Telacebec (Q203) is a new antitubercular drug with extremely potent activity against Mycobacterium ulcerans. Here, we explored the treatment-shortening potential of Q203 alone or in combination with rifampin (RIF) in a mouse footpad infection model. The first study compared Q203 at 5 and 10 mg/kg doses alone and with rifampin. Q203 alone rendered most mouse footpads culture negative in 2 weeks. Combining Q203 with rifampin resulted in a relapse-free cure 24 weeks after completing 2 weeks of treatment, compared to a 25% relapse rate in mice receiving RIF with clarithromycin, the current standard of care, for 4 weeks.
KEYWORDS: Buruli ulcer, Mycobacterium ulcerans, Q203, Telacebec
ABSTRACT
Telacebec (Q203) is a new antitubercular drug with extremely potent activity against Mycobacterium ulcerans. Here, we explored the treatment-shortening potential of Q203 alone or in combination with rifampin (RIF) in a mouse footpad infection model. The first study compared Q203 at 5 and 10 mg/kg doses alone and with rifampin. Q203 alone rendered most mouse footpads culture negative in 2 weeks. Combining Q203 with rifampin resulted in a relapse-free cure 24 weeks after completing 2 weeks of treatment, compared to a 25% relapse rate in mice receiving RIF with clarithromycin, the current standard of care, for 4 weeks. The second study explored the dose-ranging activity of Q203 alone and with RIF, including the extended activity of Q203 after treatment discontinuation. The bactericidal activity of Q203 persisted for ≥ 4 weeks beyond the last dose. All mice receiving just 1 week of Q203 at 2 to 10 mg/kg were culture negative 4 weeks after stopping treatment. Mice receiving 2 weeks of Q203 at 0.5, 2, and 10 mg/kg were culture negative 4 weeks after treatment. RIF did not increase the efficacy of Q203. A pharmacokinetics substudy revealed that Q203 doses of 2 to 10 mg/kg in mice produce plasma concentrations similar to those produced by 100 to 300 mg doses in humans, with no adverse effect of RIF on Q203 concentrations. These results indicate the extraordinary potential of Q203 to reduce the duration of treatment necessary for a cure to ≤ 1 week (or 5 doses of 2 to 10 mg/kg) in our mouse footpad infection model and warrant further evaluation of Q203 in clinical trials.
INTRODUCTION
The World Health Organization’s recommended treatment for Buruli ulcer (BU), also known as Mycobacterium ulcerans disease, recently evolved from an 8-week regimen of rifampin (RIF, R) at 10 mg/kg of body weight plus streptomycin (STR) to an 8-week regimen of RIF plus clarithromycin (CLR, C) to eliminate the need for the injectable agent STR and to avoid its related ototoxicity (1, 2). However, CLR has more limited activity than STR against M. ulcerans in mouse models of the disease, and RIF induces the metabolism of CLR, which likely limits the contribution of CLR to the regimen (3–5). Nonetheless, clinical studies have shown good efficacy of the RIF+CLR regimen (6).
Despite the success of the RIF+CLR regimen, shortening the duration of BU treatment remains an important research objective. We previously investigated replacement of STR and/or CLR with other drugs such as clofazimine and oxazolidinones in our mouse footpad infection model, as well as the impact of increasing rifamycin exposures using high-dose RIF or rifapentine (RPT), with the aim of reducing the treatment duration necessary for cure (5, 7–11). Although we identified novel combinations with efficacy superior to RIF+STR and/or RIF+CLR, none of these 2-drug combinations showed a potential to reduce the duration of treatment to less than 4 weeks in mice.
Telacebec (Q203, Q) is a new drug developed to treat tuberculosis by targeting the respiratory cytochrome bc1:aa3 complex (12). In in vitro and mouse models of tuberculosis, Q203 often exhibits bacteriostatic, rather than bactericidal, activity due to the presence of an alternative terminal oxidase, the cytochrome bd oxidase, that maintains electron transport chain (ETC) function and preserves viability (13). However, unlike Mycobacterium tuberculosis, classical strains of M. ulcerans have a naturally occurring mutation in the cydA gene that renders the cytochrome bd oxidase nonfunctional (14). Therefore, most M. ulcerans strains causing BU are exquisitely susceptible to Q203 with very low MIC values of 0.000075 to 0.00015 μg/ml (15, 16). In vivo studies also show Q203 to be a very attractive candidate for treatment of BU. Scherr et al. (16) showed that Q203 alone at a daily dose of just 0.5 mg/kg was as effective as RIF+STR and rendered 9/10 mice culture negative with 8 weeks of treatment. Seeking a novel treatment-shortening regimen, we tested Q203 at 10 mg/kg/day in 3- and 4-drug combinations with high-dose RPT and other drugs acting on the ETC and oxidative phosphorylation (clofazimine [CFZ] and bedaquiline [BDQ]) and found that mouse footpads were sterilized after just 2 weeks of treatment (15).
In the present work, we explored the treatment-shortening potential of simpler, more readily implementable regimens based on Q203 alone or in combination with RIF. Two sequential experiments in the mouse footpad infection model assessed the dose-ranging efficacy of Q203 with or without normal and high-dose rifampin in tandem with pharmacokinetics (PK) analysis to better understand the human-equivalent doses. The results demonstrate that Q203 exposures recently demonstrated in phase 1 trials (17) are capable of sterilizing mouse footpads after as little as 1 week of treatment (5 doses), making Q203 an extraordinary candidate for clinical trials to shorten BU treatment.
