. The horizontal bars indicate that the mean value and SD are shown for each population
OvCa CD8+ TIL show HLA-matched tumor reactivity
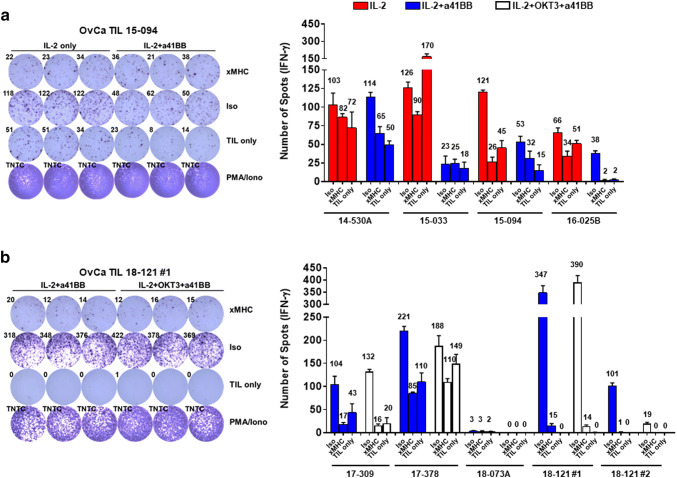
In order for TIL ACT to be an effective therapy, the TIL generation process needs to expand anti-tumor T cells. Since the three different growth methods used could potentially change the expanded TIL repertoire, tumor reactivity was used as a marker to validate preservation of tumor-reactive TIL clones. Since our main focus is reactivity of cytotoxic CD8+ T cells, each TIL line was sorted for CD8+ TIL and further expanded using the rapid expansion protocol to achieve sufficient cell numbers. Eight TIL lines were chosen to assess HLA-matched tumor reactivity that were representative of the different culture methods, primary or metastatic site, and prior chemotherapy exposure, which is summarized in Supplementary Table 1.
For the first set of four lines, reactivity was assessed comparing initial growth with IL-2 only or IL-2 + a41BB (Fig. 4a). The second set of four lines had reactivity compared between those expanded with IL-2 + a41BB or with TIL 3.0 (Fig. 4b). Prior to co-culture setup, all tumor lines were found to express robust levels of HLA class I by flow cytometry (SKOV3, MFI: 2962; COV318, MFI: 5343; COV362, MFI: 2182; Supplementary Fig. 5). As shown in Fig. 4a, upon co-culture of the CD8+ TIL with tumor targets, 2/4 of the first set (15-094 and 16-025B) demonstrated HLA-restricted anti-tumor reactivity via secretion of IFN-γ. The reactivity was split between one line grown with IL-2 + a41BB (15-094) and one with IL-2 only (16-025B). Likewise, in Fig. 4b, 3/4 of the second set of TIL lines were reactive against their HLA-matched target. One TIL line (18-121) even recognized two different tumor lines. Two TIL lines in this group (17-309 and 18-121) showed reactivity regardless of culture method while one showed reactivity only from TIL grown with IL-2 + a41BB (17-378). Overall, TIL recognition of HLA-matched tumor targets was observed across the multiple expansion platforms.
Fig. 4.
Testing reactivity of CD8+ OvCa TIL via IFN-γ ELISPOT. a Comparison of reactivity of TIL grown with either IL-2 only (red) or IL-2 + a41BB (blue) for four different lines, with a representative ELISPOT image shown. b Comparison of reactivity of TIL grown with either IL-2 + a41BB (blue) or IL-2 + OKT3 + a41BB (white) for four different lines, with a representative ELISPOT image shown. Bar graphs show average of three replicate wells with the SD and average spot value for each condition above the bars. Reactivity was considered positive if the average was ≥ twice the xMHC condition. xMHC MHCI block, Iso isotype control for MHCI blocking Ab, TIL only T cells with media only, PMA/Iono PMA and Ionomycin for positive control, TNTC too numerous to count
Discussion
In this report, we show that OvCa has a robust and activated CD8+ TIL infiltrate that is not significantly impacted by surgery site or resistance to chemotherapy. This infiltrate is greatly expanded with the addition of an agonistic 4-1BB mAb to the TIL culture. Furthermore, specific growth of CD3+CD8+ TIL is augmented by adding the anti-CD3 Ab OKT3. The 4-1BB mAb alone and together with OKT3 consistently improved the success rate of reaching a clinically relevant number of TIL. Finally, OvCa CD8+ TIL derived with either culture method showed tumor recognition via IFN-γ secretion in response to HLA-matched ovarian cancer cell lines. These results suggest that enhanced culture method can facilitate TIL ACT for OvCa by increasing the yield of a TIL product containing anti-tumor CD8+ T cells regardless of primary or metastatic site, and in a chemotherapy refractory setting.
