Abstract
Objective:
Cold and β3-adrenergic receptor (AR) agonists activate beige adipocyte biogenesis in white adipose tissue (WAT). The two stimuli also induce expression of inflammatory cytokines in WAT. The low-grade inflammation may further promote WAT browning. However, the mechanisms to reconcile these two biological processes remain to be elucidated. In this study, we aim to investigate the roles of the rate-limiting polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase (SAT1) in regulating beige adipocyte biogenesis and inflammation.
Methods:
Adipose-specific SAT1 knockout mice (SAT1-aKO) were generated by crossing adiponectin-cre to SAT1-lox/lox mice. Metabolic phenotype was investigated. Primary preadipocytes were isolated from inguinal WAT (iWAT) and differentiated to adipocytes for studying beige adipocyte biogenesis.
Result:
The expression and enzymatic activity of SAT1 were up-regulated in iWAT upon cold and β3-AR stimulation. SAT1-aKO mice developed late-onset obesity on a high-fat diet with impaired cold-induced beige adipocyte biogenesis and energy expenditure. RNA-seq analysis of iWAT from cold-challenged SAT1-aKO mice revealed that, in addition to beige adipocyte biogenesis signatures, the immune response markers were highly enriched among reduced genes. In cultured adipocytes, SAT1 overexpression or pharmacological activation with N1, N11-diethylnorspermine (DENSpm) elevated oxygen consumption and increased the expression of beige adipocyte marker UCP1 and PGC-1α. DENSpm treatment of adipocytes also increased the expression of inflammatory genes. SAT1 activation enhanced hydrogen peroxide production in adipocytes. Antioxidant N-acetylcysteine abrogated the elevated UCP1 expression and reversed some inflammatory genes induced by SAT1 activation.
Conclusions:
SAT1 activation plays a key role in cold and β3-AR agonist-induced beige adipocyte biogenesis and low-grade inflammation.
Keywords: Polyamine metabolism, beige adipocytes, inflammation, obesity, energy expenditure
1. Introduction
Polyamines including putrescine, spermidine and spermine are small polycations that are essential for multiple cellular functions affecting cells growth, cancer and ageing [1–3]. Polyamine metabolism is tightly regulated by several rate-limiting enzymes for synthesis and catabolism. Specifically, the polyamine synthesis is modulated by ornithine decarboxylase (ODC) and adenosylmethionine decarboxylase (AMD1). The catabolism is controlled by spermidine—spermine N1-acetyltransferase (SAT1), which acetylates spermidine and spermine to generate N1-acetylspermine, N1,12-diacetylspermine, and N1-acetylspermidine. These acetylated polyamines are either excreted intact in urine or oxidized by acetyl-polyamine oxidase (APAOX) [2, 4]. The oxidation of acetylpolyamines generates putrescine and spermidine as well as hydrogen peroxide (H2O2) [2, 4]. The putrescine and spermidine are then returned to the polyamine metabolic flux.
Initial polyamine research was mostly focused on cancer biology since ODC and SAT1 were found to be overexpressed in multiple cancers [2, 4–6]. Subsequent studies on genetic mouse models, especially SAT1 transgenic and knockout mice, revealed that polyamine metabolism plays a major role in regulating energy expenditure and adiposity [7–9]. Whole body SAT1 knockout mice develop late-onset obesity while systemic and adipose-specific SAT1 overexpression prevent diet-induced obesity. PGC-1α expression and AMPK activity are altered in white adipose tissue (WAT) with SAT1 overexpression or knockdown [7–9], but it is unclear to what extent they contribute to the SAT1-regulated energy metabolism and adiposity.
Brown adipose tissue (BAT) expresses high levels of uncoupling protein 1 (UCP1) in order to dissipate chemical energy to heat, therefore, plays a major role in thermogenesis. WAT is traditionally considered to be an energy storage organ. However, it is becoming clear that clusters of adipocytes in WAT undergo browning or beiging with induced expression of UCP1 in response to various environmental, hormonal or pharmacological stimuli such as cold and β3-adrenergic receptor (AR) activation [10–13]. Interestingly, cold and β3-AR also induce expression of inflammatory cytokines in adipocytes and recruit immune cells to WAT [14–16]. Although adipose inflammation has been linked to insulin resistance [17, 18], low grade inflammation appears to be required for adipose tissue remodeling and beige adipocyte biogenesis [19–21]. Therefore, cold and β3-AR agonist exert direct and indirect effects on activating WAT browning. However, the downstream signals for the dual effects remain to be fully elucidated. In the current paper, we show that adipose SAT1 is induced by cold and β3-AR agonist stimulation. Importantly, SAT1 appears to be a key mechanistic node for both beige adipocyte biogenesis and low-grade inflammation induced by cold and β3-AR stimulation.
2. Materials and Methods
2.1. Animal Studies
To generate adipose-specific SAT1 knockout mice, SAT1-flox/flox mice [22] were crossed to adiponectin-Cre mice (Jackson Lab). For diet-induced obesity, mice were fed a high fat diet containing 54.8% fat calories, 24.0% carbohydrate calories, and 21.2% protein calories (4.8 kcal/gram) (stock number TD.93075; Envigo Inc.) [23]. Body composition was analyzed using an EchoMRI 3-in-1 instrument (Echo Medical Systems, Houston, TX). Metabolic cage studies were performed as described previously [23].
For the cold-induced thermogenesis studies, mice were housed in a cold chamber (4°C) for 4, 12 or 72 hours. For the β3-adrenegic agonist treatment, mice received intraperitoneal injections of CL316, 432 (C5976; Sigma) at 1mg/kg per day for 4 days. Control mice received same volumes of phosphate-buffered saline.
Glucose tolerance test was performed by injecting glucose (1g/kg) intraperitoneally to mice with food removal for 5 hours. Blood glucose levels were measured at 0, 15, 30, 60, and 120 min. Mice were maintained under a 12hr light/12hr dark cycle at constant temperature (23°C) with free access to food and water, except for the cold exposure experiments. All mouse studies were conducted in accordance with federal guidelines and were approved by the Institutional Animal Care and Use Committee of University of California, Irvine.
