The rapid evolution of resistance in the malaria parasite to every single drug developed against it calls for the urgent identification of new molecular targets. Using a stain specific for the detection of intracellular amyloid deposits in live cells, we have detected the presence of abundant protein aggregates in Plasmodium falciparum blood stages and female gametes cultured in vitro, in the blood stages of mice infected by Plasmodium yoelii, and in the mosquito stages of the murine malaria species Plasmodium berghei.
KEYWORDS: malaria, protein aggregation
ABSTRACT
The rapid evolution of resistance in the malaria parasite to every single drug developed against it calls for the urgent identification of new molecular targets. Using a stain specific for the detection of intracellular amyloid deposits in live cells, we have detected the presence of abundant protein aggregates in Plasmodium falciparum blood stages and female gametes cultured in vitro, in the blood stages of mice infected by Plasmodium yoelii, and in the mosquito stages of the murine malaria species Plasmodium berghei. Aggregated proteins could not be detected in early rings, the parasite form that starts the intraerythrocytic cycle. A proteomics approach was used to pinpoint actual aggregating polypeptides in functional P. falciparum blood stages, which resulted in the identification of 369 proteins, with roles particularly enriched in nuclear import-related processes. Five aggregation-prone short peptides selected from this protein pool exhibited different aggregation propensity according to Thioflavin-T fluorescence measurements, and were observed to form amorphous aggregates and amyloid fibrils in transmission electron microscope images. The results presented suggest that generalized protein aggregation might have a functional role in malaria parasites. Future antimalarial strategies based on the upsetting of the pathogen’s proteostasis and therefore affecting multiple gene products could represent the entry to new therapeutic approaches.
INTRODUCTION
According to the last World Malaria Report (1), around 228 million cases of malaria occurred worldwide in 2018 (up from 216 million in 2016), and the disease led to an estimated 405,000 deaths. Although the elimination of malaria is a priority for the global health system, the drugs currently used as front-line therapy are quickly becoming obsolete due to the evolution of resistance in the parasite causing the disease, Plasmodium spp. (2). The consequence of this loss in efficacy of available antimalarial compounds is that the former decline in mortality and incidence of malaria has stalled in the last few years, which leads to an urgent need for the identification of new therapeutic targets and alternative antimalarial strategies operating through novel mechanisms (3).
The life cycle of Plasmodium in the human host begins with the bite of a parasitized female mosquito of the genus Anopheles, when it inoculates sporozoites, the infectious form of the pathogen. Sporozoites quickly reach the liver, develop asexually inside hepatocytes, and produce merozoites (4), which eventually enter the bloodstream, where they initiate the intraerythrocytic phase (5). In the red blood cell (RBC), Plasmodium grows as it develops from ring to trophozoite stages until it finally undergoes multiple asexual divisions to form schizonts containing between 8 and 36 merozoites that egress from the RBC to invade new erythrocytes. Some parasites differentiate into gametocytes, the sole form of the pathogen that can be transmitted to Anopheles during its blood meal. In the midgut of the insect fertilization takes place, and the motile zygote, termed ookinete, traverses the intestine epithelium to lodge itself on the transluminal side to form an oocyst where sporozoites develop. Through the insect’s hemolymph sporozoites migrate to the salivary glands, ready to start a new infectious cycle.
The most severe form of malaria and the majority of reported cases are caused by Plasmodium falciparum. The genome of this species (6) has some particularities, such as a clear bias in its DNA composition, which has an 80.6% AT content, comparable only to that found in Dictyostelium discoideum (7). Moreover, about one-third of the P. falciparum proteome exhibits low-complexity regions (LCRs) especially enriched in asparagine residues (8–11). Importantly, proteins with large LCR stretches having abundant glutamine/asparagine (Q/N) repeats show a strong tendency to form insoluble intracellular aggregates (12, 13). Out-of-control protein aggregation is potentially harmful for the organism and has been observed to be a characteristic feature of several pathological conditions, such as type II diabetes, systemic amyloidosis, and a number of neurodegenerative diseases (14). The aggregation of proteins can trigger aberrant molecular associations and permeate the plasma membrane, which often end up in cell death (15, 16). Evolution usually eliminates proteins that contain amino acid sequences with a high propensity to aggregate, except when these regions are required to maintain functionality (17, 18). Indeed, the aggregation of certain proteins has been found to have a functional role in several biological processes, e.g., innate immunity against certain viruses (19), the persistence of mammalian synaptic facilitation (20), and the inheritance in yeast of some particular phenotypes (21). In the course of a typical malaria infection, Plasmodium is exposed to fever episodes that can reach more than 40°C. Since protein misfolding and aggregation increase at higher temperatures, these heat shock periods could compromise parasite survival if only a fraction of its abundant asparagine repeat-containing proteins aggregated. Such havoc is avoided by the abundance in the proteome of the pathogen of chaperones which assist in protein folding (22–25).
In a previous work, an in-house-developed Python algorithm (26), which scans for consecutive 80-residue windows retrieving those containing ≥30 Q/N repeats, was applied to the P. falciparum 3D7 reference proteome containing 5,353 proteins. In good agreement with former studies (9), our algorithm identified 1,300 proteins with one or more Q/N-rich domains. These were further searched for intrinsically disordered regions with PAPA (27), obtaining 581 proteins. Finally, the pWALTZ script (28) was applied to scan within these disordered regions for the presence of soft amyloid cores, i.e., short stretches capable of facilitating the conversion of polypeptides into an amyloid-like conformation (26), resulting in a final data set of 503 proteins containing disordered regions potentially capable of nucleating aggregation events.
Encouraged by the prediction of that in silico analysis, we have evaluated here the presence of aggregative proteins in live Plasmodium cultures, using first an amyloid-specific staining for fluorescence microscopy and flow cytometry analysis. The observed existence of intracellular amyloid deposits in live parasite cells prompted the use of fractionation techniques and liquid chromatography with tandem mass spectrometry (LC-MS/MS) for the identification of individual proteins from the P. falciparum proteome.
RESULTS
Detection of protein aggregation in live Plasmodium stages.
Previous preliminary data had shown the presence of aggregated proteins in P. falciparum early trophozoite stages (26), according to staining with the red fluorescent dye ProteoStat, which can detect the presence of intracellular amyloid-like deposits in live cells with high specificity (29). ProteoStat is a molecule whose fluorophore group, when excited in solution, releases energy by rotation; however, when the dye locks into the quaternary structure of protein aggregates and cannot rotate, it becomes highly fluorescent. This property has been used to detect protein aggregates in numerous studies. Amyloid aggregates in seminal plasma, where the intensity of background noise prevented the use of Congo red or Thioflavin-T (ThT) amyloid dyes, were satisfactorily detected by ProteoStat due to its higher noise-to-signal ratio (30). Other examples of the use of this reagent to detect protein aggregates are, to name just a few, following the formation of intracellular aggregates in HeLa cells upon induction of extracellular oxidative stress to demonstrate their colocalization with the aggregate p62 protein marker (31), staining of amyloid plaques in Alzheimer’s brain sections (32), validating the binding of novel dyes to intracellular aggregates upon proteasome inhibition (33), and staining of intracellular aggregates of human α-crystallin truncated forms (34).
A detailed analysis performed in live P. falciparum cultures revealed that ProteoStat-stained protein aggregates were abundantly found in extraerythrocytic merozoites (Fig. 1A and B) and in all the blood forms except early rings, where their fluorescent signal was low or undetectable (Fig. 1C and D). ProteoStat fluorescence was detected throughout the parasite but not in the cytosol of the host RBC or in any noninfected erythrocytes. The only parasite stage capable of being transmitted from human to mosquito, the gametocyte, also showed an evident presence of aggregated proteins in all its stages (Fig. 1E to G). Female gametes, the first stage exclusive of the mosquito, have also been observed to be ProteoStat positive in P. falciparum (Fig. 1H to J). In the murine malaria parasite Plasmodium berghei, the rest of the mosquito stages were also ProteoStat positive, namely, male gametes, ookinetes, oocysts, and sporozoites (Fig. 2A to D), whereas no staining was observed in any Anopheles mosquito cells. P. falciparum sporozoites also had detectable aggregated protein deposits (see Fig. S1 in the supplemental material). P. berghei ring stages, which represent most of circulating parasitized RBCs (pRBCs) in this species (35), were negative for ProteoStat staining (Fig. S2). In another species also infecting mouse RBCs, Plasmodium yoelii, all blood stages could be observed in circulation and showed the presence of aggregated proteins (Fig. S2), with the possible exception of early rings. The existence of abundant amyloid structure in pRBCs, but not in uninfected erythrocytes, was confirmed with the use of an anti-amyloid fibril antibody (36) (Fig. S3).