It has been noted that one of the drawbacks to the early OvCa TIL ACT trials was the prolonged cell culture that limited persistence but also produced variable expansion of CD8+ TIL. Newer work by Crome et al. revealed another mechanism that could have hampered early TIL therapy pilot trials [43]. The authors observed a frequent expansion of NK cells (CD3−CD56+) within OvCa TIL cultures maintained in IL-2, associated with suppression of CD3+ T cell expansion. They documented that slow-growing TIL cultures (≤ 30 × 106 cells within 4 weeks) contained a greater number of NK cells than fast-growing cultures (> 30 × 106 cells within 4 weeks). This work indicates that a suppressive NK cell population could have been problematic for OvCa TIL cultures using IL-2 only conditioned media. The improved culture method, TIL 3.0, presented here uses 4-1BB and CD3 stimulation to selectively and robustly expand CD8+ TIL. We noted that the 4-1BB stimulation alone can cause extensive NK cell proliferation, to the point of them becoming the predominant cell type, perhaps facilitating their ability to inhibit T cell growth. This is overcome by the addition of OKT3 to specifically trigger T cell expansion in a robust and rapid manner. Concurrent engagement of the TCR and 4-1BB allows the CD8+ TIL to massively proliferate in the absence of over differentiation.
Recently, the importance of CD8+ tissue-resident memory T cells (TRM; typically defined by CD103 expression) in ovarian cancer was reported [44]. This TRM subset expressed activation and cytolytic markers and was correlated with positive prognosis. Being able to expand this TRM subset, or at least enrich for it, could allow for greater therapeutic benefit with TIL ACT. Komdeur et al. briefly explored whether CD103+TRM expanded in culture using IL-2 only, finding the population was essentially lost after 3 weeks [45]. Without TCR sequencing to track T cells over the course of the culture process, it is unclear whether the TRM subset simply does not expand or if it does but loses CD103 expression ex vivo. While the culture method used in this study enriches for CD8+ TIL, in which the TRM subset is located, further characterization is needed to determine if this culture method can specifically expand this subset [45].
HLA-matched tumor targets were used due to the difficulty of establishing autologous tumor lines. The lack of recognition displayed by some of the TIL lines could be explained by the fact that these cells may recognize an autologous target as opposed to a shared tumor-associated antigen (TAA). However, lack of an autologous target would not be an issue in the clinical setting as TIL ACT is meant to treat patients with autologous TIL. Even so, the fact that TIL reactivity was observed in many of the other lines indicates that there is recognition of shared TAA. Moreover, some of the TIL lines are matched on the same HLA allele as is the case for 15-094 & 18-121 (A*02:01) and 16-025B & 18-121 (B*18:01). This hints at the possibility for multiple patients to harbor TIL specific for the same HLA-restricted antigen. Identifying the TCR specific for a shared TAA could be an attractive therapeutic option due to its broad applicability, particularly one restricted to a prevalent HLA allele like A*02:01 (15% worldwide, 20–50% in USA, Europe, and China) [46].
