Intensive care unit (ICU) patients may experience ceftriaxone underexposure, but clinical outcomes data are lacking. The objective of this study was to determine the impact of ceftriaxone dosing on clinical outcomes among ICU patients without central nervous system (CNS) infection. A retrospective study of ICU patients receiving intravenous, empirical ceftriaxone for non-CNS infections was conducted. Patients ≥18 years of age who received ≤2 g of ceftriaxone daily for ≥72 h were included and categorized as receiving ceftriaxone 1 g or 2 g daily.
KEYWORDS: pharmacokinetics/pharmacodynamics, ceftriaxone, intensive care unit, clinical outcomes
ABSTRACT
Intensive care unit (ICU) patients may experience ceftriaxone underexposure, but clinical outcomes data are lacking. The objective of this study was to determine the impact of ceftriaxone dosing on clinical outcomes among ICU patients without central nervous system (CNS) infection. A retrospective study of ICU patients receiving intravenous, empirical ceftriaxone for non-CNS infections was conducted. Patients ≥18 years of age who received ≤2 g of ceftriaxone daily for ≥72 h were included and categorized as receiving ceftriaxone 1 g or 2 g daily. The primary, composite outcome was treatment failure, defined as inpatient mortality and/or antibiotic escalation due to clinical worsening. Propensity score matching was performed based on the probability of receiving 2 g of ceftriaxone daily. Multivariable logistic regression determined the association between ceftriaxone dose and treatment failure in a propensity-matched cohort. A total of 212 patients were included in the propensity-matched cohort. The most common diagnoses (83.0%) were pneumonia and urinary tract infection. Treatment failure occurred in 17.0% and 5.7% of patients receiving 1 g and 2 g daily, respectively (P = 0.0156). Overall inpatient mortality was 8.5%. Ceftriaxone 2 g dosing was associated with a reduced likelihood of treatment failure (adjusted odds ratio [aOR] = 0.190; 95% confidence interval [CI] = 0.059 to 0.607). Other independent predictors of treatment failure included sequential organ failure assessment score (aOR = 1.440; 95% CI = 1.254 to 1.653) and creatinine clearance at 72 h from ceftriaxone initiation (aOR = 0.980; 95% CI = 0.971 to 0.999). Therefore, ceftriaxone at 2 g daily, when used as appropriate antimicrobial coverage, may be appropriate for ICU patients with lower mortality risk.
INTRODUCTION
Ceftriaxone is a commonly prescribed β-lactam antibiotic that exhibits extensive protein binding at therapeutic concentrations (1, 2). Ceftriaxone displays time-dependent bactericidal activity and concentrations may be subtherapeutic in ICU patients, particularly those with hypoalbuminemia (3). Although pharmacokinetic data suggest ICU patients, including those with hypoalbuminemia, may experience ceftriaxone underexposure, clinical outcomes data are lacking. Within our health system, ceftriaxone dosing in ICU patients without CNS infection is variable. We sought to determine the impact of ceftriaxone dosing—1 g daily compared to 2 g daily— on clinical outcomes among ICU patients without CNS infection. We hypothesized that 2 g of ceftriaxone daily would be associated with better clinical outcomes than 1 g of ceftriaxone daily among ICU patients.