FIG 1.
ProteoStat staining for the detection of intracellular protein aggregates in live in vitro cultures of P. falciparum blood stages and female gametes. (A) RBC-invading merozoite. (B) Egressed merozoites. (C and D) Intraerythrocytic blood stages: schizont (asterisk), trophozoites (arrows), and rings (arrowheads). The black arrows in phase contrast images indicate the boundary of two RBCs infected by trophozoite stages, to highlight the lack of fluorescence in the host RBC cytosol. (E to G) Gametocytes. (H to J) Egressed female gametes (arrowheads), which lack the RBC membrane otherwise stained by Oregon Green 488.
FIG 2.
ProteoStat staining for the detection of intracellular protein aggregates in live P. berghei stages in Anopheles gambiae mosquitoes. (A) Male gametes. (B and C) Ookinetes. (D) Oocysts and sporozoites. The arrowhead indicates a stream of sporozoites leaving the oocyst; a blow-up of this region is shown in the inset of the protein aggregates panel (inset scale bar, 5 μm).
Quantitative flow cytometry analysis of ProteoStat-stained P. falciparum cultures confirmed the fluorescence microscopy observations of blood stages. The intraerythrocytic parasite population with less DNA content, which included ring stages, showed the lowest aggregated protein amounts (Fig. 3). A significant fraction of these pRBCs harboring a single parasite cell and exhibiting positive ProteoStat signal likely corresponded to late ring/early trophozoite stages, as suggested by fluorescence microscopy data.
FIG 3.

Flow cytometry analysis of ProteoStat-stained desynchronized P. falciparum cultures. The fraction of ProteoStat-positive RBCs and pRBCs is indicated (%), the latter consisting of late ring/early trophozoites and schizonts, the three stages represented in the cartoons.
Functions of the aggregation-prone proteins identified in live P. falciparum late-form blood stages.
In a first attempt to identify individual aggregation-prone proteins present in live P. falciparum, a late-stage pRBC culture homogenate was stained with ProteoStat and the positive aggregates were sorted by flow cytometry (Fig. 4). LC-MS/MS analysis provided 38 proteins present in the ProteoStat-stained aggregates (Table S1). Of these, only one was found among 342 proteins from the P. falciparum proteome that had been identified using the PLAAC algorithm to contain a prion-forming domain with strongly biased amino acid composition, most notably enriched in Q or N (Table S2). Since highly abundant and soluble proteins might be found in the protein aggregates sorted by flow cytometry, a second purification strategy was assayed with the objective of increasing the sensitivity of aggregated protein detection. This alternative approach consisted in collecting those aggregates from late-stage pRBC culture homogenates that resisted dissolution in the presence of 0.1% sodium dodecyl sulfate (SDS) (Fig. 5), which resulted in the identification of 369 parasite proteins (Table S3); of these, 85 were detected in the in silico analysis (Table 1) and 25 were captured by ProteoStat sorting (Fig. 6A).
FIG 4.
Flow cytometry sorting of ProteoStat-stained proteins in live P. falciparum blood stages. (A) Scheme of the process. (B) Histograms showing the intensity of ProteoStat signal versus the number of events, for the sample before sorting (left panel) and the resulting ProteoStat+ and ProteoStat− fractions. (C) Dot plot showing the intensity of the ProteoStat signal versus the size of each event, for the sample before sorting (left panel) and the resulting ProteoStat+ and ProteoStat− fractions. (D) To monitor ProteoStat fluorescence, pictures at ×600 magnification were taken in the bright field and fluorescence channel BP596-660 upon excitation with a 488-nm laser. (E) Silver-stained SDS-PAGE fractionation of the ProteoStat+ sample. (F) Schematic graph representing the LC-MS/MS analysis of Coomassie blue-stained bands excised from a gel run in parallel to that of panel E. The results obtained are reported in Table S1 in the supplemental material.
FIG 5.
Isolation of P. falciparum aggregative proteins insoluble in 0.1% SDS. (A) Scheme of the process. (B) Silver-stained SDS-PAGE fractionation of the 0.1% SDS-resistant sample. (C) Schematic graph representing the LC-MS/MS analysis of Coomassie blue-stained material not entering the stacking gel, excised from a gel run in parallel to that of panel B. The results obtained are reported in Table S3.
TABLE 1.
Prion-like domain (PrLD)-containing proteins found in SDS-resistant aggregates
| UniProt accession no. | Protein description | PrLD score |
|---|---|---|
| Q8IJP9 | ADA2-like protein | 289.599 |
| O96124 | Erythrocyte membrane protein 3 | 220.166 |
| Q8I398 | Nucleoporin NUP100/NSP100, putative | 188.444 |
| Q8IIS9 | Polyadenylate-binding protein-interacting protein 1, putative | 132.364 |
| Q8I207 | Uncharacterized protein | 95.427 |
| Q8ILR9 | Protein PF14_0175 | 90.871 |
| O96221 | Protein transport protein SEC31 | 84.834 |
| Q8IJG6 | Chromodomain-helicase-DNA-binding protein 1 homolog, putative | 82.683 |
| Q8ID65 | Uncharacterized protein | 80.531 |
| Q8I562 | Clustered-asparagine-rich protein | 67.567 |
| Q8IJW6 | Asparagine-rich antigen | 65.462 |
| Q8ID39 | Uncharacterized protein MAL13P1.336 | 63.816 |
| Q8ILC9 | Uncharacterized protein | 62.573 |
| Q8I4U7 | Uncharacterized protein | 55.167 |
| Q8IKH2 | Transcription factor with AP2 domain(s) | 54.992 |
| O96201 | Conserved Plasmodium protein | 54.465 |
| Q9U0K8 | Uncharacterized protein | 47.530 |
| Q8I3X9 | Uncharacterized protein | 45.295 |
| Q8I403 | Uncharacterized protein | 44.483 |
| Q8IKB6 | Histone deacetylase, putative | 43.850 |
| Q8I3V8 | Pre-mRNA-splicing factor CWC2, putative | 41.783 |
| Q8IAU1 | ATP-dependent RNA helicase DBP1, putative | 41.607 |
| O77328 | Serine/threonine protein kinase, putative | 40.696 |
| Q8IAX8 | DNA/RNA-binding protein Alba 1 | 38.471 |
| C6KT67 | Nuclear polyadenylated RNA-binding protein NAB2, putative | 35.343 |
| Q8IE71 | Uncharacterized protein | 35.082 |
| Q8IJJ2 | Conserved Plasmodium protein | 34.839 |
| Q8IB94 | E3 ubiquitin-protein ligase, putative | 34.785 |
| Q8I1X5 | Pre-mRNA-processing-splicing factor 8, putative | 33.710 |
| Q8ID63 | Uncharacterized protein | 31.733 |
| Q8IL08 | Uncharacterized protein | 31.393 |
| Q8IKJ2 | Uncharacterized protein | 31.334 |
| Q8I0W8 | Deoxyribodipyrimidine photolyase, putative | 31.172 |
| Q8IKY0 | Transcription factor with AP2 domain(s), putative | 30.505 |
| Q8I3Z1 | MATH and LRR domain-containing protein PFE0570w | 30.497 |
