Since 2012, a single low dose of primaquine (SLDPQ; 0.25 mg/kg of body weight) with artemisinin-based combination therapies has been recommended as the first-line treatment of acute uncomplicated Plasmodium falciparum malaria to interrupt its transmission, especially in low-transmission settings of multidrug resistance, including artemisinin resistance. Policy makers in Cambodia have been reluctant to implement this recommendation due to primaquine safety concerns and a lack of data on its efficacy.
KEYWORDS: direct membrane feeding assays, transmission blocking, malaria, primaquine
ABSTRACT
Since 2012, a single low dose of primaquine (SLDPQ; 0.25 mg/kg of body weight) with artemisinin-based combination therapies has been recommended as the first-line treatment of acute uncomplicated Plasmodium falciparum malaria to interrupt its transmission, especially in low-transmission settings of multidrug resistance, including artemisinin resistance. Policy makers in Cambodia have been reluctant to implement this recommendation due to primaquine safety concerns and a lack of data on its efficacy. In this randomized controlled trial, 109 Cambodians with acute uncomplicated P. falciparum malaria received dihydroartemisinin-piperaquine (DP) alone or combined with SLDPQ on the first treatment day. The transmission-blocking efficacy of SLDPQ was evaluated on days 0, 1, 2, 3, 7, 14, 21, and 28, and recrudescence by reverse transcriptase PCR (RT-PCR) (gametocyte prevalence) and membrane feeding assays with Anopheles minimus mosquitoes (gametocyte infectivity). Without the influence of recrudescent infections, DP-SLDPQ reduced gametocyte carriage 3-fold compared to that achieved with DP. Of 48 patients tested on day 0, only 3 patients were infectious to mosquitoes (∼6%). Posttreatment, three patients were infectious on day 14 (3.5%, 1/29) and on the 1st and 7th days of recrudescence (8.3%, 1/12 for each); this overall low infectivity precluded our ability to assess its transmission-blocking efficacy. Our study confirms the effective gametocyte clearance of SLDPQ when combined with DP in multidrug-resistant P. falciparum infections and the negative impact of recrudescent infections due to poor DP efficacy. Artesunate-mefloquine (ASMQ) has replaced DP, and ASMQ-SLDPQ has been deployed to treat all patients with symptomatic P. falciparum infections to further support the elimination of multidrug-resistant P. falciparum in Cambodia. (This study has been registered at ClinicalTrials.gov under identifier NCT02434952.)
INTRODUCTION
Malaria remains a major public health challenge, with an estimated 228 million cases recorded in 2018 (1). While considerable progress has been made since 2010, when the estimated case burden was 251 million, the recent emergence and spread of the Plasmodium falciparum lineage resistant to both artemisinin and piperaquine (the KEL1/PLA1 lineage) in the eastern Greater Mekong Subregion (GMS) threaten this remarkable global achievement (2–7). Eliminating rapidly multidrug-resistant (MDR) Plasmodium falciparum is therefore the top priority for countries of the GMS. Implementation of novel tools and strategies or repurposing existing tools, like primaquine, that specifically aim at interrupting malaria transmission are essential to reach this goal.
Although artemisinin derivatives are the more potent compounds in artemisinin-based combination therapies (ACTs) and are active against early gametocyte stages (8–10) and reduce transmission (11, 12), the only currently available drug effective against the transmissible mature stage V gametocytes is primaquine. In 2012, the WHO recommended the addition of a single low dose of primaquine (SLDPQ; target dose, 0.25 mg/kg of body weight) to ACTs to interrupt malaria transmission, primarily in areas with low rates of transmission of MDR P. falciparum, like GMS (13).
To date, several clinical studies conducted in Africa, Colombia, and Cambodia have assessed the safety and efficacy of SLDPQ (14–24), mainly by measuring gametocyte carriage (by microscopy or TaqMan reverse transcriptase PCR [RT-PCR]) as a surrogate marker of its transmission-blocking efficacy. This is due to the challenging logistical requirements of carrying out mosquito infectivity studies. These studies showed that SLDPQ, dosed from 0.20 to 0.75 mg/kg, clearly reduced gametocyte carriage and increased gametocyte clearance in a dose-dependent manner (15–17, 19, 21–25). A small number of studies have investigated human-to-mosquito transmission, based on infectivity measures, with variable results being found due to low infectivity before and/or after SLDPQ treatment (16, 17, 19, 22, 24). Only two studies from Mali demonstrated conclusively the transmission-blocking efficacy of SLDPQ, which produced a 92.6% to 100% within-person reduction in infectivity at day 2 versus that at baseline following treatment with 0.25 mg/kg of SLDPQ (16, 19).
In Cambodia, glucose-6-phosphate dehydrogenase deficiency (G6PDd) is a common X-linked disorder of the red blood cells, with frequencies ranging from 10.8% to 29.6% in males (26) and with a prevalence rate in malaria patients seen at health centers of 13.9% (27). This is the main reason why Cambodian policy makers have been reluctant to deploy SLDPQ without local data on the safety and the efficacy of SLDPQ. Therefore, we conducted a trial assessing the tolerability and the safety of SLDPQ (0.25 mg/kg) in Cambodia in 2015 and 2016 (28), which followed on from a safety trial of 0.75 mg/kg/week of primaquine (PQ) in vivax malaria patients (29). Here, we present the results of our investigations on the transmission-blocking efficacy of this treatment through the evaluation of gametocyte prevalence dynamics as well as infectivity measures for a subset of patients using membrane feeding assays with Anopheles minimus mosquitoes, one of the main malaria vectors in Southeast Asia.
