Flaviviruses encode one, two, or no N-linked glycosylation sites on their envelope proteins. Glycosylation can impact virus interactions with cell surface attachment factors and also may impact virion stability and virus replication. Envelope protein glycosylation has been identified as a virulence determinant for multiple flaviviruses, but the mechanisms by which glycosylation mediates pathogenesis remain unclear. In this Gem, we summarize current knowledge on flavivirus envelope protein glycosylation and its impact on viral infection and pathogenesis.
KEYWORDS: DC-SIGN, DC-SIGNR, flavivirus, West Nile virus, Zika virus, dengue virus, glycosylation
ABSTRACT
Flaviviruses encode one, two, or no N-linked glycosylation sites on their envelope proteins. Glycosylation can impact virus interactions with cell surface attachment factors and also may impact virion stability and virus replication. Envelope protein glycosylation has been identified as a virulence determinant for multiple flaviviruses, but the mechanisms by which glycosylation mediates pathogenesis remain unclear. In this Gem, we summarize current knowledge on flavivirus envelope protein glycosylation and its impact on viral infection and pathogenesis.
INTRODUCTION
The Flavivirus genus includes human pathogens such as West Nile virus (WNV), Japanese encephalitis virus (JEV), dengue virus (DENV), Zika virus (ZIKV), yellow fever virus (YFV), and tick-borne encephalitis virus (TBEV). Most flaviviruses are vector borne, transmitted primarily by mosquitoes and ticks. Many of these arboviruses are capable of causing severe diseases, such as encephalitis, hemorrhagic fever, and birth defects (1, 2). Thus, the endemic transmission of flaviviruses in certain regions of the world, as well as their emergence in new areas, represents a significant public health burden as well as a threat to livestock and wildlife (3–5). In this Gem, we discuss the current knowledge regarding flavivirus envelope protein glycosylation and how it can contribute to the viral infection cycle and pathogenesis.
Flavivirus replication.
Flaviviruses are enveloped, positive-sense single-stranded RNA viruses. The flavivirus genome is translated as a single open reading frame flanked by 5′ and 3′ untranslated regions (6). The polyprotein is cleaved by host and viral proteases into three structural proteins, the capsid (C), premembrane/membrane (prM/M), and envelope (E), as well as seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A NS4B, and NS5) (7).
Both prM and E are incorporated into the viral envelope in a highly ordered conformation. prM participates in the proper folding, maturation, and assembly of E during replication (7). E facilitates membrane fusion between the virus and host cell and is the primary viral protein against which neutralizing antibodies are produced. Flavivirus E proteins share ∼40% amino acid identity (7, 8) and are class II fusion proteins with three distinct domains (Fig. 1A) (8–10). Domains I and II are discontinuous, forming a central β-barrel and elongated dimerization region, while domain III forms a continuous immunoglobulin-like domain. E proteins form head-to-tail homodimers, 90 of which assemble to form a virion surface with 2-fold, 3-fold, and 5-fold symmetry axes (11). After virion attachment to the cell and endocytosis, E proteins undergo an irreversible pH-dependent conformational change to mediate the fusion of viral and host membranes inside the endosome (8). Flavivirus replication and assembly occur on endoplasmic reticulum (ER) membranes, with encapsidated genomes acquiring their envelope as they bud into the ER lumen. The pr peptide is positioned at the distal end of E domain II within the heterodimer, where it obscures the fusion loop on E proteins of immature virions (12). The transit of assembled virions through the acidic compartments of the trans-Golgi network results in a pH-dependent reorganization of the virion surface, allowing cleavage of pr peptide by host furin-like proteases, forming mature infectious particles (8).
FIG 1.
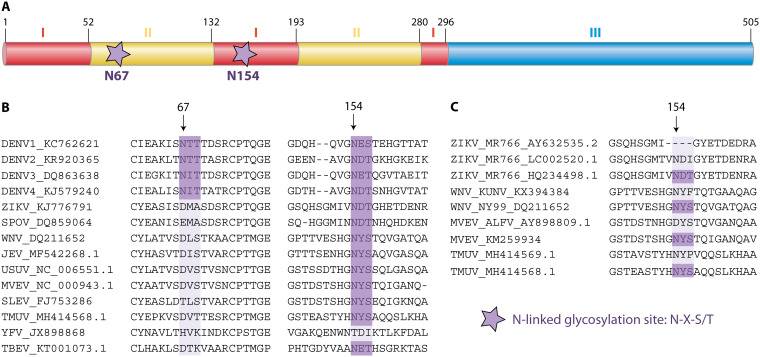
Flavivirus E protein glycosylation sites. (A) Domain organization of DENV E; stars indicate the glycosylation sites at asparagine (N) residues 67 and 154. Numbers indicate the amino acid residues comprising E domain I (red), domain II (yellow), and domain III (blue). (B) Glycosylation at N154 is highly conserved among flaviviruses, whereas glycosylation at N67 is unique to DENV. Accession numbers for each virus are indicated. (C) Glycosylation at N154 can differ among different strains of the same flavivirus. DENV, dengue virus; ZIKV, Zika virus; SPOV, Spondweni virus; WNV, West Nile virus; JEV, Japanese encephalitis virus; USUV, Usutu virus; MVEV, Murray Valley encephalitis virus; SLEV, Saint Louis encephalitis virus; TMUV, Tembusu virus; YFV, yellow fever virus; TBEV, tick-borne encephalitis virus.
Flavivirus envelope protein glycosylation.