RESULTS
Study 1, to determine the sterilizing efficacy of combining Q203 with standard and high doses of rifampin.
To determine if replacing CLR with Q203 in the RIF+CLR regimen has the potential to shorten the treatment of BU, we assessed the sterilizing efficacy of Q203 at 5 or 10 mg/kg when combined with RIF at 10- and 20-mg/kg doses.
(i) Footpad swelling and CFU counts.
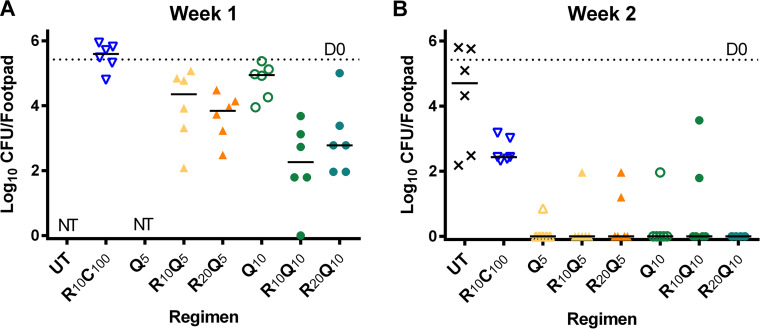
Mean (± standard deviation [SD]) footpad CFU counts on the day after infection were 2.71 ± 0.93 log10 CFU/footpad. Six weeks later, at the start of treatment (D0), the median swelling grade was ≥ 2.5 on a scale of 0 to 4 (9, 18) (Fig. 1), and the mean CFU count reached 5.42 ± 0.56 log10 CFU/footpad (Fig. 2). After 1 week of treatment, all treatment groups receiving Q203 had markedly reduced footpad swelling compared to R10C100 controls (Fig. 1) (henceforth, drugs are denoted in single letters followed by the dose in mg/kg shown in subscripts). The swelling grade in the R10C100 group remained unchanged, while mice treated with Q203-containing regimens, with the exception of Q5 alone, all had medians of ≤1 (Fig. 1). Similarly, the CFU counts at week 1 in the R10C100 group were significantly higher than those in all RQ groups except the Q10 alone group (Fig. 2A). After 2 weeks of treatment, footpads in all Q203-treated groups were almost normal compared to the R10C100 group, which still had swelling with a median grade of 2. The corresponding footpad cultures were negative in all R20Q10-treated mice and negative in nearly all other Q203-treated groups compared to 2.63 ± 0.37 log10 CFU in the R10C100 group (Fig. 2B). The limit of detection was 3 CFU per footpad. After 4 weeks of treatment, all mice had normal footpads, and no CFU were detected in any of the treatment groups tested. The limit of detection was 1 CFU per footpad. Mean CFU counts are provided in Table S1.
FIG 1.
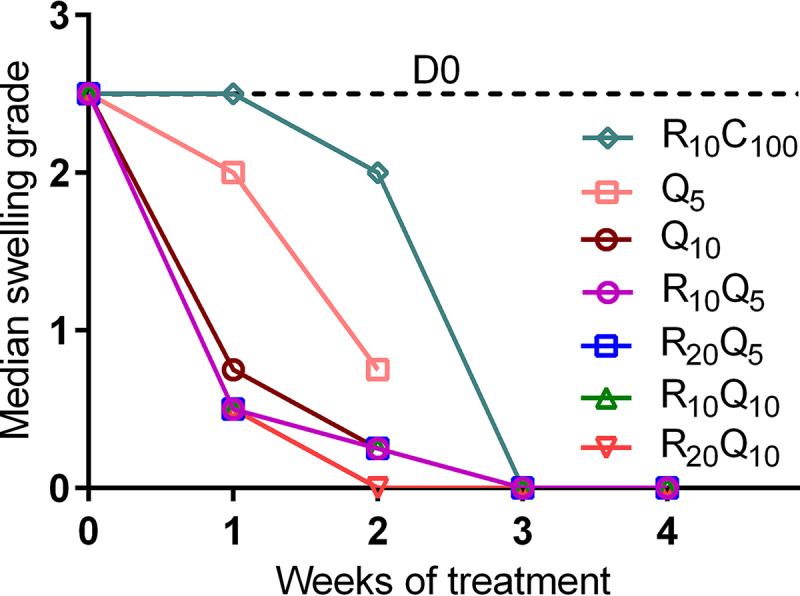
Footpad swelling grade of infected mouse footpads in response to treatment in study 1. Treatment was initiated 6 weeks after infection when swelling approached swelling grade 2.5. Swelling grade 0 corresponds to no clinically visible pathology, grade 1 infers redness of the footpad, grade 2 represents edematous swelling of the footpad, and grade 3 infers ascending swelling of the leg and impending necrosis. Data points represent medians per treatment group. Data were normalized to day 0 (beginning of treatment) by subtracting from the median swelling grade of all mice at D0 and assuming the total median as group mean for that time point. All Q203-containing regimens rapidly reduced swelling grade compared with R10C100 controls. By the end of 1 week of treatment, all Q203-containing regimens, except the lowest dose of Q203, 5 mg/kg, had reduced the swelling to below grade 1, while no change was seen in the RC treatment controls. By the end of 2 weeks, all mice treated with Q203-containing regimens had only residual swelling left; the median swelling grade was 0.25. Numbers in subscripts after drugs indicate doses in mg/kg. D, day; R, rifampin; C, clarithromycin; Q, Q203/Telacebec.
