Abstract
OBJECTIVES
The aim of this paper was to investigate whether ventricular arrhythmias in children with catecholaminergic polymorphic ventricular tachycardia (CPVT) show circadian patterns.
BACKGROUND
Circadian arrhythmic patterns have been established in long QT, Brugada, and early repolarization, but have not been investigated in CPVT.
METHODS
This is a multicenter, retrospective review of pediatric CPVT patients, age <21 years at diagnosis. Timing of ventricular tachycardia (VT ≥3 beats) was assessed during 24-h continuous monitoring (Holter, implantable loop recorder, implantable cardioverter defibrillator) and by eliminating sleep hours, in addition to sporadic exercise stress tests. Morning was defined as 6:00 am to 11:59 am, afternoon 12:00 pm to 5:59 pm, and evening 6:00 pm to 11:59 pm. Distribution of VT events was compared by time of day, day of week, age, and sex.
RESULTS
Eighty patients (53% male), 61% with an ICD, experienced 423 VT events during a median follow-up time of 6 years (interquartile range: 2 to 10 years). When compared to morning hours, VT was more likely to occur in the afternoon (odds ratio [OR]: 2.54; 95% confidence interval [CI]: 1.69 to 3.83) or evening hours (OR: 2.91; 95% CI: 1.82 to 4.67). The predominance of afternoon/evening events persisted regardless of age, gender, or day of the week. Among 50 patients who underwent exercise stress tests, VT was significantly more likely to occur in the afternoon (OR: 3.00; 95% CI: 1.39 to 6.48).
CONCLUSIONS
In pediatric CPVT patients, ventricular arrhythmias are more likely to occur in the afternoon and evening hours. Because children’s activity levels peak in both the morning and afternoon, the lack of arrhythmias in the morning hours raises questions whether factors other than adrenergic stimulation influence arrhythmia induction in pediatric patients with CPVT.
Keywords: arrhythmia, catecholaminergic polymorphic ventricular tachycardia, children, circadian
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmia disorder characterized by polymorphic or bidirectional ventricular tachycardia and ventricular fibrillation induced by elevated adrenergic states, such as exercise or emotional stress. Symptoms, typically syncope and cardiac arrest, most often have their onset in childhood or adolescence, with an average age of onset of 7.8 years (1). CPVT is the most lethal of heritable arrhythmia disorders with mortality rates reported as high as 30% to 50% by age 30 years (1,2) and a fatal event rate of 1% to 2% per year (3).
There are well-described diurnal variations among known heritable and acquired arrhythmia disorders, but there are no published reports among patients with CPVT (4–7). Notable circadian arrhythmia patterns during morning hours has been shown in patients with long QT syndromes type 1 and 2, post-myocardial infarction (MI), adults with implantable cardioverter defibrillator (ICD), and among deaths due to cardiac arrest (4,8–15). Patients with hypertrophic cardiomyopathy have a higher incidence of arrhythmias in the afternoon and evening hours (8,9).
Physiologically, there is diurnal variation in heart rate, blood pressure, QT interval, and effective refractory period of ventricular myocytes (16–18). Studies have also shown diurnal variation in ion channel expression and evidence that clock-regulated genes may play a role in arrhythmia development (19,20). In an adrenergically mediated disease process such as CPVT, these recognized circadian patterns in cardiomyocyte biology must be balanced against peak activity levels in children. In a study by Goodman et al. (21), specifically evaluating activity levels among 23,000 children, the authors noted a diurnal peak in activity between 7 am and 8 am and again between 3 pm and 4 pm (Figure 1). In CPVT, the prevailing thought is that arrhythmias most likely occur when children are most active. If this is the case, then it could be expected that children with CpVT would show bimodal peaks in ventricular arrhythmias. The purpose of this study was to determine if patterns of ventricular arrhythmias in patients with CpVT show specific patterns of circadian variation or merely reflect peak activity levels of children.
FIGURE 1. Distribution of All Events.

(A) Bimodal peaks in activity level among children. (B) The overall distribution of arrhythmia events among all continuous monitoring is shown. The graph shows a skewed distribution of arrhythmias occurring in the afternoon and evening hours that differs from bimodal peak activity levels in A. (A) Reprinted with permission by BioMed Central [Goodman et al. (21)].
METHODS
STUDY POPULATION.
