Recent studies highlight the abundance of commensal coagulase-negative staphylococci (CoNS) on healthy skin. Evidence suggests that CoNS actively shape the skin immunological and microbial milieu to resist colonization or infection by opportunistic pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), in a variety of mechanisms collectively termed colonization resistance. One potential colonization resistance mechanism is the application of quorum sensing, also called the accessory gene regulator (agr) system, which is ubiquitous among staphylococci.
KEYWORDS: antimicrobial activity, colonization resistance, polymicrobial interactions, quorum sensing
ABSTRACT
Recent studies highlight the abundance of commensal coagulase-negative staphylococci (CoNS) on healthy skin. Evidence suggests that CoNS actively shape the skin immunological and microbial milieu to resist colonization or infection by opportunistic pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), in a variety of mechanisms collectively termed colonization resistance. One potential colonization resistance mechanism is the application of quorum sensing, also called the accessory gene regulator (agr) system, which is ubiquitous among staphylococci. Common and rare CoNS make autoinducing peptides (AIPs) that function as MRSA agr inhibitors, protecting the host from invasive infection. In a screen of CoNS spent media, we found that Staphylococcus simulans, a rare human skin colonizer and frequent livestock colonizer, released potent inhibitors of all classes of MRSA agr signaling. We identified three S. simulans agr classes and have shown intraspecies cross talk between noncognate S. simulans agr types for the first time. The S. simulans AIP-I structure was confirmed, and the novel AIP-II and AIP-III structures were solved via mass spectrometry. Synthetic S. simulans AIPs inhibited MRSA agr signaling with nanomolar potency. S. simulans in competition with MRSA reduced dermonecrotic and epicutaneous skin injury in murine models. The addition of synthetic AIP-I also effectively reduced MRSA dermonecrosis and epicutaneous skin injury in murine models. These results demonstrate potent anti-MRSA quorum sensing inhibition by a rare human skin commensal and suggest that cross talk between CoNS and MRSA may be important in maintaining healthy skin homeostasis and preventing MRSA skin damage during colonization or acute infection.
INTRODUCTION
The human skin is a complex physiological barrier that is colonized by a diverse array of bacteria, archaea, fungi, and viruses (1). Several recent studies highlight coagulase-negative staphylococci (CoNS) as dominant colonizers of dry, moist, and sebaceous skin sites (1, 2). The CoNS are a heterogeneous group of approximately 40 species that remain predominantly uncharacterized genetically and functionally (3). Despite this lack of characterization, there is mounting evidence to suggest that CoNS do not passively reside on the skin but instead actively participate in shaping the composition of other microbial inhabitants and in priming the cutaneous immune response against foreign insult or injury (4). Drawing parallels to studies of the human gut microbiome, this active mechanism of barrier maintenance and protection has been termed “colonization resistance.” The majority of prior studies have focused on Staphylococcus epidermidis contributions to colonization resistance, as it is the most frequently isolated CoNS from normal skin (5). Examples include S. epidermidis-derived lipoteichoic acid suppression of inflammation during wound repair (6), education of skin-resident T lymphocytes (7), and modulation of the innate antimicrobial response to infection (8). However, S. epidermidis is also considered an “accidental pathogen” and is responsible for the majority of implant-associated infections in the United States (5, 9). Moreover, certain S. epidermidis strains have been shown to expand during atopic dermatitis (eczema) flares and could potentially exacerbate disease severity (2, 10). This dual existence as friend and foe suggests that S. epidermidis cannot be the only contributor to the maintenance of skin health. Furthermore, the underappreciated species- and strain-level diversity of CoNS on the skin suggests that other common or rare CoNS could also contribute significantly to colonization resistance.
Indeed, several recent studies highlighted the protective function of both common and rare skin commensal CoNS against colonization or infection by the opportunistic pathogen Staphylococcus aureus (11–13). S. aureus is asymptomatically carried in the anterior nares (nostrils) of at least 20% of the human population (14). While nasal carriage is significantly associated with S. aureus colonization of distal skin sites (15), S. aureus is canonically considered a poor skin colonizer and is rarely identified in analyses of the microbial flora on healthy human skin (15, 16). Yet, S. aureus, the methicillin-resistant community-associated USA300 lineage (here referred to as MRSA), continues to be the leading cause of skin and soft tissue infections in the United States (17, 18). In addition, S. aureus isolates are frequently associated with atopic dermatitis lesions, and evidence suggests that early skin colonization with S. aureus may increase the odds of subsequent atopic dermatitis onset (19, 20). Finally, the emergence and spread of MRSA in health care and community settings continue to pose a significant public health risk, demanding investigation of nonantibiotic prophylactics and therapeutics (21).
The staphylococcal quorum sensing accessory gene regulator (agr) system is necessary for MRSA-mediated injury during skin infection (22, 23). Targeting MRSA quorum sensing has been proposed as a potential alternative to antibiotic therapies, and several small-molecule inhibitors have been previously described (24–27). The agr system is conserved across all staphylococci but is best characterized in S. aureus (Fig. 1A) (28). Similar to other Gram-positive two-component systems, the agr operon (agrBDCA) is composed of a membrane-localized histidine kinase sensor AgrC that binds its cognate signal autoinducing peptide (AIP). At sufficient external concentration, AIP binding induces AgrC autophosphorylation via a conformational change and subsequent phosphorylation of the response regulator AgrA. Activated AgrA binds chromosomal promoters P2 and P3 to upregulate the transcription of the agrBDCA operon and the main effector transcript RNAIII, respectively. In S. aureus, RNAIII mediates global upregulation of a suite of virulence factors, including toxins, secreted enzymes, and immunomodulatory peptides (28).
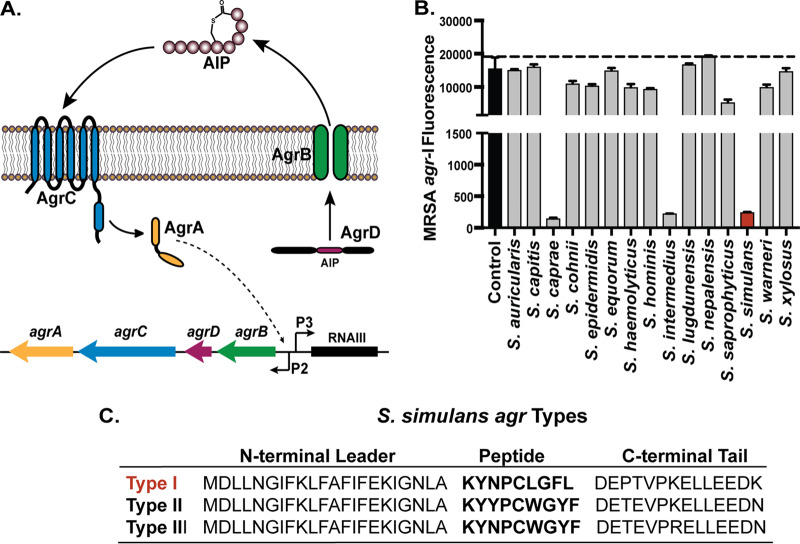
FIG 1.
The rare skin commensal S. simulans blocks MRSA agr quorum sensing. (A) Representative schematic of the staphylococcal agrBDCA operon. AgrC selectively binds its cognate AIP at sufficient external concentration and initiates an activation cascade leading to the upregulation of the main effector, RNAIII, and a positive-feedback loop of the agrBDCA operon. (B) The MRSA agr-I P3::YFP reporter was incubated with 10% spent media from each indicated coagulase-negative strain (Table 2), and fluorescence was measured at hourly intervals for 24 h. The 24-h fluorescence point is shown compared to reporter treated with media alone (control). S. simulans spent media treatment is highlighted in red. The mean ± SD of the results from three independent experiments is shown. (C) Each S. simulans agrD sequence is divided into the N-terminal leader, peptide-containing region, and C-terminal tail. From panel B, S. simulans was identified as agr type I and highlighted in red. The predicted peptide structures are in bold for each agr type. All S. simulans strains share a conserved cysteine at residue five in the peptide-containing region that mediates the formation of a thiolactone ring.
There are four allelic variants of the MRSA agr system determined by a hypervariable region spanning the agrBDC genes. As agrD encodes the AIP signal peptide, each agr allelic variant makes its own cognate AIP. All described AIPs are 7 to 12 amino acids in length, with the last 5 residues of the C terminus constrained in a thiolactone or lactone ring (28, 29). The N-terminal “tail” length is determined during AgrB-mediated AIP processing and export. Each agr type specifically responds to its cognate AIP signal, and intraspecies cross talk between noncognate S. aureus agr types has been extensively characterized (30). Furthermore, there are now several examples of human- and animal-derived CoNS AIPs that inhibit S. aureus quorum sensing via interspecies cross talk (12, 31–34). For example, quorum sensing interference by the rare skin commensal Staphylococcus caprae attenuated acute MRSA skin infection and also limited initial MRSA skin colonization (11). Earlier work on S. epidermidis also suggested that agr interference may play a role in skin competition with MRSA (32, 35). These results, together with clinical observations of CoNS dominance and the relative absence of MRSA on healthy skin, suggest that quorum sensing interference could be a strategy for CoNS colonization resistance and niche competition.
In a screen of spent media from our collection of CoNS, we observed that another rare skin commensal, Staphylococcus simulans, potently inhibited MRSA quorum sensing (Fig. 1B). Initially characterized by Kloos and Schleifer in 1975, S. simulans is an infrequent human skin colonizer isolated from the legs, arms, and heads of children (3, 36) and can also be found as a colonizer of cattle, horses, and sheep (3). Our preliminary findings that S. simulans spent media potently inhibited MRSA quorum sensing prompted us to investigate this interaction further. Here, we characterize agr cross talk between S. simulans and MRSA, solve the structure of each class of S. simulans AIP signal, and characterize intraspecies cross talk between all S. simulans agr allelic variants. Finally, we demonstrate that S. simulans AIP-I effectively reduces MRSA dermonecrotic or eczematous lesions in mouse skin infection models. Taken together, these results are an in-depth characterization of MRSA agr interference mediated by a rare skin commensal, S. simulans, and suggest that certain S. simulans strains could be protective against MRSA skin colonization or disease.
