Probiotics might provide an alternative approach for the control of oral candidiasis. However, studies on the antifungal activity of probiotics in the oral cavity are based on the consumption of yogurt or other dietary products, and it is necessary to use appropriate biomaterials and specific strains to obtain probiotic formulations targeted for local oral administration. In this study, we impregnated gellan gum, a natural biopolymer used as a food additive, with a probiotic and investigated its antifungal activity against Candida albicans.
KEYWORDS: Candida albicans, Lactobacillus paracasei, gellan gum, hydrogels, oral candidiasis
ABSTRACT
Probiotics might provide an alternative approach for the control of oral candidiasis. However, studies on the antifungal activity of probiotics in the oral cavity are based on the consumption of yogurt or other dietary products, and it is necessary to use appropriate biomaterials and specific strains to obtain probiotic formulations targeted for local oral administration. In this study, we impregnated gellan gum, a natural biopolymer used as a food additive, with a probiotic and investigated its antifungal activity against Candida albicans. Lactobacillus paracasei 28.4, a strain recently isolated from the oral cavity of a caries-free individual, was incorporated in several concentrations of gellan gum (0.6% to 1% [wt/vol]). All tested concentrations could incorporate L. paracasei cells while maintaining bacterial viability. Probiotic-gellan gum formulations were stable for 7 days when stored at room temperature or 4°C. Long-term storage of bacterium-impregnated gellan gum was achieved when L. paracasei 28.4 was lyophilized. The probiotic-gellan gum formulations provided a release of L. paracasei cells over 24 h that was sufficient to inhibit the growth of C. albicans, with effects dependent on the cell concentrations incorporated into gellan gum. The probiotic-gellan gum formulations also had inhibitory activity against Candida sp. biofilms by reducing the number of Candida sp. cells (P < 0.0001), decreasing the total biomass (P = 0.0003), and impairing hyphae formation (P = 0.0002), compared to the control group which received no treatment. Interestingly, a probiotic formulation of 1% (wt/vol) gellan gum provided an oral colonization of L. paracasei in mice with approximately 6 log CFU/ml after 10 days. This formulation inhibited C. albicans growth (P < 0.0001), prevented the development of candidiasis lesions (P = 0.0013), and suppressed inflammation (P = 0.0006) compared to the mice not treated in the microscopic analysis of the tongue dorsum. These results indicate that gellan gum is a promising biomaterial and can be used as a carrier system to promote oral colonization for probiotics that prevent oral candidiasis.
INTRODUCTION
Candida albicans is a dimorphic commensal fungus that colonizes the oral mucosa of healthy individuals but can become pathogenic when the balance between the fungal colonization and host defense mechanisms is interrupted, leading to candidiasis (1–3). The development of oral candidiasis is associated with different predisposing factors, including the use of dental prostheses, inhaled corticosteroids, reduction of salivary flow, advanced age, nutritional deficiencies, prolonged administration of broad-spectrum antibiotics, and immunosuppression associated with antineoplastic treatments, hematological diseases, or AIDS (4–7). The continuous use of therapeutic agents to control infections caused by Candida spp. in these patients has contributed significantly to the development of antifungal-resistant strains (1, 8, 9). In addition, high cost, side effects, and drug-drug interactions can limit the use of antifungal agents (10).
In this context, probiotics have been studied as an alternative approach for the control of oral candidiasis. The activity of probiotic strains against pathogenic microorganisms is mediated through competition for binding sites, depletion of nutrients, production of organic acids, production of antimicrobial compounds, and stimulation of host immune cells (11, 12). Notably, probiotics are classified by the U.S. Food and Drug Administration (FDA) as live biotherapeutic products and can be applied as foods, dietary supplements, or medicines (13, 14), facilitating the implementation of probiotics in practice.
Although probiotics have the potential to control infectious diseases, most studies related to the antifungal activity of probiotics in the oral cavity are based on the consumption of yogurt or other dietary products containing Lactobacillus spp. and Bifidobacterium spp. (15–17). However, to effectively control oral infections, it is necessary to use appropriate biomaterials and specific strains to obtain probiotic formulations targeted for local administration (18). The development of these formulations has been considered a great challenge since probiotics are biological products whose viability can be influenced by a variety of complex physiological and chemical factors (19). In addition to the cell viability and storage stability of a probiotic product, other aspects need consideration in order to achieve medical applications, such as having formulations capable of providing sufficient exposure in terms of bacterial load and time course to promote probiotic population of the oral cavity in a way that can influence the resident microbiota (19, 20).
Faced with these hurdles, the development of probiotic delivery systems for targeted sites, including the oral cavity, remains a challenge. Therefore, there is a necessity for studies that focus on different materials for the encapsulation of probiotics. Gellan gum is a natural polysaccharide produced by members of the bacterial genus Sphingomonas and is widely used in the food, pharmaceutical, and medical industries for the encapsulation of cells, antibiotics, and microparticles (21–24). Recently, different types of gellan gum preparations have been developed, ranging from large microparticles and granules to liquid formulations (25, 26). Carrier systems using gellan gum present promising results since this biopolymer does not exhibit toxicity to the oral cavity and offers protection for the drug encapsulated in the carrier. For oral administration, the drug molecules can be protected within the gellan gum, preventing degradation by the digestive process and, consequently, facilitating concentration decrease (23, 27, 28).
Considering the characteristics of biodegradability, biocompatibility, and low cost, we used gellan gum to encapsulate Lactobacillus paracasei 28.4, a strain recently isolated from the oral cavity of a healthy individual without oral caries or other lesions (29). In preliminary studies, this strain showed significant capacity to inhibit the growth of C. albicans (29) and to protect the alternative model host Galleria mellonella against C. albicans infection (30, 31). In the present study, we developed gellan gum formulations containing the probiotic for local application in the oral cavity. Using in vitro assays, we found that these probiotic formulations were able to inhibit the growth of C. albicans. Importantly, these gellan gum formulations prevented the development of oral candidiasis in a murine model.
RESULTS
Development of probiotic formulations.
Initially, we studied the capacity of gellan gum to facilitate the delivery of L. paracasei 28.4 cells in a viable state that could maintain antifungal attributes. For this, the formulations were prepared by the addition of 108 cells/ml of L. paracasei 28.4 into different concentrations of gellan gum (1.0%, 0.9%, 0.8%, 0.7%, and 0.6% [wt/vol]). Immediately after preparation of the probiotic formulations, we recovered approximately 7 log CFU/ml of L. paracasei 28.4 from all gellan gum formulations and 8 log CFU/ml of L. paracasei from the control group (L. paracasei suspension not incorporated into gellan gum). Therefore, the recovery of L. paracasei from the formulations was only 1 log less than that in the control group lacking gellan gum, indicating that gellan gum at the tested concentrations allowed for bacterial release (see Fig. S1 in the supplemental material).
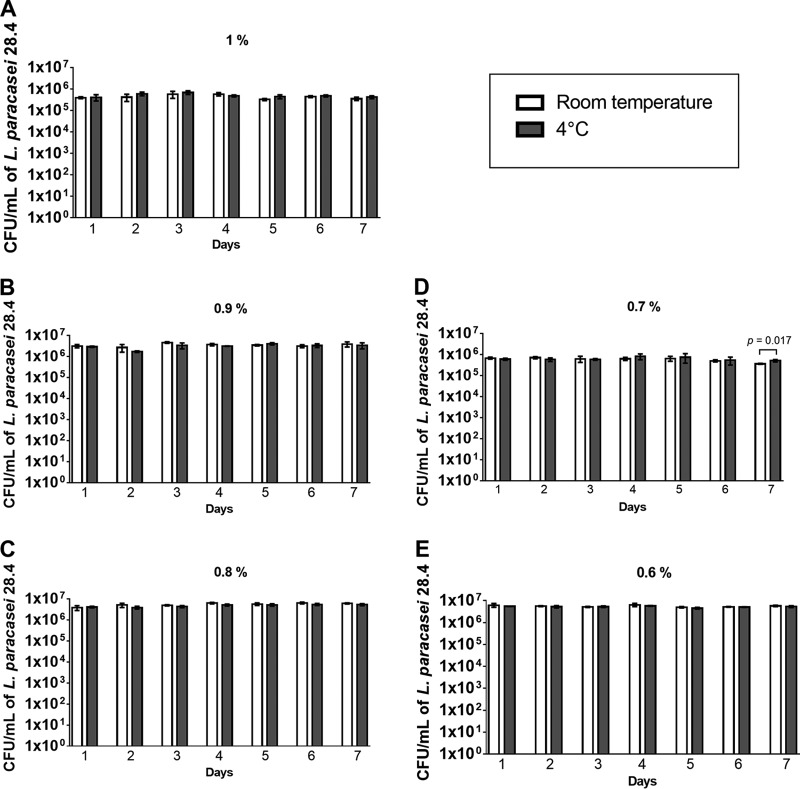
To verify the capacity of these formulations in maintaining the viability of L. paracasei 28.4, the quantity of viable cells recovered from the formulations was determined daily during a 7-day exposure period. We recovered approximately 6 log CFU/ml of L. paracasei from the gellan gum formulations for all periods of time, proving the maintenance of cell viability. In this experiment, the probiotic-impregnated gellan gum formulations were stored at room temperature or 4°C to evaluate the influence of temperature during storage periods. We verified that after 7 days of storage, the gellan gum maintained L. paracasei 28.4 viability independent of gellan gum concentrations or storage temperatures (Fig. 1).