2.2. Isolation and Culture of Primary Adipocytes
Isolation and culture of primary adipocytes were described previously [24]. Inguinal white adipose tissue excised from 4 to 5-week-old male C57BL/6 mice was minced and digested in a PBS digestion buffer containing 2.5 U/mL collagenase D (11088882001; Sigma), 2.4 U/mL Dispase II (4942078001; Sigma), and 10 mM CaCl2 at 37°C in a shaking water bath for 18 minutes. The digested tissue was filtered through 100μm nylon cell strainers (BD Biosciences). The stromal vascular fraction cells containing preadipocytes were separated from floating primary adipocytes by centrifugation at 600g for 5 minutes. The resulting pellets were resuspended and further filtered using 40μm nylon cell strainers (BD Biosciences). Stromal vascular fraction cells were maintained in DMEM/F12 GlutaMAX (Invitrogen) containing 15%FBS, 100U/mL penicillin, and 100μg/mL streptomycin. For differentiation, confluent preadipocytes were treated with medium containing 15% FBS, 0.5 mM isobutylmethylxanthine (I7018; Sigma), 1μM dexamethasone (D4902; Sigma), 2 μg/mL insulin (I0546; Sigma), and 1 μM rosiglitazone (R2408; Sigma) for 48 hours. Adipocytes were then maintained in medium containing 15% FBS and 2 μg/mL insulin. At the 7 to 8-day of differentiation, cells were incubated with CL 316,243 (C5976; Sigma) at 10μM for up to 8hrs or treated with N1, N11-Diethylnorspermine tetrahydrochloride (4A/194732; Tocris Bioscience) for 24 hours. For experiments of the PKA/CREB inhibitor, the cells were treated with 50 μM H-89 (S1582; Selleckchem) for 1 hour prior to the stimulation of the CL316, 432. In the antioxidant experiment, adipocytes were pre-treated with 100 μM N-acetylcysteine (NAC) (Sigma) for 30 mins before DENSpm treatment. Lentiviral overexpression of SAT1 was performed as previously described [25]. Oxygen consumption rate (OCR) of adipocytes was determined using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience).
2.3. SAT1 Activity Assay
Snap-frozen tissues (50–100mg) were homogenized in Tris/EDTA (pH 7.0) buffer. The soluble supernatants after centrifugation were used in activity assay. SAT1 activity was assayed radiochemically as described [5, 23] and expressed as pmol of N1-[14C]-acetylspermidine (AcSpd) generated/10 min/mg of tissue.
2.4. RNA extraction and quantitative PCR
Total RNA was extracted using the RNeasy kit (QIAGEN). cDNA was synthesized using the SuperScript III first-strand synthesis supermix for quantitative RT-PCR (qRT-PCR; Invitrogen) and used in real-time PCRs with Power SYBR Green PCR master mix (Applied Biosystems) on a 7900HT real-time PCR system (Applied Biosystems). The relative gene expression levels were calculated by the 2Ct method using Tata-binding protein (Tbp) as an internal control. Primer sequences are shown in Supplemental Table 1.
2.5. Total reactive oxygen species (ROS) and hydrogen peroxide measurements
Intracellular ROS production was measured based on the oxidation of 2′,7′-dichlorofluoresce diacetate (Invitrogen) as previously described [26]. Adipocytes were seeded into a black 96-well plate at a density of 1×105/well. After treatment, the cells were incubated with 2 ′ ,7 ′ -dichlorofluoresce diacetate at 37°C for 30 minutes, followed by washing three times with PBS.
The resultant fluorescence was measured using a fluorescent microplate reader at excitation and emission wavelengths of 485 and 528 nm, respectively.
Amplex Red assay was used to measure hydrogen peroxide levels. Adipocytes were incubated in the Krebs-Ringer phosphate (KRP) (145 mM NaCl, 5.7 mM sodium phosphate, 4.86 mM KCl, 0.54 mM CaCl2, 1.22 mM MgSO4, 5.5 mM glucose, pH 7.35) containing the Amplex Red reagent (A22188, Invitrogen). Fluorescence was then measured at excitation 485nm and emission 570nm, respectively.
2.6. RNA-seq Analyses
Total RNA isolated from iWAT of cold-treated SAT1-aKO and control mice was used for RNA-seq analyses. RNA-seq was performed at the UCI Genomic High-Throughput Facility. The Chipster software was used for quantification and statistics of the RNA-seq data [27]. Functional clustering was performed using DAVID analysis, Panther pathway and GO biological process analysis.
2.7. Immunohistochemistry
Tissues collected from cold-challenged SAT1-aKO and control mice were fixed in paraffin. Embedded sections were routinely deparaffinized and endogenous peroxidase was quenched with 3% H2O2 in 1×PBS. Then they were stained with antibody against F4/80 (Bio-Rad) to detect macrophages. Images were captured using a Zeiss Detector Imaging System.
2.8. Statistical Analysis
All data were expressed as mean ± SEM. Statistical differences were assessed using unpaired Student’s two tailed t tests for two groups and a one-way ANOVA for three groups or more. Statistical significance was assumed at P < 0.05.
3. Results
3.1. Adipose SAT1 is induced by thermogenic stimuli
Cold and β3-AR agonist are the classic stimuli to induce beige adipocyte biogenesis and BAT activity. Challenging wild type mice with cold for 72 hours strongly stimulated UCP1 and PGC-1α expression in iWAT and BAT (Supplementary Fig. 1A–1B). β3-AR agonist CL-316,243 treatment for 4 days also induced UCP1 expression in iWAT and BAT (Supplementary Fig. 1C–1D). Both Cold and CL-316,243 increased the expression of SAT1 in iWAT and BAT (Fig. 1A–1D). The polyamine synthetic enzyme ODC was elevated in iWAT and tended to be increased in BAT of mice exposed to cold. The acetylpolyamine oxidation enzyme APAOX was stimulated in iWAT, but not in BAT by cold and CL-316,243 (Fig. 1A–1D). Consistent with the increased SAT1 mRNA expression, SAT1 enzymatic activity was enhanced in iWAT and BAT by the stimulation of cold and CL-316,243 (Fig. 1E–H). It has to be noted that the baseline SAT1 activity at the room temperature in BAT was approximately 5 times higher than that in iWAT (Fig. 1E–1H). This is partially due to the higher SAT1 mRNA expression in BAT (Supplementary Fig. 1E). These results indicate that SAT1 expression and activity are linked to adipose thermogenesis.
Fig. 1-. Cold and β3-adrenergic receptor agonist stimulate adipose SAT1 expression and activity.