RESULTS
Preliminary identification of three S. simulans agr types.
Our initial screen of CoNS spent media against MRSA agr quorum sensing revealed potent inhibition by S. simulans and confirmed previously published inhibition by S. caprae and S. intermedius spent media (Fig. 1B) (11, 31, 37). Spent media from other CoNS, including Staphylococcus capitis and Staphylococcus lugdunensis, demonstrated only modest agr inhibition, as previously shown (11). We decided to investigate S. simulans further given its potent anti-MRSA agr properties. S. simulans has been shown to make at least one AIP type (38), but a search of all agrBDCA sequences in published S. simulans genomes revealed two more putative agr types present in S. simulans strains isolated from cows (Fig. 1C) (39). Other groups have investigated bovine S. simulans isolates for their anti-S. aureus activity in the context of mammary infections in dairy cows (33), but to our knowledge, this is the first in-depth characterization of multiple S. simulans agr types and their inter- and intraspecies interactions. We first sought to expand the knowledge of S. simulans agr types by transforming the representative human (AH4549 [agr-I]) and bovine (AH5532 [agr-II] and AH5533 [agr-III]) isolates with the staphylococcal agr P3::sGFP reporter plasmid pCM41 (sGFP, superfolder green fluorescent protein). Each strain grew with similar dynamics to an expected optical density of approximately 2.0, and initial P3::sGFP activation began at early exponential phase, as expected for agr expression in other staphylococci (Fig. 2A to C) (28, 40).
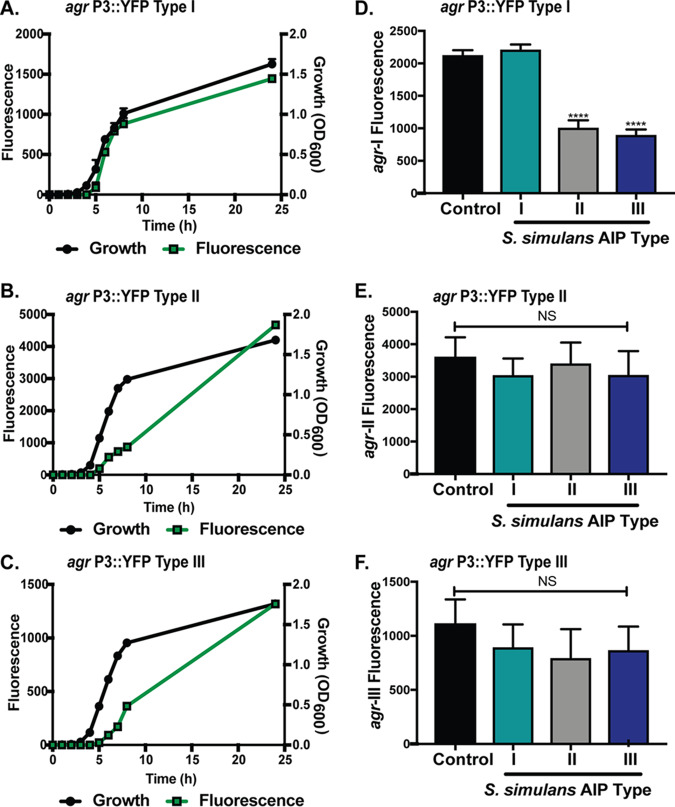
FIG 2.
S. simulans agr reporters and intraspecies cross talk. (A to C) Representative S. simulans AH4549 agr-I (A), AH5532 agr-II (B), and AH5533 agr-III (C) strains were transformed with agr P3::sGFP reporter plasmid pCM41. Growth (black circle) in TSB medium and GFP expression (green square) over 24 h is shown. (D to F) S. simulans agr-I P3::sGFP (D), agr-II P3::sGFP (E), and agr-III P3::sGFP (F) strains were grown with 10% filtered spent media from S. simulans agr types for 24 h. The 24-h fluorescence point of treated groups or reporter grown in TSB alone (control) is shown. Growth curves are representative of 3 independent experiments. Inhibition is shown as the pooled results from 3 independent experiments. Significance determined by ordinary one-way ANOVA with Dunnett’s multiple-comparison test. Mean values ± the SD are shown. ****, P < 0.0001.
S. simulans intraspecies cross talk.
S. epidermidis agr allelic variants cross-inhibit each other via “agr interference” or intraspecies cross talk (35); thus, we sought to determine if agr interference also exists between S. simulans agr types. To test this question, the S. simulans agr-I P3::sGFP (Fig. 2D), agr-II P3::sGFP (Fig. 2E), and agr-III P3::sGFP (Fig. 2F) reporters were treated with 10% (vol/vol) spent media from each S. simulans AIP type. For S. simulans agr-I, the addition of 10% AIP-I spent media modestly boosted GFP fluorescent output (Fig. 2D). The boost in fluorescence is not unusual and has been observed in S. epidermidis and S. aureus agr reporter systems under certain conditions (35, 41). The addition of spent media from either S. simulans AIP type II or type III significantly (P < 0.0001) inhibited signaling (Fig. 2D). The S. simulans agr type II reporter output was not boosted by the addition of cognate spent media nor was it significantly repressed by the addition of AIP type I or type III spent media (Fig. 2E). The lack of signal boosting could indicate maximal reporter output or a saturating concentration of AIP signal. Similar to agr-II, S. simulans agr-III fluorescence was not boosted by addition of its own spent media nor inhibited by the addition of type II or type I spent media (Fig. 2F). These results demonstrate that intraspecies cross talk between S. simulans allelic variants can occur, where agr-I is inhibited by noncognate AIPs, while agr-II and agr-III are not subject to cross-inhibition.
Interspecies cross talk between different S. simulans and MRSA agr types.
There are now several examples of human and animal CoNS AIPs that inhibit MRSA agr via interspecies cross talk (11, 12, 31, 32). To determine if S. simulans isolates can also potentiate interspecies cross talk, we tested whether S. simulans spent media could inhibit MRSA agr signaling. Filtered spent media from overnight S. simulans cultures of each AIP type was added to a panel of MRSA agr type I to -IV P3::YFP fluorescent reporters (YFP, yellow fluorescent protein). Reporter growth (optical density at 600 nm [OD600]) was not affected by addition of spent media, indicating that observed decreases in fluorescent signal were not due to bactericidal or bacteriostatic effects (see Fig. S1 in the supplemental material). Quorum sensing in MRSA agr types I, II, and III was strongly inhibited when incubated with 2.5% (vol/vol) spent media from any human skin S. simulans isolate (Fig. 3A). Of note, 2.5% spent media was selected, as higher spent media concentrations (up to 20%) resulted in complete quorum quenching for MRSA agr types I, II, and III (data not shown). MRSA agr type IV was also inhibited in the presence of 2.5% spent media from human skin S. simulans isolates, but the effect was less pronounced than with the inhibition of the other reporter types. Unlike MRSA agr types I to III, MRSA agr-IV has previously been shown to be more resistant to inhibition by CoNS AIPs, including S. epidermidis (32), S. caprae (11), and S. hominis (12). When incubated with 2.5% (vol/vol) spent media from bovine S. simulans isolates, MRSA agr inhibition was dependent on the S. simulans AIP type (Fig. 3B). S. simulans AIP-II (AH5532) was not a potent inhibitor of any MRSA agr type, suggesting either poor AIP binding to MRSA AgrC or poor AIP production by this strain. Similar to human S. simulans isolates, S. simulans AIP-III strains were effective inhibitors of MRSA agr types I, II, and III but were not effective at inhibiting MRSA agr-IV.
FIG 3.
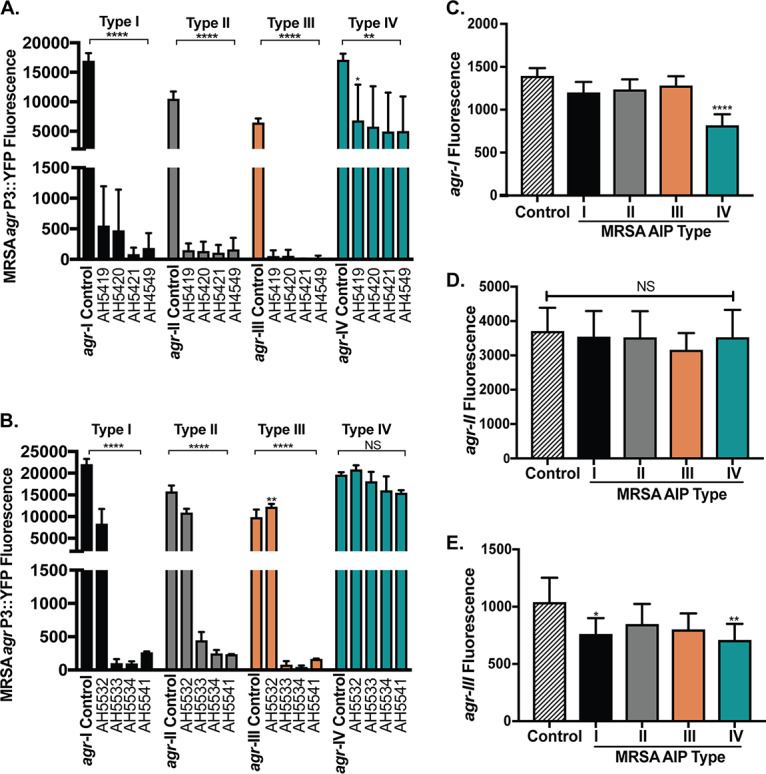
Interspecies cross talk between different MRSA and S. simulans agr types. (A) MRSA agr-I to -IV P3::YFP reporter strains were incubated with 2.5% filtered spent media from S. simulans human skin isolates. The 24-h fluorescence point is shown for treated and control groups. (B) MRSA agr-I to -IV reporter strains were incubated with 2.5% filtered spent media from S. simulans bovine and blood isolates. The 24-h fluorescence point is shown for treated and control groups. (C to E) The S. simulans agr-I (C), agr-II (D), or agr-III (E) P3::sGFP reporter strain was incubated with 10% filtered spent media from each MRSA AIP type. The 24-h fluorescence point is shown. The results are pooled from three independent experiments. Significance was determined by ordinary one-way ANOVA with Dunnett’s multiple-comparison test. Mean values ± the SD are shown. *, P < 0.05; **, P < 0.008; ****, P < 0.0001.