FIG 1.
Recovery of L. paracasei 28.4 from the probiotic-gellan gum formulations after storage for 7 days. Shown are concentrations of L. paracasei 28.4 cells (CFU per milliliter) recovered from the probiotic formulations in gellan gum (1% to 0.6% [wt/vol]) after storage at room temperature and at 4°C. Student's t test was used to compare the experimental groups (room temperature × 4°C) for each day of the experiment.
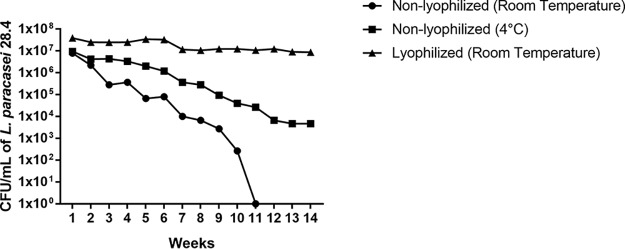
In a follow-up assay, we used probiotics incorporated into 1% gellan gum to monitor the cell viability over an extended period of time, comparing 3 different storage conditions, nonlyophilized at room temperature, nonlyophilized at 4°C, and lyophilized at room temperature. The nonlyophilized formulation at room temperature showed a reduction in viable L. paracasei 28.4 cells over time, with a total loss of viability after 11 weeks. The nonlyophilized formulation stored at 4°C also showed decreasing L. paracasei 28.4 viability over time; however, at the end of the experiment (14 weeks), approximately 4 log CFU/ml L. paracasei cells were still able to grow. However, when the probiotic formulation was lyophilized, L. paracasei 28.4 cells retained viability through the 14-week test period (Fig. 2). These results indicated that lyophilization allows L. paracasei 28.4 cell viability for at least up to 14 weeks.
FIG 2.
Recovery of L. paracasei 28.4 from the probiotic-gellan gum formulations after storage under different conditions for long periods of time. Shown are the concentrations of L. paracasei 28.4 cells (CFU per milliliter) recovered from the probiotic formulation of 1%, after 14 weeks, storage under the following conditions: nonlyophilized at room temperature, nonlyophilized at 4°C, or lyophilized at room temperature.
In addition, we evaluated the delivery system of the probiotic formulations in two different solutions, phosphate-buffered saline (PBS) and artificial saliva. We found that all gellan gum formulations (1% to 0.6%) were able to release L. paracasei 28.4 cells when deposited in either solution for 60 min. However, exposure to artificial saliva provided a higher release rate than that with the PBS suspension (Fig. 3), suggesting that artificial saliva supported L. paracasei 28.4 viability at least as well as PBS and facilitated the release of L. paracasei 28.4.
FIG 3.
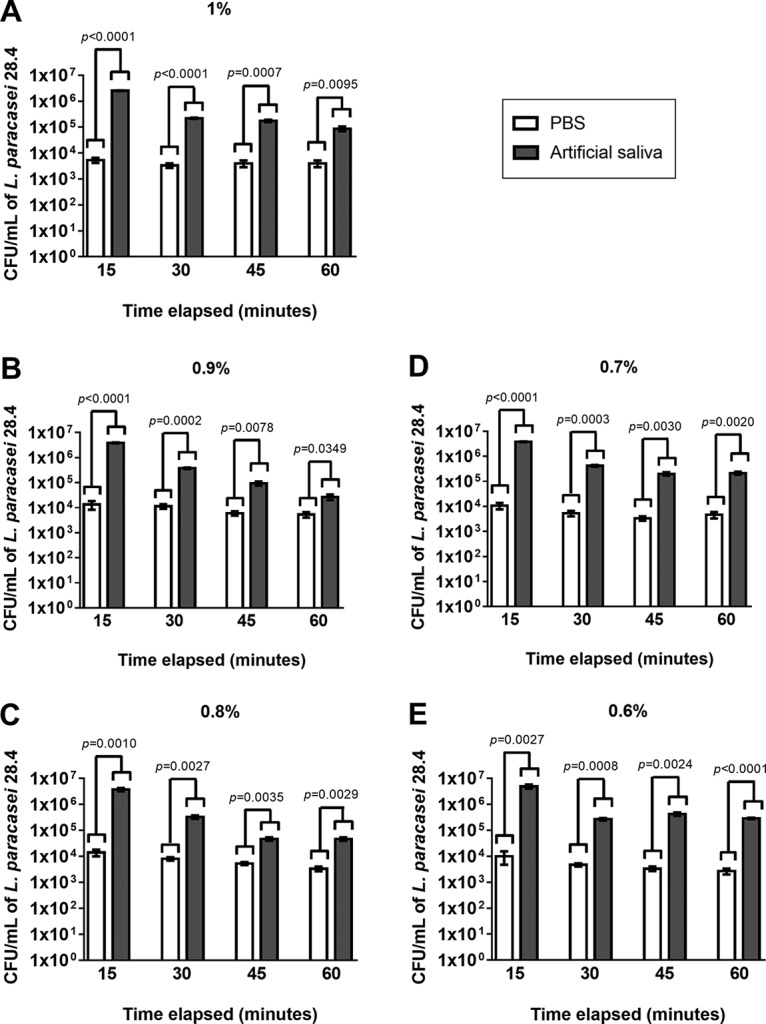
Evaluation of delivery system of the probiotic-gellan gum formulations. Shown are the concentrations of L. paracasei 28.4 cells (CFU per milliliter) released from the probiotic formulations (1 to 0.6% [wt/vol]) when in the presence of PBS or artificial saliva. The samples were followed and evaluated continuously from 15 to 60 min. Student's t test was used to compare the PBS and artificial saliva groups. OD570, optical density at 570 nm.
In vitro study of antifungal activity of probiotic formulations on C. albicans.
Since L. paracasei 28.4 cells were incorporated and maintained their viability in all gellan gum formulations (1% to 0.6%), we selected the formulations at 1% (more gelatinous consistency) and 0.6% (more liquid consistency) (Fig. S2) for further study. First, we investigated if the probiotic formulations were able to inhibit the growth of C. albicans (ATCC 18804) using the overlay agar method. For this, we tested the antifungal activity of probiotic formulations (1% and 0.6%) containing different concentrations of L. paracasei 28.4 (109 to 106 cells/ml) on C. albicans. We also performed a control group, where we only tested the gellan gum, without the presence of lactobacilli. Both formulations inhibited C. albicans growth dependent on L. paracasei 28.4 concentration, in which higher concentrations demonstrated larger zones of inhibition (Table 1), and the control group showed no inhibition against C. albicans growth. These results indicated that the cells of L. paracasei released from the gellan gum formulations into the culture medium maintained their antifungal activity against C. albicans.
TABLE 1.
Evaluation of antifungal activity using overlay agar method
| No. of cells/ml | Inhibition halo measurements (mean ± SD) (n = 6)a |
||
|---|---|---|---|
| L. paracasei 28.4 suspension (cm) | Probiotic-gellan gum formulation 1% (mm) | Probiotic-gellan gum formulation 0.6% (mm) | |
| 109 | 1.9 ± 0.12 A | 7 ± 0.71 A | 6.3 ± 0.61 A |
| 108 | 1.8 ± 0.16 A | 6.9 ± 0.66 A | 6.2 ± 0.68 A |
| 107 | 1.3 ± 0.58 B | 5.6 ± 0.66 B | 4.1 ± 0.38 B |
| 106 | 1 ± 0.16 B | 5 ± 0.63 B | 3.7 ± 0.41 B |
Different letters represent statistically significant differences between the groups, P < 0.05, ANOVA and Tukey’s test.
