Compared to its predecessor QuantiFERON-TB Gold In Tube (QFT-IT), QuantiFERON-TB Gold Plus (QFT-Plus) contains an additional antigen tube (TB2), stimulating both CD4+ and CD8+ T cells. The ability to discriminate CD4+ and CD8+ responses is suggested to be useful in differentiating stages of Mycobacterium tuberculosis infection. While QFT-Plus has already been evaluated in adults, there are not enough data in children evaluated for suspected active tuberculosis (TB) or latent TB infection (LTBI).
KEYWORDS: tuberculosis, children, interferon gamma release assay, QuantiFERON, IGRAs, QuantiFERON Plus
ABSTRACT
Compared to its predecessor QuantiFERON-TB Gold In Tube (QFT-IT), QuantiFERON-TB Gold Plus (QFT-Plus) contains an additional antigen tube (TB2), stimulating both CD4+ and CD8+ T cells. The ability to discriminate CD4+ and CD8+ responses is suggested to be useful in differentiating stages of Mycobacterium tuberculosis infection. While QFT-Plus has already been evaluated in adults, there are not enough data in children evaluated for suspected active tuberculosis (TB) or latent TB infection (LTBI). A prospective cross-sectional study was conducted among children aged 0 to 17 years who were evaluated for suspected active TB or screened for LTBI. All children underwent QFT-Plus and further clinical, radiological, and/or microbiological analyses according to clinical scenario. Of the 198 children enrolled, 43 (21.7%) were tested because of suspicion of active TB. A total of 12/43 (27.9%) were diagnosed with active TB, and among these, 10/12 (83.3%) had a positive QFT-Plus assay. Of the 155 children screened for LTBI, 18 (11.6%) had a positive QFT-Plus, and 5 (2.5%) had an indeterminate result. TB1 and TB2 quantitative responses were not able to discriminate active disease from latent infection. The percent agreement between TB1 and TB2 was 100%. QFT-Plus assay showed good sensitivity for active TB and was particularly useful for the evaluation of children with suspected LTBI, giving a low rate of indeterminate results in this group. More studies are needed to properly evaluate QFT-Plus ability in discriminating active disease from latent infection.
INTRODUCTION
Worldwide, tuberculosis (TB) is one of the top 10 causes of death and the leading cause from a single infectious agent (1). Children bear a substantial part of the TB epidemic with an estimated 1 million cases in 2015 (2), although data may not be accurate given the many difficulties associated with TB diagnosis in children and the weaknesses of surveillance systems in countries where TB is endemic. It is estimated that 25% of the global population is latently infected with Mycobacterium tuberculosis, and 2 to 8% of these will develop the disease most of the time (50%) within the first 2 years after infection. Even though they are asymptomatic and do not transmit the infection, these subjects are a reservoir that sustains TB disease (3). Importantly, the risk of TB progression is much higher in children (30 to 40% progression rate if untreated) (4). Moreover, children may present with nonspecific symptoms mimicking other childhood diseases, and microbiological confirmation is difficult due to the paucibacillary nature of TB, leading to diagnostic delay and further disease diffusion (5).
For these reasons, developing a diagnostic test that can reliably identify TB infection in children (both active TB and latent TB infection [LTBI]) is needed and considered a research priority (6). An immunological test should ideally aid in the following: early diagnosis of childhood TB; early detection and treatment of LTBI with reduction of transmission; selective treatment of those latently infected but at major risk of developing active TB, reducing adverse drug events and potentially drug resistance; and better definition of the recently defined TB spectrum (7, 8).
To date, the immunological tests used to diagnose TB infection are the tuberculin skin test (TST) and the interferon γ release assays (IGRAs). TST has well-known limitations and shortage is documented in Europe (9–11), but it still represents a reference point due to the decades of clinical experience. IGRAs are blood tests that measure the amount of interferon gamma (IFN-γ) produced after a 16 to 24 h stimulation of T cells with ESAT6 and CFP10, antigens highly specific for M. tuberculosis. IGRAs demonstrated a high negative predictive value (around 98%) for progression to active TB in low incidence countries (12), yet neither TST nor IGRAs can effectively discriminate active TB from LTBI or recognize recent M. tuberculosis infections or LTBI cases at higher risk of developing into active TB (8).