RESULTS
One hundred nine patients with uncomplicated falciparum malaria were enrolled and treated with the standard 3-day regimen of dihydroartemisinin-piperaquine (DP) alone (48.6%, 53/109) or DP-SLDPQ (51.4%, 56/109). Seven patients were lost to follow-up (6.4%). Of the remaining 102 patients, 28 (27.4%) had PCR-proven P. falciparum recrudescences; 24 occurred within the 28-day follow-up on days 12 (n = 1), 14 (n = 1), 17 (n = 2), 18 (n = 1), 21 (n = 2), 22 (n = 2), 23 (n = 1), 24 (n = 2), 25 (n = 1), 26 (n = 2), 27 (n = 1), and 28 (n = 8), and 4 occurred on days 44, 46, 52, and 124. Of the 28 patients with recrudescences, 10 were retreated with the standard 3-day DP regimen and 18 were treated with quinine or quinine plus tetracycline; 6 of the 10 patients retreated with DP experienced a second recrudescence.
At day 0, gametocytes were detected in 47/109 patients (43.1%) by TaqMan RT-PCR (Table 1) (see reference 30 for further details), with no significant difference between the two arms (P = 0.3057).
TABLE 1.
Baseline characteristics of the two study groups of patients
| Characteristic | Value for the following study group: |
|
|---|---|---|
| DP | DP-SLDPQ | |
| No. of patients enrolled | 53 | 56 |
| No. of recrudescent patients | 18 | 10 |
| No. of males, no. of females | 44, 9 | 44, 12 |
| Mean (SD) age (yr) | 25 (16) | 26 (14) |
| No. of patients G6PDa deficient, no. of patients G6PD normal | 3, 50 | 6, 50 |
| No. of gametocyte-positive slides | 14 | 7 |
| Mean (SD, range) no. of gametocytes/μl | 64 (250, 0–1,432) | 40 (149, 0–787) |
| No. of patients positive for gametocytes by TaqMan RT-PCR | 26 | 21 |
| Hemoglobin concn (g/dl) | 12.63 | 13.19 |
| No. of patients with anemiab/total no. tested | 10/51 | 9/56 |
G6PD, glucose-6-phosphate dehydrogenase.
Anemia was considered a hemoglobin concentration of <11 g/dl.
Gametocyte dynamics.
Examining the dynamics of TaqMan RT-PCR gametocyte carriage to day 28, drug arm, baseline gametocytemia, recrudescence status, the baseline hemoglobin concentration, and day of follow-up were all significant factors for gametocytemia in the univariate analysis (Table 2).
TABLE 2.
Independent factors associated with a change in gametocytemia over 28 days by univariate analysisa
| Factor | OR | 95% CI | P value |
|---|---|---|---|
| Treatment | |||
| DP-SLDPQ | |||
| DP | 5.59 | 1.71, 18.27 | 0.0044 |
| Status on day 0 | |||
| Negative | |||
| Positive | 15.83 | 4.83, 51.91 | <0.0001 |
| Patient recrudescence status | |||
| Cured | |||
| Recrudescent | 11.11 | 2.78, 44.36 | 0.0006 |
| Day 0 hemoglobin concn | 0.46 | 0.34, 0.63 | <0.0001 |
| Day of follow-up | 0.94 | 0.91, 0.96 | <0.0001 |
OR, odds ratio (an odds ratio of <1 indicates a negative association); CI, confidence interval.
The DP recipients were more likely to be gametocytemic than the DP-SLDPQ recipients during the follow-up, which was seen more clearly on day 7 and day 14 (Table 2; Fig. 1), as were patients with baseline gametocytemia (Table 2; Fig. 2) and recrudescent versus cured patients (Table 2).
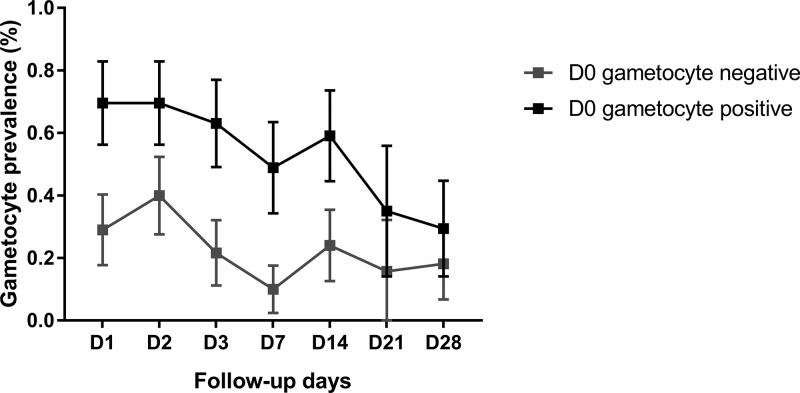
FIG 1.
Gametocyte prevalence by TaqMan RT-PCR by follow-up day and treatment group. Data show the proportion ± 95% confidence intervals. DP, dihydroartemisinin-piperaquine.
FIG 2.
Gametocyte prevalence by TaqMan RT-PCR by follow-up day and presence of gametocyte prior to treatment on day 0 (D0), detected by TaqMan RT-PCR. Data show the proportion ± 95% confidence intervals.
