Carbapenem pharmacokinetic (PK) profiles are significantly different in critically ill patients because of the drastic variability of the patients’ physiological parameters. Published population PK studies have mainly focused on specific diseases, and the majority of these studies had small sample sizes. The aim of this study was to develop a population PK model of imipenem in critically ill patients that estimated the influence of various clinical and biological covariates and the use of extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT).
KEYWORDS: imipenem, pharmacokinetics/pharmacodynamics, therapeutic drug monitoring, critically ill, ECMO
ABSTRACT
Carbapenem pharmacokinetic (PK) profiles are significantly different in critically ill patients because of the drastic variability of the patients’ physiological parameters. Published population PK studies have mainly focused on specific diseases, and the majority of these studies had small sample sizes. The aim of this study was to develop a population PK model of imipenem in critically ill patients that estimated the influence of various clinical and biological covariates and the use of extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT). A two-compartment population PK model with creatinine clearance (CLCR), body weight (WT), and ECMO as fixed effects was developed using the nonlinear mixed-effects model (NONMEM). A Monte Carlo simulation was performed to evaluate various dosing schemes and different levels of covariates based on the pharmacokinetic/pharmacodynamic index (ƒ%T>MIC) for the range of clinically relevant MICs. The results showed that there may be insufficient drug use in the clinical routine drug dose regimen, and 750 mg every 6 h (q6h) could achieve a higher treatment success rate. The blood concentrations of imipenem in ECMO patients were lower than those in non-ECMO patients; therefore, dosages may need to be increased. The dosage may need adjustment for patients with a CLCR of ≤70 ml/min, but the dose should be lowered carefully to avoid the insufficient drug exposure. Dose adjustment is not necessary for patients with WT ranging from 50 to 80 kg. Due to the large variation in PK profile of imipenem in critically ill patients, therapeutic drug monitoring (TDM) should be carried out to optimize drug regimens.
TEXT
Imipenem is the first licensed carbapenem antibiotic and has been used extensively to treat serious hospital-related infections caused by Gram-negative bacteria. Due to its broad spectrum and highly potent therapeutic effects, imipenem is usually prescribed for severe pneumonia, complicated urinary tract infections, and intra-abdominal infections in critically ill patients (1, 2).
As it is a time-dependent antibiotic, the antibacterial effects of imipenem are contingent on the time of free drug concentrations above the MIC (ƒ%T>MIC) within the dosing interval. Therefore, the pharmacokinetic (PK) and pharmacodynamic (PD) properties of imipenem determine the optimal dosing regimen, especially in specific patient populations (3, 4). The PK profile of imipenem is characterized by a low plasma protein binding rate (approximately 20%), primarily renal excretion (70%), and a short elimination half-life (1 h). However, the PK profiles of carbapenems are significantly different in intensive care unit (ICU) patients due to the drastic variability of the patients’ physiological parameters (5–9). These changes in PK parameters for hydrophilic antimicrobials such as carbapenems usually lead to a significant decrease or increase in the plasma drug concentration because of rigorous fluid resuscitation, systemic inflammatory response syndrome, and renal dysfunction. Many critically ill patients (20 to 50%, according to the DALI [defining antibiotic levels in intensive care unit patients] study [10]) have drug exposures lower than the PK/PD targets. Therefore, imipenem dosing strategies for these patients should be refined according to individual requirements.
Although there are several PK studies of imipenem in critically ill patients, optimization of imipenem administration based on therapeutic drug monitoring (TDM) remains undefined. The aforementioned PK studies focused on special diseases, and most of them had a low number of subjects (5, 7, 9, 11–17). Unfortunately, patients in critical care units have a variety of disease diagnoses and a large distribution of pathological and physiological indicators. Moreover, some of these patients receive dialysis or extracorporeal membrane oxygenation (ECMO) treatments, which lead to significant variability in PK profiles. ECMO has been shown to enhance the change of physiological characteristics in critically ill patients, leading to increased volume of distribution (V) and decreased total clearance (CL) of various antimicrobial agents (7). Imipenem PK models have rarely been tested using TDM and individualized dose adjustment, because the influence of these covariates on PK parameters has not been evaluated in the published studies. Therefore, TDM data with more subjects, different pathological states, and other covariates that may affect PK parameters should be sufficiently analyzed and evaluated in critically ill patient populations.
Some antibiotics, including aminoglycosides and vancomycin, have been traditionally monitored for individual administration. However, there is still no acknowledged procedure for the dosing adjustment of carbapenems. One of the reasons for this is that the PK/PD index ƒ%T>MIC is difficult to evaluate. As we previously reported, the TDM of imipenem is routinely performed by testing its plasma concentrations after the 4th dose and 3 h and 0.5 h before the next administration in critically ill patients in our hospital (18). The evaluation of ƒ%T>MIC was based on dose strategy, drug levels, and MIC breakpoint for each individual patient, which was estimated using a calculator developed by us. The aim of this study was to develop a population PK model of imipenem in critically ill patients, taking into account the influence of various clinical and biological covariates. A Monte Carlo simulation was performed to evaluate various dosing schemes and different levels of covariates based on the PK/PD index (ƒ%T>MIC) for the range of clinically relevant MICs.
RESULTS
Patient characteristics.