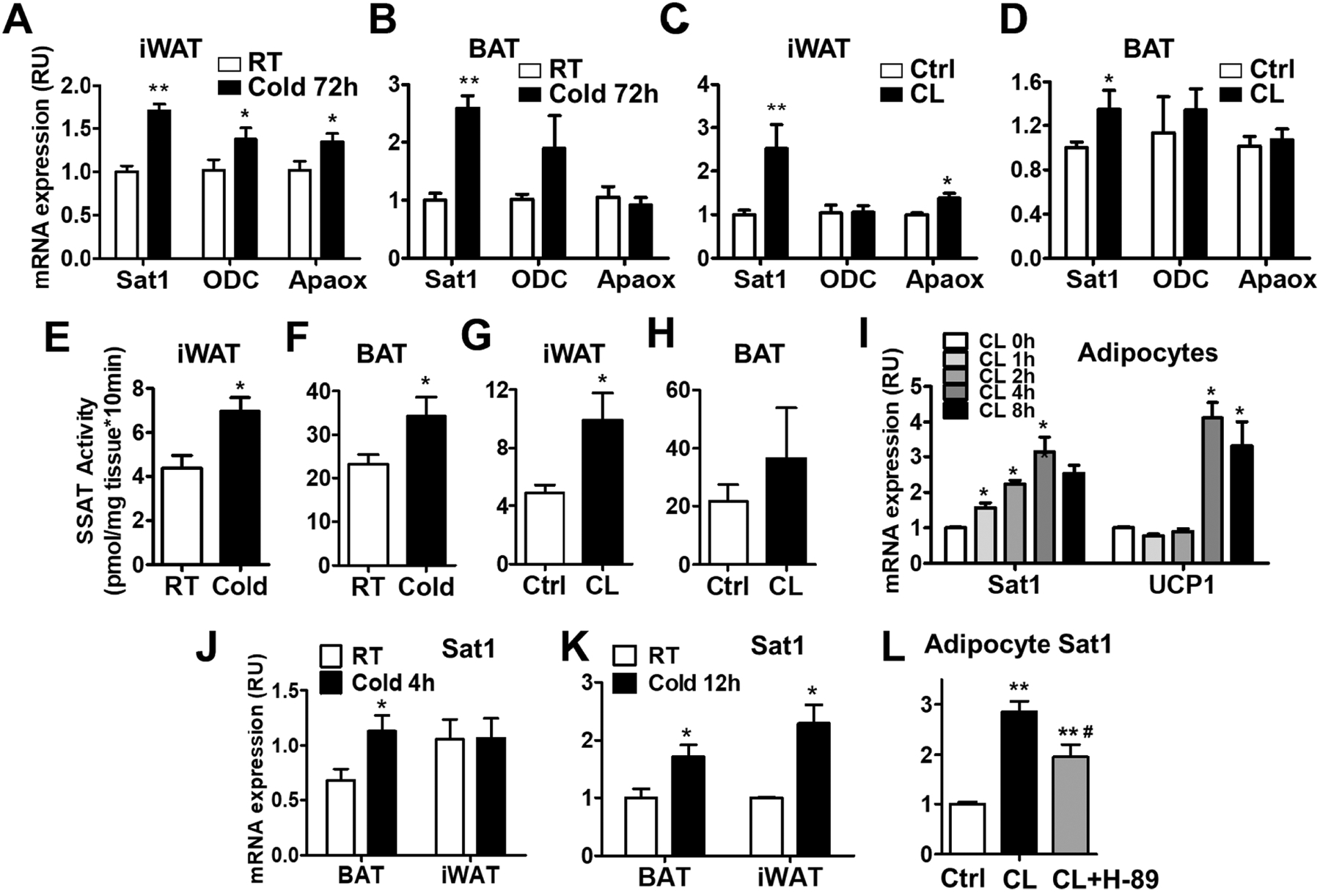
(A-B) SAT1, ornithine decarboxylase (ODC) and polyamine oxidase (APAOX) mRNA expression in inguinal (iWAT) and brown (BAT) adipose tissue of cold-challenged mice (4°C for 72 hours). (C-D) SAT1, ODC and APAOX mRNA levels in iWAT and BAT of mice treated with β3-adrenergic receptor agonist CL 316,243 (CL). (E-H) SAT1 enzymatic activity in iWAT and BAT of mice treated with cold or CL. (I) SAT1 and UCP1 expression in primary adipocytes treated with CL for the indicated times. (J-K) SAT1 expression in iWAT and BAT of mice challenged with cold for 4 hours (J) and 12 hours (K). (L) SAT1 mRNA levels in differentiated adipocytes treated with the PKA inhibitor H-89, followed by stimulation with CL. n=6–10 per group, *p<0.05, **p<0.01 vs controls (Ctrl). #p<0.05 vs CL.
To test whether these effects were cell-autonomous, we isolated preadipocytes from iWAT, differentiated the cells to adipocytes, and treated the cells with CL-316,243. SAT1 expression was rapidly induced by CL-316,243 in 1 hour. The maximum effects were at 4-hour time point. UCP1 expression, on the other hand, did not increase after 1 and 2 hours but elevated at later time points (4 and 8-hour) with CL-316,243 treatment (Fig. 1I). The rapid SAT1 induction could be recapitulated in vivo as acute cold exposure for 4 hours increased SAT1 in BAT and for 12 hours increased SAT1 in both BAT and iWAT (Fig. 1J–1K). The increased SAT1 levels were associated with elevated UCP1 expression in adipose tissue of acute cold-challenged mice (Supplementary Fig. 2A–2B). In addition to cold and adrenergic stimulation, high fat diet (HFD) feeding initially promotes the adipose UCP1 expression [28, 29] (Supplementary Fig.3A–3B). Interestingly, one-week HFD feeding also elevated adipose SAT1 expression (Supplementary Fig. 3A–3B). The SAT1 induction in adipocytes was partially caused by protein kinase A (PKA) activation because the CL-316,243-induced SAT1 elevation was attenuated by H-89, a PKA inhibitor (Fig. 1L). In addition, CL-316,243 and cold also increased the expression of adipose nuclear factor (erythroid-derived 2)-like 2 (Nrf2), the major transcription factor regulating SAT1 expression (Supplementary Fig. 4A–4B). These results indicate that thermogenic stimuli enhance polyamine catabolism in adipose tissue.