| Q8IHR4 | Dynamin-like protein | 30.270 |
| Q8I259 | Uncharacterized protein | 30.050 |
| C0H4L9 | Uncharacterized protein | 29.745 |
| Q8IBU8 | Uncharacterized protein | 29.514 |
| Q9TY99 | Knob-associated histidine-rich protein | 29.121 |
| Q8ILS4 | NOT family protein, putative | 28.802 |
| C6KTB7 | Putative E3 ubiquitin-protein ligase protein PFF1365c | 28.466 |
| Q8IKZ0 | Uncharacterized protein | 28.376 |
| Q8IM09 | Uncharacterized protein | 27.922 |
| Q8ILZ2 | WD repeat-containing protein, putative | 27.869 |
| Q8I517 | Uncharacterized protein | 27.552 |
| Q8I5Y7 | High-mobility-group protein B3, putative | 26.923 |
| C6KST7 | Uncharacterized protein | 26.876 |
| Q8IIW4 | CCR4-NOT transcription complex subunit 1, putative | 26.381 |
| Q8IHT5 | Transcription factor with AP2 domain(s) | 25.809 |
| C0H4R8 | Serine/threonine protein kinase, FIKK family | 25.312 |
| C6KSY0 | Transcription factor with AP2 domain(s) | 25.304 |
| Q8IC35 | Erythrocyte membrane-associated antigen | 25.119 |
| Q8IIS4 | Transcription factor with AP2 domain(s) | 24.751 |
| Q9U0I0 | Uncharacterized protein | 24.746 |
| Q8I3U0 | Transcription factor with AP2 domain(s) | 24.064 |
| C0H4Y0 | Ubiquitin conjugation factor E4 B, putative | 23.863 |
| Q8IKF6 | Uncharacterized protein | 23.691 |
| C0H570 | RNA-binding protein, putative | 22.853 |
| Q8IL84 | Metacaspase-like protein | 21.569 |
| C6KSS4 | Spindle assembly abnormal protein 6, putative | 21.196 |
| C0H530 | Ran-binding protein, putative | 21.141 |
| Q8IIG8 | Uncharacterized protein | 20.697 |
| Q8IE65 | Uncharacterized protein | 20.586 |
| Q8IDI3 | Inner membrane complex protein 1f, putative | 19.991 |
| Q8I3L2 | Uncharacterized protein | 19.764 |
| C6KSN4 | Uncharacterized protein | 19.745 |
| O96205 | Conserved Plasmodium protein | 19.732 |
| Q8IES7 | Uncharacterized protein | 19.294 |
| Q8II83 | Uncharacterized protein | 19.161 |
| Q8I391 | Uncharacterized protein | 18.922 |
| Q8ILQ6 | Uncharacterized protein | 18.334 |
| Q9U0J0 | Replication protein A1, large subunit | 17.760 |
| Q8I538 | Uncharacterized protein | 17.742 |
| C6KSR4 | Uncharacterized protein | 17.304 |
| Q8IJL2 | Eukaryotic translation initiation factor subunit eIF2A, putative | 17.131 |
| C6KSN9 | Transcription factor with AP2 domain(s) | 15.297 |
| O97239 | Protein dopey homolog PFC0245c | 14.519 |
| Q8IBL5 | Uncharacterized protein | 13.571 |
| Q8IM32 | Uncharacterized protein | 13.558 |
| O97298 | Uncharacterized protein | 12.842 |
| Q8I4T6 | THO complex subunit 2, putative | 12.479 |
| Q8ILJ1 | Uncharacterized protein | 12.467 |
| Q8I1N6 | AP2/ERF domain-containing protein PFD0985w | 12.226 |
| Q8ID46 | Uncharacterized protein | 12.200 |
FIG 6.

Analysis of P. falciparum aggregative proteins insoluble in 0.1% SDS. (A) Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) showing the intersection between the proteins from the P. falciparum proteome containing a prion-forming domain identified by the PLAAC algorithm and the proteins identified in 0.1% SDS-resistant aggregates and in ProteoStat-stained aggregates sorted by flow cytometry. (B to D) Gene ontology analysis of the P. falciparum proteins identified in 0.1% SDS-resistant aggregates classified according to: biological process (B), molecular function (C), and cellular component (D).
Gene ontology analysis of the proteins found in 0.1% SDS-resistant aggregates revealed an enrichment in proteins involved in nuclear import (Fig. 6B); mitotic spindle and chromosome organization, Golgi body-to-endoplasmic reticulum transport, and drug response were other biological processes well represented in the selected protein pool. The main molecular functions in which SDS-insoluble P. falciparum proteins exhibited enrichment were binding to nuclear localization sequences, to specific protein domains, and to microtubules (Fig. 6C); structural proteins, protein transporters and transcription factors were also significantly abundant. The cellular components most enriched in the proteins from Table S3 were the nuclear pore and membrane, the coat protein complex I (COPI) vesicle coat, and the nucleosome (Fig. 6D), with a smaller but yet significant representation of cytosolic ribosomal subunits.
In vitro characterization of the aggregation of peptides selected from the live P. falciparum aggregation-prone protein pool.
The proteins identified in 0.1% SDS-resistant aggregates were individually analyzed for their content in aggregation-prone amino acid sequences, and five peptides were selected to characterize their in vitro amyloid fibril forming capacity. A single copy of LQSNIG is present in a DNA-binding nucleoporin (accession number Q8I398), whose disruption might a priori be a good therapeutic target. NYN is part of the well-described self-assembling peptide NYNYNYN (37) and is found in ca. 85% of the proteome (4,533 Plasmodium proteins, in some of them more than once). The peptide NVNIYN, which is found in an uncharacterized protein (accession number C0H4L9) detected in the aggregates not solubilized by 0.1% SDS, was identified after a BLAST search to be also present as a single copy in four other proteins, among them an AP2 domain-containing transcription factor potentially implicated in heat shock responses (accession number C0H5G5). Two other single-copy peptides from this presumably essential protein which had been picked out in the in silico search, NFNNIYH and NNFYYNN, were also selected for further analysis. The abundance and aggregation propensity of the proteins containing these peptides are around the respective average values for these two parameters within the P. falciparum proteome (Fig. S4).
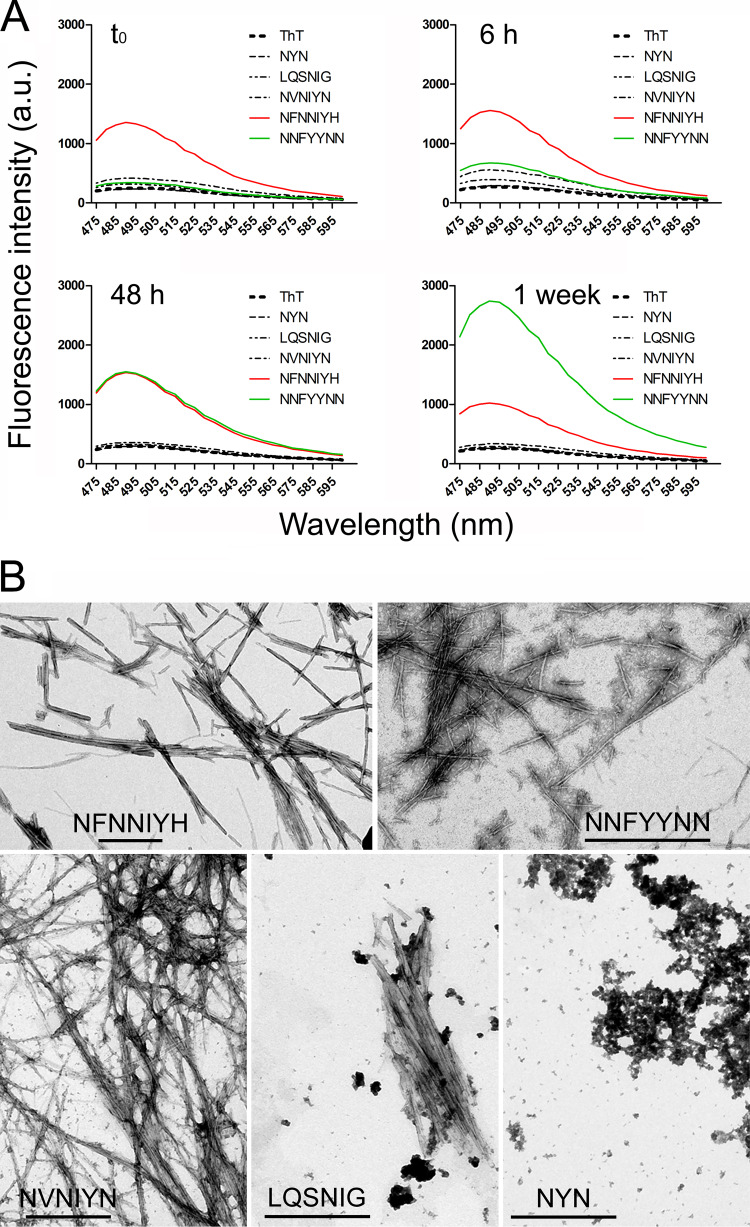
In vitro characterization of the five selected peptides revealed the existence of different aggregation behaviors. According to ThT staining (Fig. 7A), NFNNIYH fibrillated immediately upon dissolution in PBS. NNFYYNN had slower fibril-forming dynamics, but after 1 week it was the peptide with a stronger ThT fluorescence signal. NVNIYN and LQSNIG formed fibrils at a significantly slower quantitative ratio that the former two peptides, whereas the NYN spectrum did not differ from that of the ThT control in the absence of peptide. According to transmission electron microscopy analysis, the first four peptides were observed to form typical amyloid fibrils (Fig. 7B), whereas NYN samples lacked fibrils and contained only unstructured aggregates. The viability of the parasite in in vitro P. falciparum cultures was not significantly affected by any of the peptides up to a concentration of 100 μM (data not shown).