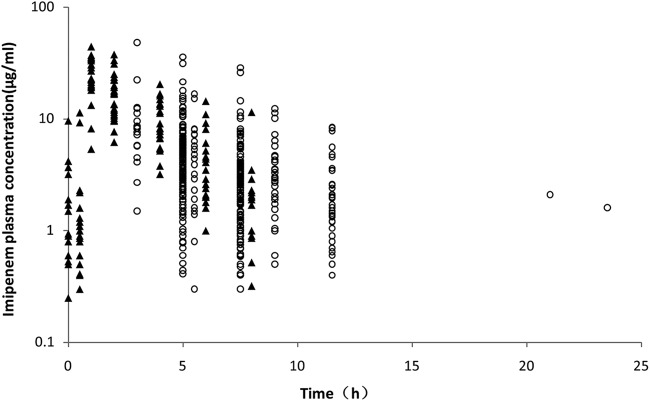
A total of 247 patients were included in the study. PK analysis incorporated data from 580 imipenem plasma assays after 17 concentrations below the limit of quantitation were excluded. Figure 1 shows the imipenem time-dependent concentrations, grouped into TDM (circles) and PK (triangles) study data. The patient-related demographic and clinical information is summarized in Table 1. Data indicate that 167 patients were male (67.6%), with ages ranging from 20 to 97 years (median, 67 years). The median body weight was 65.0 kg (range, 37.5 kg to 110 kg). Considerable variability was detected in liver and kidney functions and in several blood biochemical parameters assessed in the critical care patients (Table 1). During the sampling time, 48 patients received ECMO, including 13 patients (PK study group) who had their ECMO treatment withdrawn during the next sampling period. Continuous renal replacement therapy (CRRT) was administered to 58 patients (23.5% of all patients). Pulmonary infection was detected in 74.1% of patients. Imipenem was administered using different dosing regimens, including 500 mg every 8 h (q8h; primary dose regimen; 123 patients [49.8%]) and then 1,000 mg (q8h; 61 patients [24.7%]).
FIG 1.
Concentration-time profile of imipenem concentrations. TDM and PK study data are shown.
TABLE 1.
Characteristics of the 247 ICU patients
| Characteristic | Valuea |
|---|---|
| Sex (male/female) | 167/80 |
| Age (yrs) | 67 (20–97) |
| WT (kg) | 65.0 (37.5–110.0) |
| HT (cm) | 168 (145–187) |
| BMI (kg/m2) | 23.1 ± 4.2 |
| SCR (mmol/liter) | 87.35 (21.10–1,046.90) |
| CLCR (ml/min) | 59.07 (5.81–293.49) |
| ALT (IU/liter) | 21 (1–3,460) |
| AST (IU/liter) | 31 (5–3,130) |
| ALB (g/liter) | 31.0 (14.0–60.7) |
| TBIL (μmol/liter) | 12.10 (2.26–294.50) |
| HGB (g/liter) | 93 (45–142) |
| PLT (109/liter) | 118 (1–577) |
| ECMO (yes/no) | 48 (contained 13 PK subjects)/199 |
| CRRT (yes/no) | 58/189 |
| No. (%) with type of infection | |
| Pulmonary | 183 (74.1) |
| Abdominal and digestive tract | 16 (6.5) |
| Urinary tract | 4 (1.6) |
| Sepsis or systemic failure | 26 (10.5) |
| Skin, soft tissue, bone, and joint | 6 (2.4) |
| Unknown origin and other | 12 (4.9) |
| No. (%) receiving imipenem | |
| 500 mg q6h | 20 (8.1) |
| 500 mg q8h | 123 (49.8) |
| 500 mg q12h | 23 (9.3) |
| 500 mg qm, 250 mg qnb | 1 (0.4) |
| 250 mg q12h | 3 (1.2) |
| 1,000 mg q6h | 2 (0.8) |
| 1,000 mg q8h | 61 (24.7) |
| 1,000 mg q12h | 14 (5.7) |
Mean ± standard deviation or median (minimum–maximum) is shown for continuous variables; proportion is shown for categorical variables.
qm, every morning; qn, every night.
Population PK models.
The decreases in objective function value (OFV) were 140.66 (two-compartment model to one-compartment model) and 4.32 (three-compartment model to two-compartment model). Therefore, the two-compartment model could adequately describe the imipenem concentration-time profile, which was set to be the structural model. The parameters to be estimated were clearance (CL), central distribution volume (Vc), intercompartmental clearance (Q), and peripheral distribution volume (Vp). In the forward selection procedure, the covariates CLCR, body weight (WT), ECMO, CRRT, and aspartate transaminase (AST) were added to the parameter CL, with decreases in OFV to 75.84, 8.92, 8.38, 5.81, and 5.44, respectively. In a recursive backward elimination procedure, the increases in OFV were 57.79, 10.59, and 9.62, respectively, with the removal of CLCR, WT, and ECMO from CL. So the final model is
Residual variability was best described by a combined error model.
Estimates, relative standard errors, shrinkage of population PK parameters, and median parameter estimates (with 95% confidence intervals [CIs]) from 1,000 bootstrap replications are listed in Table 2. The PK estimates in the final model generally agreed with the median estimates and were contained within the 95% CI generated from the bootstrap results, indicating good precision in the final model. The interindividual variability (IIV) values associated with Q and Vp were fixed in the final model due to poor precision in omega estimates.
TABLE 2.