Recently, the 4th-generation QuantiFERON-TB Gold Plus (QFT-Plus) IGRA has been introduced in clinics and compared to its predecessor, the QuantiFERON-TB Gold In Tube (QFT-IT). It includes two tubes rather than one tube containing peptides of the M. tuberculosis-specific antigens EsxA and EsxB as follows: TB1, which stimulates mainly CD+ T cell responses; and TB2, which stimulates both CD4+ and CD8+ T cells. IFN-γ-producing CD8 T cells specific for these two M. tuberculosis antigens are more frequently detected in active TB patients than they are in subjects with LTBI (13, 14) and after recent infection compared to remote latent infection (15). Therefore, the QFT-Plus will ideally be useful to differentiate recent and remote LTBI, helping in the decision to start LTBI treatment.
While QFT-Plus has already been evaluated in adults, there are not enough data in children evaluated for suspected TB infection (16). In this scenario, we performed this study aiming to evaluate the accuracy of the QFT-Plus assay in children with suspected active TB/LTBI.
MATERIALS AND METHODS
A prospective cross-sectional study was conducted among children aged 0 to 17 years who were evaluated at our institution for TB infection or TB disease during the period from January 2017 to December 2018. The following categories of children were initially enrolled in the study: children with suspected active TB (symptomatic patients—children with suspected active TB) and otherwise healthy children with any risk factors for LTBI.
The following categories of children were initially enrolled in the study: children with suspected active TB (symptomatic patients—children with suspected active TB); otherwise healthy children with known exposure to an active TB adult case (therefore, children screened for LTBI); clinically healthy, nationally or internationally adopted children evaluated by a national protocol for immigrants and nationally/internationally adopted children with or without known history of contact with adult active TB cases (therefore, children screened for LTBI). This screening protocol has been approved by the National Working Group for Immigrant and Adopted Children, an official working group of the Italian Society of Pediatrics, and approved by the review board of our University; children affected by autoimmune diseases were screened for TB before starting a biological therapy.
All patients were clinically evaluated and tested by QFT-Plus. All children with a positive QFT-Plus or high clinical suspicion of TB disease underwent radiological and microbiological investigations to confirm or rule out active TB. Microbiological diagnosis included acid-fast bacilli (AFB) examination following Ziehl-Neelsen staining, culture for M. tuberculosis, and molecular detection of M. tuberculosis using the Anyplex MTB/NTM real-time detection system (Seegene) following previously indicated procedures (17). The samples were obtained on three consecutive days (either sputum or gastric gavage according to age) from all patients with suspected active TB. For those with suspected extrapulmonary TB, samples were taken from different sites according to the suspected TB localization. All children with a final diagnosis of active TB were evaluated for HIV infection. Two definitions of active TB were used as follows: definite (confirmed) and probable TB, as accepted by literature regarding pediatric TB (5, 11).
Diagnosis of LTBI was based on positive QFT-Plus and absence of any clinical, microbiological, and radiological features that would suggest active disease. Hence, according to the definitions used, the following final diagnoses were assigned for each patient at the end of clinical evaluations: active TB, LTBI, or non-TB children (no active TB or LTBI). This latter group included both healthy/asymptomatic children without LTBI (healthy) and all children with any other final diagnosis other than TB, such as bacterial, viral, fungal, or parasitic infections (disease other than TB [dotTB]). Data will be made publicly available upon publication and upon request for peer review.
QuantiFERON-TB Gold Plus.
QFT-Plus was performed according to manufacturer’s instructions. Data are presented as IU per milliliter of IFN-γ; the cutoff value for a positive test was 0.35 IU/ml.
Statistical analyses.
Data were analyzed using SPSS (SPSS, Chicago, IL) and Prism 5 software (GraphPad Software, San Diego, CA, USA). Differences in frequencies were evaluated by the Fisher exact test. The median IFN-γ production was calculated, the nonparametric Mann-Whitney U test was used to compare medians for unpaired comparisons and the Wilcoxon test for paired comparisons, and the Kruskal-Wallis test was used to compare medians among the different groups. Differences were considered significant at P values of ≤0.05.
Ethical statement.
The institutional review board of the Università Cattolica del Sacro Cuore of Rome, Italy, approved this study (prot 29213/19, ID 2555). We obtained verbal and written informed consent from the next of kin, caretakers, or guardians on behalf of the minors/children enrolled in the study.
RESULTS
Study population.
A total of 198 children were enrolled; 87 (43.9%) were female, and the mean age of the study population was 82 months (range, 0 to 224 months). Regarding age, 6/198 (3.1%) were aged 0 to 1 year, 61/198 (30.8%) were aged 2 to 5 years, 69/198 (34.8%) were aged 5 to 10 years, and 62/198 (31.3%) were older than 10 years. Most children evaluated came from western countries (western Europe and United States, 37.4%) and Eastern Europe (20.7%); 104 out of 198 (53%) patients were internationally adopted children (Table 1).