FIG 7.
Peptide characterization. (A) ThT fluorescence analysis of the peptides after different incubation times. (B) Transmission electron microscopy images of the peptides. Scale bars, 500 nm.
DISCUSSION
The amino acid sequence of the P. falciparum merozoite surface protein 2 (MSP2) has tandem repeats typical of intrinsically unstructured proteins (38, 39), in agreement with the observations that some MSP2 variants form in vitro amyloid-like fibrils (38, 40) and that merozoites bind Congo red, an amyloid-specific dye (41). The formation of MSP2 oligomers on merozoites was observed in immunofluorescence assays using a monoclonal antibody raised against the polymeric form of the protein (38). Given the evidences of amyloids interacting with RBCs (42, 43), it is reasonable to hypothesize that merozoite surface-bound MSP2 might have a role in the initial steps of erythrocyte invasion.
The presence of a clearly detectable aggregated protein content according to ProteoStat staining in egressed and invading merozoites and its absence in early intraerythrocytic ring stages suggest that the parasite’s amyloid load is lost during the RBC invasion process. This would be in agreement with the observation that MSP2 is carried into the host erythrocyte on the surface of the invading merozoite and then rapidly degraded (44) and suggests that an amyloid coat might be part of the Plasmodium invasion machinery. However, MSP2 peptides were not detected in the 0.1% SDS-insoluble fraction prepared from late-blood-stage trophozoites and schizonts, a result which might indicate that if insoluble amyloid fibrils are formed by MSP2 on the merozoite surface, this phenomenon is triggered immediately before merozoite egress from the pRBC. In agreement with this hypothesis, previous reports indicated that it was the interaction of the N-terminal 25 residues of MSP2 with membranes what induced the peptide to form β-structure and to aggregate (45).
The results presented above support the notion that most of the aggregative proteins in live Plasmodium parasites are in the cytosol and in some organelles, such as the nuclear membrane. Until future research unveils the potential physiological role of amyloids in Plasmodium, perhaps this characteristic of the parasite could be exploited for the development of new therapeutic strategies. From a generic point of view, conformational disorders occur when the load of protein aggregates surpasses the handling capacity of the cellular protein quality control machinery. Such proteostatic deregulation can be externally stimulated, as shown by the intracellular aggregation of the endogenous yeast Sup35 protein, which can be triggered by introducing in the cell aggregative seeds formed in vitro by the LCR of this protein (46). External actuation on Plasmodium proteostasis has been also shown when the function of the chaperone protein PfHsp110c was knocked down in the parasites and aggregation of LCR-containing proteins took place, which led to the pathogen’s death (24). Interestingly, the antimalarial drug methylene blue had been found to promote the formation of amyloid β peptide (Aβ) fibrils (47), and the front-line antimalarial artemisinin has been recently described to kill malaria parasites by damaging proteins and inhibiting the proteasome (48). Indeed, artemisinin resistance has been associated with increased expression of unfolded protein response pathways (49). In bacteria, the cytotoxicity of protein aggregation has been successfully used to kill the pathogen without affecting the mammalian host (50).
Several experimental evidences sustain the possibility that protein aggregation could be harnessed to be developed into new antimalarial approaches: (i) the aggregation of proteins is a generic phenomenon (14); (ii) such aggregates are often cytotoxic (51, 52); (iii) aggregation-prone regions can be reliably predicted (53), and they can trigger the aggregation of complete proteins (54, 55); (iv) most Plasmodium proteins have predicted aggregation-prone stretches (9, 24); (v) seeding primes aggregation reactions (56, 57); and (vi) homologous seeding is more efficient than heterologous seeding (56–58), thus limiting the risk of peptide cross-reactivity with proteins from the host.
The presence in the blood circulation upon Plasmodium infection of abundant proteins containing prion-like domains suggests that malaria might have to be classified as an amyloidosis, which would call for a reevaluation of potential amyloid-related pathogenic mechanisms triggered by the parasite. However, since the Plasmodium proteins identified in 0.1% SDS-resistant aggregates are involved in many critical parasite processes, it is also reasonable to hypothesize that their aggregation might be harmful for the pathogen and therefore helpful for the survival of the human host. Although this might lead one to speculate that protein aggregation can result from an anti-parasitic action of the host, some experimental evidences suggest otherwise. Protein aggregation of Plasmodium proteins has been observed in in vitro cultures, which are free from any cells or molecules related to human antimicrobial defenses. In addition, murine malaria parasites contain in vivo abundant protein aggregates that do not affect the well-known life cycle of the pathogen within the mammalian host. Finally, we have also detected aggregated proteins in the Plasmodium stages of the mosquito, whose immune system is very primitive and not expected to exert antiparasitic actions similar to those found in humans.
Preliminary growth inhibition assays indicated that aggregative peptides present in presumably essential Plasmodium proteins and selected for their aggregative capacity do not affect the parasite’s viability in in vitro cultures. Although a poor peptide entry into the cell might be responsible for the observed lack of activity, other alternative explanations must be considered. Intriguingly, the protein aggregation inhibitor rapamycin (59–61) had been observed to have antimalarial activity (62), and the antimalarial drugs curcumin and quercetin had been reported to inhibit the formation of Aβ fibrils (63, 64), suggesting the possibility that intracellular protein aggregates might actually have a functional role for the survival of the parasite. If a reduced protein aggregation can be associated with a decrease in parasite viability, then future antimalarial strategies based on the upsetting of the pathogen’s proteostasis and therefore affecting multiple gene products could represent the entry to radically new therapeutic approaches that might minimize drug resistance evolution.
MATERIALS AND METHODS
Preparation of P. falciparum blood stages and female gametes.
Human RBCs were obtained and prepared as described elsewhere (65). Cultures of the P. falciparum 3D7 and E5 strains were grown in vitro in human RBCs using previously described conditions (66). Synchronized cultures were obtained by ring-stage enrichment through 5% sorbitol lysis (67), and the medium was changed every 2 days maintaining 3% hematocrit. Late-form stages were purified in 70% Percoll (GE Healthcare) (67, 68). Previously described protocols were used for the preparation of P. falciparum merozoites (69), gametocytes (70), and female gametes (71).
Briefly, to obtain P. falciparum merozoites, a highly synchronized culture was established by performing two sorbitol lyses (days 1 and 3), a Percoll cushion purification of highly mature schizonts (day 4), and another sorbitol lysis 5 h after Percoll. This 0/5-h ring culture was then grown for 24 h until trophozoites were obtained. At this point, pRBCs were separated from parasite-free RBCs by magnetic-assisted cell sorting (VarioMACS; Miltenyi Biotec), and trophozoites were grown and monitored hourly until segmented schizonts were observed (33 h after the second sorbitol treatment). Next, 10 μM E-64 protease inhibitor was added to the culture to avoid schizont rupture, and cells were grown for 9 h (42 h after the last sorbitol treatment). Finally, merozoites were obtained by passing the synchronized schizont culture through a 1.2-μm filter (Ministart syringe filter; Sartorius). P. falciparum gametocytes were obtained using the gametocyte-generating E5 subclone of the 3D7 strain, kindly provided by Alfred Cortés. Briefly, a culture containing 10% rings was grown in Roswell Park Memorial Institute 1640 culture medium (RPMI; Gibco, Paisley, UK), supplemented with 5 mg/ml AlbuMAX II (Invitrogen) to obtain RPMI-A, and with 50 mM N-acetylglucosamine to inhibit asexual replication and to select sexually committed ring-stage parasites. After 24 h, the medium was replaced daily for 2 weeks, without further addition of fresh blood. P. falciparum female gametes were obtained from stage V gametocytes by replacing the medium by P. falciparum activation medium (RPMI containing 0.2% NaHCO3, 25 mM HEPES, 20% [vol/vol] heat-inactivated human serum, 100 μM xanthurenic acid [pH 8.0]) and incubating for 20 min at room temperature. P. falciparum sporozoites were commercially obtained (Sanaria, Inc., Rockville, MD).