Final population pharmacokinetic parameter estimates
| Parameter | Two-compartment model estimate (% RSE) [% shrinkage]a | Bootstrap (n = 1,000) |
|
|---|---|---|---|
| Median | 95% CI | ||
| CL (liters/h) | 8.88 (5) | 8.85 | 7.96–9.80 |
| Vc (liters) | 20.5 (8) | 20.0 | 16.4–23.9 |
| Q (liters/h) | 1.74 (25) | 1.77 | 1.20–5.07 |
| Vp (liters) | 8.86 (41) | 8.52 | 5.60–22.91 |
| θCLCR_CL | 0.295 (1) | 0.296 | 0.243–0.358 |
| θWT_CL | 0.306 (32) | 0.316 | 0.137–0.519 |
| θECMO_CL | 1.16 (6) | 1.17 | 1.04–1.31 |
| Interindividual variability (% CV) | |||
| CL (liters/h) | 17.7 (8.4) [9.7] | 17.7 | 12.6–25.8 |
| Vc (liters) | 14.8 (25.5) [59.1] | 15.2 | 4.2–32.8 |
| Q (liters/h) | 0 FIX | ||
| Vp (liters) | 0 FIX | ||
| Correlation | |||
| ηCLηVc | 0.102 (45) | 0.108 | 0.036–0.225 |
| Residual error (% CV if proportional, SD if additive) | |||
| Σ1, proportional | 6.2 (14.4) [23.2] | 5.9 | 4.0–7.6 |
| Σ2, additive | 0.003 (126.4) [23.2] | 0.004 | 0.000–0.044 |
% RSE, percentage of relative standard error (100 × [standard error/estimate]). FIX, parameter was fixed to a certain value during the modeling process.
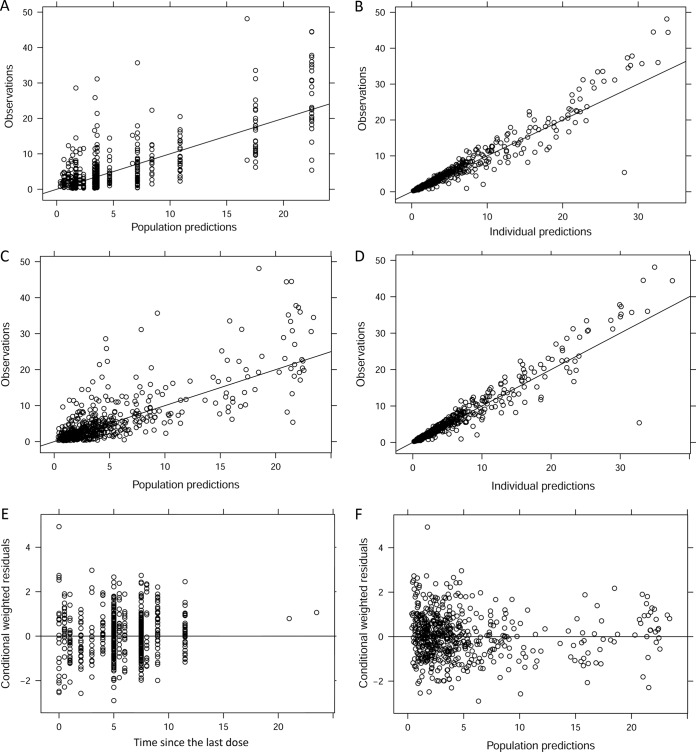
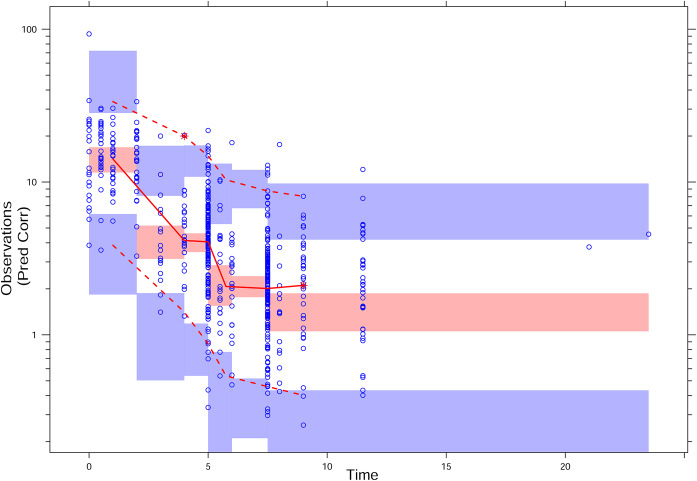
The goodness-of-fit (GOF) plots for the basic and final population model are depicted in Fig. 2. The observation-versus-population prediction plots showed an improvement in the final model over in the basic model. Observation-versus-prediction data were evenly distributed across the line of identity. Most of the conditional weighted residual values (CWRES) were within the range from −4 to 4. Using the final population PK model, a visual predictive check (VPC) with 95% CI for a simulated predicted median was calculated (Fig. 3). VPC plots demonstrate that most of the observed concentrations were overlaid within the 95% CI of the predictive interval of simulated data, suggestive of the adequate predictive performance of the final PK model. The results support a good model fit.
FIG 2.
Goodness-of-fit plots for the basic model (A and B) and the final PK model (C to F) with covariates.
FIG 3.
pcVPC plot for the final PK model. Prediction-corrected observed concentrations are shown as open circles. The lines represent the median (middle solid), 2.5th (lower dashed), and 97.5th (upper dashed) percentiles for the observed data. The shaded areas represent a 95% CI for a simulated predicted median and 2.5th and 97.5th percentiles constructed from 1,000 simulated individual data sets.
Monte Carlo simulations.
