Multidrug-resistant strains belonging to the Enterobacter cloacae complex (ECC) group, and especially those belonging to clusters C-III, C-IV, and C-VIII, have increasingly emerged as a leading cause of health care-associated infections, with colistin used as one of the last lines of treatment. However, colistin-resistant ECC strains have emerged. The aim of this study was to prove that MgrB, the negative regulator of the PhoP/PhoQ two-component regulatory system, is involved in colistin resistance in ECC of cluster C-VIII, formerly referred to as Enterobacter hormaechei subsp.
KEYWORDS: Enterobacter cloacae complex, Enterobacter hormaechei subsp. steigerwaltii, colistin resistance, MgrB, PhoPQ regulatory system
ABSTRACT
Multidrug-resistant strains belonging to the Enterobacter cloacae complex (ECC) group, and especially those belonging to clusters C-III, C-IV, and C-VIII, have increasingly emerged as a leading cause of health care-associated infections, with colistin used as one of the last lines of treatment. However, colistin-resistant ECC strains have emerged. The aim of this study was to prove that MgrB, the negative regulator of the PhoP/PhoQ two-component regulatory system, is involved in colistin resistance in ECC of cluster C-VIII, formerly referred to as Enterobacter hormaechei subsp. steigerwaltii. An in vitro mutant (Eh22-Mut) was selected from a clinical isolate of Eh22. The sequencing analysis of its mgrB gene showed the presence of one nucleotide deletion leading to the formation of a truncated protein of six instead of 47 amino acids. The wild-type mgrB gene from Eh22 and that of a clinical strain of Klebsiella pneumoniae used as controls were cloned, and the corresponding recombinant plasmids were used for complementation assays. The results showed a fully restored susceptibility to colistin and confirmed for the first time that mgrB gene expression plays a key role in acquired resistance to colistin in ECC strains.
INTRODUCTION
The genus Enterobacter is a member of the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) and grouped within the Enterobacter cloacae complex (ECC) group (1). In a seminal work, Hoffmann and Roggenkamp defined 13 genetic clusters (C-I to C-XIII) of ECC according to their hsp60 sequences (“hsp” for heat shock protein) (2). More recently, based on the analysis of 390 whole-genome sequences, ECC was placed into 18 phylogenomic groups (A to R) and two metaclusters, i.e., Enterobacter hormaechei and Enterobacter cloacae (3, 4). At present, more than 3,000 Enterobacter genomes are available in GenBank databases, and the taxonomy of this genus is still evolving (5). ECC species are opportunistic pathogens, and due to their natural and acquired resistance to many antibiotics, they are described as the leading cause of resistant nosocomial infections (1).
Colistin (polymyxin E) is considered one of the last-resort therapeutic agents for the treatment of multidrug-resistant Gram-negative bacteria (6). It is a cationic antimicrobial peptide that targets the anionic lipid A phosphate moiety of bacterial lipopolysaccharide (LPS), leading to an altered cell permeability and thereafter to cell death (6). The most common resistance mechanism in Enterobacteriaceae is attributed to the covalent modifications of LPS through the incorporation of positively charged groups such as phosphoethanolamine (pEtN) and 4-amino-4-deoxy-l-arabinose. These modifications neutralize the negative charges of LPS and subsequently reduce the binding affinity of colistin to its target (6). Plasmid-mediated colistin resistance, i.e., MCR genes encoding pEtN transferases, were described (7, 8). However, colistin resistance mechanisms are mostly attributed to chromosomal modifications in two-component regulatory systems (TCRS), namely, PmrA/PmrB and PhoP/PhoQ, which can cause constitutive expression of LPS modifications and, consequently, colistin resistance. Such chromosomal modifications were well described in Klebsiella pneumoniae (6, 9) but also in the E. cloacae complex (10). Moreover, substitution, disruption, and inactivation changes in mgrB gene encoding the negative regulator of PhoP/Q have been identified to play a prominent role in polymyxin resistance in clinical K. pneumoniae isolates (11–13). Very few reports have mentioned mgrB mutations and their possible role in colistin resistance of clinical Enterobacter spp. (14, 15), and no studies were performed to confirm its involvement in this genus.