RESULTS
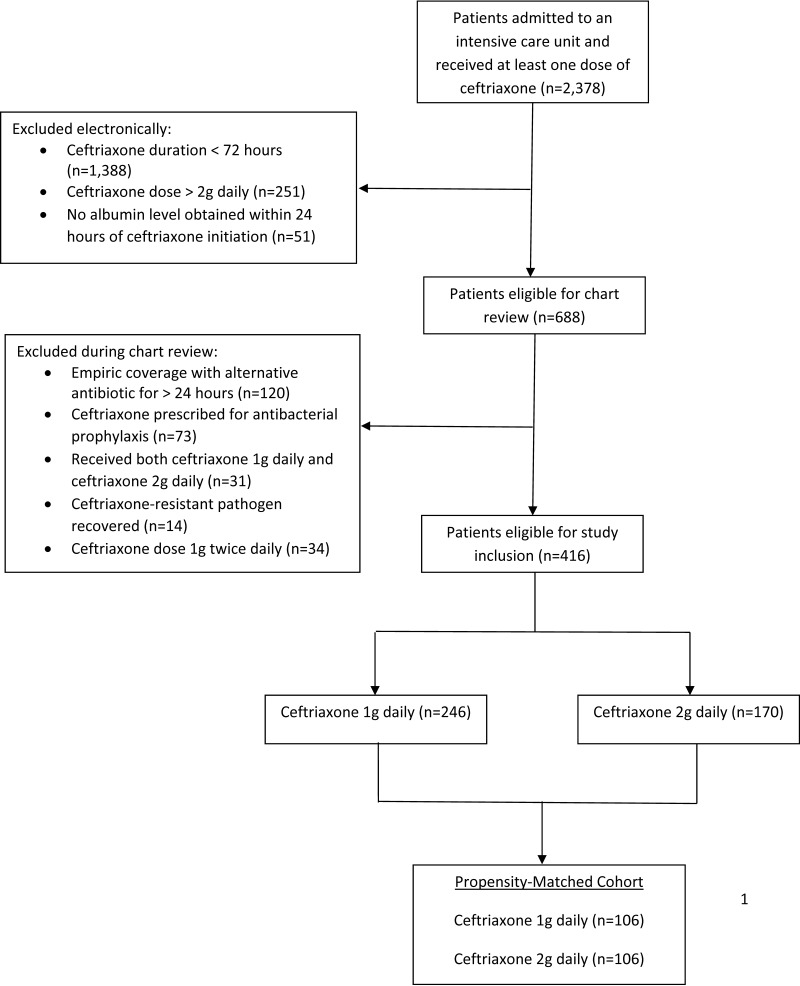
A total of 2,378 patients received ceftriaxone in an eligible ICU during the study period (Fig. 1). Of these patients, 416 were deemed study eligible and included in the propensity score matching process as follows: 246 (59.1%) patients received 1 g daily and 170 (40.9%) patients received 2 g daily. The propensity score matching resulted in 106 matched pairs (n = 212). The two groups were well matched with respect to demographics, comorbidities, infection type, and sequential organ failure assessment (SOFA) score (Table 1). Patients receiving 2 g daily had slightly higher serum creatinine values than those receiving 1g daily. A pathogen was recovered from 34% and 33% of the 1 g and 2 g ceftriaxone groups, respectively. Culture types were similar between groups. The median duration of ceftriaxone therapy was 1 day longer in the 2 g daily group (6 days compared to 5 days in the 1 g daily group; P = 0.0552).
FIG 1.
Eligibility screening.
TABLE 1.
Characteristics and outcomes among intensive care unit patients receiving ceftriaxone stratified by ceftriaxone exposure
| Variablea | Ceftriaxone daily dose: |
P value | |
|---|---|---|---|
| 1 gram (n = 106) | 2 grams (n = 106) | ||
| Median age in years (IQR) | 72 (59, 79) | 71 (59, 77) | 0.4861 |
| No. female gender (%) | 62 (58.5) | 64 (60.4) | 0.8888 |
| Median body mass index (IQR) | 27.9 (23.5, 34.6) | 28.6 (23.7, 37.3) | 0.8598 |
| Median weight in kg (IQR) | 77.3 (65.0, 104.6) | 78.9 (63.8, 106.2) | 0.9314 |
| Median height in inches (IQR) | 66 (63, 69) | 65 (62, 69) | 0.7905 |
| Median SCr baseline in mg/dL (IQR) | 0.98 (0.77, 1.42) | 1.03 (0.69, 1.34) | 0.8510 |
| Median creatinine clearance at baseline in ml/min (IQR) | 58.6 (40.7, 79.8) | 54.7 (35.0, 80.5) | 0.5732 |
| Median SCr at 72 h in mg/dl (IQR) | 0.87 (0.67, 1.22) | 0.77 (0.61, 1.02) | 0.0487 |
| Median creatinine clearance at 72 h in ml/min (IQR) | 62.8 (41.6, 101.2) | 72.6 (44.3, 104.5) | 0.3435 |