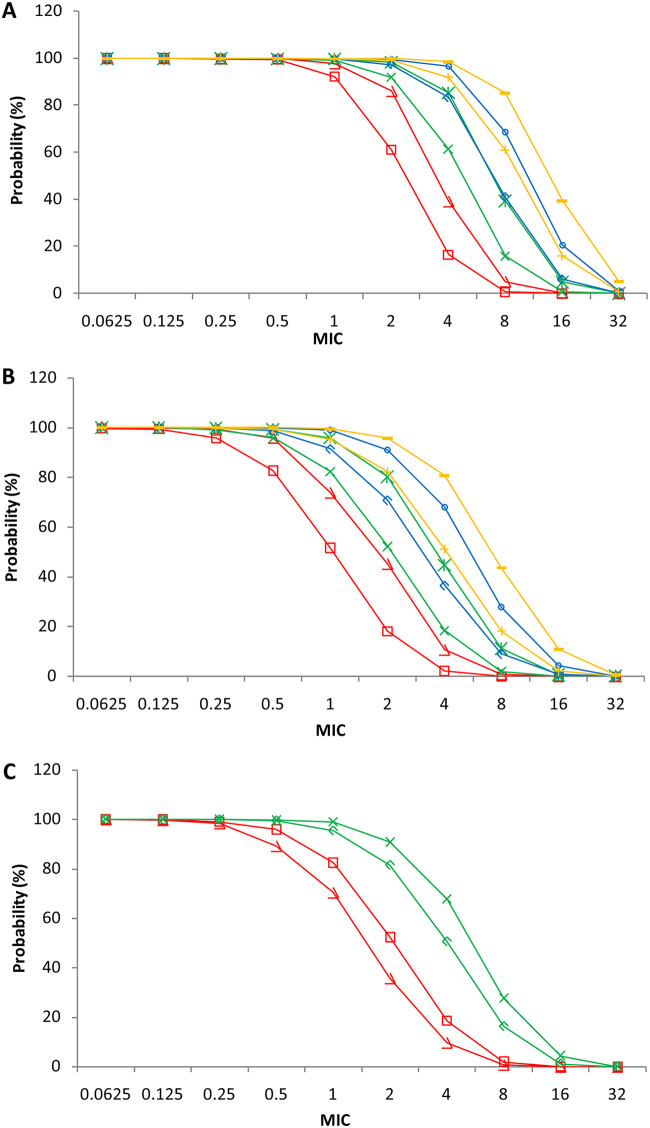
According to simulated time-dependent concentration data (at steady state), probabilities of target attainment (PTAs) (40% or 70% ƒT>MIC) was calculated for the 8 dosage regimens: 250, 500, 750, and 1,000 mg, q6h and q8h. The PTAs of 40%ƒT>MIC level are shown in Fig. 4A. In the 40%ƒT>MIC level, all the PTAs of the simulated patients were greater than 99.4% when the MIC ranged from 0.0625 to 0.5 μg/ml for the 8 dosage regimens. For a MIC of 1 μg/ml, the PTA was 92.1% with 250 mg (q8h) and 97.8 to 99.8% with the other 7 regimens. For a MIC of 2 μg/ml, PTAs were 61.0% and 86.0% with 250 mg (q8h) and 250 mg (q6h), respectively, which were below 90%. PTAs assessed for the rest of regimens were greater than 91.9%. For a MIC of 4 μg/ml, PTAs for 750 mg (q6h), 1,000 mg (q8h), and 1000 mg (q6h) exceeded 90% and were 96.5%, 91.9%, and 97.6%, respectively. For a MIC of 8 μg/ml, all of the assessed PTAs were below 90%.
FIG 4.
Simulated PTAs plotted against MIC for different imipenem dosage regimens at targets of 40% ƒ%T>MIC (A) and 70% ƒ%T>MIC (B) and for non-ECMO and ECMO patients at a target of 70% ƒ%T>MIC (C).
The PTAs of 70%ƒT>MIC are shown in Fig. 4B. At the 70%ƒT>MIC level, all of the PTAs of the simulated patients were greater than 95.9% when the MIC ranged from 0.0625 to 0.25 μg/ml (8 dosage regimens). For a MIC of 2 μg/ml, PTAs for 750 mg q6h and 1,000 mg q6h were 90.8% and 92.5%, respectively, and PTAs for the other regimens were less than 82.3% (750 mg q6h). For a MIC of 4 μg/ml, all of the measured PTAs were below 80.7%.
We also explored the impact of three significant covariates, CLCR, WT, and ECMO, on CL. As shown in Fig. 4C, we calculated the PTA reaching 70%ƒT>MIC for the dose of 500 mg (q8h, regular regimen) and 750 mg (q6h, PTA reaching over 90% for 70%ƒT>MIC when the MIC was 2 μg/ml) for ECMO or no-ECMO treatments. PTA in patients with ECMO decreased noticeably for both of the dosage regimens compared with that in patients without ECMO. PTA in patients with ECMO decreased from 90.8% (non-ECMO) to 81.5% at a MIC of 2 μg/ml. Other results, including 70%ƒT>MIC for the 500-mg (q8h) regimen with different levels of CLCR and WT, are listed in Table S1 in the supplemental material. PTAs increased with the decline of CLCR, and all PTAs were below 90% at a MIC of 2 μg/ml, with CLCR ranging from 20 to 100 ml/min. WT had a small influence on the PTA.
DISCUSSION
The emergence of carbapenem‐resistant bacteria has become a substantial worldwide problem. High rates of usage and inadequate drug exposure in critically ill patients can increase the development of resistant bacteria. The DALI study showed that 20% of patients given beta-lactam antibiotics fall below conservative PK/PD targets and 50% below suggested targets (10). Optimization of the PK exposure of antibiotics is suggested to achieve superior infection outcome in critically ill patients. In the present study, carbapenem was mainly used in the prevention and treatment of infections caused by members of the Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter. The MIC breakpoint for Enterobacteriaceae treated with imipenem was 2 μg/ml, while that for Pseudomonas and Acinetobacter was 4 μg/ml. However, the results of this study showed that the PTA for 500 mg q8h at a median CLCR of 59.1 ml/min reached the treatment target of 40% ƒ%T>MIC at MICs of 2 μg/ml and 4 μg/ml, respectively, resulting in PTA rates of 91.9% and 61.3%. The PK/PD treatment rate was not satisfactory at a MIC of 4 μg/ml, and a considerable number of patients could not meet the basic treatment requirements. For patients with severe infections, ƒ%T>MIC was expected to reach the treatment goal of 70%. At this level, the PTA rates were only 52.3% and 18.5%. Notably, the 500-mg q8h regimen was administered to 49.8% of patients in the present study, which resulted in the insufficient dosage of carbapenem antibiotics in the critically ill patients. We suggest administering imipenem at 750 mg q6h in critically ill patients. The corresponding PTAs of 40% ƒ%T>MIC reached 99.5% (MIC = 2 μg ml) and 96.5% (MIC = 4 μg/ml), and the PTAs for 70% ƒ%T>MIC were 90.8% and 67.8%. However, for insensitive bacterial infections, the dosage should be increased to 1,000 mg q6h. Given the dosages of 500 mg q6h (sensitive) and 1,000 mg q8h or q6h (insensitive) recommended by drug administration instructions for imipenem-cilastatin, the dosage for patients with severe infections should be increased appropriately.