Thus, the aim of this study was to analyze an in vitro mutant of Enterobacter hormaechei subsp. steigerwaltii containing a mutated mgrB gene and to confirm its role in colistin resistance. Our procedure was validated by comparison of results with those obtained with a clinical colistin-resistant K. pneumoniae deleted for mgrB.
RESULTS AND DISCUSSION
Eh22, a multidrug-resistant strain of E. hormaechei subsp. steigerwaltii.
A multidrug-resistant strain of ECC (named Eh22) was isolated from a patient urine sample and provided by an urban health care system (community laboratory of Djerba, Tunisia). Analysis of the hsp60 sequence revealed that Eh22 is included in the cluster C-VIII, referred to as E. hormaechei subsp. steigerwaltii (2, 16). Furthermore, in silico multilocus sequence typing (MLST) of seven housekeeping genes (dnaA, fusA, gyrB, leuS, pyrG, rplB, and rpoB) (17) and in silico multilocus sequence analysis (MLSA) of four other target genes and/or other target gene regions (gyrB, rpoB, infB, and atpD) (18), as well as the nearly complete (1,412 bp) 16S rRNA gene sequence (19), confirmed this species assignation (see Tables S1 and S2 in the supplemental material). MLST analysis also showed that Eh22 belongs to the sequence type ST177, which has a single allele different from ST93, ST294, and ST828, similarly to strains of E. hormaechei subsp. steigerwaltii for those of which the whole-genome sequencing had been performed (20, 21). Enterobacter spp. are one of the most common Enterobacteriaceae resistant to third-generation cephalosporins, along with Escherichia coli and K. pneumoniae (1). They are able to produce a low level of a chromosomal AmpC β-lactamase-type cephalosporinase that generates resistance to first-generation cephalosporins. Enterobacter spp. also often contain multiple resistance genes mediated by conjugative plasmids (1). In our study, Eh22 was multidrug resistant due, in part, to the presence of a conjugative plasmid of incompatibility group IncHI2, which carried various antibiotic resistance genes, including two extended-spectrum β-lactamases and one cephalosporinase gene (blaCTX-M-3, blaSHV-12, and blaDHA-1) (data not published, whole-plasmid sequencing in progress). Eh22 remained sensitive to carbapenems (data not shown). However, in recent years, clinical ECC isolates resistant to carbapenems have been increasingly reported through the production of plasmid-mediated carbapenemases (1). In particular, E. hormaechei subsp. steigerwaltii strains of ST177 carrying the blaNDM-1 and blaKPC-2 carbapenemases were described (20, 21). Eh22 also remained sensitive to colistin (see below) and to fosfomycin (MIC, 32 mg/liter), despite the presence of the fosA2 gene (with an identical nucleotide sequence to that of GenBank accession number CP041733, except for a T393C silent mutation). FosA is widely distributed among various Gram-negative bacilli, including Enterobacter spp. (22).
Thus, colistin is considered one of the last-resort treatments against these multidrug-resistant bacteria. Unfortunately, colistin resistance in ECC has been increasingly reported, and the resistance molecular mechanisms are less known than in K. pneumoniae (23). Very recently, mgrB mutations have been found in clinical strains of E. cloacae (14, 15). A missense mutation in MgrB (I10V) (15) as well as other mutations, e.g., C39G, N42S, I45Y, W47V, W47S, *48K, and *48Y changes, were identified (14). Thus, it is important to confirm the role of MgrB in colistin resistance and, particularly, in isolates of the C-VIII cluster, which are most frequently recovered from human clinical samples along with those of clusters C-III and C-VI (23, 24).
Selection of an mgrB mutant from E. hormaechei subsp. steigerwaltii.
The colistin MIC of Eh22 was 0.125 mg/liter without any heteroresistance phenomenon as described elsewhere (23). Using the population analysis profile (PAP) analysis (23), a colistin-resistant mutant named Eh22-Mut was selected on agar medium containing 8 mg/liter of colistin with a frequency of 1 × 10−8. The colistin MIC of Eh22-Mut was 32 mg/liter. Sequence analysis of pmrA, pmrB, phoP, and phoQ genes did not reveal any differences between Eh22 and Eh22-Mut. In contrast, the amplified region of mgrB (353 bp) spanning from 113 bp upstream of the initiation codon to 96 bp downstream of the stop codon (Fig. 1), showed one adenine deletion in the mgrB open reading frame of Eh22-Mut, leading to a truncated protein of 6 amino acids (MKKYAG) instead of 47 for the wild-type MgrB.