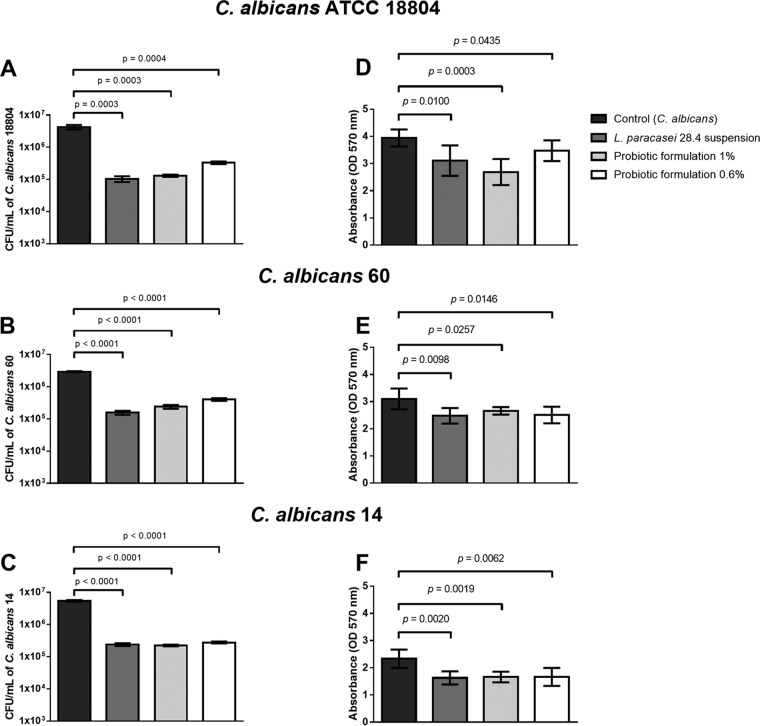
Probiotic-gellan gum formulations containing 1% or 0.6% gellan gum and 108 cells/ml of L. paracasei 28.4 were tested on biofilms formed by C. albicans strain ATCC 18804 and 2 clinical isolates (C. albicans isolates 60 and 14). Through cell counts, we verified that both probiotic-gellan gum formulations were able to inhibit the biofilm formed by all C. albicans isolates. A statistically significant reduction in the CFU per milliliter of C. albicans was observed in the groups pretreated with either probiotic-gellan gum formulations compared to the control group containing C. albicans without probiotic treatment (Fig. 4A to C). The CFU per milliliter of L. paracasei 28.4 was also determined, showing values between 106 to 107 cells/ml, confirming that L. paracasei cells were released from the gellan gum during the biofilm formation assay (Fig. S3).
FIG 4.
Analysis of C. albicans in vitro biofilms. Biofilms were formed in contact with L. paracasei suspension or probiotic-gellan gum formulations at 1 and 0.6%. (A to C) Numbers of C. albicans viable cells determined by CFU per milliliter count. (D to F) Values of optical density obtained in the biofilm biomass analysis. Student's t test was used to compare the control group with the experimental groups.
The study of biofilms was complemented with the crystal violet assay, in which we also found a reduction in C. albicans biofilm to be associated with pretreatment with probiotic formulations (Fig. 4D to F). Interestingly, the reduction in C. albicans cells (measured as CFU per milliliter) and the total biomass (measured by absorbance) reached by the probiotic were not hindered by delivery via the gellan gum formulations. Therefore, probiotic formulations at 1% or 0.6% (wt/vol) gellan gum provided a delivery system of L. paracasei 28.4 sufficient to inhibit C. albicans biofilm formation.
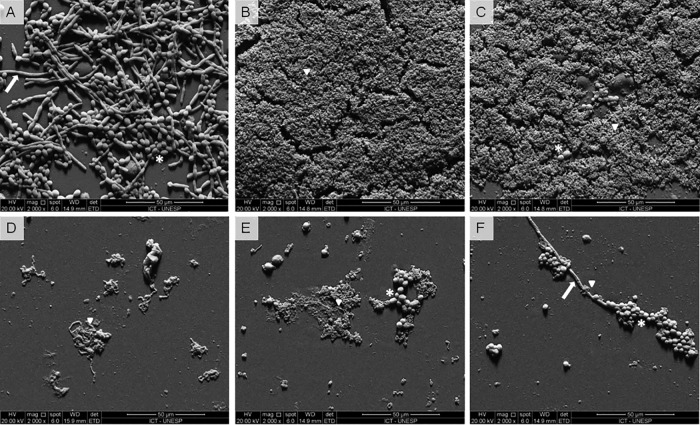
To verify the structural characteristics of biofilms, we used scanning electron microscopy (SEM) to visualize biofilms formed on the surface of acrylic resin. As expected, the group formed only by C. albicans (untreated control) showed a mature biofilm with the presence of numerous yeast cells and filaments (Fig. 5A). We also tested a group composed of an L. paracasei 28.4 suspension only (without C. albicans) showing that L. paracasei 28.4 cells were able to form biofilms with a large number of cells adhered on the resin surface (Fig. 5B). When the biofilms of C. albicans were pretreated with the L. paracasei 28.4 suspension, we found only rare C. albicans cells adhering to the resin and with no filament formation (Fig. 5C). In testing the effects of the probiotic-gellan gum formulation on the resin surface without C. albicans biofilms, L. paracasei 28.4 cells were observed, indicating that bacterial cells were released from the gellan gum and accumulated on the resin surface (Fig. 5D). L. paracasei 28.4 cells were also observed within C. albicans biofilms pretreated with probiotic formulations (1% and 0.6%); however, in these groups, the most significant characteristic was the reduction of yeast cells and filaments that adhered to resin, compared to the group of C. albicans biofilm that lacked probiotic treatment (Fig. 5E and F).
FIG 5.
(A to F) Scanning electron microscopy images of biofilms formed in vitro on resin surfaces, as follows: biofilms formed by only C. albicans (ATCC 18804) (A), biofilms formed by only L. paracasei 28.4 suspension (B), C. albicans biofilms pretreated with L. paracasei 28.4 suspension (C), biofilms formed only by probiotic formulation at 1% (D), C. albicans biofilms pretreated with probiotic formulation at 1% (E), and C. albicans biofilms pretreated with probiotic formulation at 0.6% (F). It is possible to observe the presence of L. paracasei cells (▾), yeasts (*), and hyphae (→) of C. albicans.
Study of probiotic formulations in a mouse model of oral candidiasis.
Taken in their totality, the results obtained in the in vitro assays showed that probiotic-gellan gum formulations at 1% and 0.6% provided a delivery system of L. paracasei 28.4 capable of inhibiting the growth, biofilm formation, and filamentation of C. albicans. Before examining the effects of probiotic-gellan gum formulations on oral candidiasis, we analyzed the L. paracasei 28.4 delivery and exposure to the oral microbiome. The probiotic-gellan gum formulation at 1% gellan gum was able to provide oral colonization in mice for 10 days, reaching the same cell viability found in the mice inoculated with a fresh L. paracasei suspension (7 log CFU/ml) (Fig. 6A). The recovered colonies were submitted for molecular analysis, and the results confirmed that they were L. paracasei (Fig. S6). However, it was not possible to recover L. paracasei 28.4 cells from the oral cavity when the animals were treated with the probiotic-gellan gum formulation at 0.6% gellan gum (data not shown), demonstrating that gellan gum concentration was a crucial factor for the L. paracasei 28.4 delivery system in the oral cavity of mice.
FIG 6.
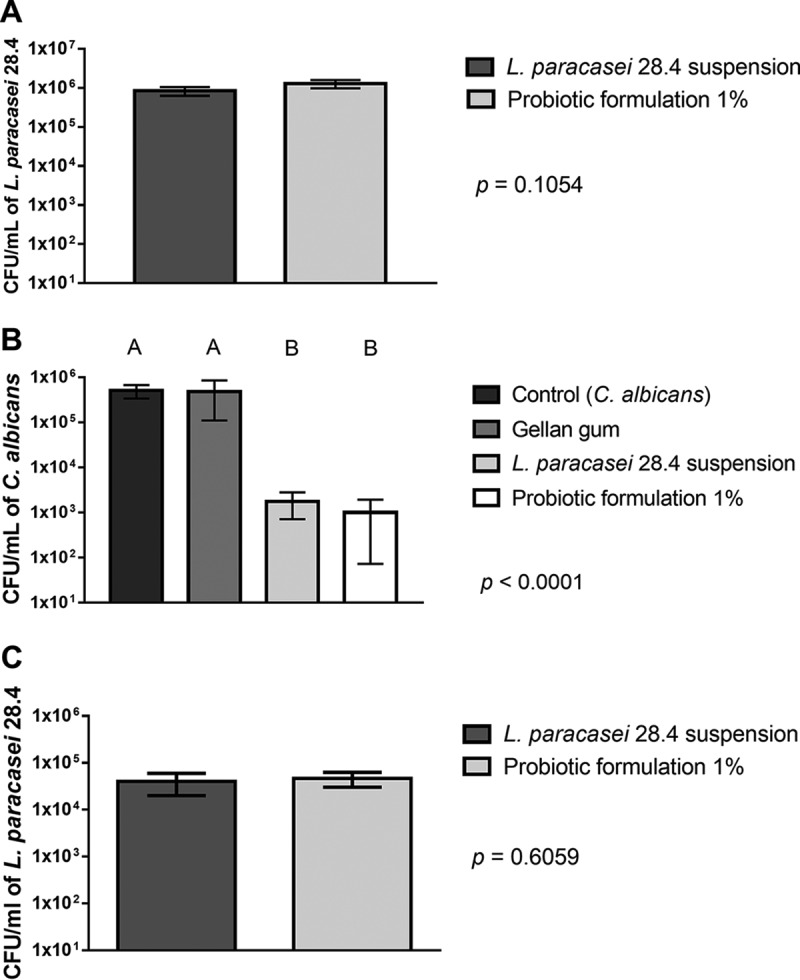
Recovery of C. albicans or L. paracasei from the oral cavity of mice. (A) Evaluation of delivery system of the probiotic-gellan gum formulation in the oral cavity of healthy mice. Concentration of L. paracasei 28.4 (CFU per milliliter) recovered after 10 days from oral cavity of mice treated with L. paracasei 28.4 suspension and probiotic formulation of 1%. Student's t test was used to compare the experimental groups. (B) C. albicans CFU/milliliter recovered from the oral cavity of mice infected by C. albicans and pretreated with PBS (control group), gellan gum formulation of 1% not incorporated with L. paracasei (gellan gum group), and L. paracasei 28.4 suspension or probiotic-gellan gum formulation of 1% (ANOVA and Tukey’s test). (C) L. paracasei 28.4 CFU per milliliter recovered from the mice infected by C. albicans and pretreated with L. paracasei 28.4 suspension or probiotic formulation of 1% (Student's t test).