| Median ideal body weight in kg (IQR) | 59.3 (52.4, 68.4) | 57.0 (50.1, 70.7) | 0.5734 |
| Median SOFA score (IQR) | 3 (0, 7) | 3.5 (1, 7) | 0.3614 |
| No. cirrhosis (%) | 3 (2.8) | 1 (0.9) | 0.6214 |
| No. immunosuppression (%) | 16 (15.1) | 12 (11.3) | 0.5434 |
| No. congestive heart failure (%) | 28 (26.4) | 26 (24.5) | 0.8749 |
| No. malignancy (%) | 18 (17.0) | 22 (20.8) | 0.5989 |
| No. chronic obstructive pulmonary disease (%) | 42 (39.6) | 42 (39.6) | >0.9999 |
| No. diabetes mellitus (%) | 43 (40.6) | 41 (38.7) | 0.8884 |
| No. mechanical ventilation (%) | 18 (17.0) | 26 (24.5) | 0.2356 |
| No. vasopressor support (%) | 22 (20.8) | 21 (19.8) | >0.9999 |
| Median albumin in g/dl (IQR) | 3.0 (2.6, 3.3) | 3.0 (2.5, 3.3) | 0.9046 |
| No. hypoalbuminemiad (%) | 23 (21.7) | 31 (29.3) | 0.2698 |
| Diagnosis | 0.8953 | ||
| No. bacteremia (%) | 12 (11.3) | 9 (8.5) | |
| No. pneumonia (%) | 71 (67.0) | 75 (70.8) | |
| No. UTI (%) | 15 (14.2) | 15 (14.2) | |
| No. other (%) | 8 (7.6) | 7 (6.6) | |
| Culture Type | 0.5389 | ||
| No. Gram-negative (%) | 30 (28.3) | 27 (25.5) | |
| No. Gram-positive (%) | 6 (5.7) | 6 (5.7) | |
| No. mixed (%) | 0 (0) | 2 (1.9) | |
| No. culture negative (%) | 70 (66.0) | 71 (67.0) | |
| Median duration of ceftriaxone therapy in days (IQR) | 5 (4, 7) | 6 (5, 7) | 0.0552 |
| Outcomes | |||
| No. treatment failureb (%) | 18 (17.0) | 6 (5.7) | 0.0156 |
| No. inpatient mortality (%) | 12 (11.3) | 6 (5.7) | 0.2172 |
| No. antibiotic escalation (%) | 10 (9.4) | 0 (0) | 0.0016 |
| No. antibiotic-related adverse eventc (%) | 2 (1.9) | 0 (0) | 0.4976 |
| Median ICU length of stay in days (IQR) | 3 (2, 5) | 3 (1, 5) | 0.4802 |
| Median hospital length of stay in days (IQR) | 7 (5, 12) | 7 (5, 11) | 0.7029 |
sCr, serum creatinine; SOFA, sequential organ failure assessment; UTI, urinary tract infection; IQR, interquartile range.
Patients could meet more than one treatment failure criterion.
All antibiotic-related adverse events were C. difficile-associated diarrhea.
Hypoalbuminemia indicates albumin at ≤ 2.5 g/dl.