Preparation of P. berghei and P. yoelii mosquito stages.
The P. berghei parasite strain used was the transgenic line CTRP-GFP (72), expressing green fluorescent protein (GFP) only during ookinete stages. To obtain gametes and ookinetes, TO-Ola mice were simultaneously injected intraperitoneally with 200 μl of phenylhydrazine (6 mg/ml in 9% NaCl; 60 mg/kg [body weight]) to induce reticulocyte production and with 108 P. berghei-infected erythrocytes. To monitor the growth of the parasite, Giemsa-stained tail blood smears and exflagellation assays were prepared 72 and 96 h after inoculation. In a typical experiment, at day 3 postinfection, average parasitemias were 10 to 20% with all parasite blood stages (rings, trophozoites, schizonts, and gametocytes) present. To assess the percentage of mature gametocytes at days 4 to 5 postinfection, exflagellation assays were performed by mixing a tail blood drop with 15 μl of exflagellating medium: cold (19°C) RPMI (pH 8.4), containing 0.2% NaHCO3, 25 mM HEPES, 50 mg/liter hypoxanthine, 2 mM glutamine, 5,000 U/ml penicillin, 5 mg/ml streptomycin, and 20% (vol/vol) heat-inactivated fetal bovine serum (Gibco, Paisley, UK). Exflagellating centers were counted microscopically at ×400 magnification. When the number of exflagellation centers per field was >10, mice were exsanguinated by cardiac puncture with a heparinized needle (30 U/ml blood). Blood was diluted 1:10 with exflagellation medium and incubated at 19°C. Gametes appeared after 20 min of incubation, whereas ookinetes were observed after 20 h. P. berghei oocysts were obtained by allowing parasites to develop in mosquito midguts. Briefly, 2-h-starved A. gambiae mosquitoes were allowed to feed for 20 min on mice infected with a high gametocytemia (>10 exflagellating centers/field at ×400 magnification) of P. berghei strain 507 (73), which constitutively expresses GFP in all parasite stages. After the blood meal, mosquitoes were maintained at 19°C for 7 to 21 days. At day 13, mosquitoes were dissected and midguts were resuspended in 200 μl of phosphate-buffered saline (PBS), proceeding immediately to fluorescence microscopy examination.
To obtain P. yoelii asexual stages, BALB/c mice were inoculated 2 × 107 red blood cells from P. yoelii yoelii 17XL-infected mice by intraperitoneal injection. Parasitemia was monitored daily by microscopic examination of Giemsa-stained thin blood smears. At day 5 after infection, mice where exsanguinated by cardiac puncture with a 10% EDTA-impregnated needle, and the blood was diluted 1:10 with RPMI-A immediately before proceeding to aggregated protein staining and fluorescence microscopy examination.
In silico analysis of the P. falciparum proteome.
The P. falciparum (isolate 3D7) reference proteome (ID UP000001450, release 2017_01), consisting of 5,369 proteins, was downloaded from UniProt (74). Protein sequences were screened with the PLAAC algorithm (75), using default parameters and the complete proteome as background, in order to identify potential prion-like domains, which rendered 342 proteins.
The abundance and aggregation propensity of each protein in the proteome were calculated and plotted as described elsewhere (76). Briefly, abundance (C) was calculated as the log10 of the protein concentration values obtained from PaxDb (77), which were normalized by rescaling them between 0 and 1 as follows:
where Cmin is the minimum value of protein concentration from the data set, Cmax is the maximum value of protein concentration from the data set, and (Ci...Cn) is each value of protein concentration from the data set.
The aggregation tendency (A) was obtained using the TANGO algorithm, which estimates the cross-beta aggregation propensity in peptides and denatured proteins (78). For the estimation, TANGO parameters were set at pH 7.4, 37°C, and 0.25 mM ionic strength, using the output parameter “cross beta-aggregation,” which was then normalized in the same manner by rescaling the values between 0 and 1 as follows:
where Amin is the minimum TANGO cross beta-aggregation score from the data set, Amax is the maximum TANGO cross beta-aggregation score from the data set, and (Ai...An) is each TANGO cross beta-aggregation score from the data set.
Analysis of ProteoStat-stained amyloid deposits in live Plasmodium parasites.
The in vivo intracellular formation of protein aggregates in the different Plasmodium stages was routinely determined with the ProteoStat aggresome detection reagent (Enzo Life Sciences, Inc.) according to the manufacturer’s instructions. As a standard protocol, 200 μl of a P. falciparum in vitro culture (3% hematocrit, 4.5% parasitemia) were harvested and washed twice with 1 ml of 7.5 mg of bovine serum albumin (BSA)/ml of PBS (PBS/BSA); the resulting cell pellet was taken up in 200 μl of PBS/BSA containing 2 μg/ml Hoechst 33342 and ProteoStat (1:3,000 or 1:5,000 stock dilution for fluorescence microscopy or flow cytometry analysis, respectively) and incubated for 30 min in the dark at room temperature before being washed again twice with 1 ml of PBS/BSA. When needed, labeling of RBC membranes was performed simultaneously by including 5 μg/ml of wheat germ agglutinin functionalized with Oregon Green 488 (Invitrogen; λex = 488 nm, λem = 510 to 550 nm). For microscopy, 10 μl of PBS/BSA washed cell suspensions were transferred into a Lab-Tek chambered cover glass (Nunc, Thermo Fisher Scientific) containing 180 μl of PBS/BSA and examined with an Olympus IX51 inverted system microscope or with a Leica TCS SP5 laser scanning confocal microscope using a ×63 immersion oil objective with 1.4 numeric aperture.
ProteoStat staining of P. yoelii and P. berghei asexual stages, P. berghei gametes, ookinetes, and oocysts, and P. falciparum sporozoites, merozoites, gametocytes, and gametes was performed as described above, using 200 μl of each preparation. Samples were viewed in a Zeiss Axioskop 2 Plus microscope fitted with an Axiovert CCD camera (Zeiss). Fluorescence was detected by excitation at 405 nm for Hoechst 33342 and 488 nm for both GFP and ProteoStat and emission collection in the ranges 415 to 500, 510 to 550, and 590 to 670 nm, respectively.
For flow cytometry, 10 μl of the cell suspension were mixed with 490 μl of sterile PBS in a disposable cytometer tube before being gated on a LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) set up with the five-laser, 20-parameter standard configuration. Forward and side scatter were used in a logarithmic scale to gate the RBC population. Acquisition was configured to stop after recording 10,000 events. Hoechst 33342 and ProteoStat fluorescence levels were detected, respectively, by excitation with a 355 nm/60 mW and 488 nm/100 mW lasers, and emissions were collected with 450/50BP nm and 610LP-610/20BP bandpass filters.
Immunocytochemical detection of amyloid fibrils.
pRBC culture smears were fixed on ice for 2 min using freshly prepared acetone:methanol (4:1). Rabbit anti-amyloid fibril OC antibody (AB2286; Merck Millipore, Darmstadt, Germany) and the secondary antibody, fluorescein-labeled AffiniPure donkey anti-rabbit IgG (711-095-152; Jackson ImmunoResearch Europe, Ltd., Cambridge, UK), were prepared at 1:200 and 1:100 dilutions, respectively, in PBS supplemented with 0.75% BSA. Smears were incubated with the primary antibody for 2 h, washed three times with PBS, incubated with the secondary antibody for 1 h, and then washed again three times with PBS. Finally, DAPI (4′,6′-diamidino-2-phenylindole; catalog no. 10236276001; Roche Applied Science, Foster City) was added to the smears at 1 μg/ml in PBS and, after a 30-min incubation, the slides were mounted with ProLong Gold antifade reagent (Life Technologies, Carlsbad, CA) and observed in a confocal microscope (TCS-SP5; Leica Microsystems, Wetzlar, Germany).
Flow cytometry sorting of ProteoStat-stained proteins in live P. falciparum blood stages.