Based on findings from the in vitro data, we examined the probiotic-gellan gum formulation at 1% gellan gum using an oral mouse candidiasis. To exclude the possibility that gellan gum interferes with oral candidiasis, a group of mice infected with C. albicans received gellan gum without L. paracasei. This group did have an alteration in the number of C. albicans cells recovered, confirming that gellan gum was unable to reduce or stimulate C. albicans growth (Fig. 6B). We also determined the number of L. paracasei 28.4 cells recovered from the oral cavity, verifying that L. paracasei cells were capable of colonizing the oral cavity of mice until the end of the experiment (15 days) (Fig. 6C).
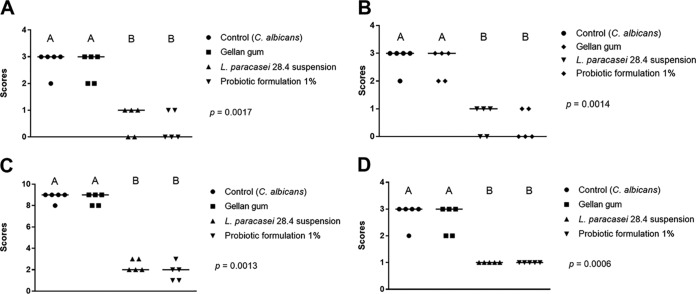
Animals pretreated with the probiotic formulation or L. paracasei 28.4 suspension had approximately a 3-log CFU/ml reduction in the number of C. albicans cells compared to the control group of mice that were not pretreated with probiotic. Interestingly, the control group showed lesions characteristic of pseudomembranous candidiasis in the dorsum tongue, while the groups treated with the probiotic-gellan gum formulation or a fresh suspension of L. paracasei 28.4 presented a significant reduction in the candidiasis lesions compared to the control group (Fig. 7A and 8A).
FIG 7.
Quantifications of candidiasis lesions formed on the dorsum tongue of mice infected with C. albicans. Mice were pretreated with PBS (control group), 1% gellan gum formulation without L. paracasei (gellan gum group), and L. paracasei 28.4 suspension with 1% probiotic-gellan gum formulation. (A to D) Scores and median obtained in the quantification of macroscopic lesions (A), count of yeasts and hyphae in histological cuts stained by PAS (B), and epithelial lesions (C) and inflammatory infiltrate (D) determined in H&E cuts (Kruskal-Wallis and Dunn’s posttest; different letters correspond to the statistically significant differences between groups).
FIG 8.
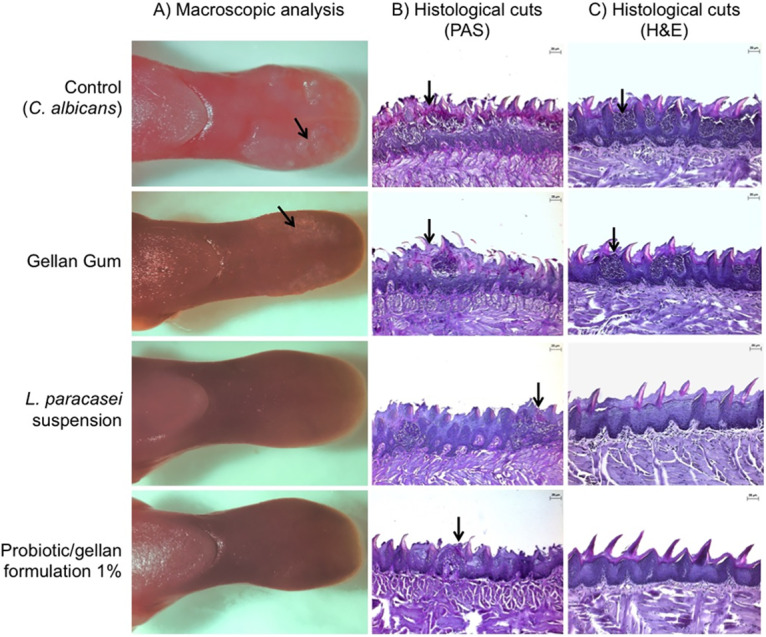
Analysis of the dorsum tongue of mice infected with C. albicans. The mice were pretreated with PBS (control group, top row), a 1% gellan gum formulation without L. paracasei (gellan gum group, second row), L. paracasei 28.4 suspension in 1% (third row), and a probiotic-gellan gum formulation (bottom row). (A) Macroscopic aspects of the dorsum tongue are the presence of white patches of candidiasis (→) in the groups not treated with L. paracasei. (B) Images of histological cuts stained by periodic acid-Schiff (PAS), where hyphae and yeast can be observed in the epithelium-keratinized layer (→). (C) Images of histological cuts stained by hematoxylin and eosin (H&E), where intraepithelial microabscesses and infiltrate inflammatory in the connective tissue were observed (→).
After euthanasia, the tongues of the 40 animals were submitted to microscopic analysis, in which the control group showed a great number of yeast and hyphae located in the keratinized layer, as well as several epithelial lesions and intense infiltrate inflammation. These lesions were characterized by a loss of papillae, desquamation, acantholysis, tissue hyperplasia, spongiosis, and intraepithelial microabscesses. In comparison, the animals pretreated with probiotic-gellan gum formulations or a fresh suspension of L. paracasei 28.4 presented fewer yeast/hyphae, epithelial lesions, and inflammatory filtrate (Fig. 7B to D and 8B and C). Taken together, the study using the oral candidiasis mouse model demonstrated that the probiotic formulation with 1% gellan gum was able to provide local delivery of L. paracasei 28.4 in the oral cavity that resulted in oral colonization, C. albicans reduction, and, importantly, diminished oral candidiasis lesions and fungal accumulation.
DISCUSSION
In this study, we used gellan gum as a carrier system to deliver L. paracasei 28.4 to the oral cavity, aiming to inhibit C. albicans growth and, consequently, the development of candidiasis. First, we defined a gellan gum composition capable of incorporating L. paracasei 28.4 cells while maintaining bacterial viability and releasing L. paracasei 28.4 once in appropriate solution. We found that formulations containing gellan gum concentrations ranging from 0.6% to 1% (wt/vol) were able to incorporate L. paracasei 28.4, as well as maintain cell viability for 7 days when stored at room temperature or 4°C. However, extended storage periods required lyophilization of the probiotic impregnated gellan gum. Interestingly, the released bacteria inhibited C. albicans growth and biofilm formation. Finally, interrogation of the probiotic-loaded gellan gum in a murine infection model confirmed the ability of oral colonization by L. paracasei sufficient to prevent C. albicans growth and symptomatic lesions.
To our knowledge, this is the first study that used gellan gum to incorporate Lactobacillus sp. cells targeted for the control of oral candidiasis. Although probiotics have been incorporated into a range of products (including alginate, chitosan, whey proteins, and cellulose acetate phthalate [32]), gellan gum was chosen as a nontoxic natural polysaccharide approved by the U.S. Food and Drug Administration (FDA) as a food additive with great potential to be employed by pharmaceutical industries in the encapsulation of antibiotics and cells (21–23). Gellan gum can provide physically cross-linked hydrogels that are mechanically robust in comparison to other polysaccharides used in pharmaceutical formulations (24).