FIG 2.
Microbiological outcome in study 1. Mice were infected with 2.71 ± 0.93 log10 CFU/footpad of M. ulcerans into both hind footpads. After 6 weeks of incubation, treatment was initiated (D0). At this time point, the CFU mean (± standard deviation [SD]) equaled 5.42 (±0.56). Groups of mice were sacrificed at week 1, week 2, and week 4, and footpads (n = 6) were dissected, minced, and plated on 7H11 selective agar for colony counting and CFU analysis. For statistical analysis, all test regimens were compared to R10C100 controls. (A) After 1 week of treatment, all Q203-containing regimens, except Q10 given alone, were significantly better than controls, with R10Q10 (P < 0.0001) and R20Q10 (P < 0.001) showing the best activity. (B) At week 2, most footpads in mice treated with Q203-containing regimens were culture negative and significantly better than R10C100 (P ≤ 0.0008). At week 4, none of the mice in the combination treatment groups, including R10C100 controls, were culture positive (data not shown). Monotherapy regimens were not tested at this time point. Numbers in subscript after drugs indicate doses in mg/kg. D, day; UT, untreated; R, rifampin; C, clarithromycin; Q, Q203/Telacebec; NT, not tested. Dashed line indicates the pretreatment CFU at D0. Horizontal lines indicate median values.
(ii) Relapse.
Relapse assessments were made 6 months after treatment completion in mice treated for 2 or 4 weeks. All mice treated with RIF+Q203 regimens showed no rebound in footpad swelling during the 6-month follow-up period, and the CFU counts were all zero (limit of detection, 1 CFU). In the R10C100 group, relapse was assessed only in mice treated for 4 weeks. Three mice experienced a rebound in footpad swelling during the 6-month follow-up period. Two of these mice required euthanasia before the planned relapse endpoint because one or both footpads had deteriorated beyond a lesion index of 3. Overall, the relapse rate in the R10C100 group was 25%, with 4/16 footpads positive for CFU (P = 0.10 versus other groups with 0/16 relapses).
Study 2, to determine the dose-ranging activity of Q203 alone and in combination with standard and high-dose rifampin, including the extended activity after treatment discontinuation.
After showing that Q203 alone at 5 to 10 mg/kg/day renders mouse footpads culture negative and combinations of RIF+Q203 sterilize footpads with just 2 weeks of treatment, we evaluated a lower dose range and shorter durations of Q203, alone and in combination with RIF. We also assessed the plasma PK of Q203 after single doses of 0.5-, 2-. and 10-mg/kg doses and determined plasma concentrations of Q203 at 3 to 4 days and 2 and 4 weeks after stopping treatment.
(i) Pharmacokinetics.
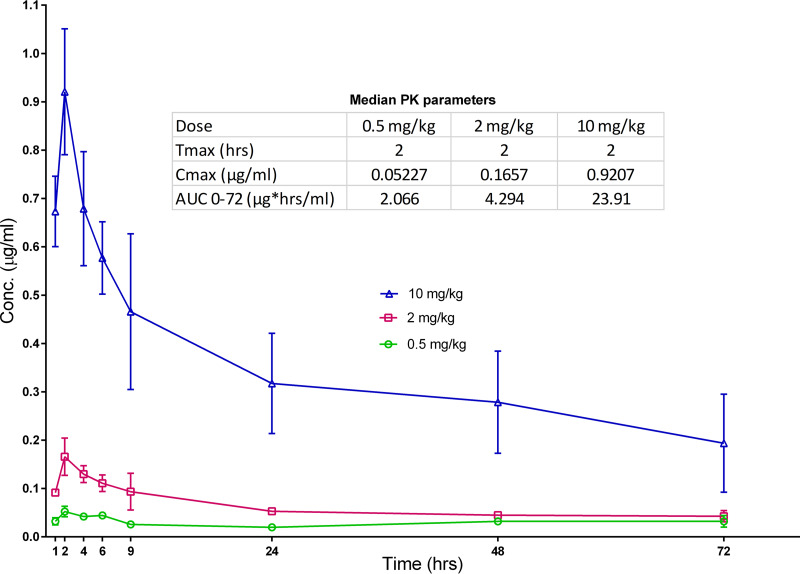
The single-dose PK results for Q203 are shown in Fig. 3. Q203 had a time to maximum concentration of drug in plasma (Tmax) of 2 h. The maximum concentration of drug in plasma (Cmax) values after 0.5-, 2-, and 10-mg/kg doses were 0.05, 0.17, and 0.92 μg/ml, respectively, indicating that even at the lowest dose, the Cmax was well above the MIC of 0.000075 to 0.00015 μg/ml. Plasma area under the concentration-time curve (AUC) values indicated dose-proportional exposures up to 2 mg/kg. After 1 week of treatment, in the lowest dose group tested, Q203 at 0.5 mg/kg, the mean plasma concentration of Q203 at 72 h postdose was 0.073 ± 0.024 μg/ml, and in the groups treated for 2 weeks, at 96 h postdose, it was 0.052 ± 0.013 μg/ml (Table S2). In both of these groups, the concentrations gradually declined during the 4-week follow-up period but remained higher than the MIC. With higher doses of Q203, more accumulation was seen, and it increased with the duration of treatment, with plasma concentrations in mice treated for 2 weeks almost twice as high as in those treated for 1 week. In mice treated with RIF+Q203, Q203 concentrations were similar to those in mice treated with Q203 alone, indicating no large effect of RIF on Q203 concentrations.