3.2. Adipose-specific SAT1 knockdown mice develop late-onset obesity
To determine the effects of adipose SAT1 on systemic energy metabolism, we generated adipose-specific SAT1 knockout mice (SAT1-aKO) by crossing adiponectin-Cre to SAT1-flox/flox mice [22]. SAT1 expression was reduced by 87% in BAT, but only 49% and 46% in iWAT and perigonadal WAT (pgWAT), with no changes in liver (Fig. 2A). The relatively modest reduction of SAT1 in WAT was likely caused by abundant SAT1 expression in stromal vascular fraction since isolated adipocytes from iWAT and pgWAT of SAT1-aKO mice showed 80–95% SAT1 reduction (Fig. 2A). The SAT1-aKO mice developed late-onset obesity after 18 weeks of high fat diet (HFD)-feeding (Fig. 2B). The increased adiposity in SAT1-aKO mice was confirmed by Echo-MRI studies showing a 20% increase in relative fat mass and a 9% decrease in relative lean mass (Fig. 2C). Consistently, the weight of iWAT and pgWAT of SAT1-aKO mice was higher than that of controls (Fig. 2D). Metabolic cage studies showed a reduction of VO2 and VCO2 in SAT1-aKO mice compared with controls when exposed to cold (Fig. 2E–2G). Glucose tolerance tended to be impaired in SAT1-aKO mice compared with controls before the body weight started to diverge (Fig. 2H–1I). However, insulin tolerance test (not shown), insulin, glucose and insulin-glucose products (Supplementary Fig. 5A–5C) were not different between SAT1-aKO and control mice fed a HFD for 22–24 weeks when the body weight was separated. These results indicate that adipose SAT1 plays a major role in regulating systemic energy metabolism.
Fig. 2-. Adipose-specific SAT1 knockout mice develop late-onset obesity.
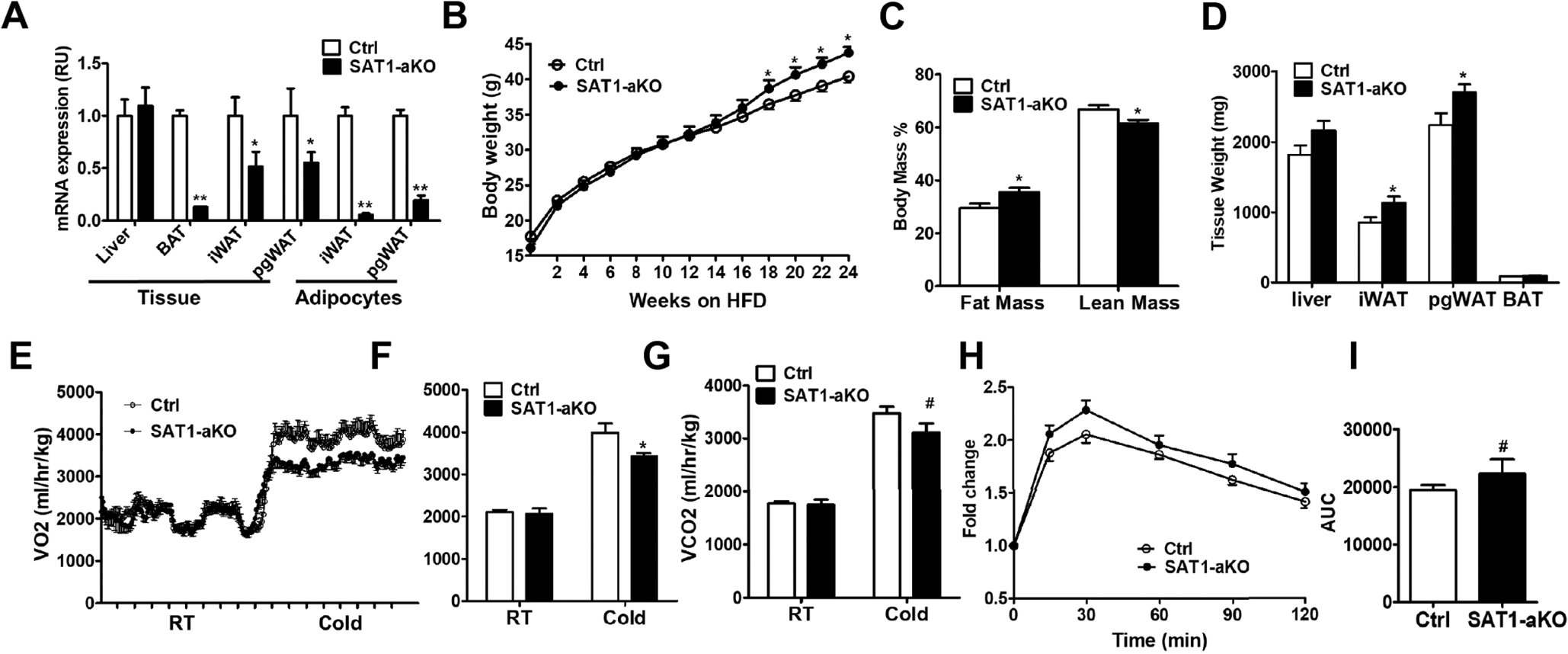
(A) SAT1 expression in adipose tissue and adipocytes, (B) body weight, (C) percent fat and lean mass, (D) metabolic organ weight in control (Ctrl) and adipose-specific knockout (SAT1-aKO) mice fed on high fat diet (HFD). (E-G) Oxygen Consumption (E-F) and CO2 production (G) in SAT1-aKO and control mice under room temperature (RT) and cold (4°C). (H) Glucose tolerance test (GTT) and (I) area under the curve (AUC) in control and SAT1-aKO mice fed a HFD for 12 weeks. n=9–15 per group, except for adipocytes in (A) with n=3, *p<0.05, **p<0.01, #p=0.2 vs Controls (Ctrl).
3.3. Adipose SAT1 regulates beige adipocyte thermogenesis
Since SAT1 was induced by cold and β3-AR agonist in BAT and iWAT and SAT1-aKO mice showed reduction in cold-induced oxygen consumption, we measured the expression of thermogenic gene markers in BAT and iWAT. Upon cold challenge, the expression of beige adipocyte markers uncoupling protein 1 (UCP1), Cidea, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), cytochrome c oxidase polypeptide 7A1 (Cox7a1), acyl-CoA Oxidase 1 (ACOX1), carnitine palmitoyltransferase 1 (Cpt1), cluster of differentiation 36 (CD36) were reduced in iWAT of SAT1-aKO mice compared with controls (Fig. 3A). Peroxisome proliferator-activated receptor gamma (PPARg) expression was not altered, suggesting SAT1 knockdown did not affect adipocyte differentiation. Interestingly, BAT thermogenic activity was not significantly affected by SAT1 knockdown (Fig. 3B). The only reduced gene in BAT of SAT1-aKO was PGC-1α while other thermogenic genes including UCP1 were not altered (Fig. 3B). Therefore, adipose SAT1 is necessary for cold-induced beige adipocyte biogenesis.
Fig. 3 -. Adipose SAT1 regulates beige adipocyte thermogenesis.