In this work, we employed the recently identified antifungal properties of L. paracasei 28.4, an isolate acquired from the oral cavity (29). The use of this orally derived probiotic is an advantage in relation to other probiotic strains isolated from the human or animal gut (33). More specifically, in our previous studies, we showed that the L. paracasei 28.4 strain has inhibitory activity on the growth of C. albicans, resulting in decreased expression of virulence genes (ALS3, HWP1, EFG1, and CPH1), biofilm deterrence, and retardation of hyphal formation (29). The antifungal effects of L. paracasei strain 28.4 were also confirmed using invertebrate model hosts (29–31, 34). Rossoni et al. (30) demonstrated that L. paracasei 28.4 was able to prolong the survival of G. mellonella larvae infected with a lethal dose of C. albicans. This effect was correlated with the capacity of L. paracasei to stimulate the immune system of G. mellonella by the recruitment of circulating hemocytes (insect defense cells) and production of elevated levels of antifungal peptides (30). de Barros et al. (34) also found a protective effect of L. paracasei 28.4 against in vivo candidiasis using a Caenorhabditis elegans model, in which the prolonged survival was accompanied by the inhibition of C. albicans filamentation. Also, Santos et al. (31) observed that the protective effects of L. paracasei 28.4 against C. albicans extended to non-albicans Candida species, including Candida glabrata, Candida krusei, and Candida tropicalis.
We demonstrate in this report that L. paracasei 28.4 cells can be incorporated into gellan gum, be released, and retain antifungal efficacy. The physical retention of cells in the hydrogel matrix depends on the encapsulation technology used, including biomaterial selection (32), process parameters (35), and the biocompatibility of polymer with the bacteria strain (36). The results obtained showed that gellan gum can incorporate L. paracasei 28.4 at consistencies ranging from liquid (0.6%) to solid (1%) formulations. In general, the encapsulation process has been associated with a decrease in cell viability of probiotic strains (37, 38). Falco et al. studied the incorporation of Lactobacillus acidophilus LA5 into chitosan-dextran sulfate hydrogel, using a layer-by-layer technique, and found a reduction of around 2 to 3 log in the quantity of viable cells after 3 h (37). Immediately after the incorporation of L. paracasei 28.4 into gellan gum formulations, we verified that the encapsulation process reduced only 1 log of viable cells.
Various external factors may impact the survival capacity of probiotic strains during storage, like time, temperature, and moisture content. In this study, gellan gum formulations showed a similar number of viable cells when stored at room temperature (22 to 25°C) or 4°C for up to 6 days. However, the impact of storage temperatures on cell viability became more evident when the probiotic formulations were stored for an extended period, in which the room temperature preserved the cell viability over 11 weeks, while the storage at 4°C maintained the cell viability over 14 weeks. Another way to preserve probiotic cells is reducing the available water content through freeze-drying (39). Therefore, in this study, we also tested lyophilized probiotic-gellan gum formulation at 1% (wt/vol) gellan gum (freeze-dried) to evaluate the capacity for long-term storage at room temperature. Interestingly, L. paracasei 28.4 maintained viability until the end of the assay period (14 weeks) with retained antifungal efficacy. Taken in their totality, these findings indicate that the drying of probiotic-gellan gum formulations can increase the stability and shelf-life of the finished pharmabiotic product. In addition, an immobilized environment provided by biocompatible materials offers protection to probiotic cells during rehydration (32).
When probiotics are encapsulated in biomaterials, it is essential to ensure that the cells are released in medium that simulates biological fluids (32). We found that the delivery system of L. paracasei cells provided by the gellan gum formulations in artificial saliva was more efficient than that in the PBS controls. Possibly, these effects occurred by the interaction of salivary enzymes present in the artificial saliva with the gellan gum hydrogel since the saliva used in this study contains glucose oxidase, lactoperoxidase, and lysozyme (Biotène dry mouth oral rinse). Polysaccharide hydrogels can undergo enzymatic hydrolysis in the presence of lysozyme (40). This enzyme breaks ether bonds connecting the structural backbone of different types of hydrogels (40). The enzymatic activity of lysozyme on gellan gum formulations was studied by Xu et al. (40), who found a degradation rate that was highly dependent on lysozyme concentration.
During the encapsulation process, probiotic cells can suffer damage in their structure and function (32, 35, 41). Thus, we performed several in vitro tests in order to confirm that L. paracasei 28.4 maintained its antifungal activity after incorporation into the gellan gum formulations. The results showed that C. albicans was inhibited by L. paracasei 28.4 is a dose-dependent manner, confirming that L. paracasei 28.4 maintained antifungal activity during the encapsulation process. We also verified that L. paracasei 28.4 incorporated in gellan gum demonstrated antifungal activity on C. albicans biofilms. Although several studies have shown the inhibitory effects of probiotics on Candida sp. biofilms, few studies were conducted with Lactobacillus sp. cells incorporated in gellan gum.
Importantly, we verified that the pretreatment with the probiotic-gellan gum formulation at 1% in mice infected by C. albicans was able to reduce the fungal colonization in the oral cavity compared to that in untreated animals. Only the probiotic formulation at 1% (wt/vol), and not the 0.6% (wt/vol), formulation, was able to establish an oral colonization of L. paracasei 28.4. These results indicate that although the concentrations of gellan gum in the formulations did not interfere with its properties of incorporation, maintenance of cell viability, or L. paracasei 28.4 release, the gellan gum concentration was a determinant factor for the oral colonization in mice. These results may be explained by the retention time of the probiotic formulation in the oral cavity influenced by the gellan gum concentration.
We cannot say how long gellan gum delivery material was retained in the mouse oral cavity. Our present goal was to determine if L. paracasei 28.4 was transferred from the gellan gum vehicle to the mouth in a means that promoted release and population. At this time, we are only able to report the release of L. paracasei from the gum, but we do not know the time interval required for release and population to occur. Also, we analyzed the quantity of L. paracasei 28.4 in the oral cavity of treated mice, verifying that their cells remained viable until the end of the experiment, and the inhibitory effect of probiotic formulation at 1% (wt/vol) gellan gum on C. albicans growth protected the mice from oral candidiasis.
In summary, the results of this study show that gellan gum is an effective biomaterial carrier for L. paracasei strain 28.4. The properties of gellan gum permit encapsulation of the Lactobacillus cells, maintain the cell viability during storage, and provide a facile delivery system to the oral cavity sufficient to interact with oral pathogens. Importantly, a gellan gum-L. paracasei preparation resulted in a significant reduction of C. albicans in the oral cavity of mice. Further, the use of probiotic-gellan gum formulations presents an alternative approach against oral infections and especially oral candidiasis.
MATERIALS AND METHODS
Microorganisms and growth conditions.
The following strains of microorganisms were used: (i) L. paracasei 28.4, a clinical isolate from the oral cavity of a caries-free individual (29); (ii) C. albicans ATCC 18804; and (iii) two clinical isolates of C. albicans, C. albicans 60 (sensitive to fluconazole) and C. albicans 14 (resistant to fluconazole), from oropharyngeal candidiasis of HIV-positive patients (42). All C. albicans isolates were cultured in yeast extract-peptone-dextrose (YPD) broth (Difco, Detroit, MI, USA) for 24 h at 37°C, and L. paracasei 28.4 was grown on Lactobacilli de Man-Rogosa-Sharpe (MRS) agar (Himedia, Mumbai, India) for 48 h at 37°C (5% CO2).
Development of probiotic-gellan gum formulations.
(i) Preparation of gellan gum formulations containing L. paracasei 28.4. We prepared the bacteria according to the methodology previously described by Coutinho et al. (21), with some modifications. An overnight L. paracasei 28.4 culture on MRS broth was centrifuged and washed with PBS solution, followed by standardization using a spectrophotometer (AJX-1900; Micronal, São Paulo, Brazil) at 600 nm to reach an optical density (OD) of 1.2, which corresponded to a concentration of 108 cells/ml of L. paracasei 28.4.
Gellan gum formulations were prepared in different hydrogel concentrations (1.0, 0.9, 0.8, 0.7, and 0.6% [wt/vol]). To achieve the designated concentrations, the gellan gum powder was added to distilled water and stirred at 85 to 90°C. The temperature was then reduced to 40°C, and 2 ml of L. paracasei 28.4 suspension containing 108 cells/ml was added into the gellan gum mixture, resulting in a probiotic formulation at a final concentration of L. paracasei 28.4 of 106 cells/ml. Finally, CaCl2 (1 mM) was added to the mixture of gellan gum and L. paracasei to promote gelation. All experiments were performed after adding CaCl2 (1 mM).
(ii) Recovery of L. paracasei 28.4 from the gellan gum formulations. The quantification of viable cells of L. paracasei 28.4 from the gellan gum formulations was performed according to the methodology described by Juarez Tomás et al. (43), with some modifications. In brief, immediately after the incorporation of L. paracasei 28.4, a 1-ml aliquot of each formulation was suspended in 9 ml of PBS, followed by stirring at room temperature. Subsequently, 1 ml of the hydrogel-bacterium suspension was serially diluted using PBS and plated on MRS agar plates. Plates were incubated at 37°C with 5% CO2 for 48 h to determine the CFU per milliliter. As a control, we used suspensions of L. paracasei 28.4 in PBS, at the same concentration of 108 cells/ml, that were not incorporated into the gellan gum.