FIG 3.
Single-dose PK for Q203. Mice were dosed with either 0.5 mg/kg (green circle), 2 mg/kg (red squares), or 10 mg/kg (blue triangles) of Q203 and the blood collected for plasma concentrations at the indicated time points. Median PK parameters shown in the inset indicate dose-proportional exposures.
(ii) Footpad swelling.
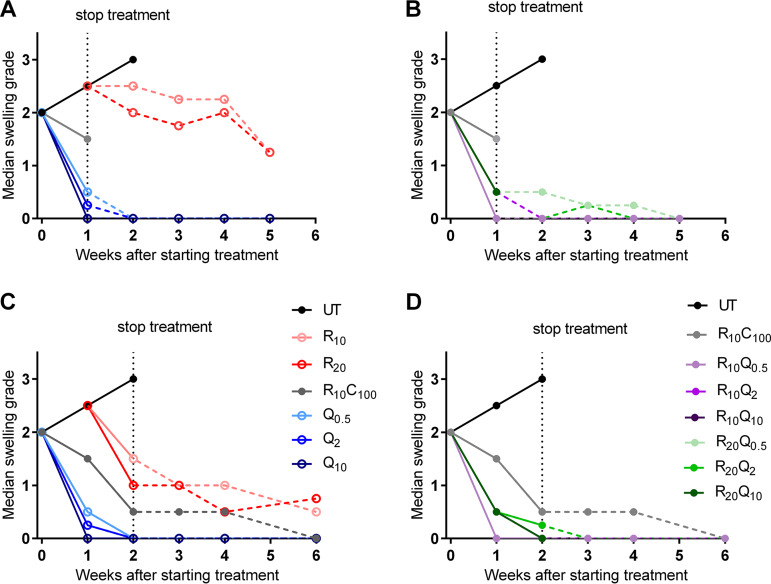
At the start of treatment, mice had a median swelling grade of 2 (Fig. 4). In untreated mice, the swelling increased to grade 2.5 and grade 3 at the end of weeks 1 and 2, respectively. All untreated mice required euthanasia at this point. In mice treated with R10C100, there was a marginal decrease to 1.5 at week 1 (Fig. 4A and B) and a further decrease to 0.5 at week 2 (Fig. 4C and D). After stopping treatment at 2 weeks, the swelling continued to decrease gradually during the 4 weeks of follow-up and reverted to baseline (Fig. 4C and D). In mice treated with RIF 10 mg/kg alone for 1 week, there was a slight decline in swelling after peaking at week 1 (Fig. 4A). At the end of 4 weeks, when all mice were sacrificed for CFU, the median swelling grade was 1.5, with one mouse showing grade 3 swelling. In mice treated with RIF 10 mg/kg for 2 weeks (Fig. 4C), the response to treatment was better than in mice treated for only 1 week. After 2 weeks of treatment, the swelling had reduced to median swelling grade of 1.5 and gradually decreased to 0.5 after 4 more weeks of follow-up without treatment. In mice treated with RIF 20 mg/kg, the response to treatment was similar to that of the 10-mg/kg group. As in the previous experiment, footpad swelling decreased rapidly in Q203-treated groups. All doses of Q203, whether given alone or in combination with RIF at 10 or 20 mg/kg, rapidly reduced footpad swelling. After 1 week of treatment, the swelling reverted to baseline in most mice, while some mice showed residual swelling (swelling grade < 1) (Fig. 4A). Irrespective of whether the treatment was stopped after 1 week or continued for an additional week, the footpads continued to improve during the follow-up period, with almost all footpads returning to baseline by week 2 and remaining free of swelling.
FIG 4.
Footpad swelling grade of infected mouse footpads in response to treatment in study 2. Treatment was initiated 6 weeks after infection when the median swelling grade approached 2. Data points represent medians per treatment group. (A and B) Swelling results in mice treated for 1 week. (C and D) Swelling results in mice treated for 2 weeks. Monotherapy groups are shown in panels A and C, while combination treatment groups are shown in panels B and D. R10C100 is the standard treatment control. Solid lines represent change in footpad swelling during treatment, while that after stopping treatment is shown by dashed lines. All Q203-containing regimens reduced footpad swelling after just 1 week of treatment and continued to show response after stopping treatment. Most footpads were at baseline levels after 2 to 3 weeks. RIF alone produced a slight decline in swelling after peaking at 1 week. The footpads never reached a median grade of 1 after 4 weeks of follow-up. As with 1 week of treatment, 2 weeks of Q203-containing regimens rapidly rendered footpads swelling free. In comparison, the RC-treated controls showed gradual decreases in footpad swelling. Numbers in subscripts after drugs indicate doses in mg/kg. D, day; UT, untreated; R, rifampin; C, clarithromycin; Q, Q203/Telacebec.
(iii) Footpad CFU counts.
At the start of treatment (D0), mean footpad CFU counts were 5.40 ± 0.39 log10. They increased to 5.71 ± 0.23 at week 1 (Fig. 5A and B) and 5.98 ± 0.23 at week 2 in untreated mice (Fig. 5C and D).
FIG 5.