This was a multicenter, international, retrospective review of patients diagnosed with CpVT from 7 institutions in the United States and Canada. The institutions and the number of patients from each center (indicated in parenthesis) are as follows: Boston Children’s Hospital (26), Texas Children’s Hospital (17), Stollery Children’s Hospital (11), Northwestern University (10), Cincinnati Children’s Medical Center (9), Oakland Children’s Hospital (6), and Alaska Children’s Heart Center (1). This study was approved by respective Institutional Review Boards at each participating institution. Inclusion criteria included: 1) age at first symptom <21 years; and 2) diagnosis of CpVT by a pediatric electrophysiologist. Diagnosis of CpVT was based on clinical history and documented polymorphic or bidirectional ventricular arrhythmias or cardiac arrest provoked by adrenergic stimulation or confirmed by genetic testing.
PATIENT CHARACTERISTICS.
Data were collected on patient demographics, dates of presentation and clinical symptoms, medication therapy and adherence, presence and date of ICD implantation, length of follow-up, family history, genetic testing, and documented arrhythmias on continuous monitoring or exercise stress testing (EST). Continuous monitoring was defined as an ICD, implantable loop recorder (ILR), or Holter monitor.
For the purposes of this study, arrhythmia events were defined as ventricular tachycardia or ventricular fibrillation of 3 or more beats on Holter, ILR, or EST. For ventricular arrhythmia events recorded by ICD devices, number of beats to trigger a stored event varied by individual patient device settings but was typically 5 to 8 beats. Efforts were made to distinguish ventricular from atrial arrhythmias. Surface and intracardiac electrograms were compared to sinus rhythm. Among patients with implanted ICD and dual-chamber systems, atrial tachycardias were excluded. For patients with single-chamber devices, events were not included if the rate of the event could be consistent with sinus tachycardia. Ventricular arrhythmias were further characterized as lasting 3 beats to 10 beats, >10 beats but <30 s, or sustained ≥30 s. Events with documented cardiac arrest requiring resuscitation in which no rhythm documentation was available were included in this study. If the timing of the cardiac arrest was available, the hour of day in which the arrest occurred was recorded. Syncopal events were excluded unless documented arrhythmia was available at the time of the event. Arrhythmia events described in the clinical record or recorded by device without a documented time of the event were excluded from this study.
To evaluate the circadian distribution of arrhythmia events, all events recorded by continuous 24-h Holter monitor, ILR, or ICD were identified. Initial analysis was performed including all events; however, to eliminate the potential bias of Holter monitors that are placed intermittently, analysis was also performed by excluding Holter events and including only continuous implantable rhythm devices (ICD, ILR). Arrhythmia events were recorded throughout the 24-h period, including during hours consistent with sleep. However, for the purposes of this study, comparison of time points was performed by comparing events that were likely to occur outside of sleeping hours. The 3 time points consisted of: morning defined as 6:00 am to 11:59 am, afternoon as 12:00 pm to 5:59 pm, and evening as 6:00 pm to 11:59 pm. Sleep hours were therefore defined as 12:00 am to 5:59 am. Weekends were defined as Saturday or Sunday.
To determine the effect of age, patients were grouped into ages as follows: <10 years, 10 years to 15 years, and >15 years. Season was defined as winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). To evaluate EST, the time of day in which the stress test was performed was recorded. ESTs without timing data were excluded. Testing was separated into EST performed during clinical hours of morning (8:00 am to 11:59 am) or afternoon (12:00 pm to 5:00 pm).
STATISTICAL ANALYSIS.
Continuous variables are presented as median with interquartile ranges (IQRs) and categorical variables are expressed as counts with percentages. To account for repeated measures, the generalized estimating equation (GEE) with Poisson distribution and log link function was applied to compare the frequencies of arrhythmia events occurring in the morning, afternoon, or evening, during weekday or weekend, and among different seasons. Age, gender, and multiple centers were adjusted in multivariate GEEs and comparisons were also analyzed by subgroups of device type, age group, and gender. To compare the frequencies of positive rates of ESTs between morning and afternoon and rates of ESTs with and without antiarrhythmic medications, the GEE with binomial distribution and logit link function was used. In addition, an interaction term of EST timing and antiarrhythmic use was included in the GEE to further determine if ESTs were affected by antiarrhythmic use between morning and afternoon hours. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all statistical analyses.
RESULTS
PATIENT CHARACTERISTICS.
A total of 80 patients (42 male, 53%) were included in this study. The median age at diagnosis of CPVT was 11 years (IQR: 7 to 14 years) with a median follow-up of 6 years (IQR: 2 to 10 years). Among the 80 patients, 29 (36%) had a history of cardiac arrest and there were 3 deaths. A majority of the patients, 49 of 80 (61%), had undergone placement of an ICD at a median age of 13.5 years (IQR: 9 to 17 years). A total of 15 (19%) patients had undergone left cardiac sympathetic denervation, 14 of whom had an ICD in place at the time of the procedure. Five patients had ILRs. Demographic data are listed in Table 1.