(iii) Recovery of L. paracasei 28.4 from gellan gum formulations after storage. After preparation, gellan gum formulations impregnated with L. paracasei 28.4 were stored at room temperature or under refrigeration (4°C). After 24 h, a 1-ml aliquot of each bacterium-impregnated gellan gum formulation (0.6%, 0.7%, 0.8, 0.9%, and 1.0% [wt/vol]) was suspended in 9 ml of PBS and agitated via stirring at room temperature for 10 min at 75 rpm. Subsequently, this suspension was serially diluted, plated on MRS agar, and incubated at 37°C for 48 h to determine the number of CFU per milliliter. This procedure was repeated daily for 7 days and compared with an aliquot of L. paracasei 28.4 that was not impregnated into gellan gum.
(iv) Recovery of L. paracasei 28.4 from gellan gum formulations after extended storage. We studied 1% gellan gum formulations to evaluate the viability of L. paracasei 28.4 after the extended storage. In this series of studies, the probiotic-infused gellan gum material was stored under 3 different conditions, room temperature, under refrigeration (4°C), and lyophilized (Terroni LS 3000, São Carlos, São Paulo, Brazil) at room temperature. After 1 week, a 1-ml aliquot of various test formulations was suspended in 9 ml of PBS, followed by stirring at room temperature for 10 min at 75 rpm. Subsequently, 1 ml was plated on MRS agar and incubated at 37°C for 48 h. This process was repeated once weekly over the course of 3 months.
(v) Evaluation of delivery system of the gellan gum formulations. The quantification of viable cells of L. paracasei 28.4 released from the formulations was evaluated using biopsy bags (0.2-mm mesh diameter; Thermo Fisher Scientific, Waltham, MA, USA). A 1-ml aliquot of each formulation was placed into biopsy bags and then transferred to tubes containing 9 ml of PBS or 9 ml of artificial saliva (Biotène dry mouth oral rinse; GSK, Middlesex, UK). The tubes were allowed to stand for 15 min and were vortexed for 10 s. The biopsy bags were then transferred to fresh tubes containing 9 ml of PBS or artificial saliva and allowed to stand again for another 15 min. This process was repeated for a total time of 1 h. After removal of the biopsy bag, a 100-μl aliquot was taken from each tube, and serial dilutions were plated on MRS agar and incubated for 48 h at 37°C (5% CO2) to determine the number of CFU per milliliter.
In vitro antifungal activity of probiotic-gellan gum formulation.
(i) Evaluation of antifungal activity using the overlay agar method. The overlay agar method was performed according to the methodology described by Son et al. (44), with some modifications. In brief, 5 μl of each probiotic-gellan gum formulation was pipetted at equidistant points, on MRS plates, which were then incubated for 24 h in a 5% CO2 at 37°C to detect the growth of lactobacilli. For this assay, we also used probiotic-gellan gum formulations containing different concentrations of L. paracasei 28.4 (109 to 106 cells/ml).
A suspension containing 106 cells/ml of C. albicans in PBS was prepared. An aliquot of 1 ml of C. albicans suspension was transferred to a tube containing 9 ml of Sabouraud agar at 55°C. This solution was vortexed and poured onto the surface of plates containing the growth of colonies from lactobacilli cells on MRS agar previously incubated in 5% CO2 at 37°C for 24 h. The plates were incubated again at 37°C for 24 h. After this period, the zone of inhibition was measured. A fresh suspension of L. paracasei 28.4 (108 cells/ml) and a gellan gum lacking probiotic were used as controls.
(ii) Analysis of antifungal activity on biofilms by determination of the number of C. albicans and L. paracasei viable cells. The biofilm assays were performed following an assay previously described by Vilela et al. (45). In brief, biofilms were formed on acrylic resin discs that were 2 mm in thickness and 5 mm in diameter. The discs were placed in 24-well microtiter plates (Kasvi, Curitiba, Brazil) containing 1.5 ml of 70% yeast nitrogen base (YNB; Difco, Detroit, MI, USA) supplemented with 100 mM glucose and 30% brain heart infusion (BHI) broth. An aliquot of 250 μl of the probiotic-impregnated 1% or 0.6% (wt/vol) gellan gum formulation was added to each well. The plates were incubated for 24 h at 37°C under 5% CO2 for the release and growth of lactobacilli. In the control wells, only a suspension of L. paracasei 28.4 was added.
After 24 h, the probiotic-gellan gum formulations were removed from the wells and 250 μl of C. albicans suspension (107 cells/ml) was added. To facilitate the formation of C. albicans biofilm, the plates were incubated with agitation at 75 rpm (Quimis, Diadema, Brazil) for 24 h at 37°C. After incubation, the resin discs were washed 3 times and transferred to a tube containing 1 ml of a NaCl solution. Biofilms were detached using an ultrasonic homogenizer (Sonopuls HD 2200; Bandelin Electronic, Berlin, Germany) at 7 W for 30 s. The suspensions were serially diluted, and aliquots were plated on Sabouraud dextrose agar with chloramphenicol to detect the number of viable C. albicans cells remaining. An additional aliquot was plated on MRS agar to visualize L. paracasei 28.4 viable cells and determine the number of CFU per milliliter.
(iii) Evaluation of antifungal activity on biofilms by quantification of the total biomass. The biofilm assays were performed using 96-well plates, according to the study of Vilela et al. (45). An aliquot of 100 μl of the probiotic-gellan gum formulation was added to each well. In the control wells, only a suspension of L. paracasei 28.4 was used. In each well, we also added 100 μl of 70% YNB supplemented with 100 mM glucose and 30% BHI broth. The plates were incubated for 24 h at 37°C under 5% CO2 to allow for the release and growth of L. paracasei 28.4 cells. After 24 h, the formulations were removed from the wells, and 100 μl of C. albicans suspension (107 cells/ml) was added. The plates were incubated for 24 h at 37°C to allow C. albicans biofilm formation. Then, 100 μl of 99% methanol (Sigma-Aldrich, St. Louis, MO, USA) was added to the wells, and after 15 min, the supernatants were removed, and the plates were air dried.
The total biomass was quantified using a crystal violet assay, as described by Rossoni et al. (29). Each well was treated with 100 μl of 1% crystal violet (CV) solution for 20 min. The residual CV solution was removed by washing with PBS. Bound CV was released by adding 150 μl of 33% acetic acid (Sigma-Aldrich, St. Louis, MO, USA), and the absorbance at 570 nm was measured (AJX-1900 spectrophotometer, Micronal, São Paulo, Brazil) to determine the total amount of biofilm dyed with CV.
(iv) Analysis of biofilms by scanning electron microscopy. Biofilms formed on resin discs were fixed in 1 ml of 2.5% glutaraldehyde for 1 h. Specimens were then dehydrated serially using increasing concentrations of ethanol (10%, 25%, 50%, 75%, and 90%) for 20 min each, followed by immersion in 100% ethanol for 1 h. The plates were kept in an incubator at 37°C for 24 h to permit total drying of the specimens. After drying, the specimens were transferred to aluminum stubs and sputter coated with gold for 160 s at 40 mA (vacuum desk II; Denton Vacuum LLC, Moorestown, NJ, USA). The specimens were examined and imaged using a JSM-5600 scanning electron microscope (JEOL USA, Inc., Peabody, MA).
(v) Effects of probiotic-gellan gum formulations on C. albicans filamentation. In a 24-well culture plate, 1 ml of deionized water was mixed with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA) and 100 μl of the standardized C. albicans suspension (107 cells/ml). Next, 50 μl of the probiotic-gellan gum formulation (1.0% or 0.6% [wt/vol]) was placed in each well. In the control groups, 50 μl of PBS or 50 μl of the hydrogel without L. paracasei 28.4 was placed in each well, depending on the experimental group. Then, the plate was incubated at 37°C under for 24 h. After incubation, 50 μl of the inoculum was transferred to glass slides with 10 previously demarcated fields on the back of the slide and observed under a light microscope at ×400 magnification. The images were analyzed, and 10 microscopic fields per slide were chosen for the quantification of hyphae. We scored hyphal formation according to criteria previously published by Ribeiro et al. (46). Then, 10 microscopic fields were analyzed per slide, and a score (0 to 5) was attributed to each field according to the number of hyphae observed, as follows: 0, no hyphae; 1, 1 to 10 hyphae; 2, 11 to 20 hyphae; 3, 21 to 30 hyphae; 4, 31 to 40 hyphae; and 5, more than 41 hyphae.
Study of probiotic-gellan gum formulations using a mouse model.
We used adult Swiss mice, weighing approximately 30 g, from the Central Animal Care Facility of UNESP (Botucatu, São Paulo, Brazil). All experiments were performed following the guidelines and approval of the Animal Research Ethics Committee of the Institute of Science and Technology (ICT/UNESP) (protocol 15/2016). Only male mice were used in order to avoid the influence of hormonal status on oral candidiasis, as per a study by Junqueira et al. (47). A total of 80 mice were used and divided, such that 40 were healthy mice not infected by C. albicans and 40 were mice infected by C. albicans.