Severe infections are often accompanied by life-threatening organ dysfunctions. Therefore, the liver and kidney function and biochemical indicators in critically ill patients are dramatically different from those in healthy people, although large individual differences are detected. In this study, we collected extensive data on imipenem TDM in patients enrolled without scope limitation for the variations in the above-mentioned indices. Therefore, the impact of various factors on PK of imipenem requires further clarification.
Our data show that CLCR can significantly affect CL, which is consistent with previous studies. In patients with a CLCR of ≤70 ml/min, the blood concentration of imipenem was increased. The corresponding PTA was also increased. For instance, when CLCR was 20 ml/min, the MIC was 4 μg/ml, and the administered dose was 500 mg (q8h), the 70%ƒT>MIC was still only 47.4%. In the case of insensitive (drug-resistant) bacteria and potential renal insufficiency, the drug dose should be lowered carefully.
CRRT was administered to 58 patients during the course of treatment and sampling, and this influenced the PK population model. However, the introduction of this factor reduced OFV only to 5.81, which was not included in the final model according to the recursive backward elimination criterion. The CLCR value was derived from serum creatinine (SCR), which can indirectly reflect the clearing effect of CRRT. Therefore, the influence of CRRT factors was indirectly reflected in the model during the optimization of CLCR. Additionally, WT partially can affect the model. However, three assessed WT levels of 50, 65, and 80 kg (10th, 50th, and 90th percentiles) in this study group showed that the differences in PTAs between groups were not significant. Accordingly, dose adjustment is not required for patients with weights in the range of 50 to 80 kg, although WT was found to have a significant influence on CL in the PK model.
Furthermore, we found that ECMO treatment had a significant effect on CL compared with no ECMO. The imipenem CL in ECMO patients was increased, while the plasma concentration of this drug was decreased. The estimate of CL in non-ECMO patients with median CLCR and WT was 8.88 liters/h, which is close to the CL in healthy volunteers (7.95 to 12.1 liters/h) (19, 20). In ECMO patients, the estimate of CL dramatically increased to 28.3 liters/h. The published PK studies of imipenem in critically ill patients are limited, and mean values of CL have obvious variance, ranging from 13.2 to 23.2 liters/h (12, 13). The ECMO circuit can increase V, changing CL and elimination of drugs because of drug sequestration and the patient’s altered physiological condition. Imipenem has been found to be hydrophilic and to have low protein binding, so sequestration may not be the main reason for change of CL in patients with ECMO. CRRT and augmented renal clearance (ARC) may cause the increase of CL, which commonly happens in ECMO patients. There were more ECMO patients receiving CRRT (27%) than non-ECMO patients (21%) in present study, and the median value of CLCR in ECMO patients (64.2 ml/min) was somewhat higher than that of non-ECMO patients (57.9 ml/min). The differences may be among the reasons why CL was higher in ECMO patients, but other reasons should be investigated in further studies. Accordingly, the PTA was also decreased. In the ECMO-treated patients, PTAs were 81.5% (MIC = 2 μg/ml) and 50.6% (MIC = 4 μg/ml) for the 750-mg q6h regimen at 70% ƒT>MIC, compared with PTAs of 90.8 and 67.8% in the patients without ECMO treatment. Therefore, in ECMO-treated patients, treatment-related failure should be expected to occur due to an insufficient dosage. It is suggested that the dosage can be increased to 1 g q6h to improve the treatment success rate.
There are limited PK studies on the dosage of imipenem in ECMO-treated patients. We found only two studies that explored the imipenem dosage and PK in critically ill patients using ECMO (7, 21, 22). One of the studies included only 10 patients, and its results showed great individual differences in the imipenem PK in this small population (7). Another case report demonstrated that the trough concentrations of imipenem were 11.3 and 2.7 mg/liter, with a large difference in the dosage and effects found in two patients with ECMO (22). The number of subjects reported in these two studies is limited, and the individual differences of imipenem PK are large. Notably, it is difficult to analyze and compare the PK differences between ECMO and non-ECMO patients accurately. In the current study, we used more TDM data, which resulted in the detection of the ECMO-related influence, although the number of ECMO patients is still limited (48 of 247 patients). This limitation may be why the influence of ECMO was not as significant as that of CLCR, so data from more ECMO subjects should be collected in further studies. Although many factors can affect imipenem PK in critically ill patients, a large set of patient data is required to deliver an accurate analysis of a factor-related impact on the imipenem PK.