TABLE 1.
Patient Characteristics (N = 80)
| Male, % | 42 (53%) |
| Age at first symptom, yrs | 11.0 (6.0-13.0) |
| Age at diagnosis, yrs | 11.0 (7.4-14.0) |
| Follow-up, yrs | 6.4 (2.2-10.0) |
| Proband | 66 (83) |
| Family history of CPVT | 19 (24) |
| History of cardiac arrest | 29 (36) |
| Positive genetic testing | 49 (80) |
| RyR2 | 45 (92) |
| CASQ2 | 2 (4) |
| KCNJ2 | 2 (4) |
| Antiarrhythmic | |
| β anti-adrenergic blocker | 78 (98) |
| Flecainide | 15 (19) |
| Calcium channel blocker | 12 (15) |
| ICD (n = 49) | |
| Age at implantation, yrs | 13.5 (9.3-16.7) |
| ICD follow up | 9.0 (6.0-12.1) |
| Dual chamber | 26 (53) |
| Left cardiac sympathetic denervation | 15 (19) |
| Deaths | 3 (4) |
Values are n (%) or median (interquartile range).
CPVT = catechotaminergic polymorphic ventricular tachycardia; ICD = implantable cardioverter defibrillator.
The majority of patients in this cohort were probands (66 of 80 patients [83%]). Genetic testing was performed in 61 of 80 patients (76%), and a probable candidate gene mutation was identified in 49 patients. Genetic variants were therefore identified in 80% of patients tested (49 of 61 patients). The majority of mutations (92%) were identified in the cardiac ryanodine receptor RyR2 gene, 2 (4%) mutations in CASQ2, and 2 (4%) had mutations in KCNJ2. Of the 45 patients with RyR2 mutations, 29 (64%) were novel.
ARRHYTHMIA EVENTS.
There were a total of 190 Holter monitors used among the 80 patients, accounting for approximately 4,500 h of monitoring. A total of 172 ESTs were conducted in 50 patients. There were 5.4 million hours of ICD recordings and 300,000 hours of ILR data.
Among the 80 patients, there were a total of 423 ventricular arrhythmia events including 299 recorded by ICD or ILR, 72 by EST, 45 by Holter, and 7 during cardiac arrest. Figure 1 shows the distribution of arrhythmia events recorded by all continuous monitoring devices (Holter, ILR, ICD). Arrhythmia events were uncommon during hours consistent with sleep. When accounting for arrhythmia events during the waking hours of 6:00 am to 11:59 pm, and broken down by morning, afternoon, and evening time blocks, arrhythmia events were significantly more likely to occur in the afternoon (odds ratio [OR]: 2.54; 95% confidence interval [CI]: 1.69 to 3.83) and evening (OR: 2.91; 95% CI: 1.82 to 4.67) when compared to morning hours (Figure 2). To remove the possible confounding effects of intermittent recording by Holter monitoring, analysis of distribution of arrhythmia events by implanted device (ICD, ILR) was performed (excluding Holter events). Similarly, arrhythmia events from implanted continuous recording devices were significantly more likely to occur in the afternoon (OR: 2.73; 95% CI: 1.66 to 4.49) and evening (OR: 3.55; 95% CI: 1.99 to 6.33) hours when compared to morning hours (Table 2, Figure 2).
FIGURE 2. Distribution of Events During Waking Hours.

Arrhythmia events are grouped by time (morning, afternoon, and evening) and separated by type of recording device (Holter monitor vs. ICD/ILR). Arrhythmia events are significantly more likely to occur in the afternoon and evening when compared to morning hours.
ICD = implantable cardioverter defibrillator; ILR = implantable loop recorder; OR = odds ratio.
TABLE 2.