The baseline hemoglobin concentration and the day of follow-up were inversely associated with the probability of being gametocytemic during the follow-up (Table 2).
When the aforementioned factors were tested in a multivariable model, all except drug arm remained significant (Table 3, model 1). However, when removing recrudescence from the multivariable model, drug arm was again a significant explanatory factor (Table 3, model 2).
TABLE 3.
Multivariable models (with and without recrudescence) of the factors associated with changes in gametocytemia over 28 daysa
| Model | Factor | Adj OR | 95% CI | P value |
|---|---|---|---|---|
| Model 1 | Treatment | 2.39 | 0.81, 7.08 | 0.1152 |
| Day 0 status | 7.35 | 2.27, 23.78 | 0.0009 | |
| Recrudescence | 6.02 | 1.70, 21.26 | 0.0053 | |
| Day 0 hemoglobin concn | 0.57 | 0.42, 0.77 | 0.0003 | |
| Day of follow-up | 0.93 | 0.90, 0.96 | <0.0001 | |
| Model 2 | Treatment | 3.15 | 1.09, 9.11 | 0.0344 |
| Day 0 status | 6.63 | 2.10, 20.87 | 0.0012 | |
| Day 0 hemoglobin concn | 0.55 | 0.41, 0.75 | 0.0001 | |
| Day of follow-up | 0.93 | 0.90, 0.96 | <0.0001 |
Adj OR, adjusted odds ratio; CI, confidence interval.
Post hoc calculation showed that there was a 99.1% power to detect a difference between the DP and DP-SLDPQ treatments with the study sample sizes (n = 49 and n = 56 on day 7 for the DP and DP-SLDPQ treatment groups, respectively).
Mosquito infectivity.
A total of 55 patients were included in the direct membrane feeding assays (DMFAs). Among the 292 feeding assays, only 113 of 14,444 (0.78%) dissected mosquitoes were infected (Table 4). Overall, 3 out of 48 (6.3% ± 6.8%) individuals infected at least one mosquito at baseline, one individual (treated with DP) was infectious on day 14, and two individuals with recrudescent infections (Tables 4 and 5) who were repeat DP treatment failures were infectious (i) at presentation on day 124 (DP-SLDPQ) and (ii) day 35 (7 days after DP was started on day 28). The three individuals infectious on day 0 were all gametocytemic by microscopy, received DP-SLDPQ, and were not infectious on day 1 or day 3 posttreatment (Table 5).
TABLE 4.
Infectivity rates defined by treatment assessed by DMFAs during the 28-day follow-upa
| Follow-up day or recrudescence, day | No. of positive isolatesb/no. of isolates tested for the following treatment: |
Infectivity rate (%) | ||
|---|---|---|---|---|
| DP | DP-SLDPQ | Total | ||
| 0 | 0/15 | 3/33 | 3/48 | 6.3 ± 6.8 |
| 1 | 0/1 | 0/6 | 0/7 | 0 |
| 2 | 0/12 | 0/26 | 0/38 | 0 |
| 3 | 0/15 | 0/31 | 0/46 | 0 |
| 7 | 0/11 | 0/24 | 0/35 | 0 |
| 14 | 1/10 | 0/19 | 1/29 | 3.5 ± 6.6 |
| 21 | 0/1 | 0/4 | 0/5 | 0 |
| 28 | 0/6 | 0/22 | 0/28 | 0 |
| Rec1, 0 | 0/4 | 1/8 | 1/12 | 8.3 ± 15.6 |
| Rec1, 3 | 0/4 | 0/6 | 0/11 | 0 |
| Rec1, 7 | 1/6 | 0/6 | 1/12 | 8.3 ± 15.6 |
| Rec1, 14 | 0/4 | 0/1 | 0/4 | 0 |
| Rec1, 28 | NA | 0/1 | 0/1 | 0 |
| Rec1, 35 | NA | 0/1 | 0/1 | 0 |
| Rec2, 0 | 0/4 | 0/1 | 0/5 | 0 |
| Rec2, 3 | 0/4 | 0/1 | 0/5 | 0 |
| Rec2, 7 | 0/4 | 0/1 | 0/5 | 0 |
| Total DMFA | 101 | 191 | 292 | 2 ± 1.6 |
DP, dihydroartemisinin piperaquine; SLDPQ, a single low dose of primaquine; Rec1 and Rec2, the first and second recrudescences, respectively; NA, data not available.
Positive isolates were isolates which were able to infect at least one mosquito from a direct membrane feeding assay (DFMA).
TABLE 5.