The analyzed imipenem PK shows a large individual variability. The coefficients of variation (CVs) of interindividual variability were 16.9%, 8.3%, 10.6%, and 15.1% in the basic model for CL, Vc, Q, and Vp, respectively, and the CVs decreased to 11.1%, 7%, 9.4%, and 11.5% in the final model. In this study, we found that CLCR, WT, and ECMO can significantly affect the drug concentration and dosage in vivo. According to our analysis, it is difficult to guarantee a high treatment success rate for patients treated with a single dose of the drug. Therefore, it is recommended to perform routine TDM and optimize an individual dose for each patient and/or a group of similar patients. We routinely monitor imipenem dosages in critically ill patients and estimate the ƒ%T>MIC based on the one-compartment PK model with two blood concentrations measured at the elimination phase. Most of the data presented here were derived from the measured TDM concentrations. The developed population PK model may be able to be further combined with Bayesian and other relevant analysis methods to optimize the individualized drug dosage regimen based on the monitored concentrations.
Conclusion.
In this study, we established a PK population model of imipenem and evaluated the effects of the physiological factors ECMO and CRRT on PK parameters. The results showed that CLCR, WT, and ECMO affected the PK parameter CL. Results also suggested that there may be insufficient drug use in clinical routine drug dose regimens. It was found that a dosage of 750 mg q6h could achieve a higher treatment success rate. The blood concentrations of imipenem in ECMO patients were lower than those in non-ECMO patients; therefore, an insufficient dosage should be paid attention to, and dosage needs to be increased. The dosage may need adjustment for patients with CLCR values of ≤70 ml/min, but doses should be lowered carefully to avoid insufficient drug exposure. Dose adjustment is not necessary for patients with WT ranging from 50 to 80 kg in spite of the significant influence of WT on CL in the PK model. Due to the large variation in individual PK levels of imipenem in critically ill patients, TDM should be carried out to optimize their drug regimens.
MATERIALS AND METHODS
Study design.
All TDM data were collected retrospectively from critically ill patients who received intravenous imipenem as part of their treatment in the China-Japan Friendship Hospital between April 2016 and June 2019. The inclusion criteria for subjects were as follows: (i) patients ≥18 years old; (ii) sex (male and female); (ii) patients who were administered imipenem after clinical diagnosis; (iv) patients without additional lifestyle risk factors, such as smoking; and (v) patients with or without ECMO. Subjects were excluded if (i) blood sampling was not available at steady state after 4 doses of imipenem and/or (ii) all of the collected concentrations were below the lower limit of quantitation (LLOQ). A total of 234 patients for whom imipenem plasma concentrations from TDM data were obtained were included in the study.
The data for another 13 patients from a previous PK study conducted in our hospital were also included. The inclusion criteria for subjects were as follows: (i) age of 18 to 80 years old; (ii) sex (male and female); (iii) ECMO treatment, followed by withdrawal of ECMO; and (iv) imipenem use both during ECMO and after withdrawal. Subjects were excluded if (i) blood sampling was not available at steady state after 4 doses of administration with or without ECMO and/or (ii) the patient died before ECMO withdrawal.
All patient demographics were collected from the electronic medical record. These demographics included sex (male = 1, female = 0), age, weight (WT), height (HT), body mass index (BMI), serum creatinine (SCR), disease diagnosis, dose strategy, alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), total bilirubin in serum (TBIL), hemoglobin (HGB), platelet count (PLT) within 3 days around imipenem TDM sampling time, whether the patient underwent continuous renal replacement therapy (CRRT) or not (yes =1, no = 0), and whether ECMO was applied (yes = 1, no = 0) within 3 days before imipenem TDM sampling time. Creatinine clearance (CLCR) was calculated according to the Cockcroft-Gault formula. This study was approved by the ethics committee of the China-Japan Friendship Hospital. Demographic and clinical information is summarized in Table 1.
Drug administration and blood sampling.
All patients received imipenem-cilastatin (Zienam; MSD, Munich, Germany) for treatment of severe Gram-negative bacterial infections in ICUs. The administration and sampling procedures generally consisted of the following two categories.
(i) TDM data were retrospectively collected. The patients were empirically administrated imipenem at the 1-h infusion dose of 250 to 1,000 mg, with intervals ranging from q6h to q12h according to CLCR levels, severity of illness, and etiology test results. Concentrations were measured after the 4th dose of the same dose regimen 3 h and 0.5 h before the next administration.
(ii) To collect PK data, patients who received ECMO during the imipenem anti-infective treatment were administrated a 500-mg q8h dose of imipenem at 0.5 h via infusion. Blood samples (2 to 4 ml) were collected immediately before administration and 0.5, 1, 2, 3, 6, and 8 h after the beginning of infusion after the 4th dose with ECMO and after its withdrawal.
Determination of total imipenem concentrations in plasma.
The blood samples with EDTA anticoagulant were immediately centrifuged at 3,000 × g for 5 min. The collected plasma samples were prepared and measured within the following 2 h using an ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method published previously by our TDM laboratory (23–25). The plasma samples were extracted with dichloromethane after precipitation of proteins by acetonitrile. Following this, the analytes were gradient eluted on a UPLC BEH (ethylene bridged hybrid) C18 column (2.1 mm by 50 mm; 1.7 μm) using UPLC-MS/MS with positive ionization. Ions monitored in multiple reaction monitoring (MRM) mode were m/z 300.0 to 171.0 for imipenem, 384.0 to 141.0 for meropenem, and 390.1 to 147.0 for meropenem-d6 (internal standard). The concentration range was 0.39 to 50 μg ml−1 of imipenem and meropenem. Concentrations below the LLOQ of 0.39 μg ml−1 were excluded. Determination of quality control samples (1.6 μg ml−1, 8.0 μg ml−1, and 40.0 μg ml−1) of imipenem and meropenem in plasma validated the LC-MS/MS method. Accuracy, extraction recovery, matrix effect, and intra-assay and interassay precision all met the requirements of quantitative analysis of in vivo concentration. This method of imipenem and meropenem determination by LC-MS/MS is considered a robust protocol that is consistent and reproducible.
