Abstract
Mosquito-transmitted Plasmodium falciparum infection can cause human cerebral malaria (HCM) with high mortality rates. The abundance of infected red blood cells that accumulate in the cerebral vasculature of patients has led to the belief that these brain-sequestered cells solely cause pathogenesis. However, animal models suggest that CD8+ T cells migrate to and accumulate in the brain, directly contributing to experimental cerebral malaria (ECM) mortality. In this issue of the JCI, Riggle et al. explored the brain vasculature from 34 children who died from HCM or other causes and frequently found CD3+ CD8+ T cells in contact with endothelial cells. Further, the authors show that coinfection with HIV enhanced such CD3+ CD8+ T cell luminal distribution. These findings suggest that the mouse model for cerebral malaria may accurately reflect human disease pathology. This study sheds new light on the mechanisms behind blood-brain barrier breakdown in this complicated neurological disease and opens up alternative approaches for treatment.
Sequestration, sequestration, sequestration
Human cerebral malaria (HCM), the most severe pathology induced by Plasmodium falciparum infection, encompasses a wide range of neurological complications, including seizures and impaired consciousness (1). Due to obvious ethical and logistical constraints, studies to understand HCM pathogenesis have largely relied on a limited number of postmortem series.
The intravascular accumulation of P. falciparum–infected red blood cells has been repeatedly reported over the years, leading to the dogma that parasite sequestration in the brain is the leading cause of HCM (2). Although necessary, sequestration does not explain the progression to pathology on its own. Several parallel contributing mechanisms have been proposed, including exaggerated immune response to P. falciparum infection and dysregulated coagulation, both impacting the integrity of the blood-brain barrier (3). Surprisingly, only a few histopathological studies reported leukocytes accumulated in the cerebrovasculature (4, 5), and thus their role in HCM pathogenesis was mostly dismissed or ignored. This starkly contrasted with experimental evidence obtained by researchers using an animal model called experimental cerebral malaria (ECM), where susceptible mouse strains such as C57BL/6 or CBA/J infected with P. berghei ANKA develop brain pathology and die with overt neurological signs between 6 and 14 days after infection (6). This model allows the investigation of parasite dynamics and distribution in vivo in the brain of infected animals, as well as parasitized erythrocyte interactions with brain-residing and immune host cells (7).
An enduring rift within the malaria research community
In 2002, a landmark study showed that CD8+ αβ T cells migrate to and sequester in the brains of mice when neurological symptoms appear, and are directly responsible for ECM mortality (8). While these observations initially suggested a potentially unifying mechanism between murine and human pathology, the use of the ECM model was deemed questionable in 2009 (9), causing an enduring rift within the malaria research community (10–12).
In this issue of the JCI, Riggle et al. bring closure to the long-standing debate of the role of CD8+ T cells in HCM (13). Using multiplexed histology with new and validated anti-CD8 antibodies, the authors elegantly and unequivocally demonstrate the frequent presence of CD3+ CD8+ T cells in the brain of a postmortem series of 31 fatal pediatric HCM cases from Malawi. A higher number of CD8+ T cells were detected in patients coinfected with HIV. A more detailed analysis showed that a substantial number of these cells were in contact with vessel walls, both in close association with the endothelium in the lumen of the venous vasculature as well as in the perivascular spaces on the abluminal side of vessels, a feature previously described in ECM (14). The observation that the apoptosis-mediating molecule, granzyme B, was distributed inside and sometimes outside the CD8+ T cells in contact with endothelial cells, suggested, for the first time, target cell recognition in HCM (13). Granzyme B is released when CD8+ T cells engage with MHC molecules that are loaded with their cognate antigens. Further, there is compelling in vivo and in vitro evidence that the endothelium is capable of acquiring and cross-presenting P. falciparum and P. berghei antigens during infection, an essential step for antigen-specific T cell receptor (TCR) ligation and cytolysis of brain microvascular endothelial cells (15). This has long been proposed as the main mechanism behind blood-brain barrier breakdown in ECM, where perforin and granzyme B are required for CD8+ T cells to disrupt the blood-brain barrier and trigger the neurological syndrome (15).