(i) Evaluation of probiotic-gellan gum formulation in the oral cavity of healthy mice. To evaluate the effects of probiotic-gellan gum formulations in the oral cavity of healthy mice, 1% (n = 10) or 0.6% gellan gum (n = 10) solutions impregnated with L. paracasei 28.4 were administered to the mice with a pipette (100 μl) once a day for 10 days. The control groups were treated by the administration of L. paracasei suspension (n = 10) or hydrogel lacking probiotic (n = 10) for the same period of time. At 24 h after the last administration, saliva samples from the oral cavity of all mice were collected by washing the animal oral cavity using 100 μl of sterile PBS and collecting back 80 μl of the liquid suspension. The samples were placed in a tube containing 920 μl of PBS for serial dilutions. Next, aliquots of the dilutions were inoculated into Rogosa agar plates (Difco, BD, NJ, USA) containing glacial acetic acid, in duplicate. The plates were incubated at 37°C for 48 h (5% CO2) and the CFU counted.
The saliva samples were also used for confirmation of the species belonging to the L. paracasei group by PCR. The chromosomal DNA of a colony was extracted using the PureLink genomic DNA kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions for PCR amplification of the intergenic segment between the 16S and 23S rRNA, as described by Song et al. (48). For this experiment, a sample of L. paracasei 28.4 was included as positive control, and a sample of Lactobacillus rhamnosus ATCC 9595 was included as a negative control.
(ii) Evaluation of probiotic-gellan gum formulation in the oral cavity of mice infected by C. albicans. Animals received probiotic-gellan gum formulation 1.0% (n = 10), L. paracasei 28.4 suspension (n = 10), hydrogel lacking L. paracasei (n = 10), or PBS solution (n = 10). These applications were performed once a day for 10 days.
For the induction of oral candidiasis, animals were immunosuppressed with the administration of 3 subcutaneous injections of prednisolone (Depo-Medrol; Laboratorios Pfizer Ltd., Guarulhos, São Paulo, Brazil) at a dose of 100 mg/kg of body weight on days alternating with the inoculation of the C. albicans 60 strain. During C. albicans inoculation, animals were sedated, and a swab previously soaked in a standard fungal suspension (108 cells/ml) was wiped on the tongue dorsum for 1 min (49).
(iii) Recovery of C. albicans and L. paracasei from the oral cavity of mice with candidiasis. C. albicans and L. paracasei were recovered by using mouthwash with 100 μl of sterile PBS. The samples were plated on Sabouraud dextrose (SD) agar with chloramphenicol to select for C. albicans, and an additional aliquot was plated on Rogosa agar to select for L. paracasei 28.4 grown to determine the number of CFU per milliliter.
(iv) Macroscopic analysis of the dorsum tongue. After euthanasia, the dorsum tongues of the mice were analyzed on a stereomicroscopy (Zeiss, Göttingen, Germany) and scored between 0 and 4 for the presence of lesions. The scoring system was as follows: 0, absence of lesions; 1, white plaques on less than 20% of the tongue surface; 2, white plates covering 21 to 90% of the surface; 3, white plaques by more than 91%; or 4, thick lesions with pseudomembrane in more than 91% (50).
(v) Microscopic analysis of the tongue dorsum. For light microscopy analysis, the tongues were fixed in 10% formalin for 24 h and hemisected in the sagittal plane. The tissue samples were mounted in paraffin, and 5-μm sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). The evaluation of the histologic sections was performed by observation of yeasts and hyphae and the presence of candidiasis lesions. The intensity of candidiasis lesions was evaluated according to epithelial alterations and to the inflammatory response of the conjunctive tissue, following the approach by Junqueira et al. (51).
Statistical analysis.
Statistical analysis was performed using the Prism 6 program (GraphPad Software, Inc., La Jolla, CA, USA), and a level of significance of 5% was adopted. Analysis of variance (ANOVA), Tukey’s test, Student's t test, and a Kruskal-Wallis test were used according to the data obtained in each experimental assay.
Supplementary Material
ACKNOWLEDGMENTS
J.C.J. was supported by the National Council for Scientific Development/CNPq (grant 306330/2018-0). B.B.F. was supported by the Brown-Brazil Initiative (Brown University). E.M. and B.B.F. are supported by COBRE grant P20 GM103430 from the NIH. A.S. acknowledges support through grants from the Office of Naval Research (U.S.) (grants N000141712120 and N000141712651). F.D.C.R. received a scholarship from the São Paulo Research Foundation/FAPESP (grant 2016/25544-1).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Thompson GR III, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, Redding SW, Patterson TF. 2010. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 109:488–495. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvatori O, Puri S, Tati S, Edgerton M. 2016. Innate immunity and saliva in Candida albicans-mediated oral diseases. J Dent Res 95:365–371. doi: 10.1177/0022034515625222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsubara VH, Bandara HM, Mayer MP, Samaranayake LP. 2016. Probiotics as antifungals in mucosal candidiasis. Clin Infect Dis 62:1143–1153. doi: 10.1093/cid/ciw038. [DOI] [PubMed] [Google Scholar]
- 4.Coronado-Castellote L, Jimenez-Soriano Y. 2013. Clinical and microbiological diagnosis of oral candidiasis. J Clin Exp Dent 5:e279–e286. doi: 10.4317/jced.51242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa AC, Pereira CA, Freire F, Junqueira JC, Jorge AO. 2013. Methods for obtaining reliable and reproducible results in studies of Candida biofilms formed in vitro. Mycoses 56:614–622. doi: 10.1111/myc.12092. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Cuesta C, Sarrion-Perez MG, Bagan JV. 2014. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent 6:e576–e582. doi: 10.4317/jced.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang LW, Fu JY, Hua H, Yan ZM. 2016. Efficacy and safety of miconazole for oral candidiasis: a systematic review and meta-analysis. Oral Dis 22:185–195. doi: 10.1111/odi.12380. [DOI] [PubMed] [Google Scholar]
- 8.Baccaglini L, Atkinson JC, Patton LL, Glick M, Ficarra G, Peterson DE. 2007. Management of oral lesions in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103(Suppl):S50.e1–S50.e23. doi: 10.1016/j.tripleo.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Moges B, Bitew A, Shewaamare A. 2016. Spectrum and the in vitro antifungal susceptibility pattern of yeast isolates in Ethiopian HIV patients with oropharyngeal candidiasis. Int J Microbiol 2016:3037817. doi: 10.1155/2016/3037817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton LL. 2016. Current strategies for prevention of oral manifestations of human immunodeficiency virus. Oral Surg Oral Med Oral Pathol Oral Radiol 121:29–38. doi: 10.1016/j.oooo.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Elahi S, Pang G, Ashman R, Clancy R. 2005. Enhanced clearance of Candida albicans from the oral cavities of mice following oral administration of Lactobacillus acidophilus. Clin Exp Immunol 141:29–36. doi: 10.1111/j.1365-2249.2005.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holgerson PL, Vestman NR, Claesson R, Ohman C, Domellof M, Tanner AC, Hernell O, Johansson I. 2013. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr 56:127–136. doi: 10.1097/MPG.0b013e31826f2bc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Hernández LA, Jave-Suarez LF, Fafutis-Morris M, Montes-Salcedo KE, Valle-Gutierrez LG, Campos-Loza AE, Enciso-Gómez LF, Andrade-Villanueva JF. 2012. Synbiotic therapy decreases microbial translocation and inflammation and improves immunological status in HIV-infected patients: a double-blind randomized controlled pilot trial. Nutr J 11:90. doi: 10.1186/1475-2891-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olle B. 2013. Medicines from microbiota. Nat Biotechnol 31:309–315. doi: 10.1038/nbt.2548. [DOI] [PubMed] [Google Scholar]
- 15.Hatakka K, Ahola AJ, Yli-Knuuttila H, Richardson M, Poussa T, Meurman JH, Korpela R. 2007. Probiotics reduce the prevalence of oral Candida in the elderly–a randomized controlled trial. J Dent Res 86:125–130. doi: 10.1177/154405910708600204. [DOI] [PubMed] [Google Scholar]