Population pharmacokinetic model.
All data were subsequently processed by the nonlinear mixed effects modeling software NONMEM 7.2. Population PK analysis was executed via PsN (version 3.4.4). Pirana 2.5.1 was used as the interface to create PK models and perform simulations. Typical values of PK parameters were evaluated using first-order conditional estimation (FOCE). Parameters were assumed to follow the log-normal distribution across the population. The residual error was characterized by a combined proportional and additive error model.
For the structural PK model, one-, two-, and three-compartment models were compared. The model was implemented in the PREDPP subroutine ADVAN3 in NONMEM. Interindividual variability (IIV) was assumed to be log-normally distributed. For example, for clearance (CL) of subject i, where CLTV is the typical value of clearance and is IIV in CL, assuming a mean of 0 and variance of Ω (2). Covariance between IIVs was estimated using a variance-covariance matrix. Additive, proportional, and combined models were tested for residual variability.
All of the potential demographic or physiologic covariates were considered, and the most significant covariate was added to the basic model; then, the other covariates were tested stepwise using a forward selection procedure. The values of individual parameters for each subject were plotted against these covariates to graphically inspect their relationships. For continuous covariates, including WT, age, and CLCR, the influence of the covariate was modeled as follows (e.g., for imipenem CL):
where Covi is the value of the covariate of the ith individual, Covm is the mean or median value of the covariate, and is the fixed effect of the covariate on CL. For categorical covariates, including sex, CRRT, and ECMO, the influence of the covariate on CL was modeled as follows: .
All of the steps in the development of the PK model were executed on the basis of the likelihood ratio test. An additional parameter was reserved if the resulting OFV decrease was greater than 3.84 (P < 0.05). Model selection was also based on graphical criteria such as goodness-of-fit plots and visual predictive checks (VPCs).The full model was constructed after all the covariates which contributed to the optimization of the model had been included. A recursive backward elimination procedure was then performed to further refine the model. The added covariates were removed from the model one by one. If the increase in OFV was less than 7.88 (P < 0.005) during the exclusion, the covariate was excluded in the final model.
The precision of the parameter estimates in the final model was evaluated using the bootstrap method. The median parameter estimates obtained from 1,000 bootstrap replicates were compared with estimates of PK parameters. Model stability was evaluated by condition number, with a condition number greater than 1,000 being considered indicative of severe collinearity. A prediction-corrected VPC (pcVPC) plot was also used for validation of the final model. We simulated 1,000 Monte Carlo samples using the model and the parameter estimates, after which the 10th, 50th, and 90th percentiles of observed data over time and their corresponding 90% prediction intervals were depicted in the VPC plot.
Monte Carlo simulation for dosage regimen evaluation.
A Monte Carlo simulation with 1,000 subjects was performed using the final PK model in NONMEM to calculate the probability of target attainment (PTA) at a specific value of a PK/PD index for different dosage regimens. We studied 8 dosage regimens, including 250 mg, 500 mg, 750 mg, and 1,000 mg. Administration was performed at q6h or q8h, and the infusion time was 1 h. For investigation of different dose regimens, each subject was given standard values of covariates included in the final model. Residual variability was also accounted for in the simulation. The influence of different levels of covariates on PTA was also evaluated. The MICs of imipenem were determined according to the Clinical and Laboratory Standards Institute (26) guidelines. The investigated MICs were 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg/ml for various bacterial infections. Imipenem showed only minimal serum protein binding (20%) (2). Therefore, we included ƒ as 0.8 in the calculation. PTA was assessed as a fraction that achieved 40% ƒ%T>MIC or 70% ƒT>MIC.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Fund of Clinical Pharmacy Research Project of Beijing Pharmaceutical Association (2019), Youth Program of National Natural Science Foundation of China (no. 81302843), National Key Research and Development Program of China (no. 2016YFC1304300), and National Natural Science Foundation of China (no. 81870072).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wu LJ, Jin HJ, Xu LP. 2014. Meta-analysis on meropenem and imipenem/cilastatin sodium in the treatment of bacterial infection. World Notes Antibiotics 35:28–31. [Google Scholar]
- 2.Kumar ST, Yassin A, Bhowmick T, Dixit D. 2017. Recommendations from the 2016 guidelines for the management of adults with hospital-acquired or ventilator-associated pneumonia. P T 42:767–772. [PMC free article] [PubMed] [Google Scholar]
- 3.China Medical Education Association, Infection Disease Committee. 2018. Expert consensus on clinical application of pharmacokinetic/pharmacodynamic theory of antibacterial drugs. Chin J Tuberc Respir Dis 41:409–446. [Google Scholar]