FIG 1.
Sequence of the Eh22 mgrB open reading frame and upstream/downstream region −113/+96. The stretch of seven adenines is boxed (a stretch of six adenines was obtained for Eh22-Mut, data not shown). The mgrB start and stop codons are indicated in bold, and the sequences of the −10 hexamer of the putative promoter and ribosome-binding site (RBS) are underlined. The open reading frame of MgrB is shown in italic font.
Detection of mgrB inactivation in a clinical strain of K. pneumoniae.
The inactivation of the PhoQ/PhoP negative regulator encoded by the mgrB gene has been identified to play a prominent role in polymyxin resistance in clinical isolates of K. pneumoniae and Klebsiella oxytoca (12). In the literature, complementation assays with mgrB genes from wild-type K. pneumoniae or K. oxytoca restored full susceptibility to colistin and confirm that MgrB expression was the key factor for this acquired resistance to colistin (11, 25, 26). These complementation assays with wild-type mgrB in K. pneumoniae were performed with recombinant plasmids as well as low or high copy numbers, without any significant differences (25). However, to check our procedure of MgrB complementation in E. hormaechei subsp. steigerwaltii with recombinant constructions using a medium-copy-number vector (pB-mfabI), we performed, in parallel and under the same conditions, the mgrB complementation of K. pneumoniae. In a previous study, we described two carbapenem-resistant strains of K. pneumoniae, Kp5196 and Kp5241, which were susceptible and resistant to colistin, respectively (27). Both strains, isolated from the same patient at a 2-month interval, belonged to the sequence type ST15 and were clonal as demonstrated by analysis of their pulsotypes (27). At that time, molecular analysis of the colistin resistance mechanism of Kp5241 had not been studied. Thus, given the data from the literature, pmrA-pmrB and phoP-phoQ genes were amplified and sequenced as described elsewhere (9, 11, 28). No changes were detected in both TCRS. The mgrB locus was targeted using three pairs of primers (see Table S3) designed to amplify the coding region and the regions flanking the mgrB gene (11). Kp5196 showed amplicons with expected sizes of 110, 253, and 1,507 bp (PCR amplifications with the primer pair mgrB_ext_F/mgrB_ext_R is depicted in Fig. S1). In contrast, Kp5241 yielded no amplification products with the former primers, suggesting a larger deletion of the locus carrying the mgrB gene (Fig. S1). Such large deletions have already been showed to be responsible for colistin resistance in K. pneumoniae (11).
Complementation of Eh22-Mut and Kp5241 with the wild-type mgrB gene.
Lippa and Goulian (29) identified MgrB homologues in the genome sequences of various enterobacteria. However, regulation of TCRS, PhoP/PhoQ, and PmrA/PmrB may be different according to Enterobacteriaceae species. Indeed, examination of the available genomes of ECC revealed the absence of a homologous gene encoding the PmrD connector, suggesting no cross talk between both TCRS (23). Thus, it is important to confirm the role of MgrB in ECC. To confirm whether the mgrB mutation found in Eh22-Mut is responsible for the colistin resistance in E. hormaechei subsp. steigerwaltii, the wild-type mgrB gene of Eh22 was inserted into pB-mfabI, giving the recombinant plasmid pB-mgrB/Eh which was used to transform Eh22-Mut. The colistin MICs of transformants showed a decreased resistance of 258-fold (0.062 mg/liter versus 16 mg/liter without the mgrB gene or with the mutated mgrB gene, respectively) (Table 1). The decrease in MIC was comparable (290-fold, 0.11 mg/liter versus 32 mg/liter) for transformants from Kp5241 with the plasmid pB-mgrB/Kp (wild-type mgrB gene of Kp5196), allowing validation of our procedure (Table 1). Thus, our results show, for the first time, that MgrB is a key target for colistin resistance in Enterobacter species.
TABLE 1.