Several groups have already shown endogenous T cell reactivity across OvCa patients to various TAA such as mesothelin [47], wild-type or mutated p53 [48–50], NY-ESO-1 [51], and Her2/neu [52] among others. However, therapies targeting a particular TAA, either through peptide vaccine or chimeric antigen receptor T cells, have had mixed results due to loss of the target antigen [51] or poor persistence [53] for example. Apart from self-antigens, finding tumor-specific mutated antigens (neoantigens) would provide a highly sought immunotherapeutic target. Recent work by Deniger et al. showed the potential of this by finding T cells from two OvCa patients that were specific for the same p53 neoepitope [49]. However, targeting neoantigens beyond a prevalently mutated gene like TP53 (> 95% of cases) could be challenging due to OvCa not having a high mutation burden [54]. An analysis of the Cancer Genome Atlas by Martin et al. underlines this issue by pointing out that only 12% of cases have a ≥ 90% chance of having a naturally presented neoantigen [55]. TIL ACT could potentially avoid these issues by providing antigen specificity against a broad range of TAA.
In summary, these results indicate that OvCa CD8+ TIL can be reproducibly and rapidly expanded to large numbers using the combination of an agnostic 4-1BB mAb and OKT3 while maintaining their anti-tumor activity in the absence of over differentiation. A Phase II clinical trial based on this work is currently open at MDACC to evaluate the feasibility of the adoptive transfer of autologous tumor-infiltrating lymphocytes in recurrent or refractory OvCa (NCT03610490).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge the patients who consented to tissue procurement for research and their caregivers. Thank you to Bristol-Myers Squibb for providing Urelumab and Prometheus for the IL-2. The authors would like to thank René J. Tavera for his early contributions to culturing OvCa TIL.
Abbreviations
- BTLA
B- and T-lymphocyte attenuator
- OvCa
Epithelial ovarian cancer
- MDACC
MD Anderson Cancer Center
- ORR
Overall response rate
- TIL-CM
Tumor-infiltrating lymphocyte complete medium
- TRM
Tissue-resident memory T cell
Author contributions
DST conducted all the experiments and data analysis. DST and MAF generated the TIL lines and prepared the figures. DST, MAF, CH, and CB were responsible for writing the manuscript, while all authors contributed to manuscript editing and approved the final manuscript version. JC oversaw the consent and collection of human samples. MAF, CH, and CB helped with interpretation of the data. JC, EH, and AAJ helped with patients’ clinical information. PH and AAJ provided support and guidance for the study. DST, MAF, CH, and CB designed the experiments and the conceptual design of the study.
Funding
This research was supported in part by the MD Anderson Cancer Center Support Grant (P30 CA016672), a T32 training grant for gynecologic oncology (CA101642), the MD Anderson Ovarian Cancer Moon Shot program, and an MD Anderson Institutional Research Grant (Amir A. Jazaeri). We also acknowledge the support of the Flow Cytometry and Cellular Imaging, Research Histology, and Characterized Cell Line cores which are also supported by the support grant (P30 CA016672).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the 1975 Helsinki declaration. Ethical approval and tissue from surgical resections used to expand TIL were both obtained under protocols (PA16-0912 and LAB02-188) approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
Informed consent
Written informed consent was obtained from all individual participants included in the study for their specimens and data to be used in research and for publication.
Cell line authentication
COV318 and COV362 were originally purchased from Sigma–Aldrich (now Millipore-Sigma, St. Louis, MO) and SKOV3 from the American Type Culture Collection (ATCC, Manassas, VA). All OvCa cell lines were HLA-typed, STR fingerprinted, and confirmed mycoplasma-free at MDACC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cara Haymaker, Phone: 713-792-1458, Email: chaymaker@mdanderson.org.
Chantale Bernatchez, Phone: 713-563-8830, Email: cbernatchez@mdanderson.org.