TABLE 1.
Study population
| Parameter | Total no. (%) (n = 198) | No. active TB (%) (n = 12) | No. LTBI (%) (n = 18) | No. dotTB (%) (n = 31) | No. healthy (%) (n = 137) | P value |
|---|---|---|---|---|---|---|
| Age group | <0.05 | |||||
| 0–12 | 6 (3.1) | 0 | 0 | 3 (9.7) | 3 (2.2) | |
| 13–60 | 61 (30.8) | 5 (41.6) | 3 (16.6) | 12 (38.7) | 41 (29.9) | |
| 61–120 | 69 (34.8) | 1 (8.4) | 4 (22.3) | 7 (22.6) | 57 (41.6) | |
| >120 Months | 62 (31.3) | 6 (50) | 11 (61.1) | 9 (29) | 36 (26.3) | |
| Origin | <0.0001 | |||||
| Western countries | 74 (37.4) | 4 (33.3) | 8 (44.4) | 27 (87) | 35 (25.5) | |
| Eastern Europe | 41 (20.7) | 1 (8.4) | 7 (38.9) | 2 (6.5) | 31 (22.6) | |
| Asia | 37 (18.7) | 2 (16.6) | 1 (5.6) | 34 (24.8) | ||
| Africa | 21 (10.6) | 4 (33.3) | 2 (11.1) | 1 (3.25) | 14 (10.2) | |
| South America | 25 (12.6) | 1 (8.4) | 0 | 1 (3.25) | 23 (16.9) | |
| QFT result | <0.0001 | |||||
| Positive | 28 (14.2) | 10 (83.3) | 18 (100) | |||
| Negative | 165 (83.4) | 2 (16.7) | 0 | 27 (87.1) | 136 (99.2) | |
| Indeterminate | 5 (2.5) | 0 | 0 | 4 (12.9) | 1 (0.8) |
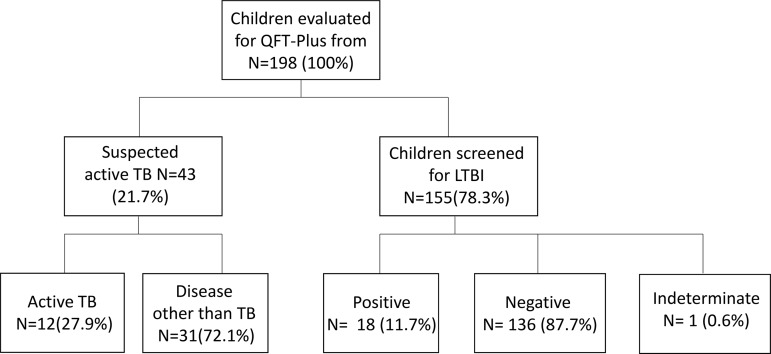
In Fig. 1, we summarized the study population, the reason for testing with QFT-Plus, and the results. In particular, 43 children (21.7%) were tested because of suspicion of active TB; among these, 12/43 (27.9%) were diagnosed with active TB disease (11 children with pulmonary TB and 1 with central nervous system [CNS] TB), and 31 (72.1%) received a final diagnosis of disease other than TB (dotTB).
FIG 1.
Study population enrolled into the study.
The remaining 155 children (78.3%) were screened for LTBI; 18 (11.6%) were eventually diagnosed as LTBI. Of these, 5/18 (27.8%) had known contact with an adult with active TB, and 10/18 (55.5%) came from countries where TB is endemic.
QFT-Plus qualitative results.
Among the 198 children tested by QFT-Plus assay, 28 (14.2%) scored positive, 165 (83.3%) scored negative, and 5 (2.5%) had indeterminate results (due to the lack of response to mitogen).
Among the 43 children evaluated for suspected active TB, 12 received a final diagnosis of active TB, and 10/12 (83.3%) had a positive QFT-Plus assay; among the 155 children screened for LTBI, 18/155 (11.6%) had a positive QFT-Plus assay and were diagnosed with LTBI and treated consequently, while 137/155 (88.3%) were QFT-Plus negative.
Among the group of children that received final diagnoses of active TB, 10/12 had a positive QFT-Plus, and 2 of these showed a negative result (sensitivity for active TB, 83.3%; specificity, 90.1%). These two children had a family member with active TB, and they received a final diagnosis of probable (microbiologically nonconfirmed) TB.
