CASE
A previously healthy 37-year-old male presented to an emergency department (ED) in central Utah with a history of recurrent fevers (>39.4°C) characterized by headaches, myalgia, and chills. The febrile episodes began during a trip to Jordan ∼3 weeks before presentation, where the patient reported receiving medical care and completing a 3-day dose of azithromycin. Upon his return to Utah, the patient had no notable outdoor exposure and denied recent history of animal or insect bites, but he described visiting archaeological sites during his trip to Jordan. Due to the repetitive nature of his fevers, ED physicians initially considered malaria as a plausible explanation for the patient’s symptoms.
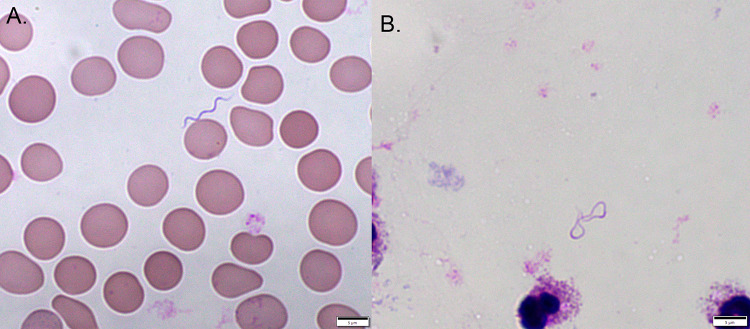
Peripheral blood was collected while the patient was experiencing a febrile episode and was submitted for parasite examination by way of Giemsa-stained thin and thick blood films. The blood films were negative for blood parasites, but long (range, 10 to 20 μm), helically coiled spirochetes were present throughout the thin films at a quantified frequency of approximately 1 or 2 bacteria per 10 high-power fields of view (Fig. 1). This finding was highly suggestive of relapsing fever Borrelia (RFB), and the specimen was reported as “spirochetes detected.” Although RFB is endemic in the mountains of central Utah, the patient’s lack of appropriate localized exposure did not conclusively support this diagnosis.
FIG 1.
Microscopic examination of extraerythrocytic Borrelia persica in the Giemsa stain of blood collected during the febrile episode. The sinusoidal morphology depicted in the thin blood smear (A) is often lost in the thick blood smear (B) (×1,000 magnification).
The treating physician ordered serological testing for Lyme disease (LD), despite the patient having no travel history to a region where LD is endemic, in an attempt to “rule in” Lyme borreliosis as a possible explanation for the peripheral spirochetemia. Total IgM/IgG antibody enzyme-linked immunosorbent assay (ELISA) results for Lyme were positive, but both confirmatory IgM and IgG blots were negative (IgM = 1 of 3 bands; IgG = 1 of 10 bands). Given the known crossreactivity of anti-RFB antibodies with commercial LD ELISAs and the lack of patient travel history to an area where LD is endemic, the results of this testing provided no additional diagnostic information (https://www.cdc.gov/relapsing-fever/). A laboratory-developed real-time PCR for RFB which specifically targets the RFB species endemic to North America (Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae) was also ordered in an attempt to provide more specific evidence of an RFB diagnosis. When molecular interrogation failed to detect RFB nucleic acid from the blood specimens, the extracted nucleic acid was amplified by conventional PCR, and Sanger sequencing of the 16S amplicon was performed. DNA sequence analysis could not distinguish between Borrelia persica and Borrelia caucasica, two species responsible for relapsing fever (RF) in Europe and the Middle East (1). These two species are more polymorphic in the conserved 16S region targeted by the RFB real-time PCR and would not be expectedly detected by the clinical assay. An ultimate resolution was provided by analysis of the Borrelia-specific flagellin (flaB) amplicon, which identified B. persica nucleic acid in the specimen. B. persica is only endemic to Jordan and other neighboring nations in the Middle East, which ultimately resolved the ambiguity of the geographic exposure.
The patient was empirically treated with oral doxycycline (100 mg twice a day [b.i.d.] for 10 days) based on the initial blood smear result and was subsequently lost to follow-up.