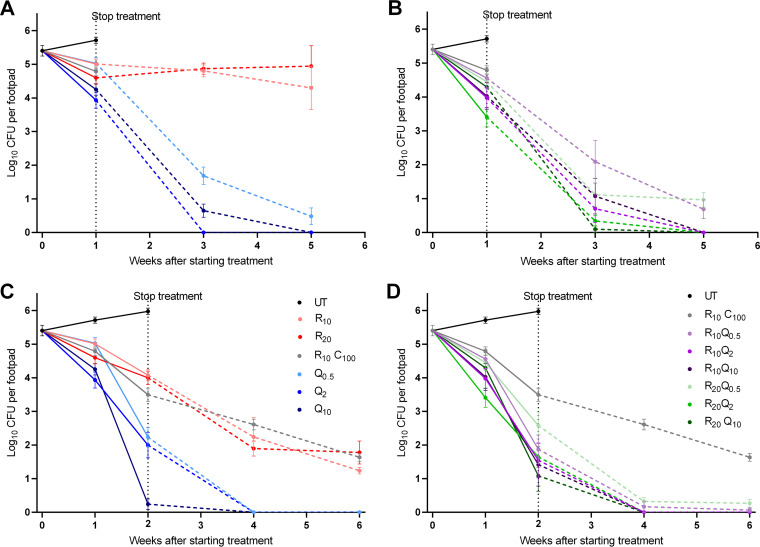
Microbiological outcome in study 2. (A and B) Response to treatment for 1 week. (C and D) Response to treatment for 2 weeks. Panels A and C show results for monotherapy, and panels B and D show combination treatment groups. Solid lines indicate fall in mean CFU (±standard error of the mean [SEM]) during treatment, and dashed lines show reduction after stopping treatment. After 1 week of treatment, Q203-containing regimens showed a marked dose response, and although CFU counts at week 1 were not significantly different than RC controls, more dramatic reductions occurred during the 4-week follow-up period after stopping treatment. All Q203-containing regimens except Q0.5 were significantly better after 1 week of treatment than RC treatment for 2 weeks. After 2 weeks of treatment, all Q203-containing regimens were significantly better than RC control after 2 weeks and rendered footpads negative at follow-up 2 weeks after stopping treatment.
In mice treated with R10C100, CFU counts decreased to 4.79 ± 0.32 log10 at week 1 and 3.50 ± 0.48 log10 at week 2. Continued killing was observed after stopping treatment, corroborating the observed reductions in footpad swelling during the 4-week follow-up period (Fig. 5C and D). In mice receiving RIF at 10 or 20 mg/kg, little change in CFU was seen after 1 week of treatment or during the 4-week follow-up period, again similar to what was seen in footpad swelling (Fig. 5A). Increasing the duration of RIF treatment to 2 weeks resulted in a 1.5 log10 CFU reduction in both dosage groups (Fig. 5C) and continued decreases to 1.24 ± 0.23 and 1.78 ± 0.83 4 weeks after completing treatment in the R10 and R20 groups, respectively.
A modest dose-dependent effect was observed in mice treated with Q203 alone for 1 week, with CFU counts falling to 5.03 ± 0.43, 3.93 ± 0.58, and 4.25 ± 0.42 log10 CFU in those receiving 0.5-, 2-, and 10-mg/kg doses, respectively. With the exception of R20Q2 (Fig. 5B) (P= 0.002), the reductions in CFU at week 1 were not significantly better than the R10C100 control. However, Q203 treatment for 1 week resulted in more dramatic reductions in CFU counts after treatment cessation. CFU counts in Q0.5-treated mice fell to 1.69 ± 0.64 and 0.48 ± 1.48 log10 CFU after 2 and 4 weeks of follow-up, respectively. No CFU were detected after 2 or 4 weeks of follow-up in mice treated with Q2 or 4 weeks after treatment with Q10 (Fig. 5A). Comparisons to the R10C100 group at the week 1+2 (where 1 represents 1-week duration of treatment and +2 represents 2 weeks of follow-up after treatment before sacrificing the mice) and week 1+4 time points were not possible since we did not include these time points for this group. However, all groups receiving Q203 doses ≥ 2 mg/kg had significantly lower CFU counts at the week 1+2 (P ≤ 0.0006) and week 1+4 (P < 0.0001) time points than R10C100 controls had at the week 2+2 and week 2+4 time points, respectively.
In mice treated for 2 weeks with Q203, a more prominent dose-response relationship was observed. In mice treated with Q203 at 10 mg/kg, the mean log10 CFU count was only 0.24 ± 0.38, with 4/6 pads negative. No CFU were detected in footpads in any Q203-treated group at 2 and 4 weeks follow-up (Fig. 5C). At this point, all Q203-containing regimens were significantly better than the R10C100 controls (P ≤ 0.05).
No benefit of adding RIF at either 10 or 20 mg/kg was seen, as CFU counts were very similar to those in groups receiving Q203 alone. Mice in the R10Q2 group received slightly more Q203 in the first week due to accidental gavage of mice with 10 mg/kg on day 4. This group was not treated on day 5 and thus received a 16 mg/kg total dose for the weeks as opposed to the intended 10 mg/kg. By the end of 2 weeks of treatment, they had received a 26 mg/kg total dose rather than the intended 20 mg/kg total dose. Mean CFU counts are given in Table S3.