Colistin MIC of strains and transformants of E. hormaechei and K. pneumoniae
| Plasmid | Mean colistin MIC (mg/liter [SD])a |
|||
|---|---|---|---|---|
|
E. hormaechei subsp. steigerwaltii |
K. pneumoniae |
|||
| Eh22 | Eh22-Mut | Kp5196 | Kp5241 | |
| Without plasmid | 0.125b | 32b | 2b,c | 64b,c |
| pB-mfabI | NDd | 16 (0)e | ND | 32 (0) |
| pB-mgrB/Eh | ND | 0.062 (0.045)f | ND | 0.11 (0.026) |
| pB-mgrB/Kp | ND | 0.043 (0.023)f | ND | 0.11 (0.031) |
Means from at least three independent experiments.
Values obtained without triclosan. For the other experiments, the values were obtained with a final concentration of 0.5 mg/liter of triclosan used for plasmid maintenance.
Values in accordance with Arpin et al. (27).
ND, not determined.
Plasmid without or with mutated mgrB.
Nonsignificant differences (P value of 0.2) by using a Student unpaired bilateral t test.
Comparison of the mgrB gene sequences.
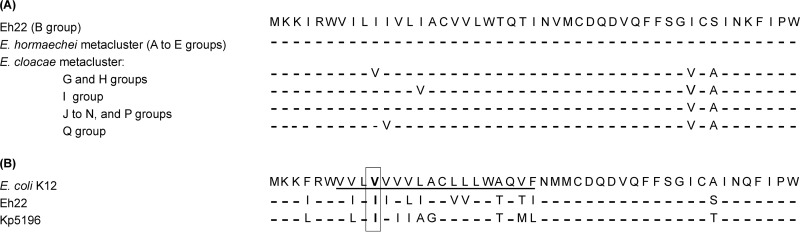
Alignment of MgrB sequences of Eh22 with 15 of 18 ECC phylogroups (A to R) publicly available in GenBank databases (3), showed a high conservation of amino acids, in particular, among the E. hormaechei metacluster to which Eh22 belongs (Fig. 2A) (4).
FIG 2.
MgrB alignment comparison of Eh22. (A) MgrB alignments of Eh22 with peptidic sequences from 15 of 18 Enterobacter cloacae complex groups as defined by Chavda et al. (3). MgrB sequences used for alignments were selected as follows: group, name of species, name of strain (strain type, when available, is designated by the superscript T), and GenBank accession number. In E. hormaechei metacluster (A to E groups): A, E. xiangfangensis LMG 27195T, NZ_CP017183; B, E. hormaechei subsp. steigerwaltii DSM 16691T, NZ_CP017179; C, E. hormaechei subsp. oharae DSM 16687T, NZ_CP017180; D, E. hormaechei subsp. hoffmannii DSM 14563T, NZ_CP017186; E, E. hormaechei ATCC 49162, AFHR01000000. In the E. cloacae metacluster (G to N, P and Q groups): G, E. cloacae subsp. cloacae ATCC 13047, NC_014121; H, E. cloacae subsp. dissolvens SDM, NC_018079; I, E. ludwigii EN-119, CP017279; J, E. asburiae ATCC 35953, CP011863; K, E. cloacae complex DC4, AZUB01000000; L, E. cloacae complex BWH 43, JMUR01000000; M, E. cloacae complex DSM 16690T, CP017184.1; N, E. cloacae complex SY-70, NZ_JALR01000000; P, E. cloacae complex JD8715, JDWG01000000; Q, E. kobei DSM 13645T, CP017181. In E. hormaechei metacluster, three groups have unavailable MgrB sequences: F (E. mori), O, and R (E. cloacae complex). (B) MgrB alignments of E. coli K-12, Eh22, and K. pneumoniae Kp5196. The putative transmembrane domain of E. coli MgrB is underlined according to Lippa and Goulian (29), and the amino acid at position 10 is indicated in bold. The MgrB sequences of Eh22 (A) and E. coli K-12 (B) are given, and only different residues are shown for the other alignments.