DISCUSSION
Vectorborne illnesses caused by members of the Borreliaceae family are found throughout most of the world and include both tick-borne relapsing fever (TBRF) and LD (1, 2). At the time of writing, TBRF Borrelia species have been reported from all continents except Antarctica (1, 2). There are 22 validly described species of RFB and 6 proposed taxa that have not been confirmed (1). The “Old World” RFB species are found primarily in Africa and the Middle East, whereas the “New World” RFB species are found exclusively in North, Central, and South America (1). Three “New World” species of RFB are endemic to the United States, namely, B. hermsii, B. turicatae, and B. parkeri. B. hermsii is the most common cause of TBRF and is endemic in the mountainous regions of the western/northwestern United States (including Utah) and Canada (3, 4). B. turicatae is endemic in the southern/central United States, western Canada, and Mexico, whereas B. parkeri is endemic in the southwestern United States, with some geographic overlap with B. hermsii (3, 4).
RFB is conventionally transmitted by soft ticks in the genus Ornithodoros, but exceptions include transmission of the phylogenetically related species Borrelia miyamotoi by Ixodes ticks (which does not cause a clinical relapsing fever syndrome) and louse-borne Borrelia recurrentis (5). In the United States, TBRF is often associated with sleeping in seasonally occupied, rodent-infested cabins, but it has also been identified following exposure to caves (1, 3; https://www.cdc.gov/relapsing-fever/). Ornithodoros ticks rarely disperse from their original habitat, as they remain close to the nest or burrow of the host animal and feed multiple times throughout a season without leaving the area. Unlike hard ticks, which require an extended feeding time to transmit infection, Ornithodoros ticks can feed in as little as 10 to 20 min, retreat back from the nest/burrow, and still transmit RFB during the feed (2, 6). This ultimately limits RFB dispersion potential, since the ticks do not remain attached to a host for extended periods (unlike the hard-shelled ticks, which attach to a host that can travel to distal locations, where the tick releases) (1, 6).
TBRF symptoms manifest ∼7 days after RFB transmission from an infected vector bite. In addition to fever, patients often display general influenza-like or nonspecific symptoms (>75% have headache, myalgia, chills, or nausea); however, infection with specific Borrelia species can present with more serious neurological involvement (1, 4). Untreated illness results in recurring febrile episodes lasting 3 to 5 days in length, which are interspaced with 5- to 7-day afebrile periods (6). The repetitive cycles of disease are the result of antigenic variation, a process through which Borrelia sequentially modifies immunodominant outer surface antigens. Only serotypes that can escape the initial IgM antibody immune response survive and give rise to subsequent peaks of spirochetemia and fever (1, 2). Overall, the number of relapses (1 to 14) and the severity of disease depends on the infecting Borrelia species, with more severe cases being associated with pregnancy or generally attributed to the human-adapted Borrelia duttonii or B. recurrentis (2, 4). B. persica typically presents with ∼1 month of symptoms if left untreated (6).
Despite the presence of bacteria in the blood, RFB is not readily culturable in blood bottles (2). The standard laboratory diagnostic test for RFB is examination of peripheral blood smears stained with Giemsa, using both thin and thick smears. Wright-Giemsa is also acceptable but not conventionally employed for parasitological examination, and its use would more likely be the result of a hematologist detecting the spirochete serendipitously during a complete cell count analysis. Dark-field microscopy is also a useful detection method for RFB; however, the blood must be freshly collected and examined in order to detect the motile spirochetes (6). Few laboratories still maintain dark-field microscopes near the point of specimen collection. RFB cells are typically >10 μm in length and display well-defined sinusoidal morphology (4). These morphological features are more consistently visualized in thin smears than in thick smears, where the RFB cells can become more irregularly curved or even “stringy” (Fig. 1B) (6).
Peripheral blood smears are often ordered due to suspicion of other endemic fever-cycling organisms such as Babesia spp. or Plasmodium spp., and the finding of RFB is often serendipitous (as was the scenario in this case, although Plasmodium spp. are not endemic in Jordan, making the diagnosis even more serendipitous). Despite being primarily used to detect blood parasites, detection of spirochetes on a peripheral Giemsa smear must always be reported. Blood smear is, on average, 80% sensitive for the detection of RFB in infected patients, but this is highly dependent on when the blood specimen is collected (6). Sensitivity is maximized when the blood is collected during a febrile episode. PCR is the most clinically sensitive test, even more so than blood smear, and is less dependent on the time of blood collection (4, 6). In our laboratory, we have regularly encountered endemic cases that were smear negative but for which the real-time PCR was positive, with very early cycling thresholds and unambiguous identification of B. hermsii by subsequent Sanger sequencing. Both the peripheral blood smear examination and PCR are appropriate detection methods for acute infection; however, obtaining an accurate travel/exposure history is paramount, since a molecular method may be optimized to detect endemic RFB only. In this case, the North American RFB species are specifically targeted by the PCR that was ordered, but in silico analysis predicts that B. recurrentis, Borrelia coriaceae, Borrelia theileri, Borrelia lonestari, and Borrelia anserine would also be detected by the assay, whereas B. persica would not be. In suspected cases of RFB with ambiguous exposure history, a blood smear should always be included in the initial laboratory orders despite the analytical sensitivity superiority of PCR.