While there are many instances of CoNS AIPs that inhibit MRSA agr signaling, it remains unclear if any MRSA AIP type can inhibit CoNS agr signaling. To this end, each S. simulans agr reporter was treated with spent media from each MRSA AIP type (Fig. 3C to E). S. simulans agr-I was inhibited by 10% (vol/vol) spent media from MRSA AIP type IV (Fig. 3C), but this was coupled with a significant S. simulans growth defect indicating some bacteriostatic agent in the MRSA spent media (Fig. S2A). At lower spent media concentrations, no significant S. simulans growth delay or agr inhibition was observed, suggesting that agr inhibition with 10% MRSA spent media was due to S. simulans growth delays and not specific interspecies cross talk (data not shown). There was no S. simulans agr-I growth defect in the presence of spent media from MRSA AIP types I to III, nor was S. simulans agr-I signaling inhibited (Fig. 3C and S2A). S. simulans agr-II signaling was not affected by the addition of 10% spent media from any MRSA AIP type, nor were significant growth defects detected (Fig. 3D and S2B). S. simulans agr-III was susceptible to inhibition by MRSA AIP types I and IV without significant growth defects (Fig. 3D and S2C). Taken together, these results suggest that only some S. simulans AIP types can inhibit MRSA quorum sensing, with type I displaying the most potent inhibition against all MRSA agr types and type II being the least potent. S. simulans agr types also display various degrees of resistance to cross-inhibition from MRSA AIPs, with type III being weakly susceptible to cross-inhibition by MRSA AIP types I and IV.
Identification and validation of S. simulans AIPs.
To identify the structures of human and bovine S. simulans AIPs, mass spectrometric analysis of spent media was performed, which resulted in the detection of the singly charged, protonated molecular ion ([M+H]+) for each AIP. For the human S. simulans isolates (AIP-I), a 9-amino-acid AIP (KYNPc[CLGFL]) was detected (Fig. 4A), with calculated and measured m/z values of 1,036.5286 and 1,036.5302 (Δ = 1.6 ppm), respectively. The S. simulans AIP-II peptide detected was also nine amino acids in length (KYYPc[CWGYF]), with a matching calculated m/z of 1,208.5234 and measured m/z of 1,208.5209 (Δ = 2.0 ppm) (Fig. 4B). Finally, AIP-III differed from AIP-II by only one amino acid (Y3N) (KYNPc[CWGYF]), with calculated and measured m/z values of 1,159.5030 and 1,159.5056 (Δ = 1.9 ppm), respectively (Fig. 4C). The structure of S. simulans AIP-I has previously been reported and is consistent with the structure reported here (38). Ours is the first report of the S. simulans AIP-II and AIP-III structures.
FIG 4.
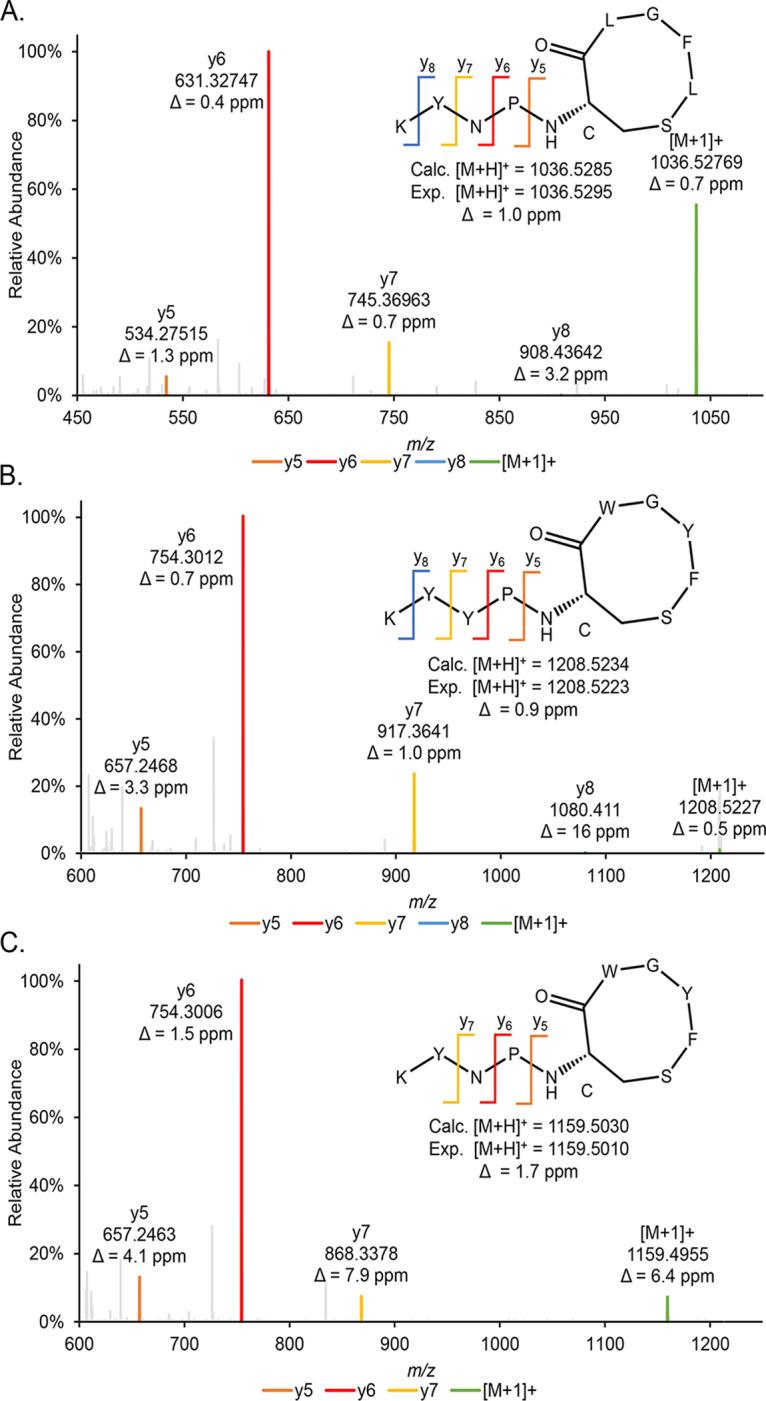
Identification and validation of S. simulans AIPs. (A to C) The identified amino acid sequence and the thiolactone ring structure for human (A) and bovine (B and C) S. simulans isolate AIPs were confirmed using MS/MS analysis. Characteristic y-ions are indicated for each AIP. The measured mass from the full-scan analysis is indicated below the predicted structure with calculated mass and mass error. Calc., calculated; Exp., experimental.
Synthetic S. simulans AIP-I, -II, and -III were used for additional validation and characterization. Liquid chromatography-mass spectrometry (LC-MS) analysis of the synthetic AIPs showed matching retention time, m/z values, and fragmentation patterns with the native AIP structure identified in each S. simulans isolate spent media, further confirming our initial identification (Fig. S3). To validate the function of the synthetic AIPs, MRSA agr P3::YFP reporter strains were treated with a dose response of each synthetic S. simulans AIP. Consistent with our initial characterization of S. simulans agr-I spent media, synthetic AIP-I potently inhibited all MRSA agr types, with 50% inhibitory concentration (IC50) values in the low-nanomolar range (Fig. S4A and Table 1). Also consistent with initial characterization, synthetic AIP-I was a more potent inhibitor of MRSA agr types I, II, and III than MRSA agr type IV. The specificity of AIP-I agr inhibition was further confirmed by a hemolysis inhibition assay. Alpha toxin is a canonical MRSA virulence factor, and its expression is controlled by agr signaling (28). Incubation with AIP-I inhibited alpha-toxin-mediated red blood cell hemolysis for all MRSA agr types, demonstrating potent inhibition of MRSA agr-regulated products at the protein level (Fig. S5A to D).
TABLE 1.
Calculated IC50 values for synthetic S. simulans AIP inhibition of MRSA quorum sensing
| MRSA agr type | IC50 (nM) by S. simulans AIP type |
||
|---|---|---|---|
| I | II | III | |
| I | 2.2 | 1.6 | 1.7 |
| II | 1.1 | 15 | 6 |
| III | 3.5 | 11.5 | 3.2 |
| IV | 23 | 40 | 48 |
Synthetic S. simulans AIP-II and AIP-III were also validated for inhibitory activity against MRSA agr. In contrast to our initial findings that spent media from S. simulans AIP-II was a poor inhibitor of MRSA agr signaling, synthetic AIP-II inhibited MRSA agr types I, II, and III with low-nanomolar IC50s (Fig. S4B and Table 1). MRSA agr type IV was also inhibited by AIP-II but with a less potent effect (IC50, 40 nM). This discrepancy between spent media and synthetic AIP-II inhibition suggests that the S. simulans agr-II strain (AH5532) may be a poor AIP producer under in vitro conditions. A comparison of the AIP peak area measured by mass spectrometry confirmed that less AIP is made by this strain than by our representative agr-I or agr-III strains (Table S1). The addition of synthetic AIP-III also confirmed our initial findings that AIP-III potently inhibited MRSA agr types I, II, and III with low-nanomolar IC50s (Fig. S4C and Table 1). Synthetic AIP-III was the least potent inhibitor of MRSA agr type IV (IC50, 48 nM). These results taken together with our initial spent medium findings confirm the inhibitory activity of S. simulans AIP-I and demonstrate the MRSA quorum sensing inhibitory activity of newly identified S. simulans AIP-II and AIP-III.