Among the 155 children tested for LTBI screening, 136/155 (87.7%) were negative, 18/155 (11.7%) were positive, and 1/155 (0.6%) was indeterminate. Of the 18 positives, 5 (28%) had a known exposure to an adult with active TB, and the remaining 7 (39%) came from countries with high TB burden.
Children tested with QFT-Plus properly respond to mitogen.
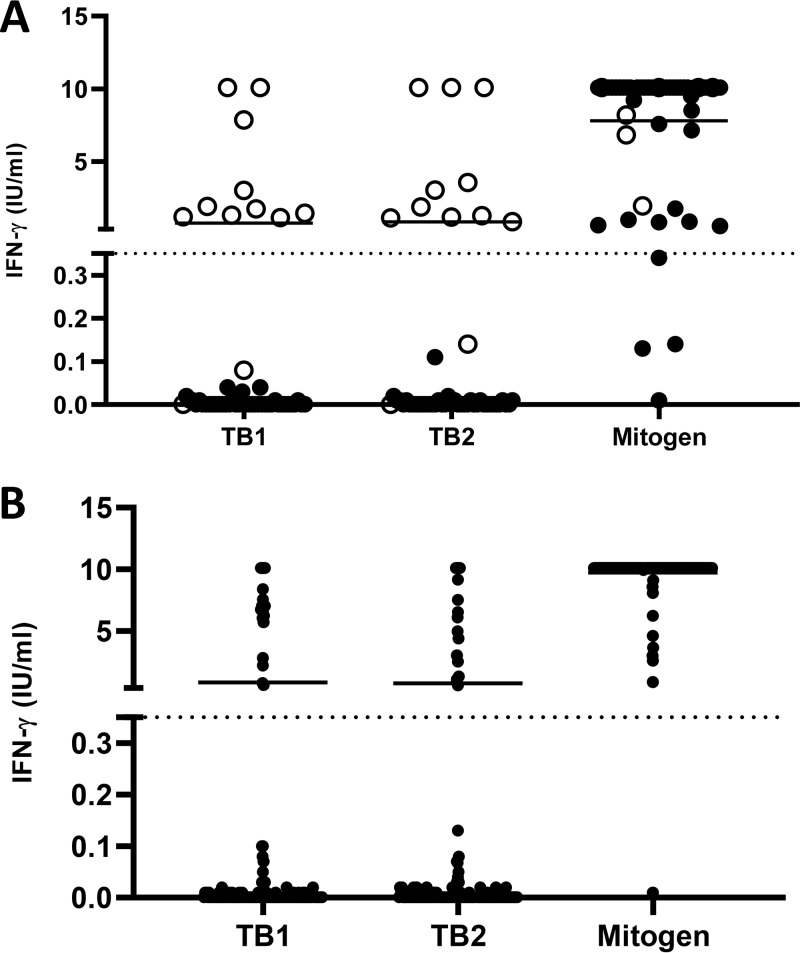
Measurement of the IFN-γ secretion in response to mitogen in IGRAs provides a useful indication of the potential ability of the immune system to respond to the antigenic stimuli. A total of 193/198 (97.5%) children along the different age groups responded to the mitogen by secreting high levels of IFN-γ (Fig. 2). Overall, only 5/198 (2.5%) had an indeterminate result, which indicates subjects unable to properly respond to the mitogen.
FIG 2.
(A) IFN-γ response to TB1 and TB2 antigens in children with suspected active TB. Each point on the graph represents an individual result. Horizontal lines indicate the median of IFN-γ production. Dotted lines indicate the cutoff for QFT-Plus (0.35 IU/ml). (B) IFN-γ response to TB1 and TB2 antigens in children screened for LTBI. IFN-γ was determined using enzyme-linked immunosorbent assay (ELISA) following whole-blood stimulation with QFT-Plus antigens for 24 h. Each point on the graph represents an individual result. Horizontal lines indicate the median of IFN-γ production. Dotted lines indicate the cutoff for QFT-Plus (0.35 IU/ml).
Among the 5 children with indeterminate results, one had congenital immunodeficiency, two had acute bacterial and viral coinfections, one child had autoimmune disorders, and only one child was otherwise healthy. There was no statistically significant effect of age or of the final diagnosis on the magnitude of the response to mitogen. Only 1/155 (0.6%) asymptomatic children screened for LTBI had indeterminate results.