Comparison of Ventricular Arrhythmias by Time of Day
| Time of Day | n (%) | OR (95% CI)* | |
|---|---|---|---|
| Total events* (ICD/ILR/Holter) | 6 am-11:59 am | 48 (14.8) | Ref. |
| 12 pm-5:59 pm | 128 (39.4) | 2.54 (1.69-3.83) | |
| 6 pm-11:59 pm | 149 (45.9) | 2.91 (1.82-4.67) | |
| ICD/ILR* | 6 am-11:59 am | 38 (13.4) | Ref. |
| 12 pm-5:59 pm | 106 (37.5) | 2.73 (1.66-4.49) | |
| 6 pm-11:59 pm | 139 (49.1) | 3.55 (1.99-6.33) | |
| Male† | 6 am-11:59 am | 21 (14.0) | Ref. |
| 12 pm-5:59 pm | 62 (41.3) | 2.49 (1.23-5.03) | |
| 6 pm-11:59 pm | 67 (44.7) | 2.72 (1.32-5.61) | |
| Female† | 6 am-11:59 am | 27 (15.4) | Ref. |
| 12 pm-5:59 pm | 66 (37.7) | 2.43 (1.57-3.77) | |
| 6 pm-11:59 pm | 82 (46.9) | 3.01 (1.69-5.34) | |
| Age <10 yrs‡ | 6 am-11:59 am | 16 (18.6) | Ref. |
| 12 pm-5:59 pm | 45 (52.3) | 2.81 (1.89-4.19) | |
| 6 pm-11:59 pm | 25 (29.1) | 1.56 (0.93-2.62) | |
| Age 10-15 yrs‡ | 6 am-11:59 am | 8 (10.0) | Ref. |
| 12 pm-5:59 pm | 39 (48.8) | 4.87 (1.96-12.14) | |
| 6 pm-11:59 pm | 33 (41.3) | 4.12 (1.59-10.67) | |
| Age >15 yrs‡ | 6 am-11:59 am | 24 (15.1) | Ref. |
| 12 pm-5:59 pm | 44 (27.7) | 1.83 (1.00-3.37) | |
| 6 pm-11:59 pm | 91 (57.2) | 3.79 (2.05-7.01) | |
| Weekday (ICD/ILR)* | 6 am-11:59 am | 27 (14.0) | Ref. |
| 12 pm-5:59 pm | 70 (36.3) | 2.56 (1.54-4.23) | |
| 6 pm-11:59 pm | 96 (49.7) | 3.47 (1.89-6.36) | |
| Weekend/holiday (ICD/ILR)* | 6 am-11:59 am | 11 (12.2) | Ref. |
| 12 pm-5:59 pm | 36 (40.0) | 3.30 (1.52-7.17) | |
| 6 pm-11:59 pm | 43 (47.8) | 3.96 (1.81-8.69) | |
Total events include ICD, ILR, Holter.
Adjusted by age, sex, and multicenters.
Adjusted by age and multicenters.
Adjusted by sex and multicenters.
CI = confidence interval; ILR = implantable loop recorder; OR = odds ratio; Ref. = reference; other abbreviation as in Table 1.
Because children’s activity levels may vary on a school day versus a weekend, we evaluated whether day of week influenced timing of arrhythmia events. Events were separated by those occurring on weekdays versus those on weekends or holidays. Taking into account only implanted recording devices, the pattern of arrhythmia events remained similar regardless of the day of week. Patients were more likely to have events in the afternoon and evening hours whether it was a weekday or weekend/holiday (Table 2) and there was no difference in this pattern by day of the week (Table 3, Figure 3).
TABLE 3.
Comparison of Ventricular Arrhythmias Weekday Versus Weekend/Holiday
| Odds Ratio (95% CI) | |
|---|---|
| Weekend/holiday vs. weekday | 1.06 (0.90-1.25) |
FIGURE 3. Distribution by Day: Weekday Versus Weekend/Holiday.

Arrhythmia events are grouped by weekdays and weekend/holiday. The pattern of arrhythmia events are not different based on day of week.
The influence of sex and age were also evaluated. There was no difference in patterns of arrhythmia events based on sex (Table 2, Figure 4). When taking into account age, arrhythmias were more common in the afternoon or evening when compared to morning in all age groups. However, with age, there are an increasing number of events during evening hours (Table 2, Figure 5).
FIGURE 4. Distribution of Events by Gender.

Arrhythmia events grouped by gender show no difference in the pattern of event timing based on sex.
FIGURE 5. Distribution by Age.