Confirmation of S. simulans intraspecies cross talk with synthetic AIPs.
From our initial spent media activity assays, we sought to confirm the pattern of intraspecies cross talk between S. simulans agr types. To accomplish this, the S. simulans agr reporters were treated with a dose response of each synthetic AIP. As demonstrated in the spent media assays, the addition of AIP-I to the S. simulans agr-I reporter boosted the fluorescent signal in a dose-dependent manner (Fig. 5A). The addition of AIP-II (Fig. 5B) repressed agr-I signaling with an AIP-II concentration of 12.5 nM or higher. AIP-III (Fig. 5C) was a slightly better inhibitor of agr-I signaling and inhibited agr-I fluorescent signal with the addition of AIP-III concentrations of 6.25 nM or higher. S. simulans agr-II reporter fluorescence was modestly boosted by increasing doses of synthetic AIP-II but was not inhibited by doses up to 100 nM AIP-I or AIP-III (Fig. S6A to C). Similarly, S. simulans agr-III reporter fluorescence was modestly boosted by the addition of 50 or 100 nM AIP-III but was not inhibited by any dose of AIP-I or AIP-II (Fig. S6D to F). These synthetic AIP findings, supported by our initial observations of S. simulans spent media, demonstrate that S. simulans agr-I is sensitive to inhibitory intraspecies cross talk from AIP-II or AIP-III, while neither bovine agr type is sensitive to cross talk (Fig. 5D).
FIG 5.
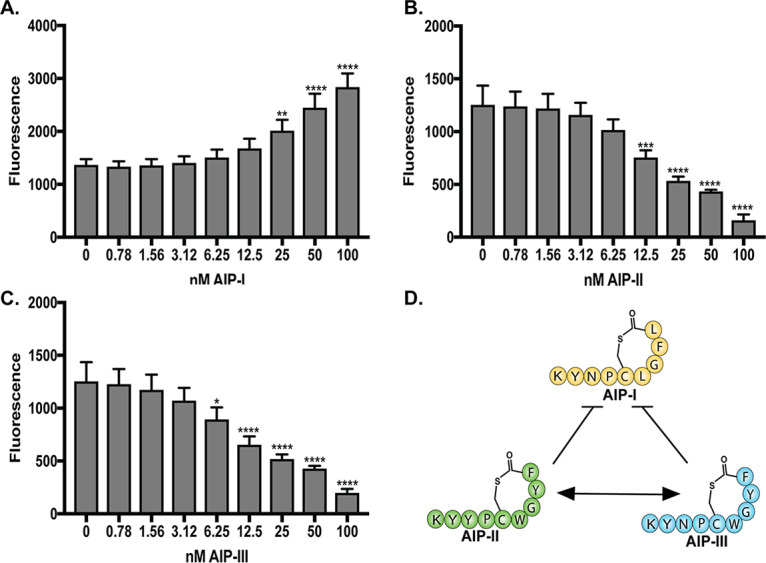
S. simulans agr-I is inhibited by noncognate S. simulans AIPs in a dose-dependent manner. (A to C) The S. simulans agr-I P3::sGFP reporter was incubated with increasing doses of synthetic S. simulans AIP-I (A), AIP-II (B), or AIP-III (C). The 24-h fluorescence point is shown. (D) Representative schematic of S. simulans intraspecies cross talk confirmed by the addition of synthetic AIP to each reporter strain. The results are pooled from three independent experiments performed in technical duplicates. Mean ± SD values are shown. Significance compared to no AIP addition was determined by ordinary one-way ANOVA with Dunnett’s multiple-corrections test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001).
S. simulans competition attenuates MRSA-associated dermonecrosis in vivo.
It is well established that MRSA agr is required for dermonecrotic skin injury (42). The significant attenuation of MRSA quorum sensing by S. simulans agr-I spent media in vitro prompted us to hypothesize that the presence of S. simulans could attenuate MRSA skin injury. To test this hypothesis, we used an established murine model of dermonecrosis (43, 44) and intradermally coinfected BALB/c mice with equal CFU of MRSA and S. simulans AH4549 (agr type I). Compared to MRSA infection alone, mice that received S. simulans at the time of MRSA infection developed significantly (P < 0.0001) smaller dermonecrotic lesions over the experimental time period (Fig. 6A and C). Additionally, coinfected mice were less moribund, as measured by changes in mouse weight, than were their MRSA-only-infected counterparts (Fig. 6B). These results demonstrate that S. simulans in competition with MRSA can attenuate acute MRSA tissue destruction during infection.
FIG 6.
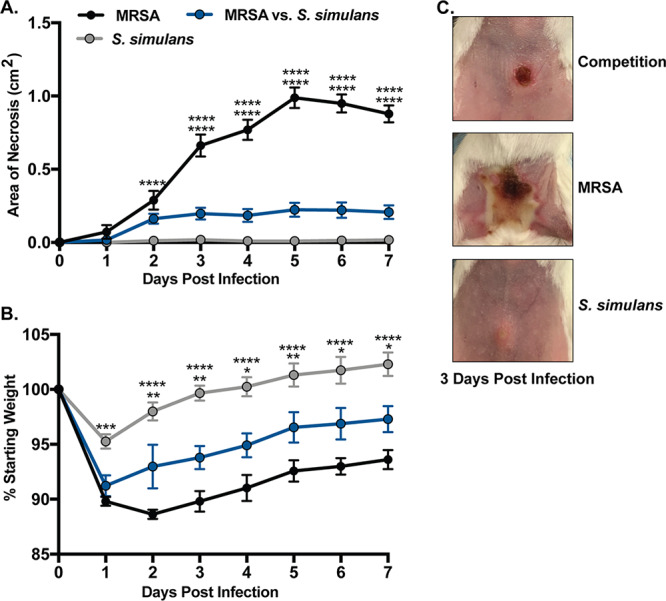
S. simulans cochallenge inhibits MRSA-mediated dermonecrosis in a murine model of soft tissue infection. (A) Dermonecrotic lesion size following infection with MRSA, equivalent CFU of MRSA and S. simulans AH4549, or S. simulans AH4549 alone. (B) Weight change following infection with the indicated groups. (C) Representative images of dermonecrotic lesions 3 days postinfection. Data are pooled from two independent experiments, n = 10 per group. Mean ± SEM values are shown. Two-way ANOVA with Dunnett’s multiple-comparison test was performed (*, P < 0.05; **, P < 0.005; ****, P < 0.0001).
S. simulans synthetic AIP-I attenuates MRSA-associated dermonecrosis in vivo.
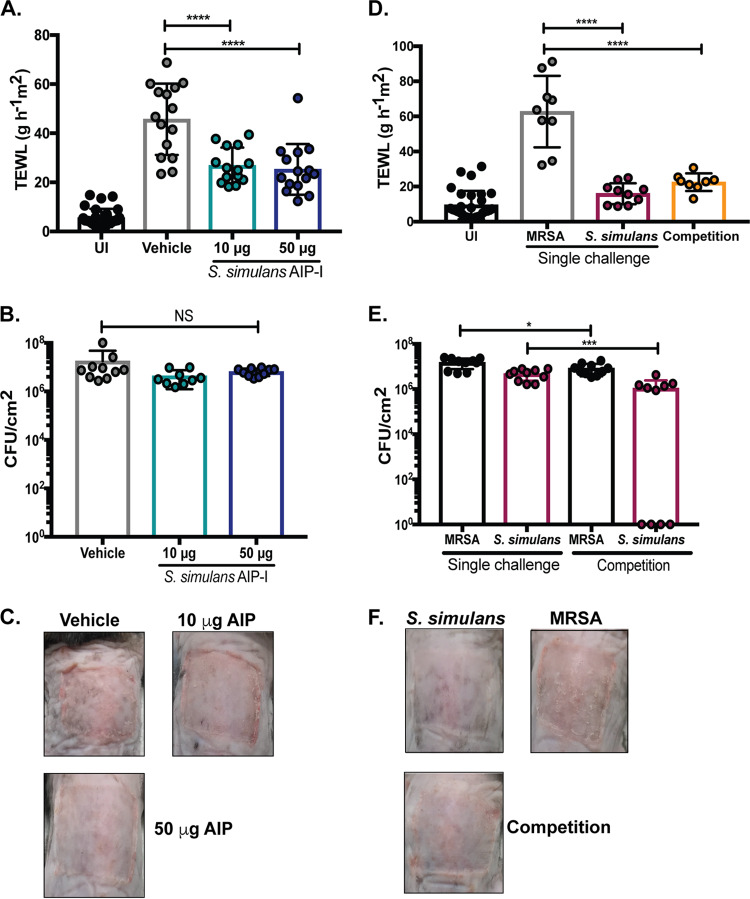
The in vitro quorum-quenching potency of synthetic S. simulans AIP-I prompted us to test its efficacy as an inhibitor of MRSA dermonecrosis in vivo. We chose to characterize in vivo potency of AIP-I given its potential relevance to human skin colonization resistance. BALB/c mice were intradermally infected with MRSA and a 10-μg or 50-μg dose of S. simulans synthetic AIP-I. Compared to vehicle treatment (DMSO), the addition of AIP-I reduced dermonecrotic lesion size and animal morbidity in a dose-dependent manner (Fig. 7A and B). In addition, mice administered a single 50-μg dose of synthetic AIP-I developed lesions similar in size to those in mice infected with an agr-null MRSA strain, highlighting its potency in vivo (Fig. 7C). These results demonstrate that S. simulans AIP-I is an effective inhibitor of MRSA quorum sensing in vivo and can protect host skin from dermonecrotic injury.