Many flaviviruses contain an N-linked glycosylation site (N-X-S/T) at amino acid N154 of E, where a glycan is added to the amide nitrogen of the asparagine (Asn [N]) residue as the emerging protein is being translated (13). However, the number and position of predicted glycosylation sites are not conserved among all flaviviruses (Fig. 1B) nor among different strains of the same virus (Fig. 1C) (14–18). For instance, most WNV strains (such as NY99) are glycosylated at N154, but some strains, such as Kunjin, are not (19, 20). Similarly, all Asian lineage ZIKV strains, such as H/PF/2013 and PRVABC59, are glycosylated at N154, whereas some African lineage ZIKV strains are not (21–23). The prototype ZIKV strain includes both glycosylated and nonglycosylated variants, all designated MR766, which can be a source of confusion in the literature (23–25). DENV is unique among flaviviruses in that its E protein contains two N-linked glycosylation sites, one at N67 and a second at N153, which are present in most strains of the four DENV serotypes (18, 26, 27). E glycosylation plays important roles in viral attachment and cell entry, replication, transmission, and pathogenesis. The effects of glycosylation are particularly important for these vector-borne viruses because the process of protein glycosylation differs between mammalian and insect cells.
Protein glycosylation in mammalian and insect cells.
In mammalian cells, glycosylation takes place in the ER-Golgi complex, where a network of glycotransferases and glycosidases enzymatically synthesize a diverse array of glycan structures (13, 28, 29). Briefly, a lipid-linked oligosaccharide, synthesized from the lipid dolichol, serves as a carrier of the glycan. Dol-PP-GlcNAc2Man5 is enzymatically synthesized on the cytoplasmic side of the ER membrane and then translocated across the membrane into the ER lumen, where further monosaccharides are added. The final glycan structure is transferred cotranslationally to the Asn residue of an appropriate N-X-S/T motif via an oligosaccharyltransferase. Further processing takes place in the ER-Golgi complex, where glycosidases trim the mannose residues before glycotransferases extend the “antennae” of the glycans to produce larger hybrid or complex structures. Many flaviviruses (e.g., WNV and JEV) have avian amplifying hosts. Although glycosylation processes in avian cells have not been described as extensively as those in mammalian cells, avian-derived viruses likely have glycan structures similar to those of mammalian-derived viruses, as influenza viruses derived from chicken eggs, human embryonic lung fibroblasts, or Madin-Darby canine kidney cells contain similar glycan structures, though at different frequencies (30, 31).
Protein glycosylation in insect cells has been best studied in organisms such as mosquitoes, fruit flies, and moths (32, 33). Ticks are another important arthropod vector of flaviviruses, but the glycosylation pathways in tick cells have not been extensively described. The N-linked glycosylation pathway in insects begins like the mammalian pathway, with the synthesis and transfer of an oligosaccharide precursor, Dol-PP-GlcNAc2Man9, to an appropriate Asn on a newly synthesized polypeptide (33). Studies of N-linked glycosylation in mosquito cells suggest that carbohydrate structures are not further processed to generate complex carbohydrates but instead remain high-mannose or paucimannose (3-mannose-residue) oligosaccharides (34–36). Because flaviviruses typically cycle between vertebrate and arthropod hosts, distinct glycan structures added by these host cells contribute to different properties of the viruses initiating each round of infection, as arthropod-derived virions with simple sugars initiate infection in vertebrate hosts, and vertebrate-derived virions with complex sugars initiate infection in arthropod vectors. This is important because the structure of the carbohydrate can have an impact on its function, such as binding to cell surface attachment factors and initiating infection (37).
Envelope protein glycosylation and attachment.
The first step in viral entry is virion binding to the cell surface. Cell surface molecules that facilitate virus binding and infection but do not trigger the fusion of viral and cellular membranes are referred to as attachment factors (10). One family of attachment factors are lectins, proteins that bind carbohydrates, many in a calcium-dependent manner (C-type lectins) (10, 38). Two extensively studied C-type lectins are dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN or CD209) and its paralog DC-SIGN-related protein (DC-SIGNR, L-SIGN, or CD209L). DC-SIGN is highly expressed in macrophages and dendritic cells (DCs), while DC-SIGNR is expressed on microvascular endothelial cells, especially in the liver sinusoids, lymph nodes, and placental villi (38).
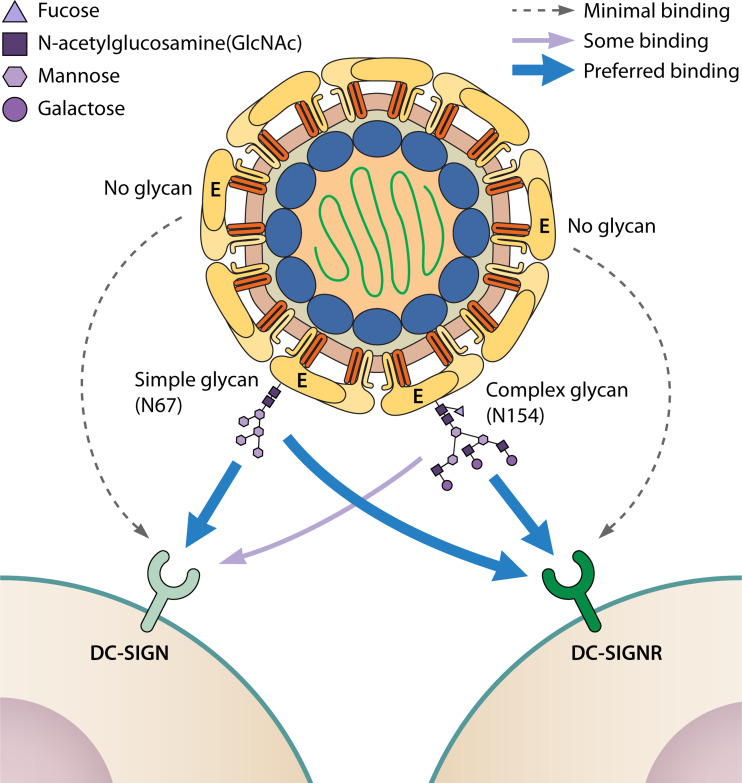
Glycosylated E protein interacts with cell surface lectins, facilitating attachment and infectivity (34–45, 83–85). Martina et al. showed that WNV strains containing E glycosylation at position N154 use DC-SIGN as an attachment factor, leading to enhanced infection compared to nonglycosylated strains (46). Davis et al. observed that DC-SIGNR promoted WNV infection more efficiently than did DC-SIGN, particularly when the virus was grown in mammalian cells, and this was dependent upon E glycosylation at N154 (40). However, mutant WNV E proteins with an N-linked glycosylation site at position N67 could interact with DC-SIGN (44). The DC-SIGN interactions were dependent on the incorporation of high-mannose sugars at position N67, whereas DC-SIGNR recognized WNV bearing either complex or high-mannose sugars (44). Likewise, JEV mutants glycosylated at position N67 showed enhanced DC-SIGN binding compared to viruses glycosylated at N154 (45). ZIKV E glycosylation at N154 facilitates the infection of cells expressing exogenous DC-SIGN and, to a greater extent, DC-SIGNR (23, 41, 86, 87). Distinct effects of glycosylation were observed in Vero and A549 cells, two epithelial cell