The alignment of MgrB from colistin-susceptible K. pneumoniae Kp5196 and E. hormaechei Eh22 showed a difference of 12 amino acids with 74% identity and 93% similarity; the N-terminal sequence being the least conserved (Fig. 2B). This region with a hydrophobic stretch may function as a transmembrane domain (29). Chavda et al. (15) identified a missense mutation in MgrB (I10V), together with PmrA/PmrB modifications in E. cloacae, suggesting that these modifications may be responsible for colistin resistance. Although a valine instead of an isoleucine is present at position 10 in the wild-type MgrB of E. coli K-12 and other ECC (G and H groups) (Fig. 2), we wanted to verify the possible consequence of such minor modifications in resistance acquisition. As indicated in Table 1, the cross-complementation of mgrB wild-type genes between Eh22-Mut (with its truncated chromosomally encoded MgrB of 6 amino acids in length) and Kp5241 (with its chromosomally encoded MgrB deletion) restored similar colistin susceptibility in both species, confirming that the amino acid differences found between their respective MgrBs have no impact on the structure and activity of the PhoP/PhoQ regulator system and suggesting that the I10V change previously found in MgrB of E. cloacae is probably not the source of acquired resistance to colistin.
Concluding remarks.
Our data confirm for the first time the role of MgrB inactivation in colistin resistance in E. hormaechei subsp. steigerwaltii (cluster C-VIII); ECC is being increasingly reported as the leading cause of resistant nosocomial infections. The knowledge of colistin resistance mechanisms is important, because very few alternative therapeutic options are available for the treatment of infection with these multidrug-resistant bacteria.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The ECC strain Eh22 was identified by using the commercialized system Api20E gallery (bioMérieux), analysis by rRNA gene 16S sequencing with the universal primers 27F/1492R (sequence overlapping the region from bases 71 to 1,482 of the complete sequence of 1,552 bp) (19), and matrix-assisted laser desorption ionization–time of flight mass spectrometry identification (MALDI-TOF MS; Bruker Daltonics). Cluster Enterobacter membership was determined by partial sequencing of the hsp60 gene (2, 16). The sequence type (ST) was determined using the genomic sequence to query the MLST database of E. cloacae (http://pubmlst.org/ecloacae/) (17), and MLSA was performed using the primers and conditions as described previously (18). Two previously described K. pneumoniae strains, Kp5196 and Kp5241, susceptible and resistant to colistin, respectively, were included in our study (27). E. coli DH5α was used for cloning and subcloning experiments of mgrB genes.
pB-mfabI is a pBR322 vector (4.4 kb; New England BioLabs), which carries the mfabI gene (mutant fabI) affecting the binding of triclosan to FabI to allow the bacteria (E. coli or E. cloacae complex) to grow in the presence of 4 mg/liter of triclosan (30). The mfabI gene was inserted between the ScaI and PvuI restriction sites of the pBR322 vector, inactivating the β-lactamase gene blaTEM-1. pBR322 is described in the literature as a medium-copy-number vector (∼15 to 20 copies by cell). The pGEM-T Easy vector (Promega) was used for PCR product cloning.
Antimicrobial susceptibility testing.
MICs of colistin (Sigma Chemical Co.) were determined by the microdilution reference method using Mueller-Hinton (MH) broth adjusted for divalent cations (CA-MHB; Bio-Rad) in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (http://www.eucast.org/). Fosfomycin MIC was determined by the Etest method (bioMérieux). Fosfomycin and colistin MICs were interpreted as indicated by the EUCAST breakpoint tables; for colistin MICs, isolates with MICs of ≤2 mg/liter were categorized as susceptible, whereas those with MICs of >2 mg/liter were categorized as resistant.
Selection of in vitro colistin mutants.
Selection of mutants from Eh22 were carried out by plating a high inoculum (∼1010 CFU) on Luria-Bertani (LB) agar plates containing 2 to 32 mg/liter of colistin, as previously described for the analysis of PAP (23).
PCR amplification, sequencing, and plasmid purification.
Plasmids were purified by using the Macherey-Nagel NucleoSpin plasmid kit according to the supplier’s recommendations. PCR experiments of pmrA, pmrB, phoP, phoQ, and mgrB genes using specific primers (see Table S3 in the supplemental material) and amplicons were verified by Sanger’s sequencing (MWG Operon Society; Eurofins Genomics Germany GmbH, Ebersberg, Germany). The nucleotide and deduced protein sequences were analyzed at the NCBI website (https://www.ncbi.nlm.nih.gov) using the Basic Local Alignment Search Tool (BLAST) program.
mgrB gene cloning and complementation experiments.