Treatment failure was more common among patients receiving 1 g of ceftriaxone (19.8%) than those receiving 2 g daily (6.6%). There were no cases of antibiotic escalation among those who received 2 g daily. Ten patients (9.4%) in the 1 g daily group required antibiotic escalation, eight for pneumonia and two for a urinary tract infection (UTI). Five patients with pneumonia were culture negative and three had ceftriaxone-susceptible pathogens (Escherichia coli, n = 2, and Viridans group Streptococcus, n = 1). Of the three patients with UTI, two had ceftriaxone-sensitive pathogens (E. coli and Klebsiella pneumoniae) and one had culture with mixed flora and no sensitivities were performed. The median duration of ceftriaxone therapy prior to escalation was 5 days (interquartile range (IQR) = 4 to 6 days). These patients were escalated to either cefepime or piperacillin-tazobactam. There was no difference in adverse effects between the two groups. Clostridioides difficile-associated diarrhea, diagnosed by PCR and confirmed by subsequent treatment with enteral vancomycin, was the only documented adverse effect (n = 2, both in the 1 g daily group). Hypoalbuminemia (albumin at ≤2.5 g/dl) was present in 54 (25.5%) patients. Among this subgroup with hypoalbuminemia, the treatment failure rate was 30.4% with 1 g daily dosing and 9.7% with 2 g daily dosing (P = 0.0779). Among patients with UTI (n = 30, 15 in each group), 33.3% and 0% of patient in the 1 g and 2 g daily groups, respectively, experienced failure (P = 0.0421). There were 23 patients with a SOFA score of >10. The failure rate among these patients was 64.3% and 22.2% with 1 g daily and 2 g daily dosing, respectively (P = 0.0487).
Table 2 lists predictor variables associated with treatment failure. Patients who experienced treatment failure had higher SOFA scores and more end organ dysfunction. Patients without hypoalbuminemia had higher rates of treatment success. The results of a multivariate analysis are shown in Table 3. Ceftriaxone at 2 g daily was independently associated with treatment success (adjusted odds ratio 0.190; 95% confidence internal 0.059 to 0.607).
TABLE 2.
Predictor variables associated with treatment failure among intensive care unit patients receiving ceftriaxone
| Variablea | Treatment outcome: |
P value | |
|---|---|---|---|
| Success (n = 188) | Failure (n = 24) | ||
| Median SOFA score (IQR) | 3 (0–6) | 9 (7–12) | <0.0001 |
| No. mechanical ventilation (%) | 33 (17.6) | 11 (45.8) | 0.0029 |
| No. vasopressor support (%) | 30 (16.0) | 13 (54.2) | <0.0001 |
| Median creatinine clearance at 72 h (IQR) | 73.0 (46.4–106.0) | 54.8 (35.0–67.3) | 0.0121 |
SOFA, sequential organ failure assessment; IQR, interquartile range.
TABLE 3.
Univariable and multivariable analyses of the association between potential predictor variables and treatment failure among intensive care unit patients receiving ceftriaxoneb
| Predictor variablea | Univariable odds ratio (95% CI) | P value | Multivariable odds ratio (95% CI) | P value |
|---|---|---|---|---|
| SOFA score | 1.409 (1.240–1.601) | <0.0001 | 1.440 (1.254–1.653) | <0.0001 |
| Creatinine clearance at 72 h | 0.985 (0.972–0.998) | 0.0205 | 0.980 (0.971–0.999) | 0.0358 |
| Ceftriaxone 2 gram dose | 0.293 (0.112–0.772) | 0.0130 | 0.190 (0.059–0.607) | 0.0051 |
SOFA, sequential organ failure assessment; CI, confidence interval.
The final model was able to distinguish between treatment failure and treatment success with a good receiver operator characteristics curve of 0.8802 (95% confidence interval of 0.8062–0.9538; P < 0.0001). None of the models reached statistical significance with the Hosmer-Lemeshow goodness-of-fit test (final model, P = 0.9033).
DISCUSSION
We retrospectively assessed ICU patients receiving empirical ceftriaxone and observed a higher rate of treatment failure among patients receiving ceftriaxone 1 g daily compared to 2 g daily in a propensity-matched cohort. This result was influenced by more empirical antibiotic escalation in the 1 g group, despite no cultures resulting in ceftriaxone-resistant organisms (as these patients were excluded). Ceftriaxone is an appropriate empirical antibiotic based on indication for many ICU patients, specifically those with pneumonia and/or urinary tract infection, which diagnoses accounted for 80% of the study population. Inpatient mortality was similar in the two ceftriaxone exposure groups. The groups were well matched owing to use of propensity-score matching. The observed imbalance in treatment failure between groups supports our hypothesis that ceftriaxone 2 g daily dosing may yield better clinical outcomes than ceftriaxone 1 g daily dosing among ICU patients without CNS infection. Our observations also suggest that ICU patients prescribed empirical ceftriaxone for a non-CNS indication may benefit from 2 g daily regardless of diagnosis and culture type. However, giving the drug more frequently, or as continuous infusion, may help maintain adequate time above the MIC (4). There was no difference in ICU length of stay or hospital length of stay between the two groups.