lines commonly used in flavivirus studies. E glycosylation partially contributed to ZIKV infection in A549 cells but was dispensable in Vero cells. Both of these cell lines lack DC-SIGN or DC-SIGNR expression, suggesting that attachment can be mediated by other lectins (23). In contrast to the single glycan on most flavivirus E proteins, DENV is glycosylated at N67 and N153 (18, 26, 27). A cryo-electron microscopy study demonstrated that the carbohydrate recognition domain of DC-SIGN binds preferentially to glycans present at N67 on DENV particles (42). Hacker et al. showed that all four DENV serotypes had N-linked glycans added to both N67 and N153 of the E protein in both mammalian and mosquito cells, and viruses derived from both cell types were equally effective at infecting DC-SIGN expressing human monocytes and DCs (39). In DENV derived from mammalian cells, the N-linked glycans were a mix of high-mannose sugars and complex sugars, while the N-linked glycans on mosquito-derived virus were a mix of high-mannose sugars and paucimannose sugars (39). Another study revealed DENV lacking E glycosylation lost the ability to infect DC-SIGN- and DC-SIGNR-expressing cells (43). Altogether, these studies show that the location of the flavivirus E protein glycosylation motif, as well as the cell type in which the virus is propagated, influence the final structure of the N-linked glycan, which in turn can influence virus binding to cell surface attachment factors and infectivity (Fig. 2).
FIG 2.
Glycan structure influences E binding to attachment factors. Flaviviruses with nonglycosylated E have minimal binding to the lectins DC-SIGN or DC-SIGNR, whereas glycosylation at N67 or N154 facilitates virus binding to cell surface lectins. Simple (high-mannose) glycans are preferentially added at N67 and facilitate binding to DC-SIGN or DC-SIGNR. In contrast, complex glycans are preferentially added at N154 and favor binding to DC-SIGNR rather than DC-SIGN.
Envelope glycosylation and replication.
E glycosylation plays a role in flavivirus attachment; however, the roles of E glycosylation in flavivirus replication and assembly are less clear. Ablating N154 glycosylation on TBEV resulted in impaired secretion of virus-like particles (VLPs) (47), as well as altered conformation of secreted E protein and a corresponding decrease in infectivity (48). This effect was observed for E protein secreted from mammalian cells but not tick cells, suggesting cell type-dependent effects of E glycosylation. Similarly, removal of the N-linked glycosylation site in E significantly reduced the release of WNV subviral particles (SVPs), but these nonglycosylated SVPs were more infectious than were glycosylated SVPs, particularly on mosquito cells (49). Glycosylation of the ZIKV E protein at N154 significantly influenced the expression, production, and secretion of the E protein ectodomain, as well as VLP production and infectivity (50). The reduction of virion or particle release may be due to impaired trafficking along the ER-Golgi secretory pathway in the absence of appropriate E protein glycosylation. It also may be the consequence of incorrect E folding or interference with E dimerization, since glycosylation at N154 may stabilize the antiparallel E dimer conformation by keeping the fusion loop in place (9). However, ZIKV mutants lacking N154 glycosylation exhibit equivalent viral replication in mammalian and mosquito cells compared to glycosylated viruses (23, 51, 52). Nonetheless, these studies suggest that flavivirus E glycosylation can influence viral particle assembly, secretion, and infectivity.
In addition to N154 glycosylation, DENV also contains a glycosylation site at N67 of the E protein. Mondotte et al. found that E glycosylation was not essential for DENV replication in insect cells, although ablating the glycosylation at N67 (N67Q), but not N153 (N153Q), resulted in a dramatic decrease in viral particle assembly or release in mammalian cells (53). Similarly, Bryant et al. observed that DENV E glycosylation at N67 was important for growth in mammalian cells (54). However, though N67Q virus could replicate in mosquito cells, a compensatory mutation arose (K64N), introducing a new glycosylation site, suggesting that glycosylation at N67 (or nearby N64) is advantageous in mosquito cells. Interestingly, nonglycosylated mutants replicated similarly in inoculated Aedes aegypti mosquitoes, with no change in their glycosylation status (54).
In contrast, Lee et al. found that N67 glycosylation was dispensable for efficient DENV release from mammalian cells, depending on the amino acid substitution introduced to abolish glycosylation (27). Changing the Thr at position 69 of the N67 N-X-S/T motif to Val or Leu retained efficient growth, whereas a change to Ala reduced virus growth compared to that of the wild-type virus (27). Additionally, the conservative substitution of Asn to Gln at position 67 was markedly less detrimental for viral growth than was the nonconservative change of Asn to Asp. Furthermore, the strain origin of the E protein influenced the impact of ablating the glycan (27). Ablating E glycosylation at N67 of the DENV2 16681 strain prevented growth in mammalian cells but was well tolerated in a chimeric virus encoding the prM and E proteins from strain PUO-218, which differs from 16681 at 4 residues. These observations highlight that it can be challenging to define whether the glycan per se mediates infection, as opposed to the specific amino acid residues that comprise the glycosylation signal. For example, different amino acid substitutions at E154/156 of WNV conferred distinct avian host and vector competence phenotypes independent of E protein glycosylation status (20). Likewise, a ZIKV mutant lacking E glycosylation via a T156I mutation was unable to bind to DC-SIGNR-expressing cells, whereas an N154Q mutant retained some binding activity (23). Thus, in addition to the specific effects of E glycosylation, the specific amino acids at or near E residues 67 and 154 also may affect viral attachment and infectivity.