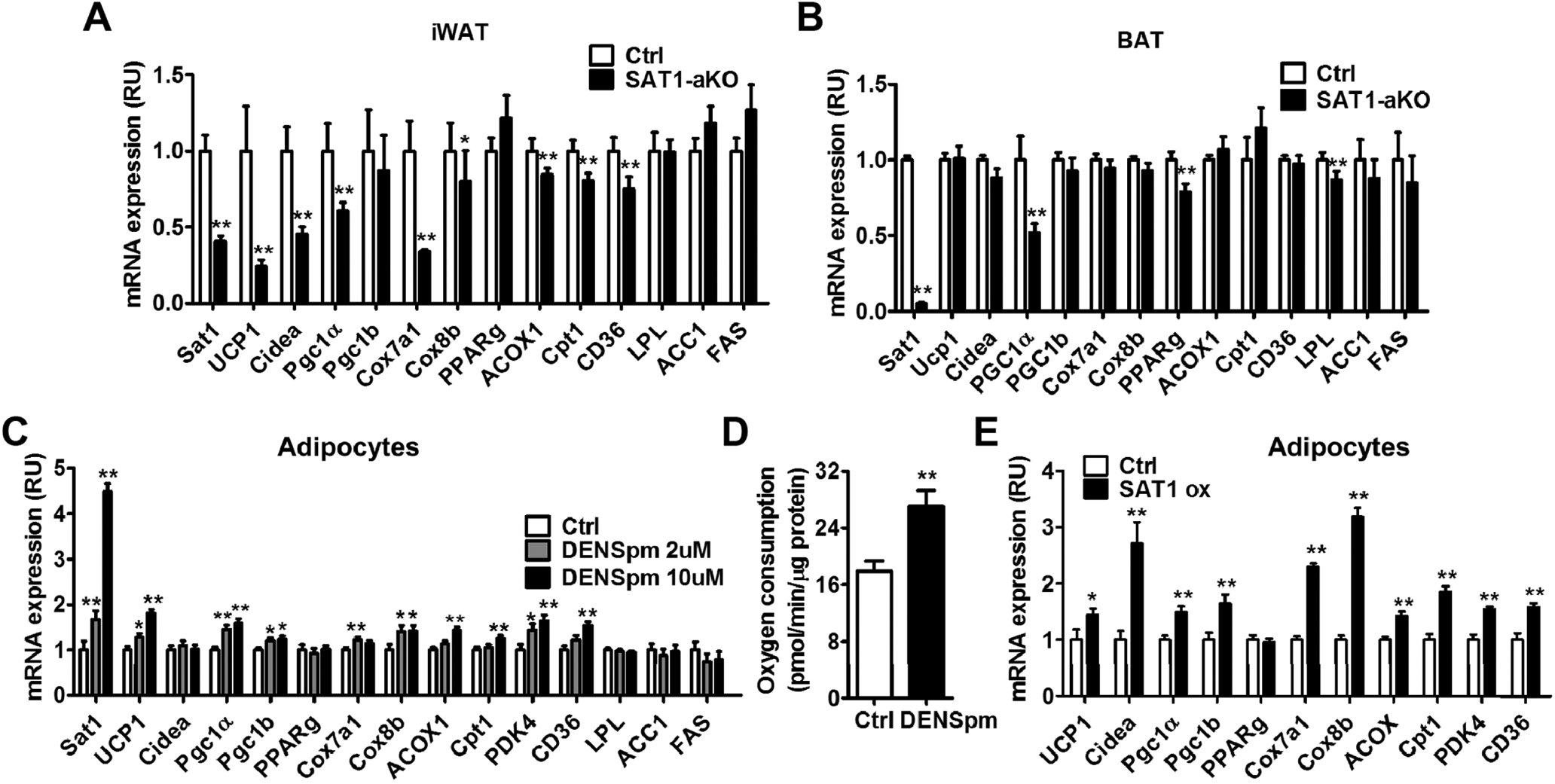
(A-B) Browning markers (UCP1, Cidea, Pgc1a, Pgc1b, PPARg, Cox7a1, Cox8b, ACOX1, Cpt1, CD36) and fatty acid synthesis gene expression (ACC, LPL and FAS) in inguinal white adipose tissue (iWAT) (A) and brown adipose tissue (BAT) (B) of cold-challenged SAT1-aKO and control mice. (C-D) Browning gene expression (C) and oxygen consumption in adipocytes treated with SAT1 agonist N1, N11-diethylnorspermine (DENSpm). (E) Browning markers in adipocytes with lentiviral SAT1 overexpression. n=3–8 per group, *p<0.05, ** p<0.01 vs Controls (Ctrl).
We next investigated whether the SAT1 directly activates beige adipocyte biogenesis. N1, N11-diethylnorspermine (DENSpm) is a polyamine analog that increases SAT1 expression and activity [2, 9]. Treating cultured adipocytes with DENSpm promoted the expression of beige adipocyte biogenesis markers and increased oxygen consumption (Fig. 3C–3D). Similarly, overexpression of SAT1 in adipocytes also stimulated beige adipocyte biogenesis (Fig. 3E, Supplementary Fig. 6). SAT1 activation did not affect adipocyte differentiation as evidence by unaltered PPARg expression. These results indicate that adipose SAT1 is necessary and sufficient to promote beige adipocyte thermogenesis.
3.4. Adipose SAT1 knockdown reduces immunity-related genes
To investigate the mechanisms for SAT1-regulated beige adipocyte thermogenesis, we performed RNA-seq and subsequent DAVID functional annotation clustering analysis as well as Panther pathway and GO biological process analysis using iWAT from cold-challenged SAT1-aKO and control mice. Both analysis tools consistently showed over-represented signatures for immunity/inflammation and mitochondrial function/TCA cycle among the downregulated genes (Fig. 4A–4B and Supplementary Table 2–3). Among the upregulated genes, the top three clusters were membrane, glycoprotein and endoplasmic reticulum (Supplementary Table 4). The immunity-related genes included cluster of differentiation (CD) markers, chemokines (C-C and C-X-C motif) and cytokines (Supplementary Table 5). These genes represent signatures of immune cells especially T-lymphocytes (CD3, CD4, Foxp3), dendritic cells (CD83), and macrophages (IL-1R), although adipocytes also express low levels of some of these genes [17, 18, 30]. Quantity RT-PCR of selected immunity-related genes confirmed the reduced expression of cluster of differentiation 14 (Cd14), tumor necrosis factor alpha (Tnf-α), chemokine (C-C motif) ligand 8 (Ccl8), forkhead box P3 (Foxp3), C-X-C chemokine receptor type 6 (Cxcr6), and C-X-C chemokine receptor type 3 (Cxcr3) identified from the RNA-seq analysis (Fig. 4C). In addition, IL-1β and IL-10 were also decreased and IL-6 and MCP-1 tended to be lower in iWAT of SAT1-aKO mice (Fig. 4C). Consistently, immunohistochemistry staining of iWAT showed a reduction of F4/80 positive macrophage infiltration in iWAT of cold-challenged SAT1-aKO mice compared with controls (Fig. 4D, Supplementary Fig. 7A). Some of the cytokines such as CD14 and IL6 were also reduced in the perigonadal WAT of SAT1-aKO mice compared with controls (Supplementary Fig. 7B). Therefore, adipose SAT1 plays a dual role in regulating beige adipocyte thermogenesis as well as immune cell infiltration.