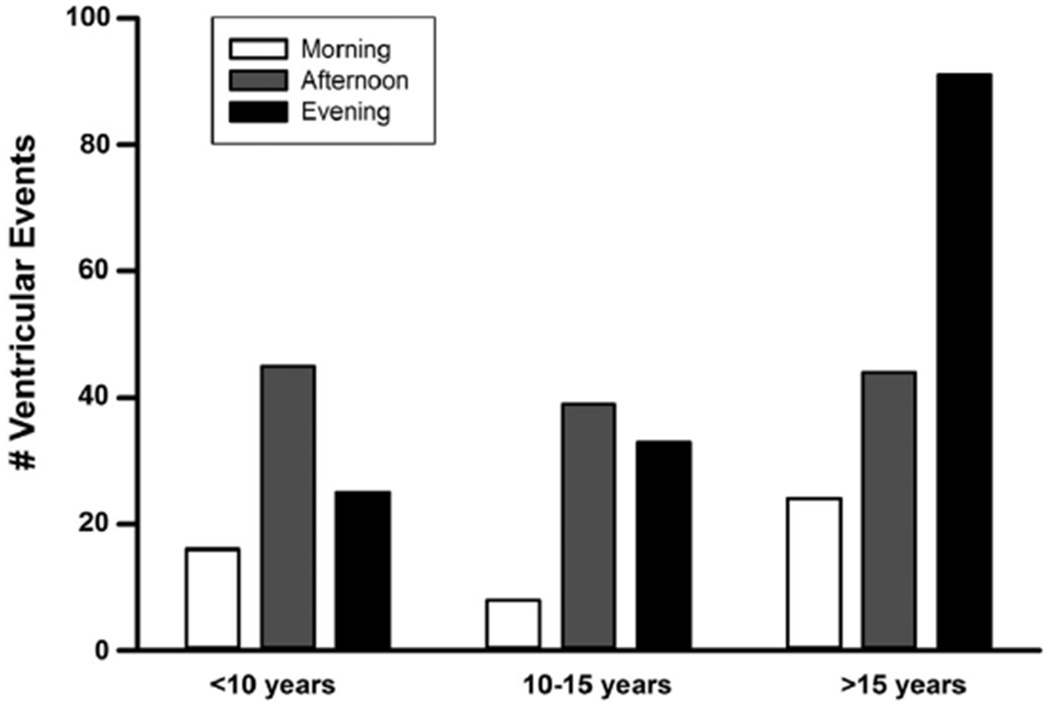
Arrhythmia events are grouped by age. Overall the least number of events occurs in the morning hours in all age groups. With increasing age, there is an increase in evening events, particularly among those >15 years of age.
To determine if there is an effect of season upon arrhythmia event timing, we evaluated the number of implanted device events that occurred during each season. Arrhythmia events were more common in the winter months when compared to other seasons of the year, although this difference was not statistically significant (Table 4, Figure 6).
TABLE 4.
Comparison of Ventricular Arrhythmias by Season
| Seasons | n (%) | p Value* | |
|---|---|---|---|
| Total | Summer | 62 (20.7) | Ref. |
| Autumn | 70 (23.4) | 0.551 | |
| Winter | 97 (32.4) | 0.243 | |
| Spring | 70 (23.4) | 0.589 | |
| Male | Summer | 33 (23.4) | Ref. |
| Autumn | 37 (26.1) | 0.624 | |
| Winter | 33 (23.2) | 0.924 | |
| Spring | 39 (27.5) | 0.456 | |
| Female | Summer | 29 (18.5) | Ref. |
| Autumn | 33 (21.0) | 0.704 | |
| Winter | 64 (40.8) | 0.139 | |
| Spring | 31 (19.8) | 0.869 | |
Adjusted by age sex, and multi-centers.
FIGURE 6. Distribution of Events by Season.

The Lowest number of events was recorded during the summer months and highest in the winter months, although this difference was not statistically significant. Abbreviation as in Figure 4.
Adherence to prescribed antiarrhythmic therapy at the time of arrhythmia events was also evaluated. Although 84% of arrhythmia events for which the medication regimen was known occurred on prescribed antiarrhythmic therapy, nonadherence was associated with 57% of arrhythmia events when antiarrhythmic adherence was reported. Among the 186 events where compliance data is known, 77 of 186 (41%) events occurred while the patient was thought to be compliant and 109 of 186 (59%) of events occurred while the patient was thought to be noncompliant. When accounting for continuously monitored events for which compliance is known, there was no difference in the pattern and timing of events (Figure 7).
FIGURE 7. Events by Arrhythmia Compliance.

Regardless of whether the patient was compliant with prescribed medications, arrhythmia events were more likely to occur in the afternoon and evening hours.
HOLTER MONITORS.
A total of 190 Holter monitors were performed among 49 patients with 25% of all Holter monitors performed when the patient was not on an antiarrhythmic medication. Of these 49 patients, 45 (92%) showed at least 1 ventricular arrhythmic event. Ventricular arrhythmias were observed on a total of 24% of all Holter monitors; 29% of arrhythmias occurred in the morning and 71% occurred in the afternoon.
EXERCISE STRESS TEST.
A total of 172 ESTs were performed among 50 patients. The average number of tests per patient was 3 (range 1 to 10 tests). Tests were performed in the morning in 47% and afternoon in 53%. Among all 172 ESTs, 20% were performed with the patient on no antiarrhythmic medication.