Envelope protein glycosylation and flavivirus transmission.
Most flaviviruses are transmitted between hosts by arthropod vectors (mosquitoes or ticks), which acquire the virus during a blood meal (55). Vector-borne transmission requires the ingested virus to cross the midgut barrier and spread to the salivary glands to be transmitted to a new vertebrate host during a subsequent feeding. Viruses must overcome multiple factors in the vector to be efficiently transmitted, including vector antiviral responses and tissue barriers (56, 57).
E protein glycosylation plays a role in flavivirus transmission via several mechanisms. Moudy et al. showed that nonglycosylated WNV replicated less efficiently than did glycosylated WNV in Culex mosquitoes and was transmitted less efficiently (58). Interestingly, nearly all of the mosquitoes infected with nonglycosylated WNV transmitted a revertant virus, suggesting a strong selective pressure favoring glycosylated WNV in mosquitoes (58). E protein glycosylation could facilitate viral transmission across vector barriers by several mechanisms. Soluble carbohydrate-binding proteins could form a link between the virus and midgut surfaces, or cell surface lectins could facilitate attachment to mosquito cells (59). In addition to facilitating transmission through attachment, the E glycan may also play a role in evading vector antiviral responses. Wen et al. found that ZIKV E N154 glycosylation promoted midgut invasion by inhibiting the reactive oxygen species (ROS) antiviral response (60). They further showed that ablating E glycosylation (T156I) prevented mosquito infection via the oral route, whereas there was no effect on infection by intrathoracic injection, which bypasses the midgut.
Fontes-Grafias et al. similarly showed that nonglycosylated ZIKV was very inefficient at infecting A. aegypti mosquitoes via a bloodmeal (52). However, in contrast to the ability of E glycosylation to facilitate attachment to mammalian cells, ZIKV with nonglycosylated E (N154Q) resulted in increased viral attachment, virion assembly, and infectivity of progeny virus in C6/36 mosquito cells compared to glycosylated virus (52, 86). Another study showed that ablating E glycosylation of DENV also increased virus entry but reduced virion release in C6/36 cells (27). These studies suggest that despite a possible detrimental role in infecting C6/36 cells in culture, flavivirus E protein glycosylation is advantageous in mosquitoes, where it aids viral transmission by subverting tissue barriers and immune responses.
Though flaviviruses primarily are transmitted by arthropod vectors, vector-independent transmission has been documented in a variety of circumstances including TBEV in goat milk, JEV among pigs, and WNV, Bagaza virus, and Tembusu virus among birds (61–66). An S156P mutation that ablated E protein glycosylation in Tembusu virus resulted in impaired transmission among ducks, suggesting that E glycosylation could facilitate vector-independent as well as vector-borne transmission of flaviviruses (67).
Envelope protein glycosylation and pathogenesis.
Since E glycosylation can play a role in flavivirus attachment, replication, and transmission, it is not surprising that it also contributes to pathogenesis for some flaviviruses. Several studies have suggested a role for E protein glycosylation as a molecular determinant of neurovirulence. The 1999 emergence of WNV in the United States was characterized by large-scale mortality in wild birds, as well as many cases of encephalitis in humans (68, 69). In studies comparing WNV strains from the 1999 outbreak to historical WNV strains, E glycosylation was associated with increased brain infection and lethality in mice (70). Beasley et al. confirmed these findings by generating infectious clones of glycosylated virulent (NY99) and nonglycosylated attenuated (ETH76a) WNV strains and measuring their lethality in mice (71). They swapped the prM-E sequences of ETH76a into the NY99 infectious clone or mutated residue N154 to abolish glycosylation in NY99, both of which resulted in attenuation of the virus to a level comparable to that of wild-type ETH76a. Furthermore, a mutation that added the glycosylation to the ETH76a E protein on the NY99 backbone yielded a virus with virulence equivalent to that of wild-type NY99, indicating that E protein glycosylation mediated the observed differences in virulence.
Several groups have generated chimeras of virulent and attenuated flaviviruses to analyze the role of viral E proteins in neuroinvasiveness (the ability of the virus to spread into the central nervous system from peripheral tissues) and neurovirulence (the ability of the virus to cause disease within the nervous system). Prow et al. generated a panel of Murray Valley encephalitis virus (MVEV) and Alfuy virus (ALFV) E protein mutants (72). MVEV causes encephalitis in humans, and isolates from clinical material or mosquitoes are highly neuroinvasive in weanling mice (73, 74). In contrast, ALFV, a subtype of MVEV, has not been associated with human disease, and its isolates are poorly neuroinvasive in weanling mice (75, 76). Their results showed that motifs within the E protein, including the absence of glycosylation and unique substitutions in the flexible hinge region, contribute to the reduced neuroinvasiveness of ALFV compared to MVEV (72).
Other studies have generated chimeras of neuroinvasive and nonneuroinvasive flaviviruses to test the role of E protein in neuroinvasion. Viruses containing the E protein of the neuroinvasive WNV, TBEV, or Langat virus on a DENV4 (nonneuroinvasive) backbone were not neuroinvasive in mice (77–79). Similarly, replacing the prM-E of YFV, which is nonneuroinvasive, with prM-E of the neuroinvasive JEV did not produce a neuroinvasive virus (80). Altogether, these data suggest that prM-E of neuroinvasive flaviviruses is not sufficient to confer neuroinvasiveness to nonneuroinvasive flaviviruses.
To address the role of JEV E protein glycosylation in pathogenesis, especially the location and number of N-linked glycosylation sites on E proteins, Liang et al. generated three JEV mutants: one with glycosylation at N67, one with glycosylation at N67 plus N154, and one with no glycosylation sites on the E protein (N154A) (45). They found that the nonglycosylated or N67-only-glycosylated virus exhibited reduced viral growth in cell culture, as well as reduced neurovirulence and neuroinvasiveness in mice, compared to the wild-type JEV with glycosylation at only N154 (45). The virus with both N67 and N154 E glycosylation exhibited efficient replication in culture and neurovirulence in mice but reduced neuroinvasiveness compared to the wild-type virus (45). The authors proposed that neurotropic flaviviruses with a single E protein glycosylation at N154 might have an enhanced ability to cross the blood-brain barrier, though the mechanism by which this would occur is unclear. Notably, DENV and YFV, which are not neuroinvasive, have two and zero E glycosylation sites, respectively.