While 1 g dosing was associated with an increased likelihood of treatment failure in the entire population, this observation was pronounced in an unadjusted, subanalysis of patients (n = 67) with hypoalbuminemia (albumin at ≤2.5 g/dl). However, it is possible that 1 g ceftriaxone daily may put ICU patients at risk for treatment failure, especially those with an albumin at ≤2.5 g/dl. The current study also indicates that ceftriaxone is well tolerated, with only three adverse effects noted in the entire sample and no appreciable difference between the two treatment arms. We acknowledge that some adverse effects may have been unaccounted for given the retrospective nature of the study. However, ceftriaxone has been shown to be well tolerated and this trial further confirms its tolerability, even at the higher 2 g dosing (5–8). Notably, recent data suggest ceftriaxone is well tolerated even at doses above 4 g per day (9).
In the multivariable analysis, three variables were independently associated with treatment failure: (i) ceftriaxone at 1 g daily dosing; (ii) an elevated SOFA score; and (iii) creatine clearance at 72 h from ceftriaxone initiation. The final model was able to distinguish between treatment failure and treatment success with a good receiver operator characteristics curve. Patients with higher SOFA scores tended to experience worse infection-related outcomes. Elevated creatinine clearance at 72 h may be a surrogate indicator for renal recovery, and prompt renal recovery may confer a mortality benefit in patients with septic shock (10). However, we did not characterize patients as having septic shock, nor did we did formally assess renal recovery, as this is challenging (11). Ceftriaxone 1 g dosing was independently associated with treatment failure, suggesting that critically ill patients may benefit from 2 g daily of ceftriaxone, regardless of the host and/or diagnosis.
Available data suggest ICU patients may experience subtherapeutic antibiotic exposure, particularly of hydrophilic antibiotics such as β-lactams (12–14). Ceftriaxone is notable given its high degree of protein binding, exempting it from renal dosing adjustments and, likely, routine clinician dose escalation in the ICU. However, increased intravascular volume combined with hypoalbuminemia may create a particularly disadvantageous scenario in which higher-than-expected concentrations of unbound ceftriaxone are not only diluted but also renally eliminated faster than anticipated. Wong and colleagues observed substantial differences between measured and predicted unbound ceftriaxone concentrations among 161 ICU patients (P < 0.05) (12). Similar findings were observed by Schlebinger et al., who found the unbound fraction of ceftriaxone to be higher in ICU patients than healthy volunteers, where the ceftriaxone volume of distribution was double compared to healthy volunteers (20 liters versus 10 liters) (13). However, ceftriaxone dosed at 2 g daily achieved sufficient unbound plasma concentrations above the EUCAST susceptibility breakpoint (≤1 mg/liter) throughout the dosing interval (15). Compared to healthy subjects, ceftriaxone clearance and volume of distribution were increased in ICU patients with severe sepsis. In this evaluation, three (25%) critically ill patients had subtherapeutic ceftriaxone plasma concentrations (14). Using a murine sepsis model, Selmi et al. demonstrated higher urinary loss of bound ceftriaxone in septic rats compared to nonseptic rats due to sepsis-induced alterations in glomerular filtration barrier permeability (16). Their findings suggest clinicians should consider urinary loss of both bound and unbound ceftriaxone during the early phases of sepsis. Patients in the current study were not characterized as meeting or not meeting sepsis criteria. However, all patients were in an ICU receiving empirical ceftriaxone therapy. Garot and colleagues have argued that ceftriaxone 1 g daily is sufficient for critically ill patients when the ceftriaxone MIC is ≤1 mg/liter, but clinical outcomes were not assessed (17). We believe our study is the first to examine clinical outcomes between 1 g and 2 g ceftriaxone dosing in ICU patients. Interestingly, in the DALI study, ceftriaxone at a median dose of 2 g per day (interquartile range = 2 to 4 g per day) had some of the highest levels of PK/PD target attainment of any β-lactam studied (18). These ICU patients had a median SOFA score of 5, which is slightly higher than in the current study.