A 3D7 P. falciparum culture (3% hematocrit, 4% parasitemia), containing ca. 6 × 1012 parasites, was spun down (300 × g, 5 min) and washed once with RPMI. To disrupt erythrocyte membranes, the resulting pellet was taken up in 10 ml of lysis solution consisting of 1.5 mg/ml saponin in complete PBS containing 1× Mini protease inhibitor cocktail (cOmplete [Roche]; one tablet in 10.5 ml for 1× concentration) and then incubated for 15 min (4°C); finally, the free parasites were spun down (10,000 × g, 3 min, 4°C) and washed three times with complete PBS. To lyse Plasmodium cells, the samples were taken up in 1 ml of 1× cOmplete in H2O, exposed to three freeze-thaw cycles (ethanol-dry ice bath for 2 min/37°C bath for 5 min), and forced 10 times through a 30-gauge needle, making fast strokes. The resulting homogenate was diluted in complete PBS, stained with ProteoStat at a final dye dilution of 1:5,000, and applied to a fluorescence-activated sorter (Aria SORP 5L) with an outlet nozzle of 130 μm. The 488-nm laser potency was adjusted to 100 mW, and the flow rate was set to the minimum (200 events/s) with a sheath pressure of 10 lb/in2. ProteoStat positive events were detected using a band pass of 610/20 and 600 LP and harvested in 5-ml polystyrene cytometry tubes (659,108 positive events in 10 ml). To verify sorting, homogenate and postsorting ProteoStat positive and negative samples were loaded on an Amnis ImageStreamX Mark II imaging flow cytometer (Luminex Corp.). Sorted aggregates were kept at −20°C for 24 h before being dialyzed (benzoylated dialysis tubing, 2-kDa molecular weight cutoff) against 2 liters of ddH2O (MilliQ system; Millipore) for 8 h, changing the water every 2 h. After dialysis, 12 ml of aggregate solution was recovered and lyophilized for 30 h, and the remaining pellet was dissolved directly in 100 μl of 1× Laemmli sample buffer and loaded in a 1.5-mm-thick 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) run for 80 min at 40 mA. Colloidal Coomassie blue G-250-stained bands were excised and subjected to LC-MS/MS analysis (79). A full description of the LC-MS/MS protocol followed is provided in the supplemental material.
Isolation of aggregative proteins insoluble in 0.1% SDS.
Aggregative proteins insoluble in 0.1% SDS were isolated as previously described (80). First, 40-ml portions of a P. falciparum preparation containing approximately 5 × 109 late-form trophozoite- and schizont-stage parasites (24 to 36 and 36 to 48 h postinvasion, respectively) that had been purified from in vitro cultures in 70% Percoll were washed with sterile PBS and spun down (300 × g, 5 min), and the the resulting cell pellet was stored at −20°C. To release cell contents, the pellet was thawed in 2 ml of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 9.4], containing 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, and 5% glycerol) supplemented with 1× cOmplete. The solution was homogenized on ice by pipetting constantly up and down for 30 min. Next, the lysate was spun (300 × g, 2.5 min) to remove debris and unbroken cells, and the supernatant was carefully loaded on top of 1 ml of 40% sucrose and ultracentrifuged (200,000 × g, 1 h) in order to pellet large insoluble aggregates. These were resuspended in 400 μl of SDS lysis buffer (PBS containing 2% SDS, 5 mM dithiothreitol, and 2 mM EDTA, supplemented with 1× cOmplete) and incubated at 37°C for 30 min, pipetting up and down every 2 min. The resulting lysate was spun down (16,000 × g, 12 min), and the supernatant was recovered and concentrated using a 3-kDa cutoff Vivaspin 500 column (15,000 × g, 30 min, room temperature). The concentrated lysate was fractionated in a 12.5% SDS-PAGE and stained with Coomassie brilliant blue R-250, and the material not entering the stacking gel was excised and subjected to LC-MS/MS analysis.
Characterization of peptide aggregation in vitro and Plasmodium growth inhibition assays.
Selected peptides were purchased from CASLO ApS c/o Scion Denmark Technical University and diluted in dimethyl sulfoxide to obtain a ≥50 mM stock solution, which was further diluted to 150 μM in PBS (pH 7.4), filtered twice through a 0.22-μm filter to ensure reproducibility in the aggregation kinetics, and incubated at 37°C and 60 rpm/min (Eppendorf ThermoMixer C) for the times indicated before proceeding to ThT measurements and transmission electron microscopy sample preparation. At different times, ThT was mixed with the sample to a final concentration of 25 μM for both peptide and ThT. Fluorescence emission was collected in the range 470 to 600 nm using an excitation wavelength of 450 nm (Infinite M200 PRO multimode microplate reader; Tecan, Switzerland). For transmission electron microscopy analysis, a carbon-coated copper grid was deposited on top of a 50-μl drop of the peptide solution (150 μM in PBS, vortexed immediately before pipetting), which had been incubated for up to 1 week. After 30 min, the excess liquid was removed with filter paper, and the grid was placed on top of a water drop for 30 s and finally negatively stained for 2 min with 20 μl of 2% uranyl acetate. Samples were observed using a JEM 1010 transmission electron microscope (JEOL, Ltd., Japan). Images were acquired using a CCD Orius camera (Gatan, Inc.).
Plasmodium growth inhibition assays were performed as previously reported (65). Briefly, sorbitol-synchronized cultures of P. falciparum were diluted with human RBCs suspended in RPMI-A growth medium to give a final concentration of 1.5% parasitemia and 3% hematocrit with more than 90% of parasites at ring stage. A total of 150 μl of these pRBC preparations were pipetted to 96-well tissue culture plates, and the required amounts of peptide seeds were added from a 500 μM stock solution in PBS. Typically, cultures were grown for one replication cycle under 5% O2, 5% CO2, and 90% N2 at 37°C for 48 h. For the determination of parasitemia, samples were diluted 1:100 in isotonic PBS and pRBC nuclei (the only nucleated cells present in the culture) were stained by addition of 0.1 μM Syto11 (Thermo Fisher Scientific, Inc.) in the final mixture before proceeding to flow cytometry analysis (81). Alternatively, microscopic counting of Giemsa-stained samples was performed (65). Growth inhibition in peptide-treated samples was defined as the percent decrease in parasitemia within the second generation of parasites relative to untreated control samples. Growth inhibition graphs and 50% inhibitory concentrations were obtained through sigmoidal fitting of growth inhibition data at different peptide concentrations.
Ethics statement.
The human blood used in this work was commercially obtained from the Banc de Sang i Teixits (www.bancsang.net). Blood was not specifically collected for this research; the purchased units had been discarded for transfusion, usually because of an excess of blood relative to anticoagulant solution. Prior to their use, blood units underwent the analytical checks specified in the current legislation. Before being delivered to us, unit data were anonymized and irreversibly dissociated, and any identification tag or label had been removed in order to guarantee the nonidentification of the blood donor. No blood data were or will be supplied, and the blood samples will not be used for studies other than those made explicit in this research. The studies reported here were performed in accordance with the current Spanish Ley Orgánica de Protección de Datos and Ley de Investigación Biomédica and under protocols reviewed and approved by the Ethical Committee on Clinical Research from the Hospital Clínic de Barcelona (Reg. HCB/2018/1223, January 23, 2019).
All animal work was carried out in full conformity with Greek regulations consisting of the Presidential Decree (160/91) and law (2015/92) which implement the directive 86/609/EEC from the European Union and the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes and the Presidential Decree 56/2013. The experiments were carried out in a certified animal facility license (EL91-BIOexp-02) and the protocol has been approved by the FORTH Ethics Committee and by the Prefecture of Crete (license number 93491, 30/04/2018).