Several recent studies with ZIKV have investigated whether N154 glycosylation contributes to neuroinvasion. The 2015–2016 ZIKV outbreak in Latin America was associated with unexpected clinical manifestations, including neurodevelopmental malformations (congenital Zika syndrome) and Guillain-Barré syndrome (2), and many studies have sought to identify the viral genetic determinants of new disease phenotypes. All contemporary ZIKV isolates encode an N-linked glycosylation site in the E protein at position N154, but this glycosylation site is absent in many historical ZIKV isolates (22, 81, 82). Nonglycosylated (N154A, T156I, and VNDT-deleted) ZIKV mutants generated on the prototype strain MR766 background were attenuated in Ifnar1−/− mice inoculated via a subcutaneous route, replicating to lower levels in serum and brain and causing less lethality than with glycosylated virus (23, 51). Similarly, nonglycosylated (N154Q and T156I) mutants generated from Asian lineage ZIKV strains (FSS13025, H/PF/2013, and PRVABC59) also produced lower viral loads in the serum and brain than did glycosylated virus and were not virulent via a subcutaneous inoculation route (23, 52). Interestingly, nonglycosylated viruses were virulent upon intracranial inoculation, and sequencing of viral RNA from the serum or brain revealed a strong selective pressure favoring glycosylated virus in peripheral tissues but not within the brain of ZIKV-infected Ifnar1−/− mice (23).
Many studies have identified a potential role for E glycosylation in flavivirus neurovirulence and pathogenesis. However, several studies conclude that the E glycosylation is a neuroinvasion determinant, implying that E glycosylation facilitates trafficking across the blood-brain barrier. Studies with WNV and chimeric viruses made this conclusion based on the difference in lethality between peripheral inoculation routes (subcutaneous, intraperitoneal, or intravenous) and intracranial inoculations, observing that mice succumb to nonglycosylated viruses via intracranial but not peripheral routes. However, many of those studies did not measure viral loads in the serum of mice infected peripherally, and those that did found lower viral loads in mice infected with nonglycosylated viruses. Such findings are similar to the results of more recent studies with ZIKV, in which nonglycosylated viruses are attenuated in the periphery, which likely contributes to lower viral loads in the brain, regardless of any effect of E glycosylation on neuroinvasion. Thus, whether E glycosylation truly mediates neuroinvasion and the mechanism by which it might do so remain open questions.
Conclusions.
N-linked glycosylation is a common posttranslational modification which has significant effects on protein conformation and function (13). It is a complex process that is highly host cell specific (13). For many flaviviruses, E glycosylation impacts viral fitness, infectivity, replication, and virulence. The producing cell type, whether from arthropod versus vertebrate cells or specific cell types within a host, likely impacts the nature of the glycan added to flavivirus E proteins, but the ultimate effect of these distinct glycan structures on viral transmission, infection, and pathogenesis remains incompletely understood. It also is unclear why DENV uniquely maintains two E glycosylation sites, or conversely, how YFV maintains efficient transmission, infection, and virulence without E glycosylation. Whether flavivirus E glycosylation is a bona fide neuroinvasive determinant or simply enhances viral replication in the periphery, resulting in higher infection levels in the brain, requires further investigation. Answers to these questions will further our knowledge of flavivirus replication and pathogenesis and potentially aid in combating these pathogenic viruses.
ACKNOWLEDGMENTS
This work was supported by grants R21AI129431 and R01AI139512 to H.M.L. D.L.C. was supported by grant T32AI007419.
REFERENCES
- 1.Gould EA, Solomon T. 2008. Pathogenic flaviviruses. Lancet 371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 2.Pierson TC, Diamond MS. 2018. The emergence of Zika virus and its new clinical syndromes. Nature 560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 3.Angenvoort J, Brault AC, Bowen RA, Groschup MH. 2013. West Nile viral infection of equids. Vet Microbiol 167:168–180. doi: 10.1016/j.vetmic.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marm Kilpatrick A, Wheeler SS. 2019. Impact of West Nile virus on bird populations: limited lasting effects, evidence for recovery, and gaps in our understanding of impacts on ecosystems. J Med Entomol 56:1491–1497. doi: 10.1093/jme/tjz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzarti E, Linden A, Desmecht D, Garigliany M. 2019. Mosquito-borne epornitic flaviviruses: an update and review. J Gen Virol 100:119–132. doi: 10.1099/jgv.0.001203. [DOI] [PubMed] [Google Scholar]
- 6.Ng WC, Soto-Acosta R, Bradrick SS, Garcia-Blanco MA, Ooi EE. 2017. The 5ʹ and 3ʹ untranslated regions of the flaviviral genome. Viruses 9:137–114. doi: 10.3390/v9060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roby JA, Setoh YX, Hall RA, Khromykh AA. 2015. Post-translational regulation and modifications of flavivirus structural proteins. J Gen Virol 96:1551–1569. doi: 10.1099/vir.0.000097. [DOI] [PubMed] [Google Scholar]
- 8.Slon Campos JL, Mongkolsapaya J, Screaton GR. 2018. The immune response against flaviviruses. Nat Immunol 19:1189–1198. doi: 10.1038/s41590-018-0210-3. [DOI] [PubMed] [Google Scholar]
- 9.Modis Y, Ogata S, Clements D, Harrison SC. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 100:6989–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly CL, Sattentau QJ. 2013. Attachment factors, p 1–23. In Pöhlmann S, Simmons G (ed), Viral entry into host cells. Springer, New York, NY. [Google Scholar]