Pharmacokinetic/pharmacodynamic data contribute to our understanding of antibiotic efficacy and toxicity, but clinical outcomes are of utmost importance. Our data suggest prescribing 2 g of ceftriaxone daily to ICU patients may improve treatment outcomes without increasing toxicity. These findings, coupled with the generic availability of ceftriaxone and the lower cost relative to many other antibiotics, make 2 g dosing in the ICU a reasonable approach. Ceftriaxone continues to be one of the most commonly prescribed antibiotics in the United States, for a variety of infections (2). Dosing ceftriaxone appropriately in the ICU will be paramount to preserving its efficacy over time. Moreover, by ensuring patients have a high likelihood of responding to ceftriaxone therapy, clinicians may avoid the use of broader-spectrum antibiotics. In the current study, patients who failed to respond to ceftriaxone at 1g daily were prescribed either cefepime or piperacillin-tazobactam. These observations are important for antimicrobial stewardship efforts in the ICU.
This study has a number of limitations. First, this evaluation was retrospective, conducted within a single health system in one U.S. state, and limited by data available in the medical record. Data elements collected represent those routinely available to clinicians and our study attempted to estimate the impact of ceftriaxone dosing among ICU patients in a real-world setting. Second, the study data cannot confirm that escalation occurred due to subtherapeutic ceftriaxone, since ceftriaxone pharmacokinetic data were not collected. We also cannot entirely confirm whether ceftriaxone-resistant organisms were implicated because many patients were culture negative. Because we focused our investigation on the index hospitalization with median 7-day length of stay in the study population, we did not collect information on infection recurrence or relapse. It is possible there were between-group differences in clinical outcomes after the index hospitalization. Additionally, while the current study included ICU patients, SOFA scores were not very high and overall inpatient mortality was only 8.5%. This is attributable to using ICU admission rather than a diagnosis of sepsis or septic shock as a requirement for study inclusion. It is possible that patients with more severe illness were less likely to receive empirical ceftriaxone. The number of treatment failure events, while different between groups, was low. Our findings should be interpreted cautiously and may not be generalizable to ICU patients with higher severity of illness and/or mortality risk. We attempted to reduce the impact of between-group differences on our outcome assessment using propensity-score matching. However, it is possible that unmeasured confounders may have contributed to the difference in outcomes observed between the two ceftriaxone-exposure groups. This study is strengthened by inclusion of over 100 propensity-matched pairs of patients with common infectious diagnoses for which ceftriaxone is prescribed in the ICU.
In conclusion, intensive care unit patients receiving ceftriaxone at 1 g daily experienced higher rates of treatment failure than those receiving 2 g daily, primarily due to escalation from ceftriaxone to an alternative antibiotic. These clinical observations correlate with existing pharmacokinetic literature suggesting a risk of ceftriaxone underexposure in ICU patients. Two grams of empirical ceftriaxone daily, when used as appropriate antimicrobial coverage, may be prudent for ICU patients without CNS infection and warrants further study in a larger clinical trial.
MATERIALS AND METHODS
Study design and patient population.
This was a retrospective review of adults admitted to one of seven ICUs within five different hospitals between January 1, 2016 and January 1, 2018, including medical, surgical, and cardiac ICUs. Patients ≥18 years of age who were admitted to one of the designated ICUs, received ≤2 g of empirical ceftriaxone daily for ≥72 h during ICU admission, and had at least one serum albumin concentration obtained within 24 h of ceftriaxone initiation were eligible for study inclusion. Patients were identified from the electronic medical record. Patients from whom a ceftriaxone-resistant pathogen was recovered were excluded. Additional exclusion criteria included a diagnosis of meningitis, pregnancy, ceftriaxone use for antibacterial prophylaxis, receipt of both 1 g daily and 2 g daily ceftriaxone during treatment, receipt of 1 g ceftriaxone twice daily, and/or empirical treatment with an alternative antibiotic for >24 h.