References
- 1.Siegel RL, Miller KD. Jemal A (2018) cancer statistics. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Zsiros E, Tanyi J, Balint K, Kandalaft LE. Immunotherapy for ovarian cancer: recent advances and perspectives. Curr Opin Oncol. 2014;26(5):492–500. doi: 10.1097/CCO.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitao MM, Chi DS. Surgical management of recurrent ovarian cancer. Semin Oncol. 2009;36(2):106–111. doi: 10.1053/j.seminoncol.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Ding M, Mei-jiao G. Immune effect of tumor-infiltrating lymphocytes and its relation to the survival rate of patients with ovarian malignancies. J Tongji Med Univ. 1991;11(4):5. doi: 10.1007/BF02888158. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58(3):449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18(12):3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 10.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ Tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schachter J, Ribas A, Long GV, Arance A, Grob J-J, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) The Lancet. 2017;390(10105):1853–1862. doi: 10.1016/s0140-6736(17)31601-x. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/s1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105(8):3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 25.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forget MA, Haymaker C, Hess KR, Meng YJ, Creasy C, Karpinets T, Fulbright OJ, Roszik J, Woodman SE, Kim YU, Sakellariou-Thompson D, Bhatta A, Wahl A, Flores E, Thorsen ST, Tavera RJ, Ramachandran R, Gonzalez AM, Toth CL, Wardell S, Mansaray R, Patel V, Carpio DJ, Vaughn C, Farinas CM, Velasquez PG, Hwu WJ, Patel SP, Davies MA, Diab A, Glitza IC, Tawbi H, Wong MK, Cain S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Wargo JA, Radvanyi LG, Torres-Cabala CA, Beroukhim R, Hwu P, Amaria RN, Bernatchez C. Prospective analysis of adoptive til therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. Clin Cancer Res. 2018;24(18):4416–4428. doi: 10.1158/1078-0432.CCR-17-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki Y, Takakuwa K, Kodama S, Tanaka K, Takahashi M, Tokunaga A, Takahashi T. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian-cancer. Can Res. 1991;51(7):1934–1939. [PubMed] [Google Scholar]
- 28.Freedman RS, Edwards CL, Kavanagh JJ, Kudelka AP, Katz RL, Carrasco CH, Atkinson EN, Scott W, Tomasovic B, Templin S, et al. Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J Immunother Emphasis Tumor Immunol. 1994;16(3):198–210. doi: 10.1097/00002371-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ikarashi H, Fujita K, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Immunomodulation in patients with epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Cancer Res. 1994;54(1):190–196. [PubMed] [Google Scholar]
- 30.Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–507. [PubMed] [Google Scholar]
- 31.Freedman RS, Kudelka AP, Kavanagh JJ, Verschraegen C, Edwards CL, Nash M, Levy L, Atkinson EN, Zhang HZ, Melichar B, Patenia R, Templin S, Scott W, Platsoucas CD. Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res. 2000;6(6):2268–2278. [PubMed] [Google Scholar]
- 32.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, Weber J, Hwu P, Pilon-Thomas S, Radvanyi L. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21(3):611–621. doi: 10.1158/1078-0432.CCR-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harao M, Forget MA, Roszik J, Gao H, Babiera GV, Krishnamurthy S, Chacon JA, Li S, Mittendorf EA, DeSnyder SM, Rockwood KF, Bernatchez C, Ueno NT, Radvanyi LG, Vence L, Haymaker C, Reuben JM. 4-1BB-Enhanced Expansion of CD8+ TIL from triple-negative breast cancer unveils mutation-specific CD8+ T cells. Cancer Immunol Res. 2017;5(6):439–445. doi: 10.1158/2326-6066.CIR-16-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakellariou-Thompson D, Forget MA, Creasy C, Bernard V, Zhao L, Kim YU, Hurd MW, Uraoka N, Parra ER, Kang Y, Bristow CA, Rodriguez-Canales J, Fleming JB, Varadhachary G, Javle M, Overman MJ, Alvarez HA, Heffernan TP, Zhang J, Hwu P, Maitra A, Haymaker C, Bernatchez C. 4-1BB agonist focuses CD8(+) Tumor-infiltrating T-Cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res. 2017;23(23):7263–7275. doi: 10.1158/1078-0432.CCR-17-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavera RJ, Forget MA, Kim YU, Sakellariou-Thompson D, Creasy CA, Bhatta A, Fulbright OJ, Ramachandran R, Thorsen ST, Flores E, Wahl A, Gonzalez AM, Toth C, Wardell S, Mansaray R, Radvanyi LG, Gombos DS, Patel SP, Hwu P, Amaria RN, Bernatchez C, Haymaker C. Utilizing T-cell activation signals 1, 2, and 3 for tumor-infiltrating lymphocytes (TIL) expansion: the advantage over the sole use of interleukin-2 in cutaneous and uveal melanoma. J Immunother. 