- 11.Kostyuchenko VA, Lim EXY, Zhang S, Fibriansah G, Ng TS, Ooi JSG, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 12.Pierson TC, Diamond MS. 2012. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aebi M. 2013. N-linked protein glycosylation in the ER. Biochim Biophys Acta 1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 15.Mandl CW, Guirakhoo F, Holzmann H, Heinz FX, Kunz C. 1989. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol 63:564–571. doi: 10.1128/JVI.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler G, Heinz FX, Kunz C. 1987. Studies on the glycosylation of flavivirus E proteins and the role of carbohydrate in antigenic structure. Virology 159:237–243. doi: 10.1016/0042-6822(87)90460-0. [DOI] [PubMed] [Google Scholar]
- 17.Post PR, Santos CN, Carvalho R, Cruz AC, Rice CM, Galler R. 1992. Heterogeneity in envelope protein sequence and N-linked glycosylation among yellow fever virus vaccine strains. Virology 188:160–167. doi: 10.1016/0042-6822(92)90745-B. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AJ, Guirakhoo F, Roehrig JT. 1994. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203:241–249. doi: 10.1006/viro.1994.1481. [DOI] [PubMed] [Google Scholar]
- 19.Alsaleh K, Khou C, Frenkiel MP, Lecollinet S, Vàzquez A, de Arellano ER, Després P, Pardigon N. 2016. The E glycoprotein plays an essential role in the high pathogenicity of European-Mediterranean IS98 strain of West Nile virus. Virology 492:53–65. doi: 10.1016/j.virol.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Maharaj PD, Langevin SA, Bolling BG, Andrade CC, Engle XA, Ramey WN, Bosco-Lauth A, Bowen RA, Sanders TA, Huang C-H, Reisen WK, Brault AC. 2019. N-linked glycosylation of the West Nile virus envelope protein is not a requisite for avian virulence or vector competence. PLoS Negl Trop Dis 13:e0007473. doi: 10.1371/journal.pntd.0007473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berthet N, Nakouné E, Kamgang B, Selekon B, Descorps-Declère S, Gessain A, Manuguerra JC, Kazanji M. 2014. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis 14:862–865. doi: 10.1089/vbz.2014.1607. [DOI] [PubMed] [Google Scholar]
- 22.Faye O, Freire CCM, Iamarino A, Faye O, de Oliveira JVC, Diallo M, Zanotto PMA, Sall AA. 2014. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl Trop Dis 8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbaugh DL, Baric RS, Lazear HM. 2019. Envelope protein glycosylation mediates Zika virus pathogenesis. J Virol 93:e00113-19. doi: 10.1128/JVI.00113-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun S, Song B, Frank JC, Julander JG, Polejaeva IA, Davies CJ, White KL, Lee Y. 2016. Complete genome sequences of three historically important, spatiotemporally distinct, and genetically divergent strains of Zika virus. Genome Announc 4:e00800-16. doi: 10.1128/genomeA.00800-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladner JT, Wiley MR, Prieto K, Yasuda CY, Nagle E, Kasper MR, Reyes D, Vasilakis N, Heang V, Weaver SC, Haddow A, Tesh RB, Sovann L, Palacios G. 2016. Complete genome sequences of five Zika virus isolates. Genome Announc 4:e00377-16. doi: 10.1128/genomeA.00377-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers TJ, Hahn CS, Galler R, Rice C. 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Leang SK, Davidson A, Lobigs M. 2010. Both E protein glycans adversely affect dengue virus infectivity but are beneficial for virion release. J Virol 84:5171–5180. doi: 10.1128/JVI.01900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rush JS, Waechter CJ. 1995. Transmembrane movement of a water-soluble analogue of mannosylphosphoryldolichol is mediated by an endoplasmic reticulum protein. J Cell Biol 130:529–536. doi: 10.1083/jcb.130.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rush JS, Waechter CJ, Wolucka B, Ouerfelli O, van Leyen K. 1998. Transbilayer movement of Glc-P-dolichol and its function as a glucosyl donor: protein-mediated transport of a water-soluble analog into sealed ER vesicles from pig brain. Glycobiology 8:1195–1205. doi: 10.1093/glycob/8.12.1195. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhong Y, Su R, Qi H, Deng W, Sun Y, Ma T, Wang X, Yu H, Wang X, Li Z. 2017. N-Glycan profiles in H9N2 avian influenza viruses from chicken eggs and human embryonic lung fibroblast cells. J Virol Methods 249:10–20. doi: 10.1016/j.jviromet.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 31.An Y, Parsons LM, Jankowska E, Melnyk D, Joshi M, Cipollo F. 2019. N-Glycosylation of seasonal influenza vaccine hemagglutinins: implication for potency testing and immune processing. J Virol 93:e01693-18. doi: 10.1128/JVI.01693-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rendić D, Wilson IBH, Paschinger K. 2008. The glycosylation capacity of insect cells. Croat Chem Acta 81:7–21. [Google Scholar]
- 33.Shi X, Jarvis D. 2007. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targets 8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh P, Robbins PW. 1984. Regulation of asparagine-linked oligosaccharide processing. J Biol Chem 259:2375–2382. [PubMed] [Google Scholar]
- 35.Hsieh P, Rosner MR, Robbins PW. 1983. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem 258:2548–2554. [PubMed] [Google Scholar]