Details of the five patients infectious for A. minimus by DMFAs during the 28-day follow-upa
| Day or recrudescence, day | Patient identifier | Treatment | Sex | Age (yr) | Gametocytemia (no. of gametocytes/μl) | Gametocyte detection by TaqMan RT-PCR | Infection prevalence (proportion of infected females ± 95% CI) | Infection intensity (mean no. of oocysts ± SE) |
|---|---|---|---|---|---|---|---|---|
| 0 | D_002 | DP-SLDPQ | F | 29 | 562 | Positive | 41.7 ± 14 | 4.7 ± 0.97 |
| 1 | 336 | Positive | 0 | NA | ||||
| 2 | 48 | Positive | 0 | NA | ||||
| 3 | 0 | Positive | 0 | NA | ||||
| 7 | NA | Negative | 0 | NA | ||||
| 14 | NA | Negative | 0 | NA | ||||
| 21 | NA | Negative | 0 | NA | ||||
| 28 | 0 | Positive | 0 | NA | ||||
| Rec1, 0 (124)b | D_002 | QN-TE | 24 | Positive | 10 ± 8.3 | 2.6 ± 1.4 | ||
| Rec1, 3 (127) | 64 | Positive | 0 | NA | ||||
| Rec1, 7 (131) | 168 | Positive | 0 | NA | ||||
| 0 | N_015 | DP-SLDPQ | M | 23 | 787 | Positive | 16 ± 10 | 2.12 ± 0.4 |
| 1 | 2,208 | Positive | 0 | NA | ||||
| 2 | 40 | Positive | 0 | NA | ||||
| 3 | 176 | Positive | 0 | NA | ||||
| 7 | NA | Negative | 0 | NA | ||||
| 14 | NA | Negative | 0 | NA | ||||
| 28 | NA | Negative | 0 | NA | ||||
| Rec1, 0 (28) | N_035 | QN | F | 8 | 1,823 | Positive | 0 | NA |
| Rec1, 3 (31) | 11,912 | Positive | 0 | NA | ||||
| Rec1, 7 (35) | 7,904 | Positive | 94.3 ± 7.7 | 82.6 ± 5.5 | ||||
| 0 | N_046 | DP | M | 8 | 1,432 | Positive | 0 | NA |
| 2 | 2,904 | Positive | 0 | NA | ||||
| 3 | 3,952 | Positive | 0 | NA | ||||
| 7 | 1,952 | Positive | 0 | NA | ||||
| 14 | 432 | Positive | 6 ± 6.5 | 1 | ||||
| 28 | 16 | Positive | 0 | NA | ||||
| 0 | N_074 | DP-SLDPQ | M | 25 | 540 | Positive | 86.3 ± 9.4 | 11.3 ± 1.7 |
| 3 | 8 | Positive | 0 | NA |
QN-TE, quinine plus tetracycline; Rec1, the first recrudescence; CI, confidence interval; NA, data not available; F, female; M, male.
The day after initial infection is indicated in parentheses.
DISCUSSION
In 2012, the WHO recommended adding 0.25-mg/kg SLDPQ to first-line treatments (ACTs) to kill mature gametocytes and block human-to-mosquito transmission (13). As no data were available at that time in Cambodia, we investigated the transmission-blocking efficacy of SLDPQ when given with DP (the recommended first-line standard ACT in 2015) by evaluating gametocyte prevalence dynamics (by TaqMan RT-PCR) and Anopheles minimus infectivity by DMFA.
Our results showed that SLDPQ, when given on the first day of the 3-day standard ACT treatment, significantly decreased gametocyte carriage over time, starting on day 3, and that gametocyte carriage was the lowest on day 7, resulting in a 5.59-fold reduced risk relative to that with treatment with DP alone over 28 days in the univariate analysis. This finding is consistent with recent reports on the efficacy of PQ in reducing gametocyte carriage (15–17, 19, 21–25, 31) and earlier reports showing a lag between reduced gametocyte carriage and rapid mosquito infectivity. The multivariable analysis revealed the significant effect of recrudescent (resistant) infections on gametocyte carriage, with a 3-fold reduction in gametocyte carriage in the absence of resistance being seen.
Our patients had a high rate of baseline gametocytemia (19% by microscopy, just under 45% by TaqMan RT-PCR), which is similar to that reported previously in 2010 in patients with artemisinin-resistant P. falciparum infections in western Cambodia (∼19%) but which is 3-fold higher than the 6% reported earlier in Ratanakiri Province, Cambodia (32). Given the high prevalence of PfKelch13 mutant P. falciparum parasites (63%) in our study and the very high recrudescent rate (~28%), our high gametocyte carriage rate is consistent with the presence of artemisinin-resistant P. falciparum, which has also led to piperaquine resistance (7). This is consistent with another study in Cambodia which showed that DP-treated patients from Oddar Meanchey province (northern Cambodia) also had prolonged gametocyte carriage that was seen in patients with recrudescent infections but was independent of the slow asexual parasite clearance time due to artemisinin-resistant P. falciparum (22, 35).
However, prolonged gametocyte carriage has also been seen in DP-treated African patients without artemisinin-resistant P. falciparum infection who, nevertheless, had low rates of human-to-mosquito transmission (16, 32, 33). Moreover, several comparative studies have found that gametocyte carriage is higher in DP-treated patients than in artemether-lumefantrine-treated patients (33–36), suggesting a reduced gametocytocidal activity of piperaquine compared to lumefantrine.
Overall, we observed a biphasic pattern of gametocyte prevalence, with a drop on day 7 and a rebound on day 14, followed by a decrease on day 21 and day 28. However, this effect was mainly driven by the DP-treated patients, as their gametocyte carriage rate was similar over the 28-day follow-up, whereas it decreased markedly in the DP-SLDPQ-treated patients (Fig. 1).