FIG 7.
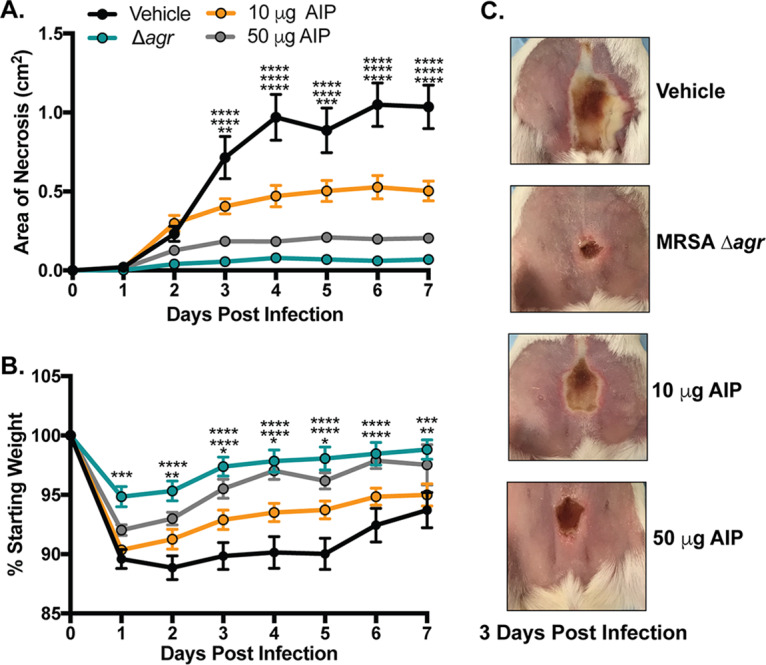
S. simulans AIP-I attenuates MRSA dermonecrosis in vivo. (A) Dermonecrotic lesion size for mice administered a 10- or 50-μg dose of S. simulans AIP-I (or vehicle) at the time of infection. (B) Weight change following infection of indicated groups. (C) Representative images of dermonecrotic lesion size and severity 3 days postinfection. Data are pooled from two independent experiments, n = 10 per group. Mean ± SEM values are shown. Two-way ANOVA with Dunnett’s multiple-comparison test was performed (*, P < 0.05; **, P < 0.009; ***, P < 0.0005; ****, P < 0.0001).
S. simulans or synthetic AIP-I reduces MRSA-associated barrier damage in vivo.
In addition to acute dermonecrosis and soft tissue injury, S. aureus skin colonization is also implicated in exacerbation of atopic dermatitis flares (45). Given that S. simulans AIP-I was a strong inhibitor of MRSA agr in vitro and in vivo, we sought to determine if the addition of S. simulans agr-I (AH4549) or synthetic AIP-I could protect the skin barrier from MRSA-mediated degradation in a murine model of epicutaneous dermatitis. C57BL/6 mice were topically associated with MRSA and a 10-μg or 50-μg dose of synthetic S. simulans AIP-I. At 48 h postinfection, skin barrier integrity was assessed by transepithelial water loss (TEWL) measurements as well as gross morphology. Mice treated with vehicle (DMSO) had significantly higher TEWL measurements (P < 0.0001) than those of mice that received either dose of synthetic AIP-I (Fig. 8A). The increased TEWL was not due to difference in bacterial numbers, as all groups had the same number of recoverable MRSA CFU at 48 h postinfection (Fig. 8B). Additionally, gross morphology of skin lesions revealed more redness and scaling on skin treated with vehicle than what was seen with either dose of AIP-I (Fig. 8C).
FIG 8.
S. simulans or AIP-I protects skin from MRSA-associated epicutaneous barrier damage in vivo. (A) Transepithelial water loss measurements at 48 h postinfection for mice challenged with MRSA and vehicle (DMSO) or MRSA and 10 or 50 μg of synthetic S. simulans AIP-I. Data are pooled from three experiments, n = 15. UI, uninfected. (B) Recovered MRSA CFU counts per cm2 of skin at 48 h postinfection. Data are from 2 independent experiments, n = 10. (C) Representative images of atopic lesions after a 48-h infection with MRSA and the indicated treatment groups. (D) Transepithelial water loss measurements 48 h postinfection for mice challenged with indicated groups. (E) Bacterial CFU counts per cm2 of skin 48 h postapplication. (F) Representative images of atopic lesions after a 48-h challenge with the indicated groups. Data are from two independent experiments, n = 9 to 10. Data points and mean ± SD values are shown. One-way ANOVA (compared to vehicle or MRSA only) was performed with Dunnett’s multiple-comparison test (****, P < 0.0001). For panel E, a Student two-tailed unpaired t test was performed (*, P = 0.03; ***, P = 0.0005).
Protective effects were also observed in mice challenged with equivalent CFU of MRSA and S. simulans (AH4549). Cochallenged mice had significantly lower TEWL measurements (P < 0.0001) than did MRSA-challenged mice (Fig. 8D). Additionally, mice challenged with S. simulans alone did not have significantly higher TEWL measurements than those of uninfected mice, suggesting that S. simulans does not degrade the skin barrier in this model. Single-challenged mice had more recoverable MRSA or S. simulans CFU than did cochallenged mice, suggesting that bacterial clearance may occur more quickly when in competition compared to single challenge (Fig. 8E). Additionally, gross morphology of atopic lesions revealed increased redness and scaling in MRSA-challenged mice compared to cochallenged or S. simulans challenge alone (Fig. 8F). These results are additional evidence of potent MRSA quorum-quenching activity in vivo and demonstrate that the addition of S. simulans (AH4549) or AIP-I can protect the skin barrier from epicutaneous MRSA damage.
DISCUSSION
MRSA skin and soft tissue infections are pervasive, often life-threatening conditions that require the development of nonantibiotic therapeutics to effectively treat while mitigating the possibility of enhanced antibiotic resistance. Here, we show that AIP-I produced by the rare skin commensal S. simulans effectively inhibits MRSA agr in vitro, reduces MRSA dermonecrosis in an acute skin infection model, and protects the outermost skin barrier from damage in a MRSA-induced epicutaneous dermatitis model (Fig. 9). Additionally, we show that S. simulans AIP-I inhibits all classes of MRSA agr with nanomolar potency. Taken together, our results demonstrate potent anti-MRSA quorum sensing activity from a human skin isolate of S. simulans and its cognate AIP-I.
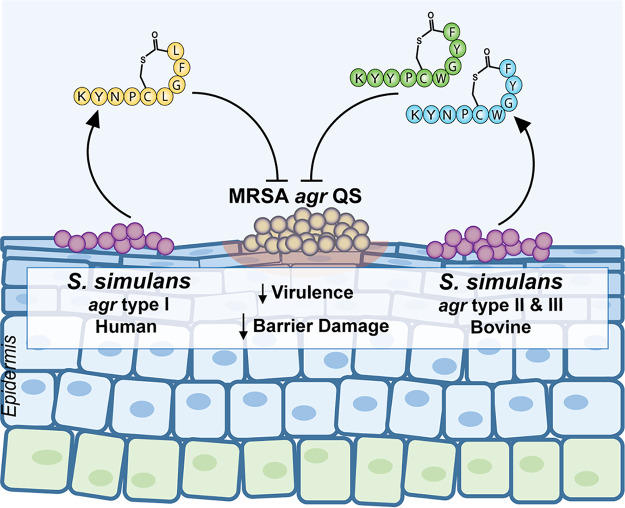
FIG 9.
S. simulans is a rare skin commensal that protects the skin barrier by blocking MRSA agr quorum sensing (QS). Confirmed human skin S. simulans AIP-I (yellow) and bovine AIP-II and AIP-III (green and blue) structures are illustrated. S. simulans AIP-I was the most potent inhibitor of all MRSA agr quorum sensing classes, with profound antivirulence effects in murine models of dermonecrotic and epicutaneous barrier injury. Bovine AIP-II and AIP-III blocked MRSA quorum sensing in vitro and cross-inhibited S. simulans agr-I signaling.
S. simulans occupies two related but distinct niches, human and livestock skin. By analyzing published S. simulans genomes, we identified three distinct agr types. While not exhaustive, our S. simulans strain library is notably divided into human skin isolates (AIP-I) and bovine isolates (AIP-II and AIP-III). Using a mass spectrometry approach, we solved the novel S. simulans AIP-II and AIP-II structures and confirmed the AIP-I structure (Fig. 9). All S. simulans AIPs were cyclic thiolactone peptides nine amino acids in length. Bovine S. simulans AIP-II and AIP-III were distinguished by a single amino acid change, while human skin S. simulans AIP-I was significantly more divergent in sequence. It is possible that human skin S. simulans isolates have a distinct agr type (I) because they colonize an immunologically, microbiologically, and structurally distinct niche compared to livestock skin. However, the underlying biological reasons for agr variation in any staphylococci remain unknown. Additionally, this study was limited by our relatively small collection of S. simulans isolates, particularly agr type II representatives.