Taking into account age, sex, multiple centers, timing of test, and whether patient was on an antiarrhythmic, there was a significantly higher likelihood of tests being positive in the afternoon versus morning (OR: 3.00; 95% CI: 1.39 to 6.48) (Table 5). Further analysis by an interaction term of testing time and whether patient was taking an antiarrhythmic suggests that antiarrhythmics reduced ventricular arrhythmias in both the morning and afternoon, with ORs of 3.90 (95% CI: 0.61 to 25.1) and 21.4 (95% CI: 5.98 to 76.4) for morning and afternoon tests performed without antiarrhythmics, respectively, when compared to morning tests performed on antiarrhythmics among all 50 patients (Table 6).
TABLE 5.
Comparison of Ventricular Arrhythmias During Exercise Stress Test
| OR (95% CI) | |
|---|---|
| Testing time: afternoon vs. morning | 3.00 (1.39-6.48) |
| Antiarrhythmics, yes vs. no | 0.16 (0.05-0.49) |
Adjusted by age, sex, and multicenters. Antiarrhythmics: whether patient was prescribed an antiarrhythmic at the time of the test.
Abbreviations as in Table 2.
TABLE 6.
Comparison of Ventricular Arrhythmias During EST Interaction between Testing Time and Antiarrhythmic Use
| OR (95% CI) | |
|---|---|
| Morning with antiarrhythmics | Ref. |
| Morning without antiarrhythmics | 3.90 (0.61-25.1) |
| Afternoon with antiarrhythmics | 2.61 (1.06-6.42) |
| Afternoon without antiarrhythmics | 21.37 (5.98-76.4) |
Adjusted by age, sex, and multicenters. Variables indicate time of EST and whether patient was prescribed an antiarrhythymic at the time of the test.
EST = exercise stress test; other abbreviations as in Table 2.
Although likely underpowered, the data suggests nearly one-half of the patients (21 of 50) performed ESTs in both the morning and afternoon periods. When evaluating only those patients who underwent EST in both periods, the afternoon predominance of positive exercise tests persisted with afternoon tests being positive 55% of the time versus 32% in the morning (Figure 8).
FIGURE 8. Patients With Both Morning and Afternoon Exercise Stress Tests.

This graph shows that, among 21 patients who underwent exercise stress tests (ESTs) in the morning and afternoon, tests were significantly more likely to be positive in the afternoon.
DEATHS AND ABORTED CARDIAC ARRESTS.
There were 7 cardiac arrest events among our cohort for which the exact timing of each event was known. Four of 7 were resuscitated and 3 resulted in death or eventual withdrawal of support. There were insufficient numbers to perform comparative analyses based on timing of arrest or death; however, 6 of 7 cases occurred in the afternoon hours.
DISCUSSION
Many heritable and acquired arrhythmia syndromes show a circadian periodicity in frequency of cardiac arrhythmic events. In a disease such as CPVT, in which arrhythmias are triggered by adrenergic states (particularly exertion), the notion that circadian patterns may be contributing to arrhythmia induction is less intuitive. In a recent study by Goodman et al. (21), the activity level of 23,188 children in 9 countries was followed using Actigraph monitoring devices (Actigraph, Pensacola, Florida). This study found a bimodal distribution in peak activity levels in children with nearly equivalent peaks in the morning (7 am to 8 am) and afternoon (3 pm to 4 pm). If arrhythmia events occurred based on children’s activity levels, in our study we should have seen a bimodal distribution of arrhythmia events peaking during both the early morning and afternoon hours. An increased arrhythmia incidence might also be expected during the naturally occurring physiologic adrenaline surge in the early morning hours. This adrenergic surge is thought to contribute to arrhythmia events in adult post-MI patients (10). This study found that arrhythmia events among CPVT children are less likely to occur during morning hours and significantly more likely to occur in the afternoon and evening.
Thus, ventricular arrhythmia events among children with CPVT may not merely reflect children’s peak activity levels and do not appear to be influenced by physiologic early morning adrenergic surges. These results suggest that the substrate or possibly the trigger underlying arrhythmogenesis may be influenced by factors other than catecholamine surge. This has important implications not only for management strategies, particularly in regard to timing of medication, but also for diagnostic testing. In CPVT, the potential morbidity and indeed mortality of a missed diagnosis is significant.
We hypothesized a higher incidence of events on weekends and holidays and during summer months when children were expected to be more active. The pattern of arrhythmia events was similar regardless of the day of week including holidays. Interestingly, arrhythmia events showed a trend towards being lowest in the summer and highest in the winter, although this did not reach statistical significance. Reasons for these findings are not clear.