We were not able to assess the transmission-blocking efficacy of SLDPQ due to the very low infectivity rate observed in only three patients (∼6%) at baseline and three infectious events during follow-up. This is a major limitation of DFMAs, as previously reported by Lin et al. (22) at baseline, using Anopheles dirus mosquitoes, and on day 7, without assessing the baseline, by Okebe et al. (24). In another study in African children, despite a higher infectivity rate at enrollment after microscopy-based selection of gametocyte-positive asymptomatic children (38%), Gonçalves et al. (17) were also unable to detect the added value of SLDPQ due to very low infectivity posttreatments in all arms. As our clinical trial aimed primarily at assessing SLDPQ tolerability and safety, we did not particularly select patent or high-density gametocyte carriers and thus would have needed substantially more individuals to assess transmission-blocking efficacy. Only two studies, which were conducted in Mali, have statistically confirmed the high anti-infectivity efficacy (>90%) at 48 h of SLDPQ, when dosed at baseline, after selecting gametocyte-positive participants by microscopy (16, 19). However, we observed that the three SLDPQ-treated infectious patients on day 0 did not infect mosquitoes on subsequent days, despite the presence of gametocytes. Several reasons may explain this finding. Following primaquine treatment, the anti-infectivity effect is rapid (≤24 h) and precedes the decline in gametocytemia (37). Male gametocytes seem to be more sensitive to primaquine than female gametocytes (38), and the P. falciparum sex ratio is biased toward females, potentially resulting in delayed gametocyte clearance. The relationship between gametocytemia and infectivity is nonlinear and is, thus, an indirect measure of infectivity (39–41).
To conclude, our study shows that the addition of SLDPQ to ACT treatment for symptomatic falciparum malaria decreases substantially gametocyte carriage in patients with MDR P. falciparum infections. Although we were unable to reconfirm the findings of the elegant studies of Dicko et al. (16, 19), SLDPQ, dosed on the first day of treatment, is likely to further decrease the transmission potential of P. falciparum patients in our setting when combined with an effective ACT.
Owing to the high failure rate of DP, Cambodia has switched to artesunate-mefloquine treatment as the first-line treatment for uncomplicated P. falciparum and in January 2018 deployed SLDPQ in the whole country, partly on the basis of the findings of the trial described here and the good tolerability of SLDPQ in G6PDd patients (28). An age-based dosing regimen of SLDPQ, designed for Cambodia, will aid elimination efforts in areas where weighing scales are unavailable (42).
MATERIALS AND METHODS
Study design.
This open-label randomized controlled trial assessing the tolerability and the safety of SLDPQ was carried out in Banlung, Rattanakiri Province, in northeastern Cambodia in 2015 and 2016 (ClinicalTrials.gov registration number NCT02434952), as described by Dysoley et al. (28). Briefly, 109 nonpregnant, non-breast-feeding patients aged ≥1 year with acute uncomplicated falciparum malaria (≥1 asexual parasites/500 white blood cells, equivalent to 16 asexual parasites/μl) and a hemoglobin concentration of ≥6 g/dl were recruited to receive either dihydroartemisinin-piperaquine (DP; dihydroartemisinin at 40 mg and piperaquine at 320 mg; Duo-Cotecxin; Zhejiang Holley Nanhu Pharmaceutical Co. Ltd., Jiaxing, Zhejiang, China) alone or combined with SLDPQ (0.25 mg/kg given with the first dose of DP, 15 mg of primaquine base; Thai Government Pharmaceutical Organization). Clinical and laboratory assessments were performed on days 0, 1, 2, 3, 7, 14, 21, and 28. Anopheles minimus mosquitoes were fed on blood samples collected from a subset of 55 patients and on the days of recurrent (Drec) falciparum parasitemia (defined by the WHO as late treatment failures), depending on mosquito availability, using direct membrane feeding assays (DMFAs).
The sample size calculation was based on the primary outcome of a reduction in the mean day 7 hemoglobin (Hb) concentration of 1 g/dl in the DP-SLDPQ arm with G6PDd patients versus that in the DP-SLDPQ arm with patients with normal G6PD levels. Assuming a mean ± standard deviation Hb concentration of 11.27 ± 1.74 g/dl (calculated from a Southeast Asian database of ~6,800 ACT-treated, P. falciparum-infected patients of all ages [W. R. Taylor, unpublished data]), a two-sided alpha value of 0.05, and a power of 80%, the sample size was 48 patients per arm, and this number was rounded up to 50. G6PD status was initially diagnosed in the field using a fluorescent spot test to allow for the allocation of SLDPQ. The results of the qualitative Carestart (AccessBio, Somerset, NJ) rapid diagnostic test were assessed in parallel.
Mosquito infection.
DMFAs were carried out to assess individual malaria infectivity as described previously (30). Briefly, 3- to 5-day-old A. minimus female mosquitoes were fed through membranes on the patients’ blood. The mosquitoes were starved for 24 h before being provided a blood meal. Venous blood samples were collected in heparinized tubes, and 400 μl of blood was made available in membrane feeders maintained at 37°C. Females were fed only once on freshly drawn blood. Postfeeding, unfed females were discarded and fed females were kept in cages (20 by 20 by 20 cm) with constant access to a 10% sucrose solution. Patient infectivity was determined by assessing infection prevalence (i.e., the proportion of infected females) and infection intensity (i.e., the number of Plasmodium oocysts in infected females). Midguts were dissected in a 1% mercurochrome stain, and the presence and the number of oocysts were determined under a microscope (×20 magnification). Dissections were performed on 7 day after the blood meal. The mean number of dissected females was 49 (range, 15 to 85; median, 50).