To our knowledge, this is the first example of intraspecies cross talk between S. simulans agr types. Intriguingly, cross talk between the three S. simulans agr types is quite similar to S. epidermidis intraspecies cross talk, where types I and II or types I and III cross-inhibit each other, while types II and III do not (35). No cross talk between S. simulans agr-II and agr-III could be attributed the similarity in AIP structure (Y3N). A similar lack of cross talk between S. aureus AIP-I and AIP-IV, which also differ by a single amino acid, has been reported (28). While the biological importance of intraspecies cross talk remains unclear, previous studies have demonstrated some links between agr type and disease state. For example, S. epidermidis agr-I was shown to be most abundant in atopic dermatitis lesions (12), while MRSA agr-IV has been associated with scalded skin syndrome (46), MRSA agr-III with toxic shock syndrome (47), and MRSA agr-I with a broad range of invasive infections (18, 42). In a screen of children with atopic dermatitis, S. aureus agr-I and agr-III were most frequently isolated (48). These observations suggest that both CoNS and S. aureus agr groups may actively exclude one another during disease progression or during certain disease states. However, additional clinical and biochemical work is needed to demonstrate that exclusion is indeed mediated by intraspecies cross talk rather than by other mechanisms. More broadly, comprehensive surveillance of S. simulans agr types, especially in husbandry practice, could reveal which S. simulans agr types are more frequently associated with commensal versus pathogenic presence on the skin.
Moreover, agr- or small-molecule-mediated intraspecies cross talk has been proposed as a potential CoNS niche competition strategy (32). It is notable that CoNS dominate healthy skin, while MRSA is most often restricted to colonization of the nares (1, 14). Several CoNS species have been shown to make non-quorum sensing small-molecule factors, including the S. epidermidis Esp serine protease (49), the S. hominis sh-lantibiotic (13), and the S. lugdunensis lugdunin cyclic peptide antibiotic (50), which have specific and potent bactericidal activity against MRSA. A recent bioinformatics analysis of bovine-isolated CoNS identified a wide array of potential bacteriocins represented within the genomes, including in S. simulans isolates (51). While we did not observe direct MRSA killing by any S. simulans isolate, these interactions could be constrained to specific environmental conditions that were not recapitulated in our study. Additionally, there is some evidence to suggest that CoNS lantibiotic production can be regulated by the agr quorum sensing system (52). Whether S. simulans skin or bovine isolates make bacteriocins, and how bacteriocin production is regulated, is an area of future investigation that may shed light on a specific mechanism of niche competition and pathogen exclusion on the skin. Additionally, lantibiotic or antibiotic MRSA killing coupled with the antivirulence activity of noncognate AIP quorum quenching represents a particularly attractive treatment alternative to current therapeutic options that often rely on last-resort antibiotic regimens for extended time periods.
In addition to the anti-infective properties of S. simulans and AIP-I, we sought to address S. simulans skin colonization competition with MRSA by using a MRSA-induced model of epicutaneous dermatitis. We have shown for the first time that S. simulans agr type I or AIP-I potently blocks MRSA agr, which protects the outermost skin barrier from MRSA agr-mediated damage. However, the current study is limited by the use of wound and barrier-defect models rather than a natural asymptomatic skin colonization model. Future work could include other skin models, such as human epidermal equivalents or porcine skin (53–55). Such models are necessary to fully understand the consequences of prophylactic CoNS application. Application of a lantibiotic-producing CoNS in a small cohort of atopic dermatitis patients was strikingly successful (13), and our current work suggests that application of S. simulans or synthetic AIP-I could also have potent anti-MRSA activity in patients by blocking agr. However, more research is critically needed to fully understand the potential benefits of such a therapeutic approach as well as the potential for innate or acquired CoNS pathogenesis. Collectively, our results indicate a novel role in colonization resistance for a rare CoNS skin commensal, enhance our knowledge of staphylococcal intra- and interspecies competition, and suggest a potential therapeutic role for noncognate AIP inhibition of MRSA quorum sensing in acute and chronic skin infections.
MATERIALS AND METHODS
Ethics statement.
Seven-week-old female BALB/c or male C57BL/6J mice were purchased from Jackson Laboratories and housed in specific pathogen-free facilities at the University of Colorado Anschutz Medical Center Animal Facility. Mice were allowed to acclimate for 1 week prior to experimentation. At experimental endpoints, mice were euthanized via CO2 inhalation, followed by cervical dislocation. All animal work was approved by and performed in accordance with the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus under protocol numbers 117217 and 00941.
Growth conditions and reagents.
The bacterial strains used in this study are listed in Table 2. All staphylococcal strains were grown in tryptic soy broth (TSB) at 37°C with shaking at 220 rpm. Escherichia coli was grown in LB at 37°C with shaking at 220 rpm. For strains with pDB59 or pCM41, chloramphenicol was added to a final concentration of 10 μg/ml. S. simulans synthetic AIPs were custom synthesized by AnaSpec, Inc.
TABLE 2.
List of strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference, source, or parent strain |
|---|---|---|
| Strains | ||
| E. coli DC10B | Restriction-modification deficient | 59 |
| S. aureus | ||
| AH1263 | USA300 CA-MRSA Erms (LAC) | 60 |
| AH1292 | AH1263 Δagr::tetM | 61 |
| AH1677 | LAC/pDB59 (agr-I) | 40 |
| AH430 | 502A/pDB59 (agr-II) | 40 |
| AH1747 | MW2/pDB59 (agr-III) | 40 |
| AH1872 | MNEV/pDB59 (agr-IV) | 40 |
| AH0845 | USA300 LAC | Parental strain of AH1677 |
| AH0408 | CA-MRSA USA400 (502A) | Parental strain of AH430 |
| AH0843 | CA-MRSA USA400 (MW2) | Parental strain of AH1747 |
| AH0407 | MNEV | Parental strain of AH1872 |
| S. simulans | ||
| AH4549 | MK148 (agr-I) | ATCC 27848 |
| AH5419 | SMU76 (agr-I) | ATCC 31432 |
| AH5420 | KL240 (agr-I) | ATCC 27850 |
| AH5421 | AW215 (agr-I) | ATCC 27849 |
| AH5532 | SNUC1535 (agr-II) | CBMRN |
| AH5533 | SNUC1396 (agr-III) | CBMRN |
| AH5534 | SNUC2478 (agr-III) | CBMRN |
| AH5541 | CNH113 (agr-III) | FDA-ARGOS |
| AH5595 | AH4549/pCM41 | This study |
| AH5596 | AH5532/pCM41 | This study |
| AH5597 | AH5533/pCM41 | This study |
| Coagulase-negative isolates | ||
| AH4551 | Staphylococcus auricularis (WH811M) | ATCC 33753 |
| AH2452 | Staphylococcus capitis | 11 |
| AH3568 | Staphylococcus caprae (DSM 20608) | 11 |
| AH4525 | Staphylococcus cohnii (MF2) | Ward lab |
| AH1740 | Staphylococcus epidermidis | ATCC 12228 |
| AH4513 | Staphylococcus equorum | L. Ackermann |
| AH4547 | Staphylococcus haemolyticus (SM131) | ATCC 29970 |
| AH4545 | Staphylococcus hominis | This study |
| AH2450 | Staphylococcus intermedius | 11 |
| AH2159 | Staphylococcus lugdunensis (N910 320) | 11 |
| AH4522 | Staphylococcus nepalensis (BHW1) | Ward lab |
| AH3604 | Staphylococcus saprophyticus (SST) | ATCC 15035 |
| AH4548 | Staphylococcus warneri (AW25) | ATCC 27836 |
| AH3603 | Staphylococcus xylosus (SXT) | ATCC 29971 |
| Plasmids | ||
| pCM41 | pRNAIII::sGFP | This study |
| pDB59 | P3::YFP | 62 |
CA-MRSA, community-acquired methicillin-resistant S. aureus; Erms, erythromycin sensitive.
S. simulans agrD sequencing.
Genomic DNA was isolated from S. simulans strains using the PureGene Yeast/Bact kit (Qiagen) with a modified protocol, in which 4 ml of cells was pelleted and resuspended in 0.5 ml phosphate-buffered saline (PBS). The suspension was homogenized in a bead beater with 1-mm zirconia-silica beads for 3 × 1-min intervals with 1 min on ice between homogenization intervals. After homogenization, the manufacturer’s protocol was continued with the omission of the RNase A step and the addition of a 1-h incubation on ice after the addition of protein precipitation solution. Samples were submitted to the Barbara Davis Center Bioresource Core for Sanger sequencing. The oligonucleotides used to sequence S. simulans agrD-I were forward, 5′-GTTCCTAATGCTTACCAACAAATG-3′, and reverse, 5′-CATCAATCCTTGATAGGTCGATATT-3′. The oligonucleotides used to sequence S. simulans agrD-II and agrD-III were forward, 5′-TCACATTACAAGGCGGTTCA-3′, and reverse, 5′-CCTTGATAAGTCGCTATAAACATACC-3′.
S. simulans agr::P3 reporter electroporation.
Plasmid pCM41 was constructed by cloning the agr P3 promoter from AH1263 into pCM29 (56). The promoter was amplified with oligonucleotides CLM469 (5′-GTTGTTGCTAGCCTGTCATTATACGATTTAGTACAATC-3′) and ARH133 (5′-GTTGTTGGTACCTTAAACAACTCATCAACTATTTTCC-3′), and the PCR product was digested with NheI and KpnI and cloned into pCM29 cut with the same enzymes. S. simulans strains AH4549, AH5532, and AH5533 were made electrocompetent following Staphylococcus epidermidis: Methods and Protocols (57). The agr reporter plasmid pCM41 was midi-prepped from the restriction-modification-deficient E. coli DC10B host with the Invitrogen PureLink midi prep kit, following the manufacturer’s protocol, and transformed into each S. simulans strain.
Fluorescent agr reporter assays.
For S. simulans spent media assays, overnight (ON) cultures of S. aureus reporter strains and S. simulans strains were grown as described. One milliliter of S. simulans culture was pelleted, and spent media was filtered through Costar Spin-X centrifuge tubes (0.22-μm cellulose acetate filter). Reporter strains were prepared by subculturing 1:500 in fresh TSB with chloramphenicol. One hundred microliters of spent media or TSB as the negative control was added at 20% (vol/vol) to a 96-well black culture plate and 10-fold serially diluted to 0.15% (vol/vol). One hundred microliters of reporter culture was added to each well for a 200-μl final volume. Cultures were grown in a Stuart humidified incubator at 37°C with shaking at 1,000 rpm. At hourly time points, the plates were measured on a Tecan Group Ltd. Infinite 200 Pro plate reader to quantify growth (optical density at 600 nm [OD600]) and YFP signal (excitation, 480 nm; emission, 515 nm). For synthetic AIP addition, peptide resuspended in DMSO or a DMSO control was added from stocks of 20 μM to indicated concentrations. S. simulans agr reporter assays were conducted in the same manner as for the S. aureus agr reporter assays.