2018 doi: 10.1097/CJI.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forget MA, Malu S, Liu H, Toth C, Maiti S, Kale C, Haymaker C, Bernatchez C, Huls H, Wang E, Marincola FM, Hwu P, Cooper LJ, Radvanyi LG. Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma. J Immunother. 2014;37(9):448–460. doi: 10.1097/CJI.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon BI, Kim TH, Seoh JY. Functional modulation of regulatory T Cells by IL-2. PLoS One. 2015;10(11):e0141864. doi: 10.1371/journal.pone.0141864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haymaker CL, Wu RC, Ritthipichai K, Bernatchez C, Forget MA, Chen JQ, Liu H, Wang E, Marincola F, Hwu P, Radvanyi LG. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology. 2015;4(8):e1014246. doi: 10.1080/2162402X.2015.1014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritthipichai K, Haymaker CL, Martinez M, Aschenbrenner A, Yi X, Zhang M, Kale C, Vence LM, Roszik J, Hailemichael Y, Overwijk WW, Varadarajan N, Nurieva R, Radvanyi LG, Hwu P, Bernatchez C. Multifaceted role of BTLA in the control of CD8(+) T-cell fate after antigen encounter. Clin Cancer Res. 2017;23(20):6151–6164. doi: 10.1158/1078-0432.CCR-16-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178(7):4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 42.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19(4):408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, Milea A, Sowamber R, Katz SR, Bernardini MQ, Clarke BA, Shaw PA, Lang PA, Berman HK, Pugh TJ, Lanier LL, Ohashi PS. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med. 2017;23(3):368–375. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 45.Komdeur FL, Wouters MC, Workel HH, Tijans AM, Terwindt AL, Brunekreeft KL, Plat A, Klip HG, Eggink FA, Leffers N, Helfrich W, Samplonius DF, Bremer E, Wisman GB, Daemen T, Duiker EW, Hollema H, Nijman HW, de Bruyn M. CD103 + intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRalphabeta + CD8alphabeta + T cells that can be targeted for cancer immunotherapy. Oncotarget. 2016;7(46):75130–75144. doi: 10.18632/oncotarget.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solberg OD, Mack SJ, Lancaster AK, Single RM, Tsai Y, Sanchez-Mazas A, Thomson G. Balancing selection and heterogeneity across the classical human leukocyte antigen loci: a meta-analytic review of 497 population studies. Hum Immunol. 2008;69(7):443–464. doi: 10.1016/j.humimm.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, Schlom J, Tsang KY. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11(17):6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, Herrin VE, Shams MA, Steinberg SM, Merino M, Gooding W, Visus C, Deleo AB, Wolf JK, Bell JG, Berzofsky JA, Whiteside TL, Khleif SN. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother. 2012;61(3):373–384. doi: 10.1007/s00262-011-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deniger DC, Pasetto A, Robbins PF, Gartner JJ, Prickett TD, Paria BC, Malekzadeh P, Jia L, Yossef R, Langhan MM, Wunderlich JR, Danforth DN, Somerville RPT, Rosenberg SA. T-cell responses to TP53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, Kaltcheva T, Leong L, Morgan R, Chung V, Tinsley R, Eng M, Wilczynski S, Ellenhorn JDI, Diamond DJ, Cristea M. p53-reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin Cancer Res. 2018;24(6):1315–1325. doi: 10.1158/1078-0432.CCR-17-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jager E. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA. 2012;109(15):5797–5802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanitis E, Smith JB, Dangaj D, Flingai S, Poussin M, Xu S, Czerniecki BJ, Li YF, Robbins PF, Powell DJ., Jr A human ErbB2-specific T-cell receptor confers potent antitumor effector functions in genetically engineered primary cytotoxic lymphocytes. Hum Gene Ther. 2014;25(8):730–739. doi: 10.1089/hum.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin SD, Brown SD, Wick DA, Nielsen JS, Kroeger DR, Twumasi-Boateng K, Holt RA, Nelson BH. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS One. 2016;11(5):e0155189. doi: 10.1371/journal.pone.0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.