Biological investigations.
Parasite RNA was extracted from TRIzol reagent (Life Technologies Holdings Pte. Ltd., Singapore)-conserved whole-blood samples using a QIAamp RNA blood minikit (Qiagen, Germany), following the protocol recommended by the manufacturer. A two-step semiquantitative real-time PCR was performed to detect malaria parasites, as previously described (43). Following PCR amplification, P. falciparum-positive samples were analyzed for the presence of gametocytes by a TaqMan RT-PCR, using primers spanning an exon-exon junction and targeting the Plasmodium falciparum meiotic recombination protein DMC1-like protein gene (GenBank accession number AF356553), as described previously (44). Gametocyte dilution series based on an in vitro-cultured local strain were used to estimate gametocyte blood concentrations.
Ethical statement.
Ethical approvals were obtained from the Cambodian National Ethics Committee for Health Research (approval number 0370NECHR), and the trial is registered at ClinicalTrials.gov (ClinicalTrials.gov registration number NCT02434952). The protocols conformed to the Helsinki Declaration on ethical principles for medical research involving human subjects (version 2002), and informed written consent was obtained from all volunteers.
Statistical analyses.
Initially, univariate generalized linear mixed models (GLMMs) with a binomial distribution were fitted to model the PCR-measured gametocyte prevalence rates. In these models, follow-up days (days 1, 2, 3, 7, 14, 21, and 28), treatment (DP versus DP-SLDPQ), the presence of gametocytes prior to treatment on day 0, recrudescence status (recrudescent versus cured patient), and the hemoglobin concentration prior to treatment on day 0 were included in the model as fixed factors. The patient identifier was coded as a random factor to account for repeated measures on the same individual. The univariate analyses were followed by analyses with a multivariable GLMM to model the dynamics of gametocyte prevalence posttreatment by including all the significant factors from the univariate analysis.
Gametocyte clearance times were not compared in the study for three principal reasons: (i) of the 21 patients with microscopically detected gametocytes on day 0, only 7 had complete follow-up microscopy data, (ii) TaqMan RT-PCR-measured gametocyte densities were highly variable during the 28 days of follow-up, and (iii) by day 28, 10/34 patients with gametocytes on day 0 by TaqMan RT-PCR were still gametocytemic (n = 1, DP-SLDPQ arm). P values of <0.05 were deemed significant. All analyses were performed in R (v3.5.1) software (45).
ACKNOWLEDGMENTS
We thank the patients for taking part in this study and the invaluable help of the village malaria health workers in helping with patient recruitment.
This work was supported by the Institut Pasteur of Cambodia, FEI 5% initiative (grant number 12INI211, Towards malaria elimination: effective strategies against transmission. The new challenges in South East Asia), the Rotary Club (grant number GG1523934, Amélioration des capacités en entomologie des acteurs luttant contre le paludisme et de de la compréhension de la transmission du paludisme), USAID/PMI/CDC through the Malaria Consortium (The tolerability and safety of low dose primaquine for transmission blocking in symptomatic falciparum infected Cambodians), and Dedonder Clayton (grant number EC/MAM/N°325/14). A.V. was supported by a postdoctoral fellowship from the International Division, Institut Pasteur, Paris, France.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We all declare that we have no conflicts of interest.
REFERENCES
- 1.World Health Organization. 2019. World malaria report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium . 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders DL, Vanachayangkul P, Lon C, U.S. Army Military Malaria Research Program, National Center for Parasitology, Entomology, and Malaria Control (CNM), Royal Cambodian Armed Forces . 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 5.Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, Kim S, Witkowski B, Duru V, Domergue A, Khim N, Ringwald P, Menard D. 2015. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother 59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White NJ. 2017. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis 17:1022–1023. doi: 10.1016/S1473-3099(17)30524-8. [DOI] [PubMed] [Google Scholar]
- 7.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, Jittamala P, Hanboonkunupakarn B, Chutasmit K, Saelow C, Runjarern R, Kaewmok W, Tripura R, Peto TJ, Yok S, Suon S, Sreng S, Mao S, Oun S, Yen S, Amaratunga C, Lek D, Huy R, Dhorda M, Chotivanich K, Ashley EA, Mukaka M, Waithira N, Cheah PY, Maude RJ, Amato R, Pearson RD, Goncalves S, Jacob CG, Hamilton WL, Fairhurst RM, Tarning J, Winterberg M, Kwiatkowski DP, Pukrittayakamee S, Hien TT, Day NP, Miotto O, White NJ, Dondorp AM. 2019. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 19:952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, International Artemisinin Study Group . 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009:CD007483. doi: 10.1002/14651858.CD007483.pub2:CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechy-Cabaret O, Benoit-Vical F. 2012. Effects of antimalarial molecules on the gametocyte stage of Plasmodium falciparum: the debate. J Med Chem 55:10328–10344. doi: 10.1021/jm3005898. [DOI] [PubMed] [Google Scholar]