Active TB and LTBI patients produce positive responses in both TB1 and TB2 tubes.
To investigate the ability to respond to the TB-specific antigens used in QFT-Plus (TB1 and TB2), we analyzed the IFN-γ response of all children according to the reason for testing (suspected active TB or screening for LTBI) (Fig. 2). In all cases, TB1 and TB2 responses were similar in each group (active TB and LTBI) and did not present statistically significant differences (P > 0.05).
Overall, all children with a positive QFT-Plus test had IFN-γ production in response to TB-specific antigens, which was always >0.5 IU/ml, whereas in cases of negative results, IFN-γ did not exceed 0.2 to 0.3 IU/ml (Fig. 2A).
In the 0- to 12-months age group, 0/6 children scored positive, and no one was diagnosed with active TB. In the 13- to 60-months age group, 8/61 children (13.1%) scored positive, and a final diagnosis of active TB was made for 5 of them. The remaining 3 children were diagnosed with LTBI. Mean values of IFN- γ production were 5.13 IU/ml for TB1 and 5.46 IU/ml for TB2 (Fig. 3).
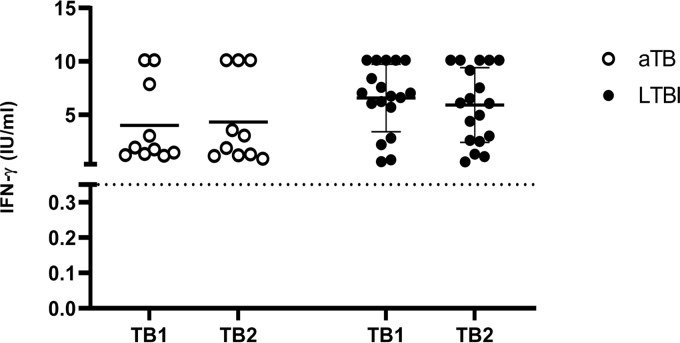
FIG 3.
Comparison in the IFN-γ response to TB1 and TB2 antigens in children with active TB and LTBI. Horizontal lines indicate the median of IFN-γ production. Dotted lines indicate the cutoff as defined in Fig. 2. aTB, active TB.
In the 61- to 120-months age group, 4/69 (5.8%) children scored QFT-Plus positive, and all of them were diagnosed as active LTBI. Mean values of IFN-γ production were 6.76 IU/ml for TB1 and 6.83 IU/ml for TB2 (Fig. 3). The child with active TB within this group had a negative QFT-Plus.
In the other group (>120 months), of the 16/62 (25.8%) children who scored positive by the QFT-Plus, 5 were diagnosed with active TB and 11 with LTBI. Mean values of IFN-γ production were 5.60 IU/ml for TB1 and 4.91 IU/ml for TB2 (Fig. 3). The percent agreement between TB1 and TB2 was 100%.
QFT-Plus does not discriminate active TB from LTBI in children.
We compared TB1 and TB2 responses in children with active TB and LTBI (Fig. 3). Active TB children had mean values of TB1 of 3.99 IU/ml and TB2 of 4.32 IU/ml (P = 0.23), while LTBI children had mean values of TB1 of 6.56 IU/ml and TB2 of 5.9 IU/ml (P = 0.24). TB1 and TB2 had similar mean values among each group. Although LTBI children had slightly higher mean values of TB1 responses over TB2 and active TB children showed the opposite pattern, we could not discriminate active TB from LTBI. Overall, the IFN-γ responses were not significantly affected by age.
DISCUSSION
In this study, we prospectively assessed the accuracy of the QFT-Plus assay to detect M. tuberculosis infection in a cohort of 198 children evaluated for either suspected active TB or LTBI. Only two studies evaluated QFT-Plus in children; therefore, our results add useful information to current literature.
First of all, we showed that 193/198 (97.5%) children in different age groups responded to the mitogen by secreting high levels of IFN-γ, indicating that the immune system is not impaired in its ability to mount an immune response, even in young children. Overall, the number of indeterminate results was low since only 5/198 (2.5%) had an indeterminate result. This rate is much smaller than what has been reported from previous studies on QFT-IT (8, 18–21) but similar to the overall percentage of indeterminate results (4%) described in a recent metanalysis about IGRAs other than QFT-Plus (22). Importantly, Nguyen et al. reported that only 2/222 children evaluated with QFT-Plus had indeterminate results, confirming our finding that QFT-Plus is also effective in children and can be a good alternative to TST (16). Among the 5 children with indeterminate results, 4/5 had comorbidities and only one child was otherwise healthy when screened for LTBI. This is an important point since QFT-Plus (and IGRAs in general) has been licensed as an aid in the diagnosis of TB and is primarily used to detect M. tuberculosis infection (23). Interestingly, children with coinfection undergoing antibiotic therapy for a disease other than TB (dotTB) had higher probability of having indeterminate results, confirming our previous data that ongoing viral infection or antibiotics can sometimes affect QFT responses (8).