This study was reviewed and approved by the Aurora Health Care Research Subject Protection Program. This research was performed in accordance with the Declaration of Helsinski and conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations for reporting observational studies (19).
Data collection.
Patient data were collected, including age, gender, height, and weight. The albumin concentration obtained closest to ceftriaxone initiation was recorded. Hypoalbuminemia was defined as an albumin level of ≤2.5 g/dl as described in the Saline versus Albumin Fluid Evaluation (SAFE) study (20). Creatinine clearance estimates upon ceftriaxone initiation and 72 h afterward were calculated using Cockcroft-Gault (21). Patient comorbidities, as documented by the treating physician, were recorded. Immunosuppression was defined as (i) exposure to at least one of the following prior to and/or during hospitalization: chronic corticosteroids (≥10 mg of prednisone daily or an alternative steroid equivalent), antineoplastic therapy, tumor necrosis factor alpha inhibitors, or calcineurin inhibitors; or (ii) a diagnosis of HIV/AIDS (22). Sequential organ failure assessment (SOFA) scores were calculated at the time of ceftriaxone initiation (23). Use of vasopressor support and/or mechanical ventilation were collected as dichotomous variables. Eligible patients were split into two treatment groups based upon empirical ceftriaxone exposure: 1 g once daily and 2 g once daily. Duration of ceftriaxone therapy was also collected.
Patient location (hospital to which the patient was admitted) and diagnoses were collected. Diagnoses were classified as either bacteremia, pneumonia, urinary tract infection (UTI), or “other.” Patients with more than one diagnosis were categorized into the most severe diagnosis category, with bacteremia being the most severe (regardless of source), followed by pneumonia, and then UTI. Patient bacterial culture data were classified as Gram-positive, Gram-negative, mixed, or culture negative.
Clinical outcomes.
The primary outcome was a composite outcome of treatment failure defined as inpatient mortality and/or antibiotic escalation. Antibiotic escalation was defined as switching from ceftriaxone to an alternative antibiotic due to clinical worsening at any time during ceftriaxone therapy, as documented by the treating provider in the electronic medical record. Secondary outcomes included ICU and hospital length of stay and adverse effects attributed to ceftriaxone, as well as treatment success in those with hypoalbuminemia and those with SOFA scores of >10. Adverse effects were assessed by searching each patient’s chart for lab abnormalities and/or notes from physicians on the day of ceftriaxone discontinuation.
Statistical analysis.
Given the nonrandom treatment assignment, a propensity score was developed for the probability of receiving ceftriaxone 2 g daily using a multivariable logistic regression model in which a binary indicator of ceftriaxone exposure (1 g or 2 g daily) was the dependent variable. The following variables were included in this model: patient age, SOFA sore, initial albumin level, culture type, location (hospital), and diagnosis. Propensity score matching was done using a 1:1 nearest neighbor approach. Treatment failure was assessed in the propensity-matched population. Univariable analyses compared patients in the 1 g and 2 g ceftriaxone exposure groups, as well as those who did and did not experience treatment failure. Categorical variables were compared by Pearson’s chi-squared test or Fisher’s exact test, and continuous variables were compared by a two-sample t test or the Mann-Whitney U test. Multivariable analyses of factors associated with treatment failure were performed using forward, stepwise, and multiple logistic regression. The criterion for model entry was α = 0.05, whereas the criterion for remaining in the model was α = 0.10. The predictors of interest were assessed for multicollinearity (tolerance statistic = <0.4). All tolerance values were >0.4. The Hosmer-Lemeshow test was utilized to evaluate goodness-of-fit. All tests were 2-tailed and a P value of ≤0.05 was considered statistically significant. SAS, version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
ACKNOWLEDGMENT
We declare no conflicts of interest nor financial disclosures to report.