A link with vasogenic edema?
The findings presented by Riggle et al. indicate that a similar mechanism may be at play in HCM, thereby contributing to blood-brain barrier disruption and the resulting vasogenic edema seen in both EMC (16) and HCM (Figure 1) (17). Coincidentally, an increase in cleaved caspase-3 was demonstrated in brain endothelial cells from a small series of fatal adult and pediatric HCM cases in Thailand, and the presence of leukocytes, albeit not phenotyped, was also reported inside the cerebrovascular lumen of patients who succumbed to the neurological syndrome (18). Previous in vitro studies showed that platelet accumulation and parasitized erythrocyte sequestration could induce human brain endothelial apoptosis (19, 20), a phenomenon that CD8+ T cells may exacerbate. The extraordinary similarities in the distribution of CD8+ T cells in the brains of children with HCM and in mice with ECM strongly suggest common pathophysiologic processes (5, 13). These new findings provide an additional piece of the puzzle by demonstrating that CD8+ T cells may indeed participate in the pathology of HCM, and further bridge human and murine disease to support murine ECM as a useful tool, without being more than what it is: a model (21). Additional studies are now warranted to assess the potential link between CD8+ T cells and microvascular brain endothelial apoptosis in HCM, evaluate its contribution to the disease, and inform new adjunct therapies targeting the induction and functions of CD8+ T cells in HCM.
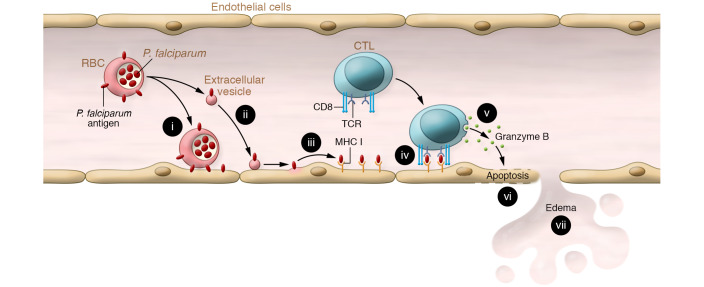
Figure 1. Proposed chain of events contributing to the blood-brain barrier breakdown in HCM.
P. falciparum–parasitized red blood cells can transfer parasite antigens to the surface of brain microvascular endothelial cells either by direct contact (22) (i), or through the production of antigen-carrying extracellular vesicles that can potentially bind the endothelial surface (ii). P. falciparum antigens are then picked up and presented by the endothelial cells through their class I MHC (23) (iii). Cytotoxic T lymphocytes (CTL) engage with antigen-presenting endothelial cells via their TCR and CD8 receptors (iv), leading to the release of granzyme B by CTL (13) (v), the apoptosis of targeted endothelial cells (18) (vi), and an alteration of the blood-brain barrier, ultimately resulting in vasogenic edema (17) (vii).
Acknowledgments
LR is supported by a core grant from the Agency of Science, Research, and Technology to the Singapore Immunology Network. GERG is supported by the Australian National Health and Medical Research Council (APP1099920) and the Australian Research Council (DP 180102741). SCW is supported by the National Institute of Allergy and Infectious Diseases of the NIH (U19AI089676 and R21AI142472) and the UK Medical Research Council (MR/S009450/1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Version 1. 02/17/2020
Electronic publication
Version 2. 03/02/2020
Print issue publication
Footnotes
Conflict of interest: LR possesses shares in Immunoscape Private Limited.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(3):1109–1111. https://doi.org/10.1172/JCI135510.
See the related article at CD8+ T cells target cerebrovasculature in children with cerebral malaria.
Contributor Information
Laurent Rénia, Email: renia_laurent@immunol.a-star.edu.sg.
Samuel C. Wassmer, Email: sam.wassmer@lshtm.ac.uk.