Murine dermonecrosis model.
To prepare bacteria for the dermonecrosis model, strains were grown ON in TSB, subcultured 1:100 in fresh TSB, and allowed to grow to early exponential phase (OD600, 0.5 to 0.7). Bacterial cells were washed and pelleted in phosphate-buffered saline (PBS) and resuspended in sterile saline to achieve an inoculum of 1 × 108 CFU in 50 μl. Inoculum concentration was verified by serial dilution, plating, and colony counting after 24 h of incubation at 37°C. One day prior to challenge, BALB/c mouse abdomens were shaved, and residual hair was removed with a 30 s application of Nair (Church & Dwight Co., Inc.). Immediately prior to injection, abdomens were sanitized with alcohol wipes. For the competition experiment, 50-μl suspensions of 1 × 108 CFU MRSA alone, equivalent CFU of MRSA and S. simulans, or 1 × 108 CFU S. simulans alone were injected intradermally. Mouse body weights were measured before infection and every day thereafter for a period of 7 days. To determine lesion size, digital images were taken using an iPhone 7 camera and analyzed using the ImageJ (NIH) software. For AIP efficacy experiments, 50-μl inoculum suspensions containing 1 × 108 CFU MRSA and either S. simulans AIP (10 μg or 50 μg in neat DMSO) or DMSO alone were injected intradermally.
Murine epicutaneous infection model.
As previously described (12), C57BL/6J mouse backs were shaved, and residual hair was removed with a 1-min application of Nair (Church & Dwight Co., Inc.). Mice were allowed to recover for 24 h following hair removal. Bacteria were applied to back skin for 48 h on a 2-cm2 piece of sterile gauze affixed with Tegaderm and covered in a Band-Aid. To prepare bacteria for the epicutaneous model, strains were grown ON in TSB, subcultured 1:50 in fresh TSB, and allowed to grow to an OD600 of 1. Bacterial cells were washed and pelleted in phosphate-buffered saline (PBS) and resuspended in sterile saline to achieve an inoculum of 1 × 108 CFU in 100 μl. Equivalent (1:1) CFU of S. simulans and S. aureus LAC or LAC and synthetic AIP-1 (10 or 50 μg) or vehicle (DMSO) were combined immediately prior to application on the gauze. Inoculum concentration was verified by serial dilution, plating, and colony counting after 24 h of incubation at 37C. A Tewameter TM300 (Courage & Khazaka Electronic GmbH) was used to determine changes to epithelial barrier integrity at 48 h postinfection. Two sites per lesion were analyzed to minimize error in measurements. To enumerate bacterial CFU on skin postchallenge, the full-thickness 2-cm2 atopic lesion was excised with sterile scissors, added to 0.5 ml PBS with 1-mm zirconia-silica homogenization beads, and homogenized for 3 × 1-min intervals. The suspension was serially diluted and plated on nonselective (TSA) and selective (mannitol salt agar [MSA] and MSA supplemented with 5.2 μg/ml cefoxitin) media. Plates were incubated overnight prior to colony counting.
Hemolysis assay.
The hemolysis assay was performed as previously described (58). Briefly, representative MRSA agr type strains were incubated shaking for 24 h with indicated doses of synthetic S. simulans AIP-I. Spent media from each culture was filter sterilized in a Costar Spin-X centrifuge tubes (0.22-μm cellulose acetate filter). Concurrently, rabbit red blood cells (RBCs) were washed in 1.3× PBS 3 to 5 times until a clear supernatant was achieved. From washed RBCs, 3% and 1% RBC solutions were made in 1.3× PBS. For MRSA agr types I, II, and IV, spent media was serially diluted 1:2 in a microtiter plate and mixed with the 3% RBC solution to achieve 70:30 RBC-spent media mix. For MRSA agr type III, spent media was serially diluted in a microtiter plate 1:2 and mixed with the 1% RBC solution to achieve a 50:50 RBC-spent media mix. All plates were incubated at room temperature for 1.5 h, and lysis was measured using a Tecan Group Ltd. Infinite 200 Pro plate reader with absorbance set to 630 nm. Data were fit to a four-parameter logistic curve with the GraphPad Prism 7 (San Diego, CA) software.
LC-MS identification of S. simulans AIPs.
For each of the representative S. simulans agr type strains, a single colony was inoculated in TSB and incubated at 37°C for with shaking of 250 rpm for 24 h. Each inoculum was then diluted 1:200 (culture/TSB) and incubated 37°C with shaking of 250 rpm for 18 to 20 h, until the OD600 for cell growth reached an initial stationary-phase value of approximately 1.5. Each culture was then centrifuged for 10 min at 10,000 rpm and vacuum filtered through a 0.22-μm surfactant-free cellulose acetate (SFCA) membrane.
A 6-ml aliquot of each spent media filtrate was subjected to solid-phase extraction (SPE) for selective isolation of AIPs from the growth media matrix. The separation was performed using Strata-X-C, strong cation exchange, reversed-phase columns, according to the manufacturer’s recommended protocol. Eluent from each step of the extraction procedure was collected, dried under nitrogen gas at room temperature, and immediately resuspended in 120 μl of an 80:20 water and methanol solution. Resuspended samples were analyzed directly via LC-MS alongside the untreated spent media filtrate samples.
All samples were analyzed using an Acquity ultrahigh-performance liquid chromatography (UPLC) system (Waters Corporation) coupled to a Q Exactive Plus hybrid quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific). A 7-μl injection of each sample was eluted from an Acquity UPLC BEH C18 1.7-μm, 2.1 by 50 mm column (Waters Corporation) at a flow rate of 0.3 ml/min using a 10-minute binary gradient of water (Optima LC-MS grade) with 0.1% formic acid and acetonitrile (Optima LC-MS grade) with 0.1% formic acid. Mass spectra were collected using two scan events utilizing positive-mode electrospray ionization, a full-scan event over a mass range of 300 to 2,000 at a resolving power of 35,000, and a data-dependent tandem mass spectrometry (MS/MS) scan event selecting the calculated m/z values of the predicted AIPs. The mass spectrometer was operated using the following settings: capillary temperature set at 300°C, S-Lens radio frequency (RF) level set at 80, spray voltage set at 4.0 kV, sheath gas flow set at 50, and auxiliary gas flow set at 15. Precursor ions detected in the full scan were selected, with an isolation window of 4 Da, and subjected to high-energy collision dissociation (HCD) fragmentation at a normalized collision energy of 25. Synthetic standards for each of the observed AIP structures were purchased from AnaSpec, EGT (Fremont, CA) and subjected to the same UPLC-MS analysis. The accurate mass, retention time, and fragmentation patterns for synthetic standards were compared to those of putative AIP ions in spent media to confirm structure.
Statistical analysis.
All analyses were performed using GraphPad Prism 7 (San Diego, CA) software. For comparisons between a single control and test group, an unpaired Student two-tailed t test was performed. For >3 comparisons, a one-way analysis of variance (ANOVA) with Dunnett’s multiple-comparison test was chosen. Animal data were analyzed with two-way ANOVA with Dunnett’s multiple-comparison test. In vitro data are presented as the mean and standard deviation (SD), and in vivo data are presented as the mean and standard error of the mean (SEM); a P value of ≤ 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
M.M.B. was supported by the NIH T32 AI052066 training grant from the National Institute for Allergy and Infectious Diseases (NIAID). A.R.H. and N.B.C. were supported by NIAID grant AI133089. A.R.H. was also supported by a Merit Award (I01 BX002711) from the Department of Veteran Affairs.
We thank Jeroen De Buck at the University of Calgary and Simon Dufour at the Canadian Bovine Mastitis Research Network (CBMRN) for help acquiring S. simulans bovine isolates. Additionally, we thank Patrick Schlievert, Laynez Ackermann, and Tim Foster for help obtaining other CoNS strains.