REFERENCES
- 1.Bos J, Prins J, Mistício M, Nunguiane G, Lang CN, Beirao JC, Mathot RAA, van Hest RM. 2018. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: a population pharmacokinetic modelling study. J Antimicrob Chemother 73:1620–1629. doi: 10.1093/jac/dky071. [DOI] [PubMed] [Google Scholar]
- 2.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. 2016. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 176:1639–1648. doi: 10.1001/jamainternmed.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JA, Boots R, Rickard CM, Thomas P, Quinn J, Roberts DM, Richards B, Lipman J. 2007. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? A randomized controlled pilot study. J Antimicrob Chemother 59:285–291. doi: 10.1093/jac/dkl478. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Ruiz G, Caballero-Lopez J, Friedland IR, Woods GL, Carides A. 2002. A study evaluating the efficacy, safety, and tolerability of ertapenem versus ceftriaxone for the treatment of community-acquired pneumonia in adults. Clin Infect Dis 34:1076–1083. doi: 10.1086/339543. [DOI] [PubMed] [Google Scholar]
- 6.Segev S, Raz R, Rubinstein E, Shmuely H, Hassin D, Rosen N, Platau E, Ben Assuli S, Pitlik S. 1995. Double-blind randomized study of 1 g versus 2 g intravenous ceftriaxone daily in the therapy of community-acquired infections. Eur J Clin Microbiol Infect Dis 14:851–855. doi: 10.1007/bf01691490. [DOI] [PubMed] [Google Scholar]
- 7.Zuck P, Rio Y, Ichou F. 1990. Efficacy and tolerance of cefpodoxime proxetil compared with ceftriaxone in vulnerable patients with bronchopneumonia. J Antimicrob Chemother 26:71–77. doi: 10.1093/jac/26.suppl_E.71. [DOI] [PubMed] [Google Scholar]
- 8.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. 1997. A prediction rule to identify low risk patients with community acquired pneumonia. N Engl J Med 336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 9.Le Turnier P, Navas D, Garot D, Guimard T, Bernard L, Tattevin P, Vandamme YM, Hoff J, Chiffoleau A, Dary M, Leclair-Visonneau L, Grégoire M, Pere M, Boutoille D, Sébille V, Dailly E, Asseray N. 2019. Tolerability of high-dose ceftriaxone in CNS infections: a prospective multicentre cohort study. J Antimicrob Chemother 74:1078–1085. doi: 10.1093/jac/dky553. [DOI] [PubMed] [Google Scholar]
- 10.Sood MM, Shafer LA, Ho J, Reslerova M, Martinka G, Keenan S, Dial S, Wood G, Rigatto C, Kumar A. 2014. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 29:711–717. doi: 10.1016/j.jcrc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M. 2017. Renal recovery after acute kidney injury. Intensive Care Med 43:855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2013. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 57:6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleibinger M, Steinbach CL, Töpper C, Kratzer A, Liebchen U, Kees F, Salzberger B, Kees MG. 2015. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 80:525–533. doi: 10.1111/bcp.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 47:421–429. doi: 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2020. Breakpoint tables for interpretation of MICs and zone diameters, Version 10.0. http://www.eucast.org.
- 16.Selmi V, Loriga B, Vitali L, Carlucci M, Di Filippo A, Carta G, Sgambati E, Tofani L, De Gaudio AR, Novelli A, Adembri C. 2016. Changes in ceftriaxone pharmacokinetics/pharmacodynamics during the early phase of sepsis: a prospective, experimental study in the rat. J Transl Med 14:316. doi: 10.1186/s12967-016-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequin PF, Le Guellec C. 2011. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 72:758–767. doi: 10.1111/j.1365-2125.2011.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 45:247–251. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S, Bellomo R, McEvoy S, Lo SK, Myburgh J, Neal B, Norton R. 2006. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the Saline versus Albumin Fluid Evaluation (SAFE) study. Bmj 333:1044. doi: 10.1136/bmj.38985.398704.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Wieland BW, Marcantoni JR, Bommarito KM, Warren DK, Marschall J. 2012. A retrospective comparison of ceftriaxone versus oxacillin for osteoarticular infections due to methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 54:585–590. doi: 10.1093/cid/cir857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]