DISCUSSION
The current treatment for BU recommended by WHO (1) is an oral regimen of RIF+CLR given daily for 8 weeks. This regimen offers advantages over the previously recommended RIF+STR combination (2). However, it remains problematic because treatment duration inversely correlates with adherence, and patients are often hospitalized until there is clear-cut evidence of treatment response, including resolution of any paradoxical reaction (19), resulting in missed school or work activities. As an extremely potent inhibitor of M. ulcerans respiration, Q203 is an exceptional candidate for treatment-shortening regimens. Recently, we described 3-drug combinations of drugs active on the ETC with and without rifapentine that appeared capable of shortening the treatment of BU (15). Q203-containing regimens proved to be most effective and cured all mice after treatment for just 2 weeks. However, none of the companion drugs in those regimens is currently used in the treatment of BU. Reasoning that RIF is already a core component of BU treatment regimens, we aimed to test Q203 alone and in combination with RIF, comprising regimens easier to implement in the clinical setting. Our results show that regimens of Q203 alone or in combination with RIF are clearly superior to RIF+CLR and may be capable of reducing the duration of BU treatment to 1 to 2 weeks.
Little information about the PK of Q203 in humans exists in the public domain (17), and published PK data from mice report very different drug exposures for the same or similar doses (12, 16). To better understand the dose-response profile of Q203 in mice and the human-equivalent doses of the Q203 doses evaluated in our model, we evaluated Q203 doses ranging from 0.5 to 10 mg/kg and included PK analyses. Remarkably, Q203 alone at 2 mg/kg rendered mouse footpads culture negative after just 5 daily doses. The median plasma Cmax and AUC from 0 to 72 hours (AUC0–72) values after a single oral dose in mice were 0.17 μg/ml and 4.3 μg-h/ml, respectively. These results are in line with mouse PK results from Pethe et al. (12) and, more importantly, comparable to the plasma Cmax and AUC0–∞ values of 0.38 μg/ml and 6.3 μg-h/ml, respectively, after a single dose of 100 mg in fed human (17) subjects. Considering that we observed dose-proportional PK in mice, Q203 doses of 2 to 10 mg/kg in mice likely correspond well to the daily doses of 100 to 300 mg that were recently reported to be well tolerated and safe in phase 1 trials and TB patients over 14 days of dosing in a recent phase 2a trial (22), provided that the drug is administered with food. Therefore, we predict that these doses can safely shorten the treatment of BU to 5 doses or less.
The extreme treatment-shortening effects of Q203 we observed in mice were the result of persistent killing of M. ulcerans that extended well beyond the end of dosing, even at the lowest dose of 0.5 mg/kg. This persistent killing is likely a function of multiple phenomena: exceptionally potent activity (e.g., very low MIC), low clearance (e.g., long plasma half-life), favorable partitioning into tissue (e.g., lung:plasma concentration ratio of 2 to 3) (12), and a postantibiotic effect (e.g., continued antimicrobial effect against M. ulcerans after plasma concentrations fall below the MIC). While the roles of the first 2 phenomena are self-evident from the PK/pharmodynamic (PD) data generated in study 2, more evidence is needed to confirm the partitioning of Q203 into mouse footpads and the presence of a postantibiotic effect. Treatment with 0.5 mg/kg for 1 to 2 weeks resulted in persistent killing for at least 4 weeks beyond the end of dosing. Although plasma concentrations were approximately 5 to 10 times higher than MIC at 2 weeks posttreatment and in the MIC range at 4 weeks posttreatment, Q203 is 99.8% protein bound in mouse plasma. Therefore, it seems likely that free drug concentrations at the site of infection fell below the MIC during the 4-week follow-up period, thus suggesting the presence of a postantibiotic effect. M. ulcerans may be especially vulnerable to postantibiotic effects in vivo if drug treatment shuts down production of the immunosuppressive mycolactone toxin, allowing a more effective host immune response to develop and enhance bacterial clearance. Indeed, even RIF+CLR exhibited persistent effects after the end of dosing in study 2.
Another surprising finding of these experiments was that the addition of RIF did not significantly increase the treatment efficacy of Q203. Other than modest additive effects of RIF with Q203 at 0.5 mg/kg at the week 1 and week 2 time points, there was no evidence of additive activity, especially at 2 mg/kg and above. Our PK results did not show any significant differences in Q203 plasma concentrations when RIF and Q203 were coadministered compared to Q203 given alone. These results raise the prospect of using Q203 as monotherapy, a scenario that may be defensible because the spontaneous frequency of Q203 resistance mutations in M. ulcerans appears to be very low (16) and M. ulcerans is not transmitted from person to person, making resistance development both unlikely to occur as well as unlikely to have any impact beyond the affected individual.
In summary, we have demonstrated the extraordinary potential of Q203 to reduce the duration of treatment for BU to 1 week (or 5 doses of 2 to 5 mg/kg) in our mouse footpad infection model. As these doses appear to be a good representation of doses recently tested successfully in humans, they warrant consideration for further evaluation in clinical trials for BU treatment. Importantly, we did not define the shortest duration of Q203 treatment needed to eradicate M. ulcerans from mouse footpads. Studies evaluating even shorter durations of Q203 with and without additional companion drugs are underway.
MATERIALS AND METHODS
Bacterial strain.
M. ulcerans strain 1059, originally obtained from a patient in Ghana, was used for the study (20).
Antibiotics.
RIF was purchased from Sigma. CLR was purchased from the Johns Hopkins Hospital pharmacy. Q203 was kindly provided by the Global Alliance for TB Drug Development. RIF and CLR were prepared in sterile 0.05% (wt/vol) agarose solution in distilled water. Q203 was formulated in 20% (wt/wt) D-α tocopheryl polyethylene glycol 1000 (Sigma) succinate solution.
Mouse infection.
BALB/c mice (Charles River Laboratories) were inoculated subcutaneously in both hind footpads with 0.03 ml of a culture suspension containing M. ulcerans 1059. Treatment began 6 to 7 weeks (D0) after infection when the mice had footpad swelling of grade ≥2.