The overall sensitivity of QFT-Plus for active TB was 83.3% and specificity was 90.1%. These rates are similar to what have been reported from previous studies on QFT-IT (8) but higher compared with rates of the only extensive pediatric study published so far on QFT-Plus that reported an overall sensitivity of 54.5% (16). Nevertheless, sensitivity raised to 84.2% when they exclusively evaluated pulmonary TB. In our series, only one child had extrapulmonary TB, and he had a negative QFT-Plus result and high mitogen response. Kay et al. described twelve children with active TB showing that 3 had negative results and 4 indeterminate results, therefore, having a sensitivity of 41.6%; however, in this series, 5 out of 12 children were HIV positive (24). Other QFT-Plus studies on adults also described good sensitivities for pulmonary TB (from 83% to 98.9%) (1, 18).
We evaluated QFT-Plus on a large group of children with possible LTBI (due to either known exposure or risk factors). The lack of a gold standard for LTBI prevents proper assessment of QFT-Plus performance for this group. Nevertheless, it is remarkable that a total of 12/18 children (66.6%) scoring positive to QFT-Plus, and therefore diagnosed with LTBI, had at least one risk factor for TB infection (known exposure with an adult TB index case, being born in a country where TB is endemic, or having parents born in a country where TB is endemic), supporting M. tuberculosis infection. Importantly, only 1/155 (0.6%) child of otherwise healthy children screened for LTBI had an indeterminate result, indicating that QFT-Plus can be used for this group of patients without concerns.
The manufacturer and regulatory agencies introduced QFT-Plus in clinical practice since there is evidence of different CD4+ and CD8+ T cell responses in active TB versus LTBI. Higher M. tuberculosis-specific CD8+ T cell responses are associated with higher M. tuberculosis burden, such as active TB disease or with a recent M. tuberculosis infection (25, 26). In our study, we found that children with LTBI produced, in general, higher amounts of IFN-γ from both tubes than children with active TB (mean TB1 value of 3.99 IU/ml and TB2 value of 4.32 IU/ml for active TB; TB1 value of 6.56 IU/ml and TB2 value of 5.9 IU/ml for LTBI). Within each group, the mean values of IFN-γ were very similar, although the active TB group had a slightly higher CD8+ T cell responses (TB2) while LTBI showed a slightly higher response for CD4+ (TB1). These differences, which support an increased secretion of IFN-γ by CD8+ T cells in active TB, were minimal and not statistically significant, preventing us from drawing any conclusion that QFT-Plus is able to distinguish active TB from LTBI in children. Studies on new markers to be potentially associated with QFT-Plus, such as the recently described combined use of QFT and heparin-binding hemagglutinin (HBHA)-IGRA (27), are still needed. Importantly, differently from adults, the CD4+/CD8+ ratio changes during child growth (28), potentially affecting TB1 and TB2 responses. Future studies should also compare IFN-γ responses with CD4+ and CD8+ total number along different age groups.
In our study, we found that TB1 and TB2 responses had a 100% concordance. In the single available study evaluating TB1/TB2 concordance in the pediatric population, among 45 positive QFT-Plus results, 37 were identified by both TB1 and TB2, 7 were identified by TB2 only, and 1 was identified by TB1 only; the TB1/TB2 agreement was 96.4% (95% confidence interval [CI], 93.9 to 98.9%) (16). According to the manufacturer instructions, QFT-Plus is considered positive when either TB1 or TB2 IFN-γ values (IU/ml) reach the assay cutoff. However, there are different views on how to interpret a discordant result. On this regard, Moon and colleagues compared QFT and QFT-Plus results in low-risk health care workers (HCWs) at a single U.S. center (25). They found that 2.1% and 3.0% of 626 HCWs with no identifiable risk factors for LTBI had positive QFT and QFT-Plus results, respectively. The higher positivity rate with QFT-Plus was more frequently due to positive results with TB2 than TB1 (2.4% versus 1.6%). Follow-up data were available for 11 of the 13 HCWs with discordant QFT-Plus results. No HCWs developed active TB during the follow-up period. At 9 to 13 months follow-up, 10 HCWs had a negative QFT, and 6 of 7 HCWs had a negative QFT-Plus (TB1 or TB2). One HCW, who was positive with QFT and QFT-Plus TB2 on enrollment, was subsequently positive with QFT and QFT-Plus (TB1 and TB2) and diagnosed with LTBI and treated accordingly. Although there are no similar studies evaluating the TB1/TB2 concordance in the pediatric populations (either active TB or infected/exposed children), these data suggest that our pediatric QFT-Plus-positive responses are real M. tuberculosis infection due to the 100% concordance rate.