Fig. 4 -. SAT1 knockdown reduces immunity-related genes in adipose tissue.
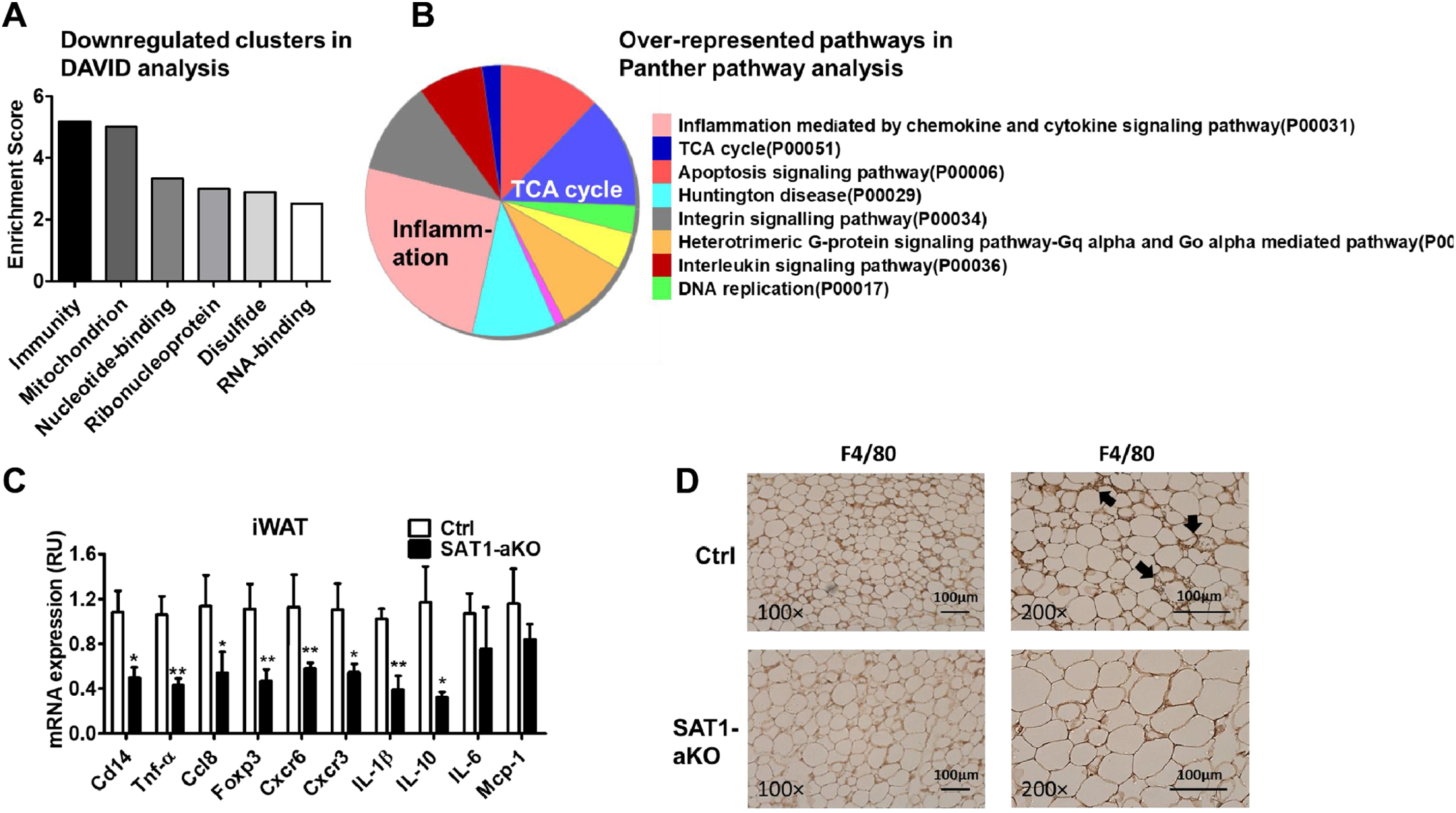
(A-B) DAVID analysis (A) and Panther pathway analysis (B) of RNA-seq results from iWAT of SAT1-aKO and control mice exposed to cold. (C) qPCR measurements of immunity-related gene expression. (D) F4/80 staining of iWAT from cold-challenged SAT1-aKO and control mice. n=6 per group, *p<0.05, ** p<0.01 vs Controls (Ctrl).
3.5. SAT1-induced reactive oxygen species regulate beige adipocyte thermogenesis and inflammation
SAT1-activated polyamine flux generates H2O2 from oxidation of acetylpolyamines [2, 4]. Reactive oxygen species (ROS) has been shown to mediate beige adipocyte biogenesis and UCP1 expression/activation. ROS also induces cellular inflammation in adipocytes [31, 32]. Consistently, treatment of cultured adipocytes with DENSpm increased H2O2 and total ROS levels, both were neutralized by antioxidant agent N-acetylcysteine (NAC) (Fig. 5A). The elevated H2O2/ROS was necessary for the UCP1 expression induced by SAT1 activation since NAC abolished the elevated UCP1 induced by DENSpm (Fig. 5B). SAT1 activation by DENspm also increased Cd14, Tnf-α, Mcp-1 and IL-6 expression in adipocytes (Fig. 5C). NAC reversed the elevated expression of CD14, and tended to reduce Tnf-α and IL-6 elevation (Fig.5C). DENspm-induced expression of UCP1, Tnf-α, Mcp-1 and IL-6 was attenuated by SAT1 siRNA knockdown, indicating the specificity of DENspm-activated SAT1 in stimulating beige adipocyte biogenesis and inflammation (Supplementary Fig. 8A–8B). These data indicate that SAT1-induced ROS production is involved in beige adipocyte thermogenesis and inflammation.
Fig. 5 -. SAT1-induced reactive oxygen species regulate beige adipocyte thermogenesis and inflammation.