Treatment.
Mice were treated 5 days per week with 0.2 ml by gavage. Drug doses were chosen based on mean plasma exposures (i.e., similar area under the concentration-time curve [AUC] over 24 h postdose in blood) compared to human doses (7, 15). All animal procedures were conducted according to relevant national and international guidelines and approved by the Johns Hopkins University Animal Care and Use Committee.
(i) Study 1.
Mice were randomized to one of the seven treatment groups (Table S4). Control regimens included R10C100, Q5 alone, or Q10 alone, where the subscript represents the dose in mg per kg of body weight. Test regimens consisted of either R10Q5, R20Q5, R10Q10, or R20Q10, and mice were treated for either 2 or 4 weeks. Mice treated with Q203 alone were treated for only 2 weeks since they were only included in the experiment to inform the contribution of Q203, and we did not initially intend to explore the use of Q203 alone as monotherapy. CFU counts were performed after 1, 2, and 4 weeks of treatment to determine the response to treatment. To determine the sterilizing activity of each test regimen, mice were held without treatment for 6 months after completing 2 and 4 weeks of treatment. Relapse assessment for the R10C100 control group was done only after 4 weeks of treatment.
(ii) Study 2.
Mice were randomized to one of 12 treatment groups, which included Q203 at doses of 0.5, 2, and 10 mg/kg given alone or in combination with RIF at 10 or 20 mg/kg. Control groups were untreated or received R10 or R20 alone, or R10C100, which is the current standard of care (Table S5). Mice were treated for either 1 or 2 weeks. R10Q2-group mice were accidentally gavaged with Q203 at 10 mg/kg dose on day 4 of treatment. These mice were not gavaged on the following day and therefore received a cumulative dose of 16 mg/kg instead of the intended 10-mg/kg dose for the first week. By the end of 2 weeks of treatment, these mice had received a total dose of 26 mg/kg instead of the intended 20-mg/kg dose. Footpad CFU counts were done at treatment completion and also at 2 and 4 weeks after stopping treatment in each treatment group to determine the continued bactericidal activity of Q203-containing regimens.
Pharmacokinetics.
Intensive PK evaluation was done for groups receiving Q203 alone and in combination with RIF. After a single dose on D0, small-volume blood samples were collected in EDTA-containing tubes at 1, 2, 4, 6, 9, 24, 48, and 72 h postdose from the submandibular vein. To assess the clearance of Q203 after stopping treatment, samples were obtained at 72 h after the final dose in mice treated for 1 week and 96 h after the final dose in mice treated for 2 weeks. Blood samples were also obtained at the 2- and 4-week follow-up time points in mice treated with Q203 alone for 1 and 2 weeks and in mice treated with RIF+Q203 for 2 weeks. Samples from the R10Q2 group were excluded because mice in this group were accidentally gavaged with a 10-mg/kg dose on day 4 of treatment.
Evaluation of treatment response.
Treatment outcomes were evaluated based on (i) decrease in footpad swelling, denoted as swelling grade, and (ii) decrease in CFU counts. The swelling grade was scored as described previously (7). Briefly, the presence and the degree of inflammatory swelling of the infected footpad were assessed weekly and scored from 0 (no swelling) to 4 (inflammatory swelling extending to the entire limb) for all surviving mice. For CFU counts, six footpads (from three mice) were evaluated on the day after infection (D42) and at the start of treatment (D0) to determine the infectious dose and the pretreatment CFU counts, respectively. The response to treatment was determined by plating 6 footpads (from 3 mice) from each treatment group at predetermined time points. Footpad tissue was harvested after thorough disinfection with 70% alcohol swabs and then homogenized by fine mincing before suspending in sterile phosphate-buffered saline (PBS). Ten-fold serial dilutions and undiluted fractions of homogenate were plated in 0.5-ml aliquots on selective 7H11 agar and incubated at 32°C for up to 12 weeks before CFU counts were enumerated. In the second study, homogenates were plated on 7H11 agar supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) and 5% bovine plasma albumin to reduce any potential effects of Q203 carryover due to its long half-life (21).
To determine the sterilization activity of each test regimen in study 1, mice were held for relapse assessment for 6 months after completing 2 and 4 weeks of treatment. Results were compared to those from mice treated with R10C100 for 4 weeks. Footpads were inspected every 2 weeks for any signs of reswelling after stopping treatment. When reswelling was observed, mice were sacrificed when the swelling reached a lesion index ≥ 3 and the footpads were harvested and plated for CFU counts. At the end of the 6-month follow-up period, all remaining mice were sacrificed, and their footpads (16 footpads in each group) were harvested and plated for CFU. In study 2, instead of conducting relapse assessment at 6 months, we held mice without treatment for an additional 2 or 4 weeks after treatment completion before harvesting and plating for determination of CFU counts.
Statistical analysis.
GraphPad Prism 6 software was used to compare mean CFU counts in Q203-containing groups to the R10C100 control group using two-way analysis of variance with Bonferroni’s posttest to adjust for multiple comparisons. Proportions were compared using Fisher’s exact test.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (R01-AI113266).