In conclusions, our study suggests that the QFT-Plus assay is accurate in pediatric practice including very young children, showing good sensitivity for active TB (particularly pulmonary TB). QFT-Plus can be particularly useful for the evaluation of children with suspected LTBI, giving a very low rate of indeterminate results in this group. More studies are needed to properly evaluate QFT-Plus ability in discriminating active TB from LTBI in children of different age groups.
ACKNOWLEDGMENT
This work has been supported by the Università Cattolica del Sacro Cuore (Linea D3.2 awarded to G.D.).
We declare no conflict of interest.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. 2017. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet GlobHealth 5:e898–e906. doi: 10.1016/S2214-109X(17)30289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smits K, Corbiere V, Dirix V, Mekkaoui L, Wyndham-Thomas C, Libin M, Dreesman A, Loyens M, Payen MC, Singh M, Locht C, Mascart F. 2015. Immunological signatures identifying different stages of latent Mycobacterium tuberculosis infection and discriminating latent from active tuberculosis in humans. J Clin Cell Immunol 6:4. doi: 10.4172/2155-9899.1000341. [DOI] [Google Scholar]
- 4.Venturini E, Tersigni C, Chiappini E, De MM, Galli L. 2017. Optimizing the management of children with latent tuberculosis infection. Expert Rev Anti Infect Ther 15:341–349. doi: 10.1080/14787210.2017.1279541. [DOI] [PubMed] [Google Scholar]
- 5.Buonsenso D, Lancella L, Delogu G, Krzysztofiak A, Testa A, Ranno O, D’Alfonso P, Valentini P. 2012. A 20-year retrospective study of pediatric tuberculosis in two tertiary hospitals in Rome. Pediatr Infect Dis J 31:1022–1026. doi: 10.1097/INF.0b013e3182615270. [DOI] [PubMed] [Google Scholar]
- 6.Ciofi Degli Atti ML, Castelli GG, Ciliento G, Lancella L, Russo C, Coltella L, Vinci MR, Zaffina S, Raponi M. 2011. Prolonged in-hospital exposure to an infant with active pulmonary tuberculosis. Epidemiol Infect 139:139–142. doi: 10.1017/S0950268810001809. [DOI] [PubMed] [Google Scholar]
- 7.Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, Ma S, Meermeier E, Lewinsohn DM, Sherman DR. 2018. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 31:e00021-18. doi: 10.1128/CMR.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sali M, Buonsenso D, Goletti D, D’Alfonso P, Zumbo A, Fadda G, Sanguinetti M, Delogu G, Valentini P. 2015. Accuracy of QuantiFERON-TB Gold test for tuberculosis diagnosis in children. PLoS One 10:e0138952. doi: 10.1371/journal.pone.0138952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D, Sanduzzi A, Delogu G. 2014. Performance of the tuberculin skin test and interferon-gamma release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl 91:24–31. doi: 10.3899/jrheum.140099. [DOI] [PubMed] [Google Scholar]
- 10.Tebruegge M, on behalf of the Paediatric Tuberculosis Network European Trials Group (ptbnet), Buonsenso D, Brinkmann F, Noguera-Julian A, Pavic I, Arbore A, Vancikova Z, Velizarova S, Welch SB, Ritz N. 2016. European shortage of purified protein derivative and its impact on tuberculosis screening practices. Int j Tuber Lung Dis 20:1293–1299. doi: 10.5588/ijtld.15.0975. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull L, Bell C, Child F. 2017. Tuberculosis (NICE clinical guideline 33). Arch Dis Child Educ Pract Ed 102:136–142. doi: 10.1136/archdischild-2016-310870. [DOI] [PubMed] [Google Scholar]