- 11.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis 183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 12.Drakeley CJ, Jawara M, Targett GA, Walraven G, Obisike U, Coleman R, Pinder M, Sutherland CJ. 2004. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop Med Int Health 9:53–61. doi: 10.1046/j.1365-3156.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. 2012. Updated WHO policy recommendation (October 2012). Single dose primaquine as gametocytocide in Plasmodium falciparum malaria. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.Mwaiswelo R, Ngasala BE, Jovel I, Gosling R, Premji Z, Poirot E, Mmbando BP, Björkman A, Mårtensson A. 2016. Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania. Malar J 15:316. doi: 10.1186/s12936-016-1341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J, Grignard L, Lanke KHW, Wanzira H, Mpimbaza A, Nsobya S, White NJ, Webb EL, Staedke SG, Drakeley C. 2014. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 14:130–139. doi: 10.1016/S1473-3099(13)70268-8. [DOI] [PubMed] [Google Scholar]
- 16.Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, Sanogo K, Koita F, Keita S, Traore SF, Chen I, Poirot E, Hwang J, McCulloch C, Lanke K, Pett H, Niemi M, Nosten F, Bousema T, Gosling R. 2016. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis 16:674–684. doi: 10.1016/S1473-3099(15)00479-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves BP, Tiono AB, Ouedraogo A, Guelbeogo WM, Bradley J, Nebie I, Siaka D, Lanke K, Eziefula AC, Diarra A, Pett H, Bougouma EC, Sirima SB, Drakeley C, Bousema T. 2016. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med 14:40. doi: 10.1186/s12916-016-0581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen I, Diawara H, Mahamar A, Sanogo K, Keita S, Kone D, Diarra K, Djimde M, Keita M, Brown J, Roh ME, Hwang J, Pett H, Murphy M, Niemi M, Greenhouse B, Bousema T, Gosling R, Dicko A. 2018. Safety of single-dose primaquine in G6PD-deficient and G6PD-normal males in Mali without malaria: an open-label, phase 1, dose-adjustment trial. J Infect Dis 217:1298–1308. doi: 10.1093/infdis/jiy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dicko A, Roh ME, Diawara H, Mahamar A, Soumare HM, Lanke K, Bradley J, Sanogo K, Kone DT, Diarra K, Keita S, Issiaka D, Traore SF, McCulloch C, Stone WJR, Hwang J, Muller O, Brown JM, Srinivasan V, Drakeley C, Gosling R, Chen I, Bousema T. 2018. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect Dis 18:627–639. doi: 10.1016/S1473-3099(18)30044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastiaens GJH, Tiono AB, Okebe J, Pett HE, Coulibaly SA, Gonçalves BP, Affara M, Ouédraogo A, Bougouma EC, Sanou GS, Nébié I, Bradley J, Lanke KHW, Niemi M, Sirima SB, d'Alessandro U, Bousema T, Drakeley C. 2018. Safety of single low-dose primaquine in glucose-6-phosphate dehydrogenase deficient falciparum-infected African males: two open-label, randomized, safety trials. PLoS One 13:e0190272. doi: 10.1371/journal.pone.0190272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tine RC, Sylla K, Faye BT, Poirot E, Fall FB, Sow D, Wang D, Ndiaye M, Ndiaye JL, Faye B, Greenwood B, Gaye O, Milligan P. 2017. Safety and efficacy of adding a single low dose of primaquine to the treatment of adult patients with Plasmodium falciparum malaria in Senegal, to reduce gametocyte carriage: a randomized controlled trial. Clin Infect Dis 65:535–543. doi: 10.1093/cid/cix355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JT, Lon C, Spring MD, Sok S, Chann S, Ittiverakul M, Kuntawunginn W, My M, Thay K, Rahman R, Balasubramanian S, Char M, Lanteri CA, Gosi P, Ubalee R, Meshnick SR, Saunders DL. 2017. Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: an open-label randomized trial. PLoS One 12:e0168702. doi: 10.1371/journal.pone.0168702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arroyo-Arroyo M, Arango E, Carmona-Fonseca J, Aristizabal B, Yanow S, Maestre A. 2017. Efficacy of different primaquine regimens to control Plasmodium falciparum gametocytemia in Colombia. Am J Trop Med Hyg 97:712–718. doi: 10.4269/ajtmh.16-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okebe J, Bousema T, Affara M, Di Tanna GL, Dabira E, Gaye A, Sanya-Isijola F, Badji H, Correa S, Nwakanma D, Van Geertruyden J-P, Drakeley C, D'Alessandro U. 2016. The gametocytocidal efficacy of different single doses of primaquine with dihydroartemisinin-piperaquine in asymptomatic parasite carriers in the Gambia: a randomized controlled trial. EBioMedicine 13:348–355. doi: 10.1016/j.ebiom.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Syafruddin D, Kusriastuti R, Hawley WA, Lobo NF, Ter Kuile FO. 2013. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South Sumatra, western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis 56:685–693. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- 26.Bancone G, Menard D, Khim N, Kim S, Canier L, Nguong C, Phommasone K, Mayxay M, Dittrich S, Vongsouvath M, Fievet N, Le Hesran J-Y, Briand V, Keomany S, Newton PN, Gorsawun G, Tardy K, Chu CS, Rattanapalroj O, Dong LT, Quang HH, Tam-Uyen N, Thuy-Nhien N, Hien TT, Kalnoky M, Nosten F. 2019. Molecular characterization and mapping of glucose-6-phosphate dehydrogenase (G6PD) mutations in the Greater Mekong Subregion. Malar J 18:20. doi: 10.1186/s12936-019-2652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khim N, Benedet C, Kim S, Kheng S, Siv S, Leang R, Lek S, Muth S, Chea N, Chuor CM, Duong S, Kerleguer A, Tor P, Chim P, Canier L, Witkowski B, Taylor WR, Menard D. 2013. G6PD deficiency in Plasmodium falciparum and Plasmodium vivax malaria-infected Cambodian patients. Malar J 12:171. doi: 10.1186/1475-2875-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dysoley L, Kim S, Lopes S, Khim N, Bjorges S, Top S, Huch C, Rekol H, Westercamp N, Fukuda MM, Hwang J, Roca-Feltrer A, Mukaka M, Menard D, Taylor WR. 2019. The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians. BMC Infect Dis 19:250. doi: 10.1186/s12879-019-3862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheng S, Muth S, Taylor WR, Tops N, Kosal K, Sothea K, Souy P, Kim S, Char CM, Vanna C, Ly P, Ringwald P, Khieu V, Kerleguer A, Tor P, Baird JK, Bjorge S, Menard D, Christophel E. 2015. Tolerability and safety of weekly primaquine against relapse of Plasmodium vivax in Cambodians with glucose-6-phosphate dehydrogenase deficiency. BMC Med 13:203. doi: 10.1186/s12916-015-0441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vantaux A, Samreth R, Piv EP, Khim N, Kim S, Berne L, Chy S, Lek D, Siv S, Taylor WR, Menard D. 2018. Contribution to malaria transmission of symptomatic and asymptomatic parasite carriers in Cambodia. J Infect Dis 217:1561–1568. doi: 10.1093/infdis/jiy060. [DOI] [PubMed] [Google Scholar]
- 31.Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo APP, Naing AL, Nyo MY, Myint NZH, Imwong M, Ashley E, Lee SJ, White NJ. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, Manjurano A, Kavishe RA, Beshir KB, Yussuf RU, Omar SA, Hermsen CC, Okell L, Schallig H, Sauerwein RW, Hallett RL, Bousema T. 2013. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis 207:1637–1645. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 34.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menéndez C, Nambozi M, Valéa I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A, D'Alessandro U. 2009. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One 4:e7871. doi: 10.1371/journal.pone.0007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onyamboko MA, Fanello CI, Wongsaen K, Tarning J, Cheah PY, Tshefu KA, Dondorp AM, Nosten F, White NJ, Day NP. 2014. Randomized comparison of the efficacies and tolerabilities of three artemisinin-based combination treatments for children with acute Plasmodium falciparum malaria in the Democratic Republic of the Congo. Antimicrob Agents Chemother 58:5528–5536. doi: 10.1128/AAC.02682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WWARN Gametocyte Study Group. 2016. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med 14:79. doi: 10.1186/s12916-016-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess RW, Bray RS. 1961. The effect of a single dose of primaquine on the gametocytes, gametogony and sporogony of Laverania falciparum. Bull World Health Organ 24:451–456. [PMC free article] [PubMed] [Google Scholar]
- 38.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White N, Ashley E, Recht J, Delves M, Ruecker A, Smithuis F, Eziefula A, Bousema T, Drakeley C, Chotivanich K, Imwong M, Pukrittayakamee S, Prachumsri J, Chu C, Andolina C, Bancone G, Hien T, Mayxay M, Taylor W, von Seidlein L, Price R, Barnes K, Djimde A, ter Kuile F, Gosling R, Chen I, Dhorda M, Stepniewska K, Guerin P, Woodrow C, Dondorp A, Day N, Nosten F. 2014. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J 13:483. doi: 10.1186/1475-2875-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery GM, Eyles DE. 1955. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am J Trop Med Hyg 4:781–789. doi: 10.4269/ajtmh.1955.4.781. [DOI] [PubMed] [Google Scholar]
- 41.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, Basáñez M-G. 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife 2:e00626. doi: 10.7554/eLife.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leang R, Khu NH, Mukaka M, Debackere M, Tripura R, Kheang ST, Chy S, Kak N, Buchy P, Tarantola A, Menard D, Roca-Felterer A, Fairhurst RM, Kheng S, Muth S, Ngak S, Dondorp AM, White NJ, Taylor WR. 2016. An optimised age-based dosing regimen for single low-dose primaquine for blocking malaria transmission in Cambodia. BMC Med 14:171. doi: 10.1186/s12916-016-0701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canier L, Khim N, Kim S, Sluydts V, Heng S, Dourng D, Eam R, Chy S, Khean C, Loch K, Ken M, Lim H, Siv S, Tho S, Masse-Navette P, Gryseels C, Uk S, Van Roey K, Grietens KP, Sokny M, Thavrin B, Chuor CM, Deubel V, Durnez L, Coosemans M, Ménard D. 2013. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J 12:405. doi: 10.1186/1475-2875-12-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawaly YR, Sakuntabhai A, Marrama L, Konate L, Phimpraphi W, Sokhna C, Tall A, Diène Sarr F, Peerapittayamongkol C, Louicharoen C, Schneider BS, Levescot A, Talman A, Casademont I, Menard D, Trape J-F, Rogier C, Kaewkunwal J, Sura T, Nuchprayoon I, Ariey F, Baril L, Singhasivanon P, Mercereau-Puijalon O, Paul R. 2010. Heritability of the human infectious reservoir of malaria parasites. PLoS One 5:e11358. doi: 10.1371/journal.pone.0011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. 2018. R: a language and environment for statistical computing. https://www.R-project.org/. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]