After ligation into the pGEM-T Easy vector, PCR products of mgrB obtained after amplification with primer pairs EhmgrB_F113 and EhmgrB_R96 (for Eh22) and KpmgrB_F112 and KpmgrB_R88 (for Kp5196) (Table S3) were used to transform E. coli DH5α chemically competent cells. The selection of recombinant clones was carried out on LB agar medium supplemented with 100 mg/liter of ampicillin (Sigma Chemical Co.), 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Eurobio), and 80 mg/liter of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; MP Biomedical). After plasmid extraction and EcoRI digestion (Promega), fragments containing mgrB gene were purified on agarose gel and extracted using the Gel and PCR cleanup kit (Macherey-Nagel). The insert was cloned into the EcoRI site of the linearized and dephosphorylated plasmid pB-mfabI using T4 DNA ligase (Promega). After E. coli DH5α transformation, bacteria were selected on LB agar medium with tetracycline (15 mg/liter). Recombinant plasmids, with mgrB inserted in the same orientation, were verified by sequencing analysis using the primers Tetra-161_F and Tetra+94_R (Table S3). Eh22-Mut (colistin-resistant mutant of Eh22) and Kp5241 were transformed with mgrB constructs using chemically competent cells prepared by CaCl2 treatment, followed by heat shock. Transformants were selected on MH agar plates containing 4 mg/liter of triclosan.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sabine Aillerie and Nathalie Peyron for their technical assistance. We thank Véronique Dubois for allowing us access to the device (MALDI-TOF) of the Bacteriology Laboratory of University Hospital of Bordeaux. We also thank Christine Reix for the English language correction.
This work was supported by the University of Bordeaux and the National Center of Scientific Research (France) and by the Ministry of Higher Education and Research Scientific (Tunisia).
We have no relevant financial disclosures or funding to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Davin-Regli A, Lavigne J-P, Pagès J-M. 2019. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev 32:e00002-19. doi: 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann H, Roggenkamp A. 2003. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol 69:5306–5318. doi: 10.1128/aem.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, Bonomo RA, Adams MD, Kreiswirth BN. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. mBio 7:e02093-16. doi: 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrouthy R, Barets M, Marion E, Dananché C, Dauwalder O, Robin F, Gauthier L, Jousset A, Dortet L, Guérin F, Bénet T, Cassier P, Vanhems P, Bonnet R. 2018. Novel Enterobacter lineage as leading cause of nosocomial outbreak involving carbapenemase-producing strains. Emerg Infect Dis 24:1505–1515. doi: 10.3201/eid2408.180151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Wu W, Wei L, Feng Y, Kang M, Xie Y, Zong Z. 2020. Enterobacter wuhouensis sp. nov. and Enterobacter quasihormaechei sp. nov. recovered from human sputum. Int J Syst Evol Microbiol 70:874–881. doi: 10.1099/ijsem.0.003837. [DOI] [PubMed] [Google Scholar]
- 6.Baron S, Hadjadj L, Rolain J-M, Olaitan AO. 2016. Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int J Antimicrob Agents 48:583–591. doi: 10.1016/j.ijantimicag.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Kieffer N, Royer G, Decousser J-W, Bourrel A-S, Palmieri M, Ortiz De La Rosa J-M, Jacquier H, Denamur E, Nordmann P, Poirel L. 2019. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother 63:e00965-19. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan Y, Li Y, Wang G, Li C, Xiang L, She J, Yang Y, Zhong F, Zhang L. 2019. Coproduction of MCR-9 and NDM-1 by colistin-resistant Enterobacter hormaechei isolated from bloodstream infection. Infect Drug Resist 12:2979–2985. doi: 10.2147/IDR.S217168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannatelli A, D'Andrea MM, Giani T, Di Pilato V, Arena F, Ambretti S, Gaibani P, Rossolini GM. 2013. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother 57:5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang KN, Klein DR, Kazi MI, Guérin F, Cattoir V, Brodbelt JS, Boll JM. 2019. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol Microbiol 111:1604–1616. doi: 10.1111/mmi.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannatelli A, Giani T, D'Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini GM, COLGRIT Study Group . 