- 12.Abubakar I, Drobniewski F, Southern J, Sitch AJ, Jackson C, Lipman M, Deeks JJ, Griffiths C, Bothamley G, Lynn W, Burgess H, Mann B, Imran A, Sridhar S, Tsou C-Y, Nikolayevskyy V, Rees-Roberts M, Whitworth H, Kon OM, Haldar P, Kunst H, Anderson S, Hayward A, Watson JM, Milburn H, Lalvani A, Adeboyeku D, Bari N, Barker J, Booth H, Chua F, Creer D, Darmalingam M, Davidson RN, Dedicoat M, Dunleavy A, Figueroa J, Haseldean M, Johnson N, Losewicz S, Lord J, Moore-Gillon J, Packe G, Pareek M, Tiberi S, Pozniak A, Sanderson F. 2018. Prognostic value of interferon-gamma release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis 18:1077–1087. doi: 10.1016/S1473-3099(18)30355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M. 2013. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Ohmiti K, Goletti D, Bart PA, Hanekom W, Scriba TJ, Nicod L, Pantaleo G, Harari A. 2015. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis 60:432–437. doi: 10.1093/cid/ciu795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, Codecasa LR, Cugnata F, Di SC, Ferrarese M, Goletti D, Lipman M, Rancoita PM, Russo G, Tadolini M, Vanino E, Cirillo DM. 2016. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48:1411–1419. doi: 10.1183/13993003.00510-2016. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DT, Phan H, Trinh T, Nguyen H, Doan H, Pham N, Nguyen H, Nguyen H, Nguyen HV, Le HV, Nguyen N, Graviss EA. 2019. Sensitivity and characteristics associated with positive QuantiFERON-TB Gold-Plus assay in children with confirmed tuberculosis. PLoS One 14:e0213304. doi: 10.1371/journal.pone.0213304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sali M, De Maio F, Caccuri F, Campilongo F, Sanguinetti M, Fiorentini S, Delogu G, Giagulli C. 2016. Multicenter evaluation of Anyplex Plus MTB/NTM MDR-TB assay for rapid detection of Mycobacterium tuberculosis complex and multidrug-resistant isolates in pulmonary and extrapulmonary specimens. J Clin Microbiol 54:59–63. doi: 10.1128/JCM.01904-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergamini BM, Losi M, Vaienti F, D'Amico R, Meccugni B, Meacci M, De Giovanni D, Rumpianesi F, Fabbri LM, Balli F, Richeldi L. 2009. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 123:e419–e424. doi: 10.1542/peds.2008-1722. [DOI] [PubMed] [Google Scholar]
- 19.Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. 2008. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One 3:e2624. doi: 10.1371/journal.pone.0002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, Nienhaus A, Ruhwald M, Wagner D, Zellweger JP, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 37:88–99. doi: 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- 21.Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, Novelli V, Dixon GL. 2009. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J 28:669–673. doi: 10.1097/INF.0b013e3181a16394. [DOI] [PubMed] [Google Scholar]
- 22.Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M, Ritz N. 2019. Risk factors for indeterminate interferon-gamma release assay for the diagnosis of tuberculosis in children-a systematic review and meta-analysis. Front Pediatr 7:208. doi: 10.3389/fped.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delogu G, Sali M, Fadda G. 2013. The biology of mycobacterium tuberculosis infection. Mediterr J Hematol Infect Dis 5:e2013070. doi: 10.4084/mjhid.2013.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay AW, DiNardo AR, Dlamini Q, Kahari J, Mndzebele T, Mtetwa G, Ustero P, Maphalala G, Mandalakas AM. 2019. Evaluation of the QuantiFERON-Tuberculosis Gold Plus assay in children with tuberculosis disease or following household exposure to tuberculosis. Am J Trop Med Hyg 100:540–543. doi: 10.4269/ajtmh.18-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. 2017. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657. doi: 10.1128/JCM.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2017. Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 106:38–43. doi: 10.1016/j.tube.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Sali M, Buonsenso D, D'Alfonso P, De Maio F, Ceccarelli M, Battah B, Palucci I, Chiacchio T, Goletti D, Sanguinetti M, Valentini P, Delogu G. 2018. Combined use of Quantiferon and HBHA-based IGRA supports tuberculosis diagnosis and therapy management in children. J Infect 77:526–533. doi: 10.1016/j.jinf.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Tosato F, Bucciol G, Pantano G, Putti MC, Sanzari MC, Basso G, Plebani M. 2015. Lymphocytes subsets reference values in childhood. Cytometry 87:81–85. doi: 10.1002/cyto.a.22520. [DOI] [PubMed] [Google Scholar]