Serology for B. hermsii is commercially available in the United States and is considered crossreactive for other RFB species (multiple laboratories interpret a positive result as indicative of RFB exposure), but it cannot be used for acute diagnosis and should only be used for epidemiological studies or retrospective diagnosis of resolved clinical syndromes (2, 4). Lyme Borrelia total antibody ELISAs will detect crossreacting anti-RFB antibodies in patient serum (6; https://www.cdc.gov/relapsing-fever/), and thus physicians should take great care in only ordering LD antibody testing if the patient has been in regions where Lyme is endemic and LD is high on the differential diagnosis.
The 3-day azithromycin course taken by the patient while in Jordan was not an effective treatment; TBRF is treated empirically or based on positive laboratory tests with doxycycline or erythromycin when tetracyclines are contraindicated. The treatment length and dosing frequency is not well standardized; however, 2,000 mg/day (either 1,000 mg twice a day [b.i.d.] or 500 mg four times a day [q.i.d.]) for 7 to 10 days is generally recommended and is highly effective for rapid, sustained clearance of RFB (https://www.cdc.gov/relapsing-fever/). Most cases of TBRF are thought to spontaneously resolve after ∼4 weeks; however, some species are capable of causing longer clinical syndromes, in excess of 3 months, without appropriate therapy (6).
In this case, even though the patient resided in an area where RFB is endemic, the infection was acquired during travel to Jordan, likely during exposure to archeologic sites, as B. persica infections are associated with exposure to caves and archeological dig sites where the vector, Ornithodoros tholozani, lives (6). This case highlights the importance of collecting a thorough travel history, understanding the inclusivity of molecular assays, and ensuring that standard blood smears are ordered based on these aforementioned variables.
SELF-ASSESSMENT QUESTIONS
- Which of the following tests is not recommended for detection of acute tick-borne relapsing fever (TBRF)?
-
a.Serology
-
b.PCR
-
c.Giemsa smear of peripheral blood
-
d.Dark-field microscopy of peripheral blood
-
a.
- What is the typical morphology of RFB on Giemsa-stained thick smears?
-
a.Short, curved rods
-
b.Long (>10 μm), sinusoidal spirochetes
-
c.Long (>10 μm), irregularly curved or “stringy” spirochetes
-
d.Long (>10 μm), straight, boxy rods
-
a.
- What is the most sensitive method for detection of acute phase TBRF from blood specimens?
-
a.Culture of peripheral blood
-
b.PCR of peripheral blood
-
c.Giemsa smear of peripheral blood
-
d.Dark-field microscopy of peripheral blood
-
a.
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.01622-19 in this issue.
REFERENCES
- 1.Talagrand-Reboul E, Boyer PH, Bergstrom S, Vial L, Boulanger N. 2018. Relapsing fevers: neglected tick-borne diseases. Front Cell Infect Microbiol 8:98. doi: 10.3389/fcimb.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler SJ. 2015. Relapsing fever borreliae: a global review. Clin Lab Med 35:847–865. doi: 10.1016/j.cll.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Forrester JD, Kjemtrup AM, Fritz CL, Marsden-Haug N, Nichols JB, Tengelsen LA, Sowadsky R, DeBess E, Cieslak PR, Weiss J, Evert N, Ettestad P, Smelser C, Iralu J, Nett RJ, Mosher E, Baker JS, Van Houten C, Thorp E, Geissler AL, Kugeler K, Mead P, Centers for Disease Control and Prevention. 2015. Tickborne relapsing fever—United States, 1990–2011. MMWR Morb Mortal Wkly Rep 64:58–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin MS, Schwan TG, Anderson DE Jr, Borchardt SM. 2008. Tick-borne relapsing fever. Infect Dis Clin North Am 22:449–468, viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. 2013. Human Borrelia miyamotoi infection in the United States. N Engl J Med 368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assous MV, Wilamowski A. 2009. Relapsing fever borreliosis in Eurasia—forgotten, but certainly not gone! Clin Microbiol Infect 15:407–414. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]