- 4.Wang H, Zhang B, Ni YX, Kuti JL, Chen BY, Chen MJ, Nicolau DP. 2007. Pharmacodynamic target attainment of seven antimicrobials against Gram-negative bacteria collected from China in 2003 and 2004. Int J Antimicrob Agents 30:452–457. doi: 10.1016/j.ijantimicag.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Lipš M, Siller M, Strojil J, Urbánek K, Balík M, Suchánková H. 2014. Pharmacokinetics of imipenem in critically ill patients during empirical treatment of nosocomial pneumonia: a comparison of 0.5-h and 3-h infusions. Int J Antimicrob Agents 44:358–362. doi: 10.1016/j.ijantimicag.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 6.He J, Xu SL, Shao H, Zhan Y, Hu RR, Hu LL. 2018. Optimization of the concentration monitoring method of meropenem in critically ill patients and its clinical application. Chin Hosp Pharm J 38:416–419. [Google Scholar]
- 7.Jaruratanasirikul S, Vattanavanit V, Samaeng M, Nawakitrangsan M, Sriwiriyajan S. 2019. Pharmacokinetics of imipenem in critically ill patients with life-threatening severe infections during support with extracorporeal membrane oxygenation. Clin Drug Invest 39:787–798. doi: 10.1007/s40261-019-00796-3. [DOI] [PubMed] [Google Scholar]
- 8.Ye LQ, Shi LH, Qiu XH, Jin YH, Cai T. 2015. Pharmacodynamics of prolonged dosing regimens of four β-lactam antibiotics against severe infection. Chin J Clin Pharmacol Ther 20:51–55. [Google Scholar]
- 9.Xu B, Guo SW, Li X, Zhou GQ, Zhu YQ, Li Y. 2016. Therapeutic drug monitoring coupled with Bayesian forecasting-based pharmacokinetic/pharmacodynamic analysis of meropenem injection. Chin J Clin Pharmacol 32:2141–2144. [Google Scholar]
- 10.Roberts JA, DALI Study, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, Margarit Ribas A, De Waele JJ, et al. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 11.Xu GQ, Zhu LQ, Liu W, Liao SS, Ge TY, Yang JW. 2015. Optimizing dosage regimens of imipenem in multiple organ dysfunction failure patients receiving continuous veno-venous hemofiltration. Chin J New Drugs Clin Rem 34:802–806. [Google Scholar]
- 12.Couffignal C, Pajot O, Laouénan C, Burdet C, Foucrier A, Wolff M, Armand-Lefevre L, Mentré F, Massias L. 2014. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator-associated pneumonia and evaluation of dosage regimens. Br J Clin Pharmacol 78:1022–1034. doi: 10.1111/bcp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaruratanasirikul S, Wongpoowarak W, Jullangkoon M, Samaeng M. 2015. Population pharmacokinetics and dosing simulations of imipenem in serious bacteraemia in immunocompromised patients with febrile neutropenia. J Pharmacol Sci 127:164–169. doi: 10.1016/j.jphs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Boucher BA, Hudson JQ, Hill DM, Swanson JM, Wood GC, Laizure SC, Arnold-Ross A, Hu ZY, Hickerson WL. 2016. Pharmacokinetics of imipenem/cilastatin burn intensive care unit patients undergoing high-dose continuous venovenous hemofiltration. Pharmacotherapy 36:1229–1237. doi: 10.1002/phar.1866. [DOI] [PubMed] [Google Scholar]
- 15.Kong L, Xu H, Wu C, Zhao X, Wu X. 2018. Pharmacokinetics of imipenem in plasma and cerebrospinal fluid in patients with intracerebral hemorrhage. Eur J Clin Pharmacol 74:1193–1195. doi: 10.1007/s00228-018-2488-3. [DOI] [PubMed] [Google Scholar]
- 16.Wen A, Li Z, Yu J, Li R, Cheng S, Duan M, Bai J. 2016. Clinical validation of therapeutic drug monitoring of imipenem in spent effluent in critically ill patients receiving continuous renal replacement therapy: a pilot study. PLoS One 11:e0153927. doi: 10.1371/journal.pone.0153927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournier A, Eggimann P, Pagani JL, Revelly JP, Decosterd LA, Marchetti O, Pannatier A, Voirol P, Que YA. 2015. Impact of the introduction of real-time therapeutic drug monitoring on empirical doses of carbapenems in critically ill burn patients. Burns 41:956–968. doi: 10.1016/j.burns.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen WQ, Hang YF, Zhang D, Li PM, Guo DJ, Wang XX, Kong XD, Zhang XL. 2018. Development of f%T>MIC calculation model and its application in individualized medication optimization of carbapenems in critical care patients. Chin Pharm J 53:1313–1317. [Google Scholar]
- 19.Jaruratanasirikul S, Raungsri N, Punyo J, Sriwiriyajan S. 2005. Pharmacokinetics of imipenem in healthy volunteers following administration by 2 h or 0.5 h infusion. J Antimicrob Chemother 56:1163–1165. doi: 10.1093/jac/dki375. [DOI] [PubMed] [Google Scholar]
- 20.Lee LS, Kinzig-Schippers M, Nafziger AN, Ma L, Sörgel F, Jones RN, Drusano GL, Bertino JS Jr.. 2010. Comparison of 30-min and 3-h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation. Diagn Microbiol Infect Dis 68:251–258. doi: 10.1016/j.diagmicrobio.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Hahn J, Choi JH, Chang MJ. 2017. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther 42:661–671. doi: 10.1111/jcpt.12636. [DOI] [PubMed] [Google Scholar]
- 22.Welsch C, Augustin P, Allyn J, Massias L, Montravers P, Allou N. 2015. Alveolar and serum concentrations of imipenem in two lung transplant recipients supported with extracorporeal membrane oxygenation. Transpl Infect Dis 17:103–‐105. doi: 10.1111/tid.12327. [DOI] [PubMed] [Google Scholar]
- 23.Li PM, Liu X, Liu JY, Chen WQ, Wang XX, Zhang XL. 2014. Rapid determination of meropenem concentration in human plasma by column-switching-ultra high performance liquid chromatography. Chin Pharm J 49:776–780. [Google Scholar]
- 24.Wang XX, He J, Cui G, Li PM, Kong WH, Zhang XL, Zhao T. 2014. Analysis of electrospray mass spectrum fragmentation regularity of carbapenem antibiotics. China Pharm 25:4294–4296. [Google Scholar]
- 25.Kong WH, Ju HS, Wang XX, Shao H, Zhang XL. 2014. Determination of imipenem, meropenem, panipenem, faropenem concentrations in human plasma by HPLC. Chin Pharm J 49:1247–1251. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed. (M100-S26). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.