References
- 1.[No authors listed] Severe malaria. Trop Med Int Health. 2014;19 Suppl 1:7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 2.White NJ, Turner GD, Day NP, Dondorp AM. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208(2):192–198. doi: 10.1093/infdis/jit116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. doi: 10.1146/annurev-pathmechdis-012419-032640. Moxon CA, Gibbins MP, McGuinness D, Milner DA, Marti M. New insights into malaria pathogenesis [published online October 24, 2019]. Annu Rev Pathol . [DOI] [PubMed]
- 4.Wassmer SC, et al. Fatal cerebral malaria: distinct microvascular pathologies in children and adult patients. Int J Parasitol. 2008;38:S44. [Google Scholar]
- 5.Barrera V, et al. Comparison of CD8+ T cell accumulation in the brain during human and murine cerebral malaria. Front Immunol. 2019;10:1747. doi: 10.3389/fimmu.2019.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engwerda C, Belnoue E, Grüner AC, Rénia L. Experimental models of cerebral malaria. Curr Top Microbiol Immunol. 2005;297:103–143. [PubMed] [Google Scholar]
- 7.Claser C, et al. CD8+ T cells and IFN-γ mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS ONE. 2011;6(4):e18720. doi: 10.1371/journal.pone.0018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belnoue E, et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169(11):6369–6375. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 9.White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26(1):11–15. doi: 10.1016/j.pt.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137(5):755–772. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 11.Hunt NH, et al. Murine cerebral malaria: the whole story. Trends Parasitol. 2010;26(6):272–274. doi: 10.1016/j.pt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Rénia L, Grüner AC, Snounou G. Cerebral malaria: in praise of epistemes. Trends Parasitol. 2010;26(6):275–277. doi: 10.1016/j.pt.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Riggle BA, et al. CD8+ T cells target cerebrovasculature in children with cerebral malaria. J Clin Invest. 2020;130(3):1128–1138. doi: 10.1172/JCI133474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strangward P, et al. Targeting the IL33-NLRP3 axis improves therapy for experimental cerebral malaria. Proc Natl Acad Sci U S A. 2018;115(28):7404–7409. doi: 10.1073/pnas.1801737115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howland SW, Claser C, Poh CM, Gun SY, Rénia L. Pathogenic CD8+ T cells in experimental cerebral malaria. Semin Immunopathol. 2015;37(3):221–231. doi: 10.1007/s00281-015-0476-6. [DOI] [PubMed] [Google Scholar]
- 16.Penet MF, et al. Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci. 2005;25(32):7352–7358. doi: 10.1523/JNEUROSCI.1002-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohanty S, et al. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere. 2017;2(3):e00193-17. doi: 10.1128/mSphere.00193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punsawad C, Maneerat Y, Chaisri U, Nantavisai K, Viriyavejakul P. Nuclear factor kappa B modulates apoptosis in the brain endothelial cells and intravascular leukocytes of fatal cerebral malaria. Malar J. 2013;12:260. doi: 10.1186/1475-2875-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006;74(1):645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wassmer SC, de Souza JB, Frère C, Candal FJ, Juhan-Vague I, Grau GE. TGF-beta1 released from activated platelets can induce TNF-stimulated human brain endothelium apoptosis: a new mechanism for microvascular lesion during cerebral malaria. J Immunol. 2006;176(2):1180–1184. doi: 10.4049/jimmunol.176.2.1180. [DOI] [PubMed] [Google Scholar]
- 21.Craig AG, et al. The role of animal models for research on severe malaria. PLoS Pathog. 2012;8(2):e1002401. doi: 10.1371/journal.ppat.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jambou R, Combes V, Jambou MJ, Weksler BB, Couraud PO, Grau GE. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PLoS Pathog. 2010;6(7):e1001021. doi: 10.1371/journal.ppat.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howland SW, et al. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med. 2013;5(7):984–999. doi: 10.1002/emmm.201202273. [DOI] [PMC free article] [PubMed] [Google Scholar]