- 36.Butters TD, Hughes RC, Vischer P. 1981. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta 640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- 37.Yap SSL, Nguyen-Khuong T, Rudd PM, Alonso S. 2017. Dengue virus glycosylation: what do we know? Front Microbiol 8:1415. doi: 10.3389/fmicb.2017.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoo US, Chan KYK, Chan VSF, Lin C. 2008. DC-SIGN and L-SIGN: the SIGNs for infection. J Mol Med (Berl) 86:861–874. doi: 10.1007/s00109-008-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hacker K, White L, De Silva AM. 2009. N-Linked glycans on dengue viruses grown in mammalian and insect cells. J Gen Virol 90:2097–2106. doi: 10.1099/vir.0.012120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis CW, Nguyen H-Y, Hanna SL, Sánchez MD, Doms RW, Pierson TC. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goo L, Demaso CR, Pelc RS, Kuhn RJ, Pierson TC, Ledgerwood JE, Graham BS. 2018. The Zika virus envelope protein glycan loop regulates virion antigenicity. Virology 515:191–202. doi: 10.1016/j.virol.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Pokidysheva E, Hendrickson WA, Battisti AJ, Bator-Kelly CM, Zhang Y, Rossmann MG, Gregorio GG, Chipman PR, Kuhn RJ, Xiao C. 2006. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Alen MMF, Dallmeier K, Balzarini J, Neyts J, Schols D. 2012. Crucial role of the N-glycans on the viral E-envelope glycoprotein in DC-SIGN-mediated dengue virus infection. Antiviral Res 96:280–287. doi: 10.1016/j.antiviral.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Davis CW, Mattei LM, Nguyen HY, Ansarah-Sobrinho C, Doms RW, Pierson TC. 2006. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin). J Biol Chem 281:37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- 45.Liang J-J, Chou M-W, Lin Y-L. 2018. DC-SIGN binding contributed by an extra N-linked glycosylation on Japanese encephalitis virus envelope protein reduces the ability of viral brain invasion. Front Cell Infect Microbiol 8:239. doi: 10.3389/fcimb.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martina BEE, Koraka P, van den Doel P, Rimmelzwaan GF, Haagmans BL, Osterhaus A. 2008. DC-SIGN enhances infection of cells with glycosylated West Nile virus in vitro and virus replication in human dendritic cells induces production of IFN-α and TNF-α. Virus Res 135:64–71. doi: 10.1016/j.virusres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Goto A, Yoshii K, Obara M, Ueki T, Mizutani T, Kariwa H, Takashima I. 2005. Role of the N-linked glycans of the prM and E envelope proteins in tick-borne encephalitis virus particle secretion. Vaccine 23:3043–3052. doi: 10.1016/j.vaccine.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 48.Yoshii K, Yanagihara N, Ishizuka M, Sakai M, Kariwa H. 2013. N-linked glycan in tick-borne encephalitis virus envelope protein affects viral secretion in mammalian cells, but not in tick cells. J Gen Virol 94:2249–2258. doi: 10.1099/vir.0.055269-0. [DOI] [PubMed] [Google Scholar]
- 49.Hanna SL, Pierson TC, Sanchez MD, Ahmed AA, Murtadha MM, Doms RW. 2005. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J Virol 79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mossenta M, Marchese S, Poggianella M, Slon Campos JL, Burrone OR. 2017. Role of N-glycosylation on Zika virus E protein secretion, viral assembly and infectivity. Biochem Biophys Res Commun 492:579–586. doi: 10.1016/j.bbrc.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Annamalai AS, Pattnaik A, Sahoo BR, Muthukrishnan E, Natarajan SK, Steffen D, Vu HLX, Delhon G, Osorio FA, Petro TM, Xiang S-H, Pattnaik AK. 2017. Zika virus encoding nonglycosylated envelope protein is attenuated and defective in neuroinvasion. J Virol 91:e01348-17. doi: 10.1128/JVI.01348-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontes-Garfias CR, Shan C, Luo H, Muruato AE, Medeiros DBA, Mays E, Xie X, Zou J, Roundy CM, Wakamiya M, Rossi SL, Wang T, Weaver SC, Shi P-Y. 2017. Functional analysis of glycosylation of Zika virus envelope protein. Cell Rep 21:1180–1190. doi: 10.1016/j.celrep.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondotte JA, Lozach P-Y, Amara A, Gamarnik AV. 2007. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J Virol 81:7136–7148. doi: 10.1128/JVI.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryant JE, Calvert AE, Mesesan K, Crabtree MB, Volpe KE, Silengo S, Kinney RM, Huang CYH, Miller BR, Roehrig JT. 2007. Glycosylation of the dengue 2 virus E protein at N67 is critical for virus growth in vitro but not for growth in intrathoracically inoculated Aedes aegypti mosquitoes. Virology 366:415–423. doi: 10.1016/j.virol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Braack L, Gouveia De Almeida AP, Cornel AJ, Swanepoel R, De Jager C. 2018. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasit Vectors 11:29. doi: 10.1186/s13071-017-2559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, Johnson N, Kocan KM, Mansfield KL, Nijhof AM, Papa A, Rudenko N, Villar M, Alberdi P, Torina A, Ayllón N, Vancova M, Golovchenko M, Grubhoffer L, Caracappa S, Fooks AR, Gortazar C, Rego R. 2017. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol 7:114. doi: 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franz AWE, Kantor AM, Passarelli AL, Clem RJ. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses 7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moudy RM, Zhang B, Shi PY, Kramer LD. 2009. West Nile virus envelope protein glycosylation is required for efficient viral transmission by Culex vectors. Virology 387:222–228. doi: 10.1016/j.virol.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinglasan RR, Jacobs-Lorena M. 2005. Insight into a conserved lifestyle: protein-carbohydrate adhesion strategies of vector-borne pathogens. Infect Immun 73:7797–7807. doi: 10.1128/IAI.73.12.7797-7807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen D, Li S, Dong F, Zhang Y, Lin Y, Wang J, Zou Z, Zheng A. 2018. N-glycosylation of viral E protein is the determinant for vector midgut invasion by flaviviruses. mBio 9:e00046-18. doi: 10.1128/mBio.00046-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banet-Noach C, Simanov L, Malkinson M. 2003. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol 32:489–494. doi: 10.1080/0307945031000154080. [DOI] [PubMed] [Google Scholar]