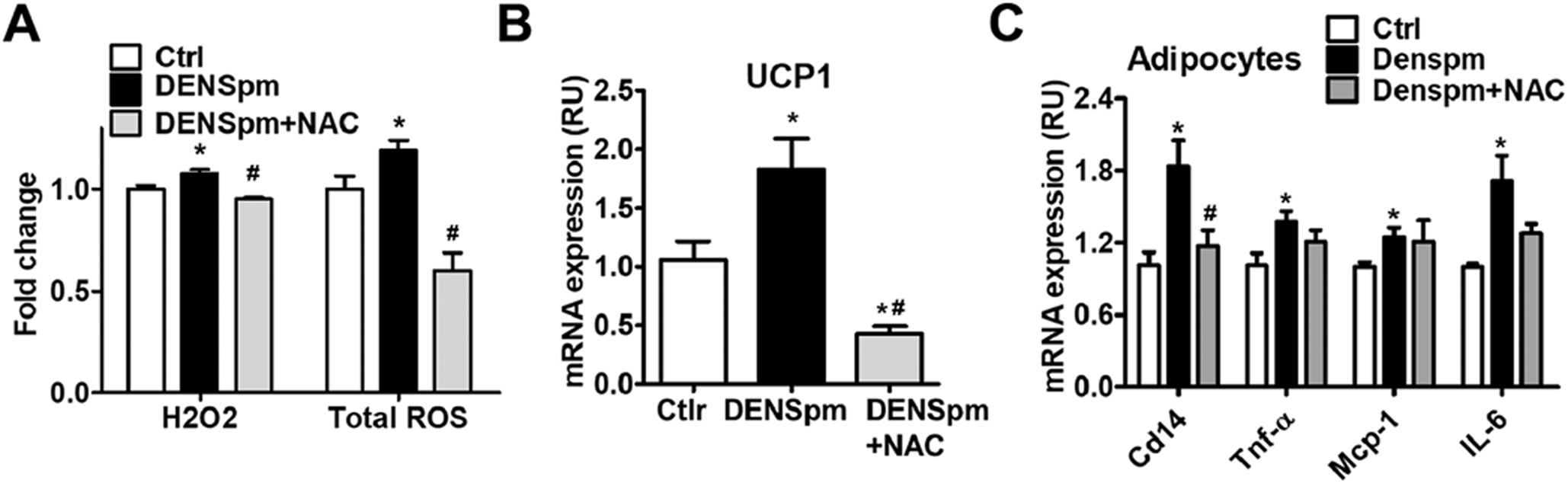
(A) H2O2 levels and total ROS production, (B) UCP1 expression and (C) inflammation-related gene expression in adipocytes treated with SAT1 agonist DENSpm with or without antioxidant N-acetylcysteine (NAC). n=4–8 per group, * p<0.05 vs, ** p<0.01 vs controls (Ctrl), #p<0.05 vs DENSpm.
4. Discussion
Adipose SAT1 plays a major role in systemic energy metabolism. However, it is not clear how SAT1 is regulated in adipose tissue. We found that thermogenic stimuli such as cold and β3-AR agonist induce SAT1 expression and activity in adipose tissue. This appears to be partially mediated by PKA/CREB signal pathway since PKA inhibitor H-89 reduces SAT1 elevation stimulated by β3-AR agonist (Fig. 1J). Consistently, there is a consensus CREB binding site in the SAT1 promoter [2]. Cold and β3-AR agonist also stimulate the expression of Nrf2 (supplementary Fig. 1E–1F), a major transcription factor that binds to the polyamine-responsive element of the SAT1 promoter [2]. The elevated Nrf2 likely represents a response to intracellular ROS stimulated by cold and β3-AR in adipose tissue [33, 34]. Interestingly, SAT1 activation also generates ROS. Therefore, Nrf2 and SAT1 may form a regulatory loop in adipose tissue upon cold or β3-AR stimulation.
Four SAT1-related mouse models have been reported. Whole body SAT1 knockout mice are obese, while systemic SAT1 overexpression causes leanness [7–9, 35, 36]. Mouse aP2 promoter driven SAT1 transgenic and knockout show similar adiposity phenotype as that in systemic transgenic and knockout mice [8, 35]. However, aP2 promoter activity is not adipose-specific and drives target gene expression in non-adipose tissues/cells such as macrophages [37]. In the current studies, we used adiponectin-Cre to generate truly adipocyte-specific SAT1 knockout mice. The fact that SAT1-aKO developed late-onset obesity provided a conclusive evidence that SAT1 in adipocytes plays a critical role in energy metabolism and adiposity. However, the weight gain of our SAT1-aKO mice appears to be milder than that of whole body and aP2-Cre driven SAT1 knockout mice. It therefore remains possible that SAT1 in other tissues/cells might also play some roles in regulating energy expenditure and adiposity.
One major contribution of our study is the elucidation of the novel role of SAT1 in beige adipocyte biogenesis. SAT1 is sufficient and necessary for beige adipocyte biogenesis. Since SAT1 expression and activity are induced by cold and β3-AR agonist, SAT1 activation is a critical downstream mediator of cold and β3-AR stimulation for beige adipocyte biogenesis. This is partially mediated by the direct effects of SAT1 on adipocytes as SAT1 activation increased UCP1 expression and oxygen consumption (Fig. 3C–3E). SAT1 activation generates H2O2 and ROS through APAOX and spermine oxidase (SMOX) [1–3]. This appears to be a mechanism for the direct effects of SAT1 on beige adipocyte biogenesis since neutralizing H2O2 with NAC abolishes UCP1 elevation induced by SAT1 activation. Although pathologically high ROS are detrimental to cellular function, physiological ROS are important second messengers for multiple biological processes including beige adipocyte biogenesis and activity [31]. For example, overexpression of Sestrin2, an antioxidant protein, suppresses ROS and inhibits UCP1 expression in adipose tissue [32]. A more recent study show that ROS may induce UCP1 cysteine-253 sulfenylation, thereby promote adipocyte thermogenesis [34]. Therefore, SAT1-activated ROS production is an important mechanism for beige adipocyte biogenesis.