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 58:5696–5703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olaitan AO, Morand S, Rolain J-M. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayol A, Poirel L, Dortet L, Nordmann P. 2016. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro Surveill 21:30339. doi: 10.2807/1560-7917.ES.2016.21.37.30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawfal Dagher T, Al-Bayssari C, Chabou S, Baron S, Hadjadj L, Diene SM, Azar E, Rolain J-M. 12 December 2019. Intestinal carriage of colistin resistant Enterobacteriaceae at Saint Georges Hospital in Lebanon. J Glob Antimicrob Resist doi: 10.1016/j.jgar.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Chavda B, Lv J, Hou M, Chavda KD, Kreiswirth BN, Feng Y, Chen L, Yu F. 2018. Coidentification of mcr-4.3 and blaNDM-1 in a clinical Enterobacter cloacae isolate from China. Antimicrob Agents Chemother 62:e00649-18. doi: 10.1128/AAC.00649-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Monget D, Pierard D, Ziesing S, Heesemann J, Roggenkamp A, Schleifer KH. 2005. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J Clin Microbiol 43:3297–3303. doi: 10.1128/JCM.43.7.3297-3303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. doi: 10.1371/journal.pone.0066358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady C, Cleenwerck I, Venter S, Coutinho T, De Vos P. 2013. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst Appl Microbiol 36:309–319. doi: 10.1016/j.syapm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Usadel B. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (eds), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, UK. [Google Scholar]
- 20.Yang B, Feng Y, McNally A, Zong Z. 2018. Occurrence of Enterobacter hormaechei carrying blaNDM-1 and blaKPC-2 in China. Diagn Microbiol Infect Dis 90:139–142. doi: 10.1016/j.diagmicrobio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, Feng Y, Carattoli A, Zong Z. 2015. Characterization of an Enterobacter cloacae strain producing both KPC and NDM carbapenemases by whole-genome sequencing. Antimicrob Agents Chemother 59:6625–6628. doi: 10.1128/AAC.01275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito R, Mustapha MM, Tomich AD, Callaghan JD, McElheny CL, Mettus RT, Shanks RMQ, Sluis-Cremer N, Doi Y. 2017. Widespread fosfomycin resistance in Gram-negative bacteria attributable to the chromosomal fosA gene. mBio 8:e00749-17. doi: 10.1128/mBio.00749-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guérin F, Isnard C, Sinel C, Morand P, Dhalluin A, Cattoir V, Giard J-C. 2016. Cluster-dependent colistin hetero-resistance in Enterobacter cloacae complex. J Antimicrob Chemother 71:3058–3061. doi: 10.1093/jac/dkw260. [DOI] [PubMed] [Google Scholar]
- 24.Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, Tazi A, Anract P, Courpied J-P, Poyart C, Dumaine V. 2009. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol 47:2489–2495. doi: 10.1128/JCM.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel L, Jayol A, Bontron S, Villegas M-V, Ozdamar M, Türkoglu S, Nordmann P. 2015. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother 70:75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 26.Jayol A, Poirel L, Villegas M-V, Nordmann P. 2015. Modulation of mgrB gene expression as a source of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents 46:108–110. doi: 10.1016/j.ijantimicag.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Arpin C, Noury P, Boraud D, Coulange L, Manetti A, André C, M'Zali F, Quentin C. 2012. NDM-1-producing Klebsiella pneumoniae resistant to colistin in a French community patient without history of foreign travel. Antimicrob Agents Chemother 56:3432–3434. doi: 10.1128/AAC.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayol A, Poirel L, Brink A, Villegas M-V, Yilmaz M, Nordmann P. 2014. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob Agents Chemother 58:4762–4766. doi: 10.1128/AAC.00084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippa AM, Goulian M. 2009. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet 5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang C-W, Magnuson T. 2013. A novel selection marker for efficient DNA cloning and recombineering in E. coli. PLoS One 8:e57075. doi: 10.1371/journal.pone.0057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.