M.M.B. and A.R.H. wrote the manuscript. A.R.H., M.M.B., and J.M.K. designed the experimental plan. M.M.B. performed in vitro microbiological assays, all in vivo studies, and relevant data analysis. A.S. and L.M.C. designed and performed all mass spectrometric identification and validation of AIP structures. N.B.C. and D.A.T. assisted with mass spectrometry analysis and data interpretation. All authors contributed to the revision and final review of the manuscript.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Byrd AL, Belkaid Y, Segre JA. 2018. The human skin microbiome. Nat Rev Microbiol 16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 2.Oh J, Byrd AL, Park M, NISC Comparative Sequencing Program, Kong HH, Segre JA. 2016. Temporal stability of the human skin microbiome. Cell 165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parlet CP, Brown MM, Horswill AR. 2019. Commensal staphylococci influence Staphylococcus aureus skin colonization and disease. Trends Microbiol 27:497–507. doi: 10.1016/j.tim.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto M. 2009. Staphylococcus epidermidis–the “accidental” pathogen. Nat Rev Microbiol 7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, Von Aulock S, Radek KA, Huang CM, Ryan AF, Gallo RL. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y, Cogen AL, Radek KA, Park HJ, MacLeod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. 2010. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira WF, Silva PMS, Silva RCS, Silva GMM, Machado G, Coelho L, Correia M. 2018. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect 98:111–117. doi: 10.1016/j.jhin.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, NISC Comparative Sequencing Program, Belkaid Y, Segre JA, Kong HH. 2017. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 9:eaal4651. doi: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paharik A, Parlet C, Chung N, Todd D, Rodriguez E, Van Dyke M, Cech N, Horswill A. 2017. Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22:746–756. doi: 10.1016/j.chom.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Sanford JA, O’neill AM, Liggins MC, Nakatsuji T, Cech NB, Cheung AL, Zengler K, Horswill AR, Gallo RL. 2019. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med 11:eaat8329. doi: 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim J-N, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DYM, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakr A, Brégeon F, Mège JL, Rolain JM, Blin O. 2018. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol 9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh J, Conlan S, Polley EC, Segre JA, Kong HH. 2012. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med 4:77. doi: 10.1186/gm378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CM, Price LB, Hungate BA, Abraham AG, Larsen LA, Christensen K, Stegger M, Skov R, Andersen PS. 2015. Staphylococcus aureus and the ecology of the nasal microbiome. Sci Adv 1:e1400216. doi: 10.1126/sciadv.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 19.Williams MR, Gallo RL. 2017. Evidence that human skin microbiome dysbiosis promotes atopic dermatitis. J Invest Dermatol 137:2460–2461. doi: 10.1016/j.jid.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, Vial Y, Prod’hom G, Greub G, Kypriotou M, Christen-Zaech S. 2017. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol 137:2497–2504. doi: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- 21.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 22.Wright JS, Jin R, Novick RP. 2005. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A 102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwan WR, Langhorne MH, Ritchie HD, Stover CK. 2003. Loss of hemolysin expression in Staphylococcus aureus agr mutants correlates with selective survival during mixed infections in murine abscesses and wounds. FEMS Immunol Med Microbiol 38:23–28. doi: 10.1016/S0928-8244(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 24.Parlet CP, Kavanaugh JS, Crosby HA, Raja HA, El-Elimat T, Todd DA, Pearce CJ, Cech NB, Oberlies NH, Horswill AR. 2019. Apicidin attenuates MRSA virulence through quorum-sensing inhibition and enhanced host defense. Cell Rep 27:187–198.e6. doi: 10.1016/j.celrep.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhs A, Lyles JT, Parlet CP, Nelson K, Kavanaugh JS, Horswill AR, Quave CL. 2017. Virulence inhibitors from Brazilian peppertree block quorum sensing and abate dermonecrosis in skin infection models. Sci Rep 7:42275. doi: 10.1038/srep42275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. 2014. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harraghy N, Kerdudou S, Herrmann M. 2007. Quorum-sensing systems in staphylococci as therapeutic targets. Anal Bioanal Chem 387:437–444. doi: 10.1007/s00216-006-0860-0. [DOI] [PubMed] [Google Scholar]
- 28.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. 2011. Peptide signaling in the Staphylococci. Chem Rev 111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T, Tal-Gan Y, Paharik AE, Horswill AR, Blackwell HE. 2016. Structure-function analyses of a Staphylococcus epidermidis autoinducing peptide reveals motifs critical for AgrC-type receptor modulation. ACS Chem Biol 11:1982–1991. doi: 10.1021/acschembio.6b00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu Rev Genet 42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 31.Fetzner S, Collin M, Canovas J, Baldry M, Ms B, Canovas J, Baldry M, Bojer MS, Andersen PS, Grzeskowiak PK, Stegger M, Damborg P, Olsen CA, Ingmer H. 2016. Cross-talk between Staphylococcus aureus and other staphylococcal species via the agr quorum sensing system. Front Microbiol 7:1733. doi: 10.3389/fmicb.2016.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otto M, Echner H, Voelter W, Götz F. 2001. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 69:1957–1960. doi: 10.1128/IAI.69.3.1957-1960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmmod YS, Klaas IC, Svennesen L, Pedersen K, Ingmer H. 2018. Communications of Staphylococcus aureus and non-aureus Staphylococcus species from bovine intramammary infections and teat apex colonization. J Dairy Sci 101:7322–7333. doi: 10.3168/jds.2017-14311. [DOI] [PubMed] [Google Scholar]
- 34.Peng P, Baldry M, Gless BH, Bojer MS, Espinosa-Gongora C, Baig SJ, Andersen PS, Olsen CA, Ingmer H. 2019. Effect of co-inhabiting coagulase negative staphylococci on S. aureus agr quorum sensing, host factor binding, and biofilm formation. Front Microbiol 10:2212. doi: 10.3389/fmicb.2019.02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson ME, Todd DA, Schaeffer CR, Paharik AE, Van Dyke MJ, BüTtner H, Dunman PM, Rohde H, Cech NB, Fey PD, Horswill AR. 2014. Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J Bacteriol 196:3482–3493. doi: 10.1128/JB.01882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloos WE, Schleifer KH. 1975. Isolation and characterization of staphylococci from human skin. Descriptions of four new species: Staphylococcus warneri, Staphylococcus capitis, Staphylococcus hominis, and Staphylococcus simulans. Int J Syst Bacteriol 25:62–79. doi: 10.1099/00207713-25-1-62. [DOI] [Google Scholar]
- 37.Ji G, Pei W, Zhang L, Qiu R, Lin J, Benito Y, Lina G, Novick RP. 2005. Staphylococcus intermedius produces a functional agr autoinducing peptide containing a cyclic lactone. J Bacteriol 187:3139–3150. doi: 10.1128/JB.187.9.3139-3150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gless BH, Bojer MS, Peng P, Baldry M, Ingmer H, Olsen CA. 2019. Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation. Nat Chem 11:463–469. doi: 10.1038/s41557-019-0256-3. [DOI] [PubMed] [Google Scholar]
- 39.Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, Etienne J, Lina G. 2002. High genetic variability of the agr locus in Staphylococcus species. J Bacteriol 184:1180–1186. doi: 10.1128/jb.184.4.1180-1186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. 2009. Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods 77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malone CL, Boles BR, Horswill AR. 2007. Biosynthesis of Staphylococcus aureus autoinducing peptides by using the Synechocystis DnaB mini-intein. Appl Environ Microbiol 73:6036–6044. doi: 10.1128/AEM.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung GYC, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwiecinski J, Jin T, Josefsson E. 2014. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. APMIS 122:1240–1250. doi: 10.1111/apm.12295. [DOI] [PubMed] [Google Scholar]
- 44.Quave CL, Lyles JT, Kavanaugh JS, Nelson K, Parlet CP, Crosby HA, Heilmann KP, Horswill AR. 2015. Castanea sativa (European chestnut) leaf extracts rich in ursene and oleanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. PLoS One 10:e0136486. doi: 10.1371/journal.pone.0136486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangel SM, Paller AS. 2018. Bacterial colonization, overgrowth, and superinfection in atopic dermatitis. Clin Dermatol 36:641–647. doi: 10.1016/j.clindermatol.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Lamand V, Dauwalder O, Tristan A, Casalegno JS, Meugnier H, Bes M, Dumitrescu O, Croze M, Vandenesch F, Etienne J, Lina G. 2012. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clin Microbiol Infect 18:E514–E521. doi: 10.1111/1469-0691.12053. [DOI] [PubMed] [Google Scholar]
- 47.Lyon GJ, Novick RP. 2004. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides 25:1389–1403. doi: 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Chung HJ, Jeon HS, Sung H, Kim MN, Hong SJ. 2008. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from children with eczematous atopic dermatitis lesions. J Clin Microbiol 46:991–995. doi: 10.1128/JCM.00698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 50.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brötz-Oesterhelt H, Grond S, Peschel A, Krismer B. 2016. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 51.Carson DA, Barkema HW, Naushad S, De Buck J. 2017. Bacteriocins of non-aureus staphylococci isolated from bovine milk. Appl Environ Microbiol 83:e01015-17. doi: 10.1128/AEM.01015-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kies S, Hille M, Vuong C, Meyer C, Götz F, Otto M, Peschel A. 2003. Control of antimicrobial peptide synthesis by the agr quorum sensing system in Staphylococcus epidermidis: activity of the lantibiotic epidermin is regulated at the level of precursor peptide processing. Peptides 24:329–338. doi: 10.1016/S0196-9781(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 53.Summerfield A, Meurens F, Ricklin ME. 2015. The immunology of the porcine skin and its value as a model for human skin. Mol Immunol 66:14–21. doi: 10.1016/j.molimm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 54.Popov L, Kovalski J, Grandi G, Bagnoli F, Amieva MR. 2014. Three-dimensional human skin models to understand Staphylococcus aureus skin colonization and infection. Front Immunol 5:41. doi: 10.3389/fimmu.2014-00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cadau S, Valla-Dury L, Cenizo V, Le-Beux C, Gault M, Vianney A, Andre-Frei V. 2017. Studying microbiote competition and skin interaction using organotypic 3D skin models. Adv Tissue Eng Regen Med Open Access 2:233–234. doi: 10.15406/atroa.2017.02.00041. [DOI] [Google Scholar]
- 56.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. 2010. Agr-dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun 2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maliszewski KL, Nuxoll AS. 2014. Use of electroporation and conjugative mobilization for genetic manipulation of Staphylococcus epidermidis, p 125–134. In Fey P. (ed), Staphylococcus epidermidis: methods and protocols, vol 1106. Humana Press, Springer Science and Business Media LLC, New York, NY. [DOI] [PubMed] [Google Scholar]
- 58.Daly SM, Elmore BO, Kavanaugh JS, Triplett KD, Figueroa M, Raja HA, El-Elimat T, Crosby HA, Femling JK, Cech NB, Horswill AR, Oberlies NH, Hall PR. 2015. ω-Hydroxyemodin limits staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob Agents Chemother 59:2223–2235. doi: 10.1128/AAC.04564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boles BR, Thoende M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, Griffith S, Kiedrowski MR, Flack CE, Kavanaugh JS, Kreiswirth BN, Horswill AR, Voyich JM. 2013. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. 2004. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.