- 62.Ricklin ME, García-Nicolás O, Brechbühl D, Python S, Zumkehr B, Nougairede A, Charrel RN, Posthaus H, Oevermann A, Summerfield A. 2016. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun 7:10832–10839. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Elizalde M, Figuerola J, Soriguer RC, Jiménez-Clavero MÁ. 2015. Bagaza virus is pathogenic and transmitted by direct contact in experimentally infected partridges, but is not infectious in house sparrows and adult mice. Vet Res 46:93. doi: 10.1186/s13567-015-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brockmann SO, Oehme R, Buckenmaier T, Beer M, Jeffery-Smith A, Spannenkrebs M, Haag-Milz S, Wagner-Wiening C, Schlegel C, Fritz J, Zange S, Bestehorn M, Lindau A, Hoffmann D, Tiberi S, Mackenstedt U, Dobler G. 2018. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Euro Surveill 23:pii=00336 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.15.17-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hudopisk N, Korva M, Janet E, Simetinger M, Grgič-Vitek M, Gubenšek J, Natek V, Kraigher A, Strle F, Avšič-Županc T. 2013. Tick-borne encephalitis associated with consumption of raw goat milk, Slovenia, 2012. Emerg Infect Dis 19:806–808. doi: 10.3201/eid1905.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Shi Y, Liu Q, Wang Y, Li G, Teng Q, Zhang Y, Liu S, Li Z. 2015. Airborne transmission of a novel Tembusu virus in ducks. J Clin Microbiol 53:2734–2736. doi: 10.1128/JCM.00770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan D, Shi Y, Wang H, Li G, Li X, Wang B, Su X, Wang J, Teng Q, Yang J, Chen H, Liu Q, Ma W, Li Z. 2018. A single mutation at position 156 in the envelope protein of Tembusu virus is responsible for virus tissue tropism and transmissibility in ducks. J Virol 92:e00427-18. doi: 10.1128/JVI.00427-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garmendia AE, Van Kruiningen HJ, French RA. 2001. The West Nile virus: its recent emergence in North America. Microbes Infect 3:223–229. doi: 10.1016/s1286-4579(01)01374-0. [DOI] [PubMed] [Google Scholar]
- 69.Hadfield J, Brito AF, Swetnam DM, Vogels CBF, Tokarz RE, Andersen KG, Smith RC, Bedford T, Grubaugh ND. 2019. Twenty years of West Nile virus spread and evolution in the Americas visualized by Nextstrain. PLoS Pathog 15:e1008042. doi: 10.1371/journal.ppat.1008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, Takashima I. 2004. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol 85:3637–3645. doi: 10.1099/vir.0.80247-0. [DOI] [PubMed] [Google Scholar]
- 71.Beasley DWC, Whiteman MC, Zhang S, Huang C-H, Schneider BS, Smith DR, Gromowski GD, Higgs S, Kinney RM, Barrett A. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prow NA, May FJ, Westlake DJ, Hurrelbrink RJ, Biron RM, Leung JY, McMinn PC, Clark DC, Mackenzie JS, Lobigs M, Khromykh AA, Hall RA. 2011. Determinants of attenuation in the envelope protein of the flavivirus Alfuy. J Gen Virol 92:2286–2296. doi: 10.1099/vir.0.034793-0. [DOI] [PubMed] [Google Scholar]
- 73.Broom AK, Lindsay MDA, Wright AE, Smith DW, Mackenzie JS. 2018. Epizootic activity of Murray Valley encephalitis and Kunjin viruses in an aboriginal community in the Southeast Kimberley Region of Western Australia: results of mosquito fauna and virus isolation studies. Am J Trop Med Hyg 69:277–283. doi: 10.4269/ajtmh.2003.69.277. [DOI] [PubMed] [Google Scholar]
- 74.Lobigs M, Marshall ID, Weir RC, Dalgarno L. 1988. Murray Valley encephalitis virus field strains from Australia and Papua New Guinea: studies on the sequence of the major envelope protein gene and virulence for mice. Virology 165:245–255. doi: 10.1016/0042-6822(88)90678-2. [DOI] [PubMed] [Google Scholar]
- 75.Mackenzie JS, Williams DT. 2009. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health 56:338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 76.May FJ, Lobigs M, Lee E, Gendle DJ, Mackenzie JS, Broom AK, Conlan JV, Hall RA. 2006. Biological, antigenic and phylogenetic characterization of the flavivirus Alfuy. J Gen Virol 87:329–337. doi: 10.1099/vir.0.81252-0. [DOI] [PubMed] [Google Scholar]
- 77.Pletnev AG, Bray M, Lai CJ. 1993. Chimeric tick-borne encephalitis and dengue type 4 viruses: effects of mutations on neurovirulence in mice. J Virol 67:4956–4963. doi: 10.1128/JVI.67.8.4956-4963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pletnev AG, Karganova GG, Dzhivanyan TI, Lashkevich VA, Bray M. 2000. Chimeric Langat/dengue viruses protect mice from heterologous challenge with the highly virulent strains of tick-borne encephalitis virus. Virology 274:26–31. doi: 10.1006/viro.2000.0426. [DOI] [PubMed] [Google Scholar]
- 79.Pletnev AG, Putnak R, Speicher J, Wagar EJ, Vaughn DW. 2002. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc Natl Acad Sci U S A 99:3036–3041. doi: 10.1073/pnas.022652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chambers TJ, Nestorowicz A, Mason PW, Rice CM. 1999. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 73:3095–3101. doi: 10.1128/JVI.73.4.3095-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu ZY, Shi WF, Qin CF. 2019. The evolution of Zika virus from Asia to the Americas. Nat Rev Microbiol 17:131–139. doi: 10.1038/s41579-018-0134-9. [DOI] [PubMed] [Google Scholar]
- 82.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. 2012. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Desprès P. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Hu K, Luo S, Zhang M, Deng X, Li C, Jin W, Hu B, He S, Li M, Du T, Xiao G, Zhang B, Liu Y, Hu Q. 2016. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology 488:108–119. doi: 10.1016/j.virol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Gong D, Zhang T-H, Zhao D, Du Y, Chapa TJ, Shi Y, Wang L, Contreras D, Zeng G, Shi P-Y, Wu T-T, Arumugaswami V, Sun R. 2018. High-throughput fitness profiling of Zika virus E protein reveals different roles for glycosylation during infection of mammalian and mosquito cells. iScience 1:97–111. doi: 10.1016/j.isci.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau V-M, Choumet V, Briant L, Desprès P, Amara A, Yssel H, Missé D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]