Supplementary Material
ACKNOWLEDGMENTS
X.F.-B. was funded by (i) Spanish Ministry of Science, Innovation and Universities (http://www.ciencia.gob.es/), grant numbers PCIN-2017-100 and RTI2018-094579-B-I00 (which included FEDER funds), and (ii) ERA-NET Cofund EURONANOMED (http://euronanomed.net/), grant number 2017-178 (NANOpheles). I.S.-K. was supported within the NANOpheles European project from Greek national funds cofinanced by the European Union (project number T8EPA2-00026). S.V. was funded by (i) Ministerio de Ciencia, Innovación y Universidades, Spain (http://www.ciencia.gob.es/), grant number BIO2016-78310-R, and (ii) Generalitat de Catalunya, Spain (http://agaur.gencat.cat/), grant number 2017-SGR-908. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ISGlobal and IBEC are members of the CERCA Program, Generalitat de Catalunya. We acknowledge support from the Spanish Ministry of Science, Innovation and Universities through the Centro de Excelencia Severo Ochoa 2019-2023 Program (CEX2018-000806-S). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria, which is partially supported by the Fundación Ramón Areces.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2019. World malaria report, 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. 2015. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso PL, Tanner M. 2013. Public health challenges and prospects for malaria control and elimination. Nat Med 19:150–155. doi: 10.1038/nm.3077. [DOI] [PubMed] [Google Scholar]
- 4.Prudêncio M, Rodriguez A, Mota MM. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol 4:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 5.Cowman AF, Crabb BS. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DMA, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichinger L, Pachebat JA, Glöckner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, van Driessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R, Hauser H, James K, Quiles M, Babu MM, Saito T, Buchrieser C, Wardroper A, Felder M, Thangavelu M, Johnson D, Knights A, Loulseged H, Mungall K, Oliver K, Price C, Quail MA, Urushihara H, Hernandez J, Rabbinowitsch E, Steffen D, Sanders M, Ma J, Kohara Y, Sharp S, Simmonds M, Spiegler S, Tivey A, Sugano S, White B, Walker D, Woodward J, Winckler T, Tanaka Y, Shaulsky G, Schleicher M, Weinstock G, Rosenthal A, Cox EC, Chisholm RL, Gibbs R, Loomis WF, Platzer M, Kay RR, Williams J, Dear PH, Noegel AA, Barrell B, Kuspa A. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature 435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravind L, Iyer LM, Wellems TE, Miller LH. 2003. Plasmodium biology: genomic gleanings. Cell 115:771–785. doi: 10.1016/s0092-8674(03)01023-7. [DOI] [PubMed] [Google Scholar]
- 9.Singh GP, Chandra BR, Bhattacharya A, Akhouri RR, Singh SK, Sharma A. 2004. Hyper-expansion of asparagines correlates with an abundance of proteins with prion-like domains in Plasmodium falciparum. Mol Biochem Parasitol 137:307–319. doi: 10.1016/j.molbiopara.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Feng ZP, Zhang X, Han P, Arora N, Anders RF, Norton RS. 2006. Abundance of intrinsically unstructured proteins in Plasmodium falciparum and other apicomplexan parasite proteomes. Mol Biochem Parasitol 150:256–267. doi: 10.1016/j.molbiopara.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.DePristo MA, Zilversmit MM, Hartl DL. 2006. On the abundance, amino acid composition, and evolutionary dynamics of low-complexity regions in proteins. Gene 378:19–30. doi: 10.1016/j.gene.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia GG, Cavalli A, Pellarin R, Caflisch A. 2005. Prediction of aggregation rate and aggregation-prone segments in polypeptide sequences. Protein Sci 14:2723–2734. doi: 10.1110/ps.051471205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halfmann R, Alberti S, Krishnan R, Lyle N, O’Donnell CW, King OD, Berger B, Pappu RV, Lindquist S. 2011. Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol Cell 43:72–84. doi: 10.1016/j.molcel.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiti F, Dobson CM. 2017. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 15.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. 2011. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Seuring C, Greenwald J, Wasmer C, Wepf R, Saupe SJ, Meier BH, Riek R. 2012. The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol 10:e1001451. doi: 10.1371/journal.pbio.1001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Dokholyan NV. 2008. Natural selection against protein aggregation on self-interacting and essential proteins in yeast, fly, and worm. Mol Biol Evol 25:1530–1533. doi: 10.1093/molbev/msn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsellier E, Chiti F. 2007. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep 8:737–742. doi: 10.1038/sj.embor.7401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. 2011. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. 2010. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell 140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Patino MM, Liu JJ, Glover JR, Lindquist S. 1996. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- 22.Muralidharan V, Goldberg DE. 2013. Asparagine repeats in Plasmodium falciparum proteins: good for nothing? PLoS Pathog 9:e1003488. doi: 10.1371/journal.ppat.1003488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przyborski JM, Diehl M, Blatch GL. 2015. Plasmodial HSP70s are functionally adapted to the malaria parasite life cycle. Front Mol Biosci 2:34. doi: 10.3389/fmolb.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muralidharan V, Oksman A, Pal P, Lindquist S, Goldberg DE. 2012. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat Commun 3:1310. doi: 10.1038/ncomms2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acharya P, Kumar R, Tatu U. 2007. Chaperoning a cellular upheaval in malaria: heat shock proteins in Plasmodium falciparum. Mol Biochem Parasitol 153:85–94. doi: 10.1016/j.molbiopara.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Pallarès I, de Groot NS, Iglesias V, Sant’Anna R, Biosca A, Fernàndez-Busquets X, Ventura S. 2018. Discovering putative prion-like proteins in Plasmodium falciparum: a computational and experimental analysis. Front Microbiol 9:1737. doi: 10.3389/fmicb.2018.01737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toombs JA, Petri M, Paul KR, Kan GY, Ben-Hur A, Ross ED. 2012. De novo design of synthetic prion domains. Proc Natl Acad Sci U S A 109:6519–6524. doi: 10.1073/pnas.1119366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabate R, Rousseau F, Schymkowitz J, Ventura S. 2015. What makes a protein sequence a prion? PLoS Comput Biol 11:e1004013. doi: 10.1371/journal.pcbi.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro S, Ventura S. 2014. Fluorescent dye ProteoStat to detect and discriminate intracellular amyloid-like aggregates in Escherichia coli. Biotechnol J 9:1259–1266. doi: 10.1002/biot.201400291. [DOI] [PubMed] [Google Scholar]
- 30.Usmani SM, Zirafi O, Müller JA, Sandi-Monroy NL, Yadav JK, Meier C, Weil T, Roan NR, Greene WC, Walther P, Nilsson KP, Hammarström P, Wetzel R, Pilcher CD, Gagsteiger F, Fändrich M, Kirchhoff F, Münch J. 2014. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun 5:3508. doi: 10.1038/ncomms4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jena KK, Kolapalli SP, Mehto S, Nath P, Das B, Sahoo PK, Ahad A, Syed GH, Raghav SK, Senapati S, Chauhan S, Chauhan S. 2018. TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy. EMBO J 37:e98358. doi: 10.15252/embj.201798358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. 2012. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes da Silva R, Costa CC, Santos MJG, Alves MQ, Braga SS, Vieira SI, Rocha J, Silva AMS, Guieu S. 2019. Fluorescent light-up probe for the detection of protein aggregates. Chem Asian J 14:859–863. doi: 10.1002/asia.201801606. [DOI] [PubMed] [Google Scholar]
- 34.Raju I, Kumarasamy A, Abraham EC. 2011. Multiple aggregates and aggresomes of C-terminal truncated human αA-crystallins in mammalian cells and protection by αB-crystallin. PLoS One 6:e19876. doi: 10.1371/journal.pone.0019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franke-Fayard B, Fonager J, Braks A, Khan SM, Janse CJ. 2010. Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria? PLoS Pathog 6:e1001032. doi: 10.1371/journal.ppat.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. 2007. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Díaz-Caballero M, Navarro S, Fuentes I, Teixidor F, Ventura S. 2018. Minimalist prion-inspired polar self-assembling peptides. ACS Nano 12:5394–5407. doi: 10.1021/acsnano.8b00417. [DOI] [PubMed] [Google Scholar]