- 16.Hu H, Merenstein DJ, Wang C, Hamilton PR, Blackmon ML, Chen H, Calderone RA, Li D. 2013. Impact of eating probiotic yogurt on colonization by Candida species of the oral and vaginal mucosa in HIV-infected and HIV-uninfected women. Mycopathologia 176:175–181. doi: 10.1007/s11046-013-9678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgieva R, Yocheva L, Tserovska L, Zhelezova G, Stefanova N, Atanasova A, Danguleva A, Ivanova G, Karapetkov N, Rumyan N, Karaivanova E. 2015. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol Biotechnol Equip 29:84–91. doi: 10.1080/13102818.2014.987450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft-Bodi E, Jorgensen MR, Keller MK, Kragelund C, Twetman S. 2015. Effect of probiotic bacteria on oral Candida in frail elderly. J Dent Res 94:181S–186S. doi: 10.1177/0022034515595950. [DOI] [PubMed] [Google Scholar]
- 19.Wescombe PA, Hale JD, Heng NC, Tagg JR. 2012. Developing oral probiotics from Streptococcus salivarius. Future Microbiol 7:1355–1371. doi: 10.2217/fmb.12.113. [DOI] [PubMed] [Google Scholar]
- 20.Devine DA, Marsh PD, Meade J. 2015. Modulation of host responses by oral commensal bacteria. J Oral Microbiol 7:26941. doi: 10.3402/jom.v7.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutinho DF, Sant SV, Shin H, Oliveira JT, Gomes ME, Neves NM, Khademhosseini A, Reis RL. 2010. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials 31:7494–7502. doi: 10.1016/j.biomaterials.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Arrigo G, Navarro G, Di Meo C, Matricardi P, Torchilin V. 2014. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: a multi-drug delivery system for a combination therapy in cancer treatment. Eur J Pharm Biopharm 87:208–216. doi: 10.1016/j.ejpb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Ao J, Li P. 2015. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: in vitro and in vivo evaluation. Drug Des Devel Ther 9:3943–3949. doi: 10.2147/DDDT.S87368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla S, Shukla A. 2018. Tunable antibiotic delivery from gellan hydrogels. J Mater Chem B 6:6444–6458. doi: 10.1039/C8TB00980E. [DOI] [PubMed] [Google Scholar]
- 25.Agnihotri SA, Jawalkar SS, Aminabhavi TM. 2006. Controlled release of cephalexin through gellan gum beads: effect of formulation parameters on entrapment efficiency, size, and drug release. Eur J Pharm Biopharm 63:249–261. doi: 10.1016/j.ejpb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Mahdi MH, Conway BR, Smith AM. 2015. Development of mucoadhesive sprayable gellan gum fluid gels. Int J Pharm 488:12–19. doi: 10.1016/j.ijpharm.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki S, Aoyama H, Kawasaki N, Kubo W, Attwood D. 1999. In situ-gelling gellan formulations as vehicles for oral drug delivery. J Control Release 60:287–295. doi: 10.1016/S0168-3659(99)00084-X. [DOI] [PubMed] [Google Scholar]
- 28.Kubo W. 2003. Oral sustained delivery of paracetamol from in situ-gelling gellan and sodium alginate formulations. Int J Pharm 258:55–64. doi: 10.1016/S0378-5173(03)00163-7. [DOI] [PubMed] [Google Scholar]
- 29.Rossoni RD, de Barros PP, de Alvarenga JA, Ribeiro FC, Velloso MDS, Fuchs BB, Mylonakis E, Jorge AOC, Junqueira JC. 2018. Antifungal activity of clinical Lactobacillus strains against Candida albicans biofilms: identification of potential probiotic candidates to prevent oral candidiasis. Biofouling 34:212–225. doi: 10.1080/08927014.2018.1425402. [DOI] [PubMed] [Google Scholar]
- 30.Rossoni RD, Fuchs BB, de Barros PP, Velloso MD, Jorge AO, Junqueira JC, Mylonakis E. 2017. Lactobacillus paracasei modulates the immune system of Galleria mellonella and protects against Candida albicans infection. PLoS One 12:e0173332. doi: 10.1371/journal.pone.0173332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos RB, Scorzoni L, Namba AM, Rossoni RD, Jorge AOC, Junqueira JC. 2019. Lactobacillus species increase the survival of Galleria mellonella infected with Candida albicans and non-albicans Candida clinical isolates. Med Mycol 57:391–394. doi: 10.1093/mmy/myy032. [DOI] [PubMed] [Google Scholar]
- 32.Gbassi GK, Vandamme T. 2012. Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharmaceutics 4:149–163. doi: 10.3390/pharmaceutics4010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradeep K, Kuttappa MA, Prasana KR. 2014. Probiotics and oral health: an update. SADJ 69:20–24. [PubMed] [Google Scholar]
- 34.de Barros PP, Scorzoni L, Ribeiro FC, Fugisaki LRO, Fuchs BB, Mylonakis E, Jorge AOC, Junqueira JC, Rossoni RD. 2018. Lactobacillus paracasei 28.4 reduces in vitro hyphae formation of Candida albicans and prevents the filamentation in an experimental model of Caenorhabditis elegans. Microb Pathog 117:80–87. doi: 10.1016/j.micpath.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Šipailienė A, Petraityte S. 2018. Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob Proteins 10:1–10. doi: 10.1007/s12602-017-9347-x. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez-Pranteda ML, Poncelet D, Náder-Macías ME, Arcos A, Aguilera M, Monteoliva-Sánchez M, Ramos-Cormenzana A. 2012. Stability of lactobacilli encapsulated in various microbial polymers. J Biosci Bioeng 113:179–184. doi: 10.1016/j.jbiosc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Yucel Falco C, Falkman P, Risbo J, Cardenas M, Medronho B. 2017. Chitosan-dextran sulfate hydrogels as a potential carrier for probiotics. Carbohydr Polym 172:175–183. doi: 10.1016/j.carbpol.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Santivarangkna C, Higl B, Foerst P. 2008. Protection mechanisms of sugars during different stages of preparation process of dried lactic acid starter cultures. Food Microbiol 25:429–441. doi: 10.1016/j.fm.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Broeckx G, Vandenheuvel D, Henkens T, Kiekens S, van den Broek MFL, Lebeer S, Kiekens F. 2017. Enhancing the viability of Lactobacillus rhamnosus GG after spray drying and during storage. Int J Pharm 534:35–41. doi: 10.1016/j.ijpharm.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z, Li Z, Jiang S, Bratlie KM. 2018. Chemically modified gellan gum hydrogels with tunable properties for use as tissue engineering scaffolds. ACS Omega 3:6998–7007. doi: 10.1021/acsomega.8b00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nivoliez A, Camares O, Paquet-Gachinat M, Bornes S, Forestier C, Veisseire P. 2012. Influence of manufacturing processes on in vitro properties of the probiotic strain Lactobacillus rhamnosus Lcr35. J Biotechnol 160:236–241. doi: 10.1016/j.jbiotec.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Junqueira JC, Vilela SFG, Rossoni RD, Barbosa JO, Costa A, Rasteiro VMC, Suleiman J, Jorge A. 2012. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop S Paulo 54:17–24. doi: 10.1590/S0036-46652012000100004. [DOI] [PubMed] [Google Scholar]
- 43.Juárez Tomás MS, De Gregorio PR, Leccese Terraf MC, Nader-Macías MEF. 2015. Encapsulation and subsequent freeze-drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur J Pharm Sci 79:87–95. doi: 10.1016/j.ejps.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Son SH, Jeon HL, Yang SJ, Lee NK, Paik HD. 2017. In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb Pathog 112:135–141. doi: 10.1016/j.micpath.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 45.Vilela SF, Barbosa JO, Rossoni RD, Santos JD, Prata MC, Anbinder AL, Jorge AO, Junqueira JC. 2015. Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. albicans and attenuates the experimental candidiasis in Galleria mellonella. Virulence 6:29–39. doi: 10.4161/21505594.2014.981486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribeiro FC, de Barros PP, Rossoni RD, Junqueira JC, Jorge AO. 2017. Lactobacillus rhamnosus inhibits Candida albicans virulence factors in vitro and modulates immune system in Galleria mellonella. J Appl Microbiol 122:201–211. doi: 10.1111/jam.13324. [DOI] [PubMed] [Google Scholar]
- 47.Junqueira JC, Colombo CED, Martins J. d S, Ito CYK, Carvalho YR, Jorge AOC. 2005. Experimental candidosis and recovery of Candida albicans from the oral cavity of ovariectomized rats. Microbiol Immunol 49:199–207. doi: 10.1111/j.1348-0421.2005.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Kato N, Liu C, Matsumiya Y, Kato H, Watanabe K. 2000. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett 187:167–173. doi: 10.1111/j.1574-6968.2000.tb09155.x. [DOI] [PubMed] [Google Scholar]
- 49.Rossoni RD, Barbosa JO, Vilela SF, dos Santos JD, de Barros PP, Prata MC, Anbinder AL, Fuchs BB, Jorge AO, Mylonakis E, Junqueira JC. 2015. Competitive interactions between C. albicans, C. glabrata and C. krusei during biofilm formation and development of experimental candidiasis. PLoS One 10:e0131700. doi: 10.1371/journal.pone.0131700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, Abe S. 2003. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol 47:321–326. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 51.Junqueira JC, da Silva Martins J, Faria RL, Colombo CED, Jorge AOC. 2009. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med Sci 24:877–884. doi: 10.1007/s10103-009-0673-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.