RNA-seq analyses surprisingly reveal highly enriched immunity-related genes in addition to mitochondrial function/beige adipocyte markers among the reduced genes in adipose tissue of cold-challenged SAT1-aKO compared with control mice (Fig. 4). The gene markers of T-lymphocytes, dendritic cells, and macrophages are reduced, suggesting decreased immune cell infiltration into adipose tissue of SAT1-aKO mice. Interestingly, knocking out SAT1 from renal proximal tubule also decreases immune cell infiltration and inflammatory response to ischemia/reperfusion injury [38]. The mechanism for the reduced immune cell infiltration is not very clear. SAT1 activation induces ROS and cytokine production in adipocytes. This may trigger immune cell infiltration as a compensatory mechanism. Alternatively, SAT1 activation may decrease intracellular polyamines which have anti-inflammatory properties [1]. Regardless of mechanisms, cold and β3-AR stimulation increase SAT1 activity and promote immune cell infiltration and cytokine expression in adipose tissue [14]. Extensive adipose inflammation is generally considered to be detrimental to insulin sensitivity, BAT activity and beige adipocyte biogenesis. However, low level inflammation is necessary for adipose remodeling and beige adipocyte activation [19]. SAT1 activation during cold and β3-AR stimulation may contribute to the low-grade inflammation in adipose tissue, which indirectly promotes beige adipocyte biogenesis.
In summary, SAT1-mediated polyamine catabolism is sufficient and necessary for beige adipocyte biogenesis. This is mediated by the direct effects of SAT1-induced ROS production in adipocytes. SAT1-induced inflammation and immune cell infiltration may also contribute to the beige adipocyte biogenesis indirectly. Thus, the current study identifies SAT1-mediate polyamine catabolism as a novel mechanistic node for beige adipocyte biogenesis during cold and β3-AR stimulation.
Supplementary Material
Highlights:
Polyamine catabolic enzyme spermidine-spermine acetyltransferase (SAT1) is induced in adipose tissue upon cold and β3-adrenergic stimulation.
Adipose-specific SAT1 knockout mice develop late-onset obesity with impaired cold-induced beige adipocyte biogenesis.
SAT1 activation promotes beige adipocyte biogenesis.
Reactive oxygen species and inflammation induced by SAT1 activation contribute to beige adipocyte biogenesis.
SAT1 activation is a critical mechanistic node for beige adipocyte biogenesis induced by cold and 3-adrenergic stimulation.
Funding
The study was supported by grants from the NIH (R01 DK100385) to Q.Y.; the National Science Foundation of China (No. 81270428 and No. 81470501) to X.L.; the National Natural Science Foundation of China (No. 81273713 and No. 81573909) to Z.F.; the Postgraduate Research and Innovation Project in Jiangsu Province, China (JX22013318) and the China Scholarship Council (1410150039) to W.G; scholarship from the China Scholarship Council (201608320234) to C.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- [1].Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359. [DOI] [PubMed] [Google Scholar]
- [2].Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–1010. [DOI] [PubMed] [Google Scholar]
- [3].Wang Y, Casero RA Jr. Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J Biochem. 2006;139:17–25. [DOI] [PubMed] [Google Scholar]
- [4].Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421:323–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bernacki RJ, Oberman EJ, Seweryniak KE, Atwood A, Bergeron RJ, Porter CW. Preclinical antitumor efficacy of the polyamine analogue N1, N11-diethylnorspermine administered by multiple injection or continuous infusion. Clin Cancer Res. 1995;1:847–57. [PubMed] [Google Scholar]
- [6].Nowotarski SL, Woster PM, Casero RA, Jr. Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev Mol Med. 2013;15:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jell J, Merali S, Hensen ML, Mazurchuk R, Spernyak JA, Diegelman P, et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem. 2007;282:8404–13. [DOI] [PubMed] [Google Scholar]
- [8].Liu C, Perez-Leal O, Barrero C, Zahedi K, Soleimani M, Porter C, et al. Modulation of polyamine metabolic flux in adipose tissue alters the accumulation of body fat by affecting glucose homeostasis. Amino Acids. 2014;46:701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pirinen E, Kuulasmaa T, Pietila M, Heikkinen S, Tusa M, Itkonen P, et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol. 2007;27:4953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. [DOI] [PubMed] [Google Scholar]
- [11].Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–43. [DOI] [PubMed] [Google Scholar]
- [13].Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luo X, Jia R, Zhang Q, Sun B, Yan J. Cold-Induced Browning Dynamically Alters the Expression Profiles of Inflammatory Adipokines with Tissue Specificity in Mice. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roth Flach RJ, Matevossian A, Akie TE, Negrin KA, Paul MT, Czech MP. beta3-Adrenergic receptor stimulation induces E-selectin-mediated adipose tissue inflammation. J Biol Chem. 2013;288:2882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta. 2010;1801:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity. 2017;47:406–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19:512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, et al. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;285:4637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zahedi K, Barone SL, Xu J, Steinbergs N, Schuster R, Lentsch AB, et al. Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G546–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gao W, Wang H, Zhang L, Cao Y, Bao JZ, Liu ZX, et al. Retinol-Binding Protein 4 Induces Cardiomyocyte Hypertrophy by Activating TLR4/MyD88 Pathway. Endocrinology. 2016;157:2282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, et al. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2004;15:1844–52. [DOI] [PubMed] [Google Scholar]
- [26].Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal. 2014;20:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kallio MA, Tuimala JT, Hupponen T, Klemela P, Gentile M, Scheinin I, et al. Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genomics. 2011;12:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1–8. [DOI] [PubMed] [Google Scholar]
- [29].Watson PM, Commins SP, Beiler RJ, Hatcher HC, Gettys TW. Differential regulation of leptin expression and function in A/J vs. C57BL/6J mice during diet-induced obesity. Am J Physiol Endocrinol Metab. 2000;279:E356–65. [DOI] [PubMed] [Google Scholar]
- [30].Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–87. [DOI] [PubMed] [Google Scholar]
- [31].Chouchani ET, Kazak L, Spiegelman BM. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: Bridging physiology and mechanisms. J Biol Chem. 2017;292:16810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ro SH, Nam M, Jang I, Park HW, Park H, Semple IA, et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci U S A. 2014;111:7849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ortega SP, Chouchani ET, Boudina S. Stress turns on the heat: Regulation of mitochondrial biogenesis and UCP1 by ROS in adipocytes. Adipocyte. 2017;6:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Koponen T, Cerrada-Gimenez M, Pirinen E, Hohtola E, Paananen J, Vuohelainen S, et al. The activation of hepatic and muscle polyamine catabolism improves glucose homeostasis. Amino Acids. 2012;42:427–40. [DOI] [PubMed] [Google Scholar]
- [36].Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, et al. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med. 2006;10:933–45. [DOI] [PubMed] [Google Scholar]
- [37].Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zahedi K, Barone S, Wang Y, Murray-Stewart T, Roy-Chaudhury P, Smith RD, et al. Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS One. 2014;9:e110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