This pattern of afternoon arrhythmias is in contrast to descriptions of nocturnal ventricular arrhythmias in other heritable channelopathies, such as LQTS3 (4) and Brugada syndrome (5), as well as predominantly morning arrhythmias in LQTS2, post-MI patients, and adults with ICDs, and among all sudden cardiac death (10–15). These results could thus represent a circadian influence that is unique to the CPVT population.
There are proposed mechanisms for circadian influence of arrhythmogenesis. The expression of approximately 10% to 15% of genes in mouse cardiomyocytes exhibit diurnal variation, including several circadian clock transcription factors, and this expression pattern can change in response to several metabolic and disease states (22). If, for example, clock-regulated transcription factors for beta-adrenergic receptors or RyR2 kinases or phosphatases showed diurnal variation, then it could be hypothesized that presence of catecholamines and RyR2 leak may be affected by time of day. Hence, adrenaline surges at certain times may require higher thresholds for arrhythmia induction. The idea that threshold for arrhythmias may vary by time of day is interesting when considering our findings during EST. Among patients not on antiarrhythmics, we found that the likelihood of a positive EST was significantly higher in the afternoon. Although our patient cohort was small, the findings are intriguing and raise questions about timing of an EST for diagnostic testing. Our findings warrant replication and further investigation. We included all ventricular arrhythmia events (≥3 beats or based on minimum detection by implantable devices) and hence our findings are not reflective of life-threatening events and thus do not indicate a higher incidence of sudden death in the afternoon or evening hours. Ultimately, understanding whether CPVT patients are at higher risk during certain times of the day is important. Among 7 patients in whom the exact time of cardiac arrest or death was available, 6 occurred in the afternoon. Therefore, although timing of nonsustained ventricular arrhythmias captured in this study may not correlate with sudden death, further studies investigating timing of arrhythmic events including cardiac arrest and sudden death are warranted.
STUDY LIMITATIONS.
This is a retrospective study. We only included events for which the timing was documented, and thus had to exclude events with unknown timing that would ideally be included in our analysis. Similarly, data on medication adherence was only available for a portion of the events, and treating physicians may have different sensitivity to the degree of nonadherence associated with each arrhythmia event. This study included all ventricular arrhythmias, even those that are non-sustained; hence our data may not be reflective of risk of life-threatening events. Patient activity level was not recorded at the time of the arrhythmia events, hence we cannot associate arrhythmia occurrence with activity level. Genetic testing was not performed on all patients diagnosed with clinical CPVT, and although 80% of our patients who underwent genetic testing returned with positive results, only 61% of our total patient cohort had genetic confirmation of CPVT.
CONCLUSIONS
Patients with CPVT are more likely to have arrhythmia events in the afternoon and evening hours. It remains unclear whether arrhythmogenesis in CPVT is regulated by circadian control; however, the data in this study might suggest that catecholamine exposure alone may not fully explain susceptibility to arrhythmias among patients with CPVT. Regardless of mechanism, patients with CPVT appear to have more ventricular arrhythmias in the afternoon and evening hours. These findings may have implications for management strategies, including potential timing of peak antiarrhythmic drugs levels. Further investigation is warranted to determine the relationship between activity patterns and arrhythmia occurrence in CPVT and to further evaluate the optimal timing of ESTs.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
The findings from this study raise awareness about timing of potential life-threatening events among children with CPVT. Regardless of mechanism (i.e., innate vs. extrinsic factors), patients with CPVT appear to have more ventricular arrhythmias in the afternoon and evening hours in comparison to the morning hours. This may suggest that factors other than levels of circulating catecholamines may be responsible for arrhythmia events. Thus, it may be reasonable to focus management strategies in the future to address circadian variation in the patient population. For example, physicians caring for pediatric CPVT patients often try to improve patient compliance and limit side effects of beta anti-adrenergic medications by prescribing once daily drugs administered before bedtime. This may result in trough drug levels the following afternoon and evening at the time when children may be most susceptible to arrhythmic events. As such, physicians may want to consider timing of peak concentrations of antiarrhythmic medications. This study was not powered to assess whether patient activity level directly affected arrhythmia occurrence and hence we cannot comment whether patient activity is safer at specific times of the day. This study warrants replication and further investigation into the clinical and molecular reasons behind arrhythmia variation seen in CPVT and other inheritable arrhythmia syndromes.