- 38.Adda CG, Murphy VJ, Sunde M, Waddington LJ, Schloegel J, Talbo GH, Vingas K, Kienzle V, Masciantonio R, Howlett GJ, Hodder AN, Foley M, Anders RF. 2009. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol Biochem Parasitol 166:159–171. doi: 10.1016/j.molbiopara.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Low A, Chandrashekaran IR, Adda CG, Yao S, Sabo JK, Zhang X, Soetopo A, Anders RF, Norton RS. 2007. Merozoite surface protein 2 of Plasmodium falciparum: expression, structure, dynamics, and fibril formation of the conserved N-terminal domain. Biopolymers 87:12–22. doi: 10.1002/bip.20764. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Adda CG, Keizer DW, Murphy VJ, Rizkalla MM, Perugini MA, Jackson DC, Anders RF, Norton RS. 2007. A partially structured region of a largely unstructured protein, Plasmodium falciparum merozoite surface protein 2 (MSP2), forms amyloid-like fibrils. J Pept Sci 13:839–848. doi: 10.1002/psc.910. [DOI] [PubMed] [Google Scholar]
- 41.Marques J, Moles E, Urbán P, Prohens R, Busquets MA, Sevrin C, Grandfils C, Fernàndez-Busquets X. 2014. Application of heparin as a dual agent with antimalarial and liposome targeting activities towards Plasmodium-infected red blood cells. Nanomedicine 10:1719–1728. doi: 10.1016/j.nano.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Jayakumar R, Kusiak JW, Chrest FJ, Demehin AA, Murali J, Wersto RP, Nagababu E, Ravi L, Rifkind JM. 2003. Red cell perturbations by amyloid beta-protein. Biochim Biophys Acta 1622:20–28. doi: 10.1016/s0304-4165(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 43.Murali J, Koteeswari D, Rifkind JM, Jayakumar R. 2003. Amyloid insulin interaction with erythrocytes. Biochem Cell Biol 81:51–59. doi: 10.1139/o03-009. [DOI] [PubMed] [Google Scholar]
- 44.Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, Beeson JG. 2014. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun 82:924–936. doi: 10.1128/IAI.00866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu C, Zheng X, Zhang W, Zhao H, MacRaild CA, Norton RS, Zhuang Y, Wang J, Zhang X. 2019. Interaction of merozoite surface protein 2 with lipid membranes. FEBS Lett 593:288–295. doi: 10.1002/1873-3468.13320. [DOI] [PubMed] [Google Scholar]
- 46.King CY, Diaz-Avalos R. 2004. Protein-only transmission of three yeast prion strains. Nature 428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 47.Necula M, Breydo L, Milton S, Kayed R, van der Veer WE, Tone P, Glabe CG. 2007. Methylene blue inhibits amyloid Aβ oligomerization by promoting fibrillization. Biochemistry 46:8850–8860. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- 48.Bridgford JL, Xie SC, Cobbold SA, Pasaje CF, Herrmann S, Yang T, Gillett DL, Dick LR, Ralph SA, Dogovski C, Spillman NJ, Tilley L. 2018. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat Commun 9:3801. doi: 10.1038/s41467-018-06221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, Chotivanich K, Imwong M, Pukrittayakamee S, Dhorda M, Nguon C, Lim P, Amaratunga C, Suon S, Hien TT, Htut Y, Faiz MA, Onyamboko MA, Mayxay M, Newton PN, Tripura R, Woodrow CJ, Miotto O, Kwiatkowski DP, Nosten F, Day NPJ, Preiser PR, White NJ, Dondorp AM, Fairhurst RM, Bozdech Z. 2015. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bednarska NG, van Eldere J, Gallardo R, Ganesan A, Ramakers M, Vogel I, Baatsen P, Staes A, Goethals M, Hammarström P, Nilsson KP, Gevaert K, Schymkowitz J, Rousseau F. 2016. Protein aggregation as an antibiotic design strategy. Mol Microbiol 99:849–865. doi: 10.1111/mmi.13269. [DOI] [PubMed] [Google Scholar]
- 51.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. 2002. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 52.Bucciantini M, Calloni G, Chiti F, Formigli L, Nosi D, Dobson CM, Stefani M. 2004. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J Biol Chem 279:31374–31382. doi: 10.1074/jbc.M400348200. [DOI] [PubMed] [Google Scholar]
- 53.Belli M, Ramazzotti M, Chiti F. 2011. Prediction of amyloid aggregation in vivo. EMBO Rep 12:657–663. doi: 10.1038/embor.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ventura S, Zurdo J, Narayanan S, Parreno M, Mangues R, Reif B, Chiti F, Giannoni E, Dobson CM, Aviles FX, Serrano L. 2004. Short amino acid stretches can mediate amyloid formation in globular proteins: the Src homology 3 (SH3) case. Proc Natl Acad Sci U S A 101:7258–7263. doi: 10.1073/pnas.0308249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanova MI, Sawaya MR, Gingery M, Attinger A, Eisenberg D. 2004. An amyloid-forming segment of β2-microglobulin suggests a molecular model for the fibril. Proc Natl Acad Sci U S A 101:10584–10589. doi: 10.1073/pnas.0403756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabaté R, Espargaró A, de Groot NS, Valle-Delgado JJ, Fernàndez-Busquets X, Ventura S. 2010. The role of protein sequence and amino acid composition in amyloid formation: scrambling and backward reading of IAPP amyloid fibrils. J Mol Biol 404:337–352. doi: 10.1016/j.jmb.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 57.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM. 2004. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci 13:1933–1938. doi: 10.1110/ps.04707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright CF, Teichmann SA, Clarke J, Dobson CM. 2005. The importance of sequence diversity in the aggregation and evolution of proteins. Nature 438:878–881. doi: 10.1038/nature04195. [DOI] [PubMed] [Google Scholar]
- 59.Wyttenbach A, Hands S, King MA, Lipkow K, Tolkovsky AM. 2008. Amelioration of protein misfolding disease by rapamycin: translation or autophagy? Autophagy 4:542–545. doi: 10.4161/auto.6059. [DOI] [PubMed] [Google Scholar]
- 60.Ravikumar B, Duden R, Rubinsztein DC. 2002. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 61.King MA, Hands S, Hafiz F, Mizushima N, Tolkovsky AM, Wyttenbach A. 2008. Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol 73:1052–1063. doi: 10.1124/mol.107.043398. [DOI] [PubMed] [Google Scholar]
- 62.Bell A, Wernli B, Franklin RM. 1994. Roles of peptidyl-prolyl CIS-trans isomerase and calcineurin in the mechanisms of antimalarial action of cyclosporin A, FK506, and rapamycin. Biochem Pharmacol 48:495–503. doi: 10.1016/0006-2952(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 63.Espargaró A, Ginex T, Vadell MD, Busquets MA, Estelrich J, Muñoz-Torrero D, Luque FJ, Sabate R. 2017. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J Nat Prod 80:278–289. doi: 10.1021/acs.jnatprod.6b00643. [DOI] [PubMed] [Google Scholar]
- 64.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. 2005. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 65.Urbán P, Estelrich J, Cortés A, Fernàndez-Busquets X. 2011. A nanovector with complete discrimination for targeted delivery to Plasmodium falciparum-infected versus noninfected red blood cells in vitro. J Control Release 151:202–211. doi: 10.1016/j.jconrel.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. 1997. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg 91:363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 67.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 68.Radfar A, Méndez D, Moneriz C, Linares M, Marín-García P, Puyet A, Diez A, Bautista JM. 2009. Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat Protoc 4:1899–1915. doi: 10.1038/nprot.2009.198. [DOI] [PubMed] [Google Scholar]
- 69.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, de la Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 70.Saliba KS, Jacobs-Lorena M. 2013. Production of Plasmodium falciparum gametocytes in vitro. Methods Mol Biol 923:17–25. doi: 10.1007/978-1-62703-026-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP, Kafatos FC. 2004. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol 6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 73.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. 2006. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 74.UniProt Consortium. 2015. UniProt: a hub for protein information. Nucleic Acids Res 43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lancaster AK, Nutter-Upham A, Lindquist S, King OD. 2014. PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 30:2501–2502. doi: 10.1093/bioinformatics/btu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carija A, Pinheiro F, Iglesias V, Ventura S. 2019. Computational assessment of bacterial protein structures indicates a selection against aggregation. Cells 8:E856. doi: 10.3390/cells8080856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang M, Weiss M, Simonovic M, Haertinger G, Schrimpf SP, Hengartner MO, von Mering C. 2012. PaxDb, a database of protein abundance averages across all three domains of life. Mol Cell Proteomics 11:492–500. doi: 10.1074/mcp.O111.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L. 2004. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat Biotechnol 22:1302–1306. doi: 10.1038/nbt1012. [DOI] [PubMed] [Google Scholar]
- 79.Moles E, Urbán P, Jiménez-Díaz MB, Viera-Morilla S, Angulo-Barturen I, Busquets MA, Fernàndez-Busquets X. 2015. Immunoliposome-mediated drug delivery to Plasmodium-infected and noninfected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J Control Release 210:217–229. doi: 10.1016/j.jconrel.2015.05.284. [DOI] [PubMed] [Google Scholar]
- 80.Kryndushkin D, Pripuzova N, Burnett BG, Shewmaker F. 2013. Non-targeted identification of prions and amyloid-forming proteins from yeast and mammalian cells. J Biol Chem 288:27100–27111. doi: 10.1074/jbc.M113.485359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis BH. 2001. Diagnostic utility of red cell flow cytometric analysis. Clin Lab Med 21:829–840. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.