We gratefully acknowledge TB Alliance for providing Q203 and Qurient for quantifying Q203 in mouse plasma. We thank Kingsley Asiedu for fruitful discussions and encouragement at the beginning of this study.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2017. Report from the meeting of the Buruli ulcer Technical Advisory Group. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. 2014. Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 8:e2739. doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dega H, Robert J, Bonnafous P, Jarlier V, Grosset J. 2000. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 44:2367–2372. doi: 10.1128/aac.44.9.2367-2372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentoucha A, Robert J, Dega H, Lounis N, Jarlier V, Grosset J. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 45:3109–3112. doi: 10.1128/AAC.45.11.3109-3112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. 2011. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans disease in mice. PLoS Negl Trop Dis 5:e933. doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips RO, Robert J, Abass KM, Thompson W, Sarfo FS, Wilson T, Sarpong G, Gateau T, Chauty A, Omollo R, Ochieng Otieno M, Egondi TW, Ampadu EO, Agossadou D, Marion E, Ganlonon L, Wansbrough-Jones M, Grosset J, Macdonald JM, Treadwell T, Saunderson P, Paintsil A, Lehman L, Frimpong M, Sarpong NF, Saizonou R, Tiendrebeogo A, Ohene SA, Stienstra Y, Asiedu KB, van der Werf TS, study team . 12 March 2020. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet doi: 10.1016/S0140-6736(20)30047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida DV, Omansen TF, Li SY, Lee J, Grosset JH, Converse PJ, Nuermberger EL. 2019. Oxazolidinones can replace clarithromycin in combination with rifampin in a mouse model of Buruli ulcer. Antimicrob Agents Chemother 63:e02171-18. doi: 10.1128/AAC.02171-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida DV, Converse PJ, Li S, Tyagi S, Nuermberger EL, Grosset JH. 2013. Bactericidal activity does not predict sterilizing activity: the case of rifapentine in the murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 7:e2085. doi: 10.1371/journal.pntd.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omansen TF, Almeida D, Converse PJ, Li SY, Lee J, Stienstra Y, van der Werf T, Grosset JH, Nuermberger EL. 2018. High-dose rifamycins enable shorter oral treatment in a murine model of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 63:e01478-18. doi: 10.1128/AAC.01478-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Converse PJ, Tyagi S, Xing Y, Li SY, Kishi Y, Adamson J, Nuermberger EL, Grosset JH. 2015. Efficacy of rifampin plus clofazimine in a murine model of Mycobacterium ulcerans disease. PLoS Negl Trop Dis 9:e0003823. doi: 10.1371/journal.pntd.0003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Converse PJ, Almeida DV, Tasneen R, Saini V, Tyagi S, Ammerman NC, Li S, Anders NM, Rudek MA, Grosset JH, Nuermberger EL. 2018. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl Trop Dis 12:e0006728. doi: 10.1371/journal.pntd.0006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han SJ, No Z, Lee J, Brodin P, Cho SN, Nam K, Kim J. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 13.Kalia NP, Shi Lee B, Ab Rahman NB, Moraski GC, Miller MJ, Pethe K. 2019. Carbon metabolism modulates the efficacy of drugs targeting the cytochrome bc1:aa3 in Mycobacterium tuberculosis. Sci Rep 9:8608–8609. doi: 10.1038/s41598-019-44887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PD, Davies JK, Jenkin GA, Small PL, Jones LM, Tekaia F, Laval F, Daffe M, Parkhill J, Cole ST. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Converse PJ, Almeida DV, Tyagi S, Xu J, Nuermberger EL. 2019. Shortening Buruli ulcer treatment with combination therapy targeting the respiratory chain and exploiting Mycobacterium ulcerans gene decay. Antimicrob Agents Chemother 63:e00426-19. doi: 10.1128/AAC.00426-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherr N, Bieri R, Thomas SS, Chauffour A, Kalia NP, Schneide P, Ruf MT, Lamelas A, Manimekalai MSS, Gruber G, Ishii N, Suzuki K, Tanner M, Moraski GC, Miller MJ, Witschel M, Jarlier V, Pluschke G, Pethe K. 2018. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer. Nat Commun 9:5370–5378. doi: 10.1038/s41467-018-07804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J. 2017. Q203 update. In Critical PAth to Tuberculosis Regimens (CPTR) Workshop. CPTR Initiative, Bethesda, MD. [Google Scholar]
- 18.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother 46:3193–3196. doi: 10.1128/aac.46.10.3193-3196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frimpong M, Agbavor B, Duah MS, Loglo A, Sarpong FN, Boakye-Appiah J, Abass KM, Dongyele M, Amofa G, Tuah W, Frempong M, Amoako YA, Wansbrough-Jones M, Phillips RO. 2019. Paradoxical reactions in Buruli ulcer after initiation of antibiotic therapy: relationship to bacterial load. PLoS Negl Trop Dis 13:e0007689. doi: 10.1371/journal.pntd.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson HR, Benbow ME, Nguyen KD, Beachboard DC, Kimbirauskas RK, McIntosh MD, Quaye C, Ampadu EO, Boakye D, Merritt RW, Small PL. 2008. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl Trop Dis 2:e205. doi: 10.1371/journal.pntd.0000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lounis N, Gevers T, Van Den Berg J, Verhaeghe T, van Heeswijk R, Andries K. 2008. Prevention of drug carryover effects in studies assessing antimycobacterial efficacy of TMC207. J Clin Microbiol 46:2212–2215. doi: 10.1128/JCM.00177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jager VR, Dawson R, van Niekerk C, Hutchings J, Kim J, Vanker N, van der Merwe L, Choi J, Nam K, Diacon AH. 2020. Telacebec (Q203), a new antituberculosis agent. N Engl J Med 382:1280–1281. doi: 10.1056/NEJMc1913327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.