TRANSLATIONAL OUTLOOK:
The findings of these studies warrant further investigation in both clinical and basic research. Determining if arrhythmia occurrences are regulated or influenced by circadian or clock genes will provide insight into disease pathology and alternative targets for treatment. CPVT is the most lethal of all heritable arrhythmia syndromes. At this time, although beta anti-adrenergic medications are first-line therapy, neither medications, nor ICDs have been shown to be 100% effective. Thus, further understanding of how to effectively prevent arrhythmias and death among CPVT patients is critical.
Acknowledgments
Supported by PACES, Pediatric and Adult Congenital Electrophysiology Society Paul C. Gillette grant funding, NIH-NHLBI grants (R01-HL089598, R01-HL091947, R01-HL117641, R41-HL129570), NIH-LRP grant funding, the American Heart Association (13EIA14560061), and the Juanita P. Quigley endowed chair in cardiology. Dr. Kim has received fellowship support from Medtronic Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Miyake and Asaki contributed equally to this this paper and are co-first authors.
ABBREVIATIONS AND ACRONYMS
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- EST
exercise stress test
- ICD
implantable cardioverter-defibrillator
- ILR
implantable loop recorder
REFERENCES
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation 1995; 91:1512–9. [DOI] [PubMed] [Google Scholar]
- 2.Swan H, Piippo K, Viitasalo M, et al. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol 1999; 34:2035–42. [DOI] [PubMed] [Google Scholar]
- 3.Sy RW, Gollob MH, Klein GJ, et al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2011;8:864–71. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long QT-syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 5.Baron RC, Thacker SB, Gorelkin L, Vernon AA, Taylor WR, Choi K. Sudden death among Southeast Asian refugees: an unexplained nocturnal phenomenon. JAMA 1983;250: 2947–51. [PubMed] [Google Scholar]
- 6.Matsuo K, Kurita T, Inagaki M, et al. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J 1999;20:465–70. [DOI] [PubMed] [Google Scholar]
- 7.Kim S-H, Nam G-B, Baek S, et al. Circadian and seasonal variations of ventricular tachyarrhythmias in patients with early repolarization syndrome and Brugada syndrome: analysis of patients with implantable cardioverter defibrillator. J Cardiovasc Electrophysiol 2012;23:757–63. [DOI] [PubMed] [Google Scholar]
- 8.Kiernan TJ, Weivoda PL, Somers VK, Ommen SR, Gersh BJ. Circadian rhythm of appropriate implantable cardioverter defibrillator discharges in patients with hypertrophic cardiomyopathy. PACE 2008;31:1253–8. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Semsarian C, Shen W-K, et al. Circadian patterns in the occurrence of malignant ventricular tachyarrhythmias triggering defibrillator interventions in patients with hypertrophic cardiomyopathy. Heart Rhythm 2009;6:599–602. [DOI] [PubMed] [Google Scholar]
- 10.Willich SN, Maclure M, Mittleman M, Arntz H-R, Muller JE. Sudden cardiac death: support for a role of triggering in causation. Circulation 1993;87:1442–50. [DOI] [PubMed] [Google Scholar]
- 11.Tofler GH, Gebara OCE, Mittleman MA, et al. Morning peak in ventricular tachyarrhythmias detected by time of implantable cardioverter/defibrillator therapy. Circulation 1995;92:1203–8. [DOI] [PubMed] [Google Scholar]
- 12.Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75:131–8. [DOI] [PubMed] [Google Scholar]
- 13.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol 1987;60:801–6. [DOI] [PubMed] [Google Scholar]
- 14.Willich SN, Goldberg RJ, Maclure M, Perriello, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol 1992;70:65–8. [DOI] [PubMed] [Google Scholar]
- 15.Takigawa M, Kawamura M, Noda T, et al. Seasonal and circadian distributions of cardiac events in genotyped patients with congenital long QT syndrome. Circ J 2012;76:2112–8. [DOI] [PubMed] [Google Scholar]
- 16.Bexton RS, Vallin HO, Camm AJ. Diurnal variation of the QT interval – influence of the autonomic nervous system. Br Heart J 1986;55:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong TQ, Goldberger JJ, Parker M, Want T, Kadish AH. Circadian variation in human ventricular refractoriness. Circulation 1995;92: 1507–16. [DOI] [PubMed] [Google Scholar]
- 18.Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res 2009;105:1047–61. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, Sekiguchi A, Iwasaki YK, et al. Circadian variation in cardiac K+ channel gene expression. Circulation 2003;107:1917–22. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaraj D, Haldar SM, Wan X, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012;483:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman A, Page AS, Cooper AR. Daylight saving time as a potential public health intervention: an observational study of evening day light and objectively-measured physical activity among 23, 000 children from 9 countries. Int J Behav Nutr Phys